Abstract
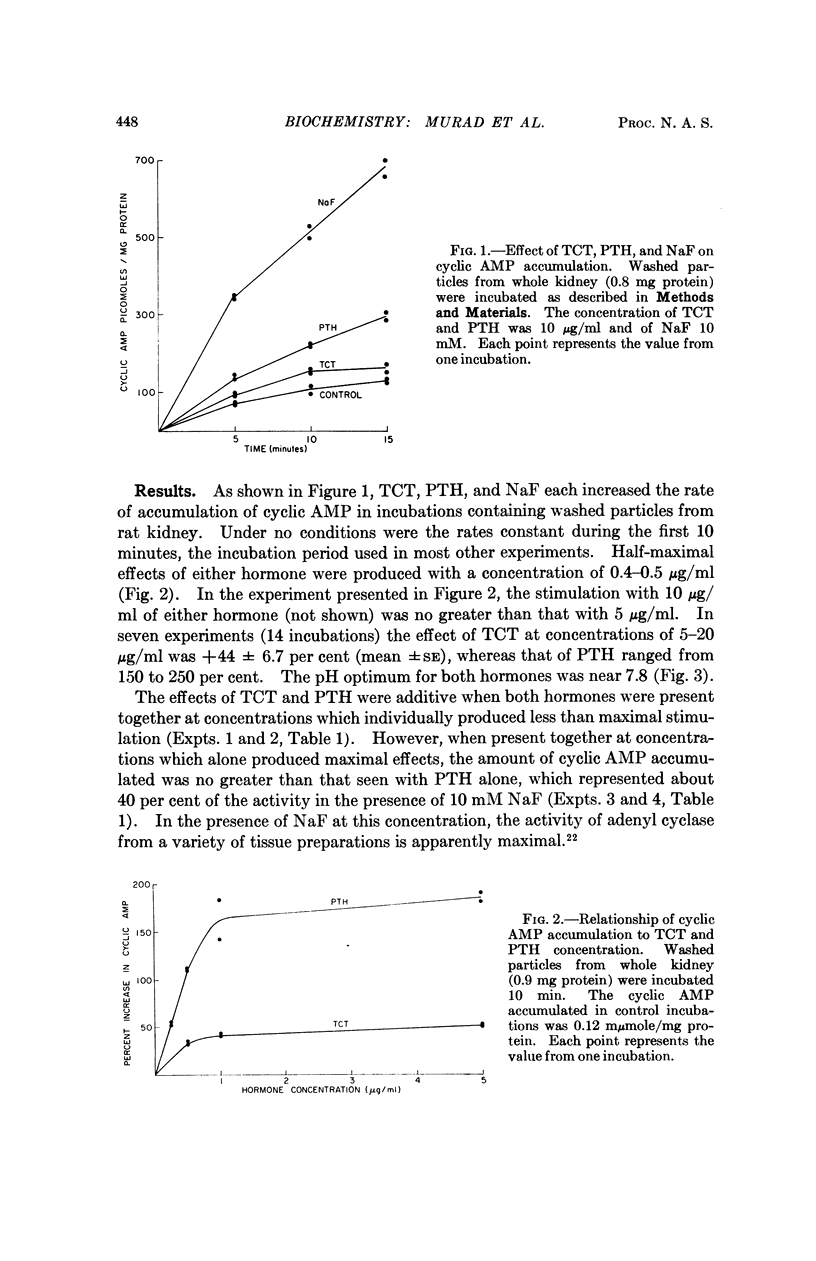
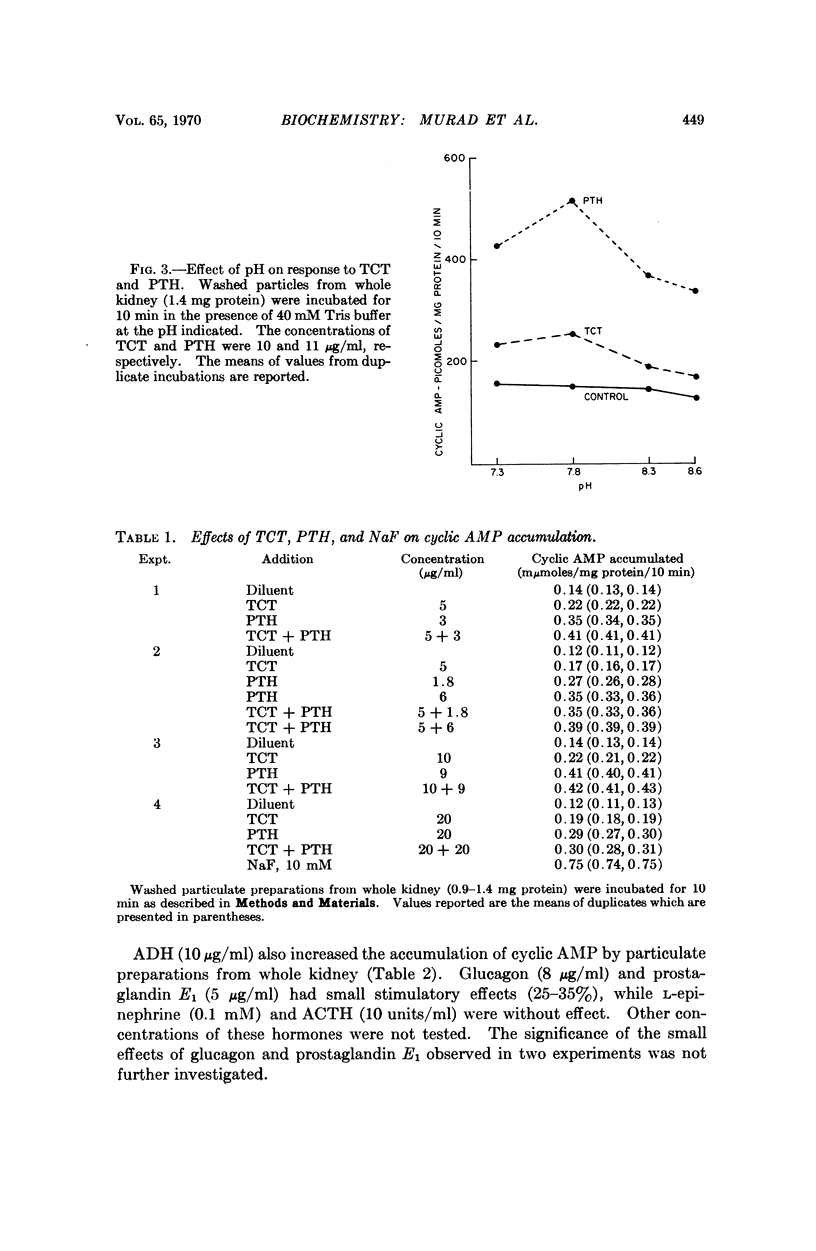
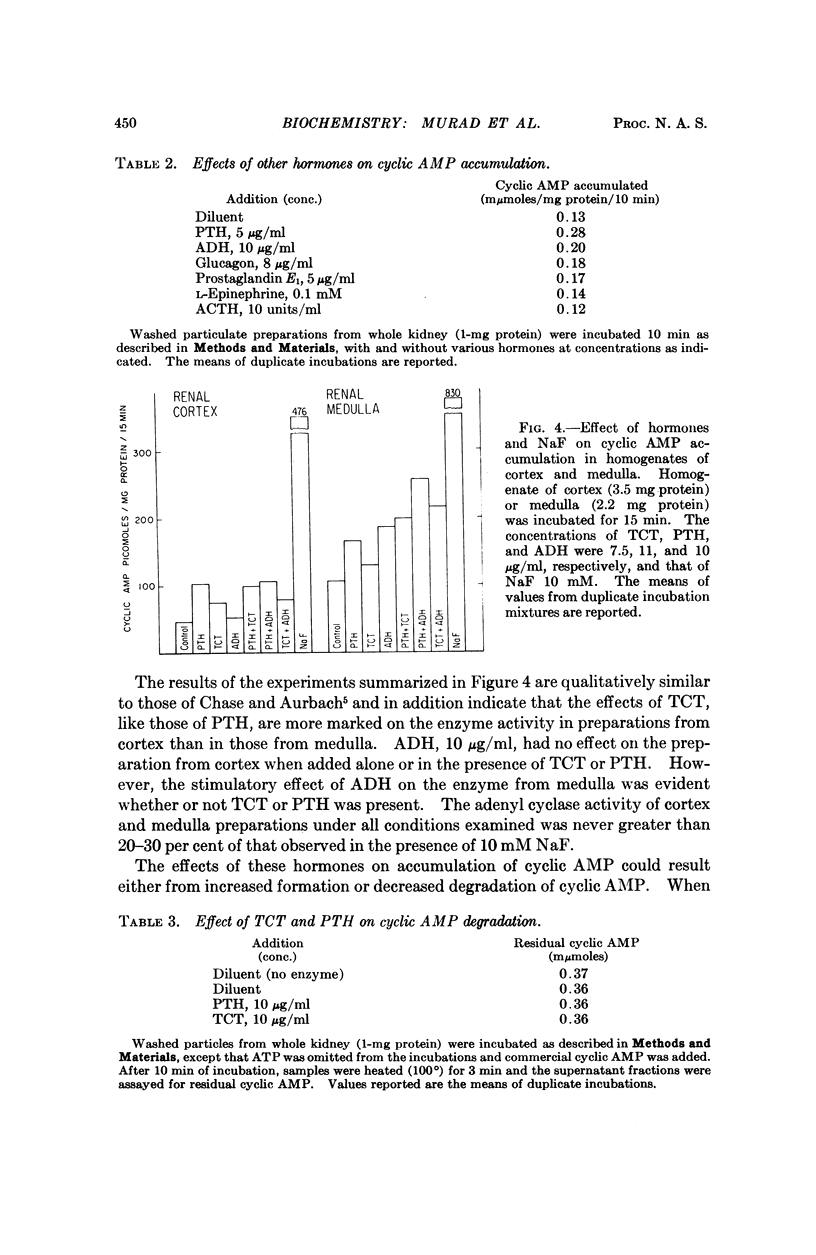
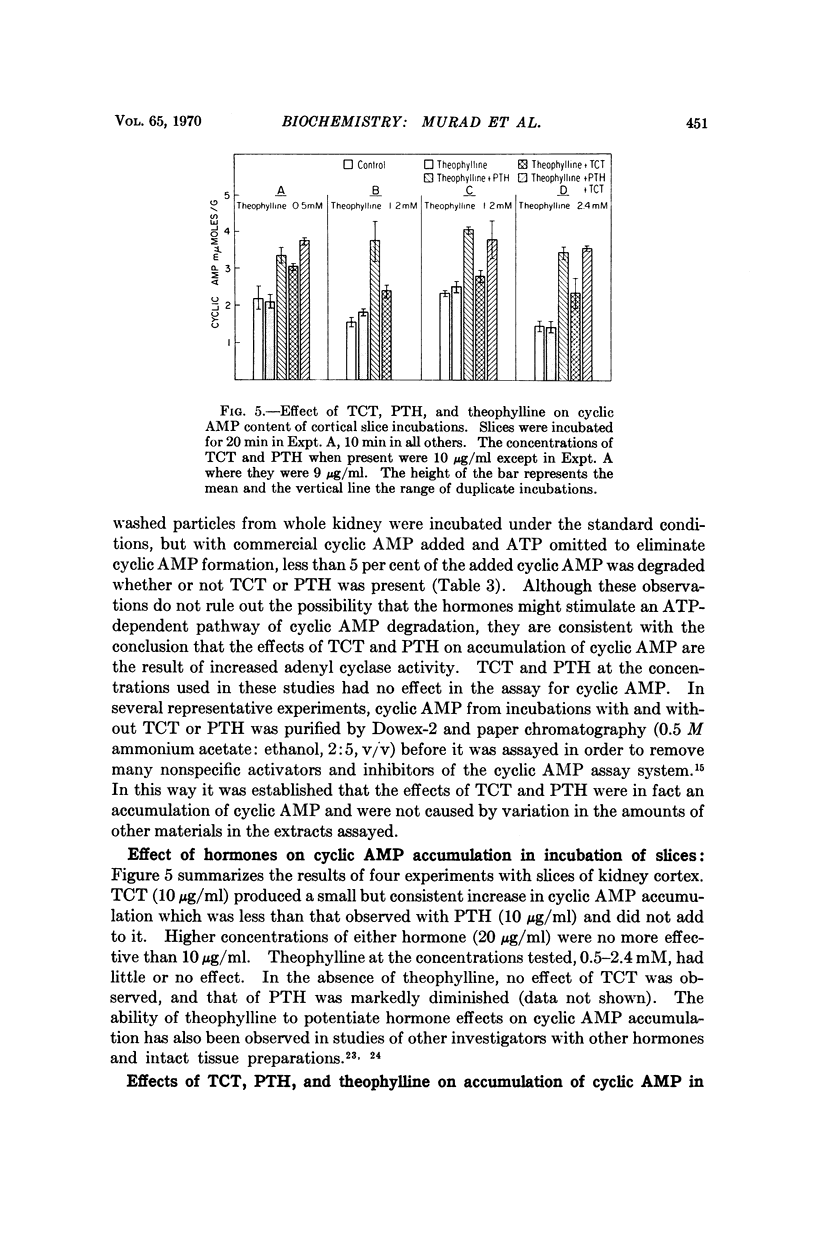
Thyrocalcitonin (TCT) increased the rate of accumulation of adenosine 3′:5′-cyclic phosphate (cyclic AMP) when added to incubations containing washed particles from whole rat kidney, adenosine triphosphate (ATP), MgSO4, and caffeine. The maximum stimulatory effect of TCT, 44 ± 6.7 per cent, was always less than the 150 to 250 per cent increase produced by parathyroid hormone (PTH). The effect of both hormones together was no greater than that of PTH alone when each was present at a maximally effective concentration. Since neither TCT nor PTH altered the rate of degradation of cyclic AMP by the kidney preparation, it may be inferred that their effects on cyclic AMP accumulation are the result of increased formation of cyclic AMP. Adenyl cyclase activity in homogenates of renal cortex was stimulated to a greater extent by TCT and PTH than was that of medulla, whereas, as reported earlier, the effect of vasopressin was much larger with homogenates of medulla. The accumulation