Introduction
The discovery of leptin fifteen years ago generated great excitement that the treatment for obesity had been found, and thus, this prototypical adipocyte-secreted protein/cytokine was named leptin after the Greek word “leptos” for thin. It also pioneered the concept that adipose tissue is not an inert energy storage organ but an active endocrine organ. Subsequent clinical trials led to initial disappointment, however, when leptin was eventually found to be ineffective for the treatment of obesity (1). Research efforts have since expanded to elucidating leptin's role in human physiology and have resulted in a fundamentally renewed understanding of its role in regulation of energy homeostasis, neuroendocrine function, and metabolism, mainly in states of energy deficiency and not energy excess (i.e. obesity). In this review, we summarize the biology and physiology of leptin, its role in the pathophysiology of several disorders, and the emerging therapeutic applications of recombinant human leptin.
The biology of Leptin
Leptin, a 167-amino-acid product of the human leptin gene, was originally discovered through positional cloning of ob/ob mice, a mouse model of obesity found serendipitously at Jackson Laboratories (2). These mice were found to have a homozygous mutation of the leptin gene resulting in complete leptin deficiency, which manifested with hyperphagia, extreme obesity, diabetes, neuroendocrine abnormalities, and infertility.
Leptin is secreted mainly by white adipose tissue, and levels are positively correlated with the amount of body fat (3). Like many other hormones, leptin is secreted in a pulsatile fashion and has a significant diurnal variation with higher levels in the evening and early morning hours (4, 5). Circulating leptin levels reflect primarily the amount of energy stored in fat and secondarily acute changes in caloric intake (4-8) (Table 1).
Table 1. Factors that regulate circulating leptin levels.
| Factors promoting leptin secretion |
|---|
| * Excess energy stored as fat (obesity) |
| * Overfeeding |
| Glucose |
| Insulin |
| Glucocorticoids |
| Estrogens‡ |
| Inflammatory cytokines, including Tumor Necrosis Factor-α and Interleukin-6 (acute effect) |
| Factors inhibiting leptin secretion |
| * Low energy states with decreased fat stores (leanness) |
| * Fasting |
| Catecholamines and adrenergic agonists |
| Thyroid hormones |
| Androgens ‡ |
| Peroxisome Proliferator-activated Receptor-γ (PPARγ) agonists† |
| Inflammatory cytokines, including Tumor Necrosis Factor-α (prolonged effect) |
Denotes major factor influencing leptin levels.
Unlike animals, in humans PPARγ agonists decrease leptin gene expression but increase subcutaneous fat mass. Thus, the net effect is null.
Leptin mediates its effects by binding to specific leptin receptors (ObRs) expressed in the brain as well as in peripheral tissues. Alternative splicing generates several isoforms of ObRs. The ObRa isoform (the short leptin receptor isoform) is thought to play an important role in transporting leptin across the blood–brain barrier (11). The ObRb isoform (the long leptin receptor isoform) mediates signal transduction and is strongly expressed in the hypothalamus, an important site for the regulation of energy homeostasis and neuroendocrine function (12-14).
The binding of leptin to the ObRb receptor activates several signal transduction pathways, including Janus Kinase-Signal Transducer and Activator of Transcription-3 (JAK-STAT3), which is important for regulation of energy homeostasis (15), and Phosphatidylinositol 3-Kinase (PI3K), which is important for regulation of both food intake and glucose homeostasis (16). Other pathways, including Mitogen-activated Protein Kinase (MAPK), 5′Adenosine Monophosphate-activated Protein Kinase (AMPK), and the Mammalian Target of Rapamycin (mTOR), have been proposed to be downstream of leptin and are under investigation (17).
Homozygous mutations of the leptin gene leading to complete leptin deficiency have been described in extremely rare cases of obese humans. The vast majority of obese humans, however, have high circulating leptin levels (3) and are either resistant or tolerant to its weight-reducing effects (18). Proposed hypothalamic mechanisms underlying leptin resistance include a) defects at or downstream of the ObRb receptor, b) induction of inhibitors of leptin signaling (e.g. Suppressor of Cytokine Signaling-3 (SOCS-3) (19)), and c) alterations in the transport of leptin across the blood-brain barrier (18, 20). More studies are needed to fully elucidate leptin's signaling pathways and the mechanisms underlying leptin resistance or tolerance in humans, which in turn may lead to the development of novel treatment options for obesity and the metabolic syndrome.
The Role of Leptin in Human Physiology and Pathophysiology
The most significant roles of leptin include regulation of energy homeostasis, neuroendocrine function, and metabolism. Other effects of leptin involving regulation of immune function (21, 22) and bone metabolism are under intense investigations but are beyond the scope of this clinical review.
The role of leptin in energy homeostasis
The circulating leptin level serves as a gauge for energy reserves and directs the central nervous system to adjust food intake and energy expenditure accordingly. Leptin exerts immediate effects by acting on the brain to regulate appetite (Figure 1). Via ObRb-receptor binding in the hypothalamus, leptin activates a complex neural circuit comprising of anorexigenic (i.e. appetite-diminishing) and orexigenic (i.e. appetite-stimulating) neuropeptides to control food intake. Outside of the hypothalamus, leptin interacts with the mesolimbic dopamine system, which is involved in motivation for and reward of feeding, and the nucleus of the solitary tract of the brainstem to contribute to satiety (17).
Figure 1.