of cyclic AMP in incubations of rat kidney cortex slices was increased 20 to 60 per cent by TCT and 50 to 140 per cent by PTH. The accumulation of cyclic AMP in incubations of rat calvaria was increased about threefold with TCT and nine to tenfold with PTH, while reduced and alkylated TCT had less than 10 per cent of the activity of TCT. These observations are consistent with the view that the physiological effects of TCT and PTH in kidney and bone are secondary to the enhanced formation of cyclic AMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Borle A. B. Effects of thyrocalcitonin on calcium transport in kidney cells. Endocrinology. 1969 Aug;85(2):194–199. doi: 10.1210/endo-85-2-194. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Keutmann H. T., Potts J. T., Jr, Reisfeld R. A., Schlueter R., Munson P. L. Isolation and chemical properties of porcine thyrocalcitonin. J Biol Chem. 1968 Nov 10;243(21):5739–5747. [PubMed] [Google Scholar]
- Brewer H. B., Jr, Ronan R. Amino acid sequence of bovine thyrocalcitonin. Proc Natl Acad Sci U S A. 1969 Jul;63(3):940–947. doi: 10.1073/pnas.63.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- Butcher R. W., Robison G. A., Hardman J. G., Sutherland E. W. The role of cyclic AMP in hormone actions. Adv Enzyme Regul. 1968;6:357–389. doi: 10.1016/0065-2571(68)90023-x. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Renal adenyl cyclase: anatomically separate sites for parathyroid hormone and vasopressin. Science. 1968 Feb 2;159(3814):545–547. doi: 10.1126/science.159.3814.545. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Fedak S. A., Aurbach G. D. Activation of skeletal adenyl cyclase by parathyroid hormone in vitro. Endocrinology. 1969 Apr;84(4):761–768. doi: 10.1210/endo-84-4-761. [DOI] [PubMed] [Google Scholar]
- Cooper C. W., Hirsch P. F., Toverud S. U., Munson P. L. An improved method for the biological assay of thyrocalcitonin. Endocrinology. 1967 Sep;81(3):610–616. doi: 10.1210/endo-81-3-610. [DOI] [PubMed] [Google Scholar]
- Dousa T., Rychlík I. The effect of parathyroid hormone on adenyl cyclase in rat kidney. Biochim Biophys Acta. 1968 Jun 24;158(3):484–486. doi: 10.1016/0304-4165(68)90309-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Rall T. W. Factors influencing adenosine 3',5'-phosphate accumulation in bovine thyroid slices. J Biol Chem. 1968 Nov 25;243(22):5867–5871. [PubMed] [Google Scholar]
- Hirsch P. F., Munson P. L. Thyrocalcitonin. Physiol Rev. 1969 Jul;49(3):548–622. doi: 10.1152/physrev.1969.49.3.548. [DOI] [PubMed] [Google Scholar]
- Kenny A. D., Heiskell C. A. Effect of crude thyrocalcitonin on calcium and phosphorus metabolism in rats. Proc Soc Exp Biol Med. 1965 Oct;120(1):269–271. doi: 10.3181/00379727-120-30508. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murad F., Rall T. W., Vaughan M. Conditions for the formation, partial purification and assay of an inhibitor of adenosine 3',5'-monophosphate. Biochim Biophys Acta. 1969 Dec 30;192(3):430–445. doi: 10.1016/0304-4165(69)90392-4. [DOI] [PubMed] [Google Scholar]
- Murad F., Strauch B. S., Vaughan M. The effect of gonadotropins on testicular adenyl cyclase. Biochim Biophys Acta. 1969 May 6;177(3):591–598. doi: 10.1016/0304-4165(69)90324-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Vaughan M., Murad F. Adenyl cyclase activity in particles from fat cells. Biochemistry. 1969 Jul;8(7):3092–3099. doi: 10.1021/bi00835a060. [DOI] [PubMed] [Google Scholar]



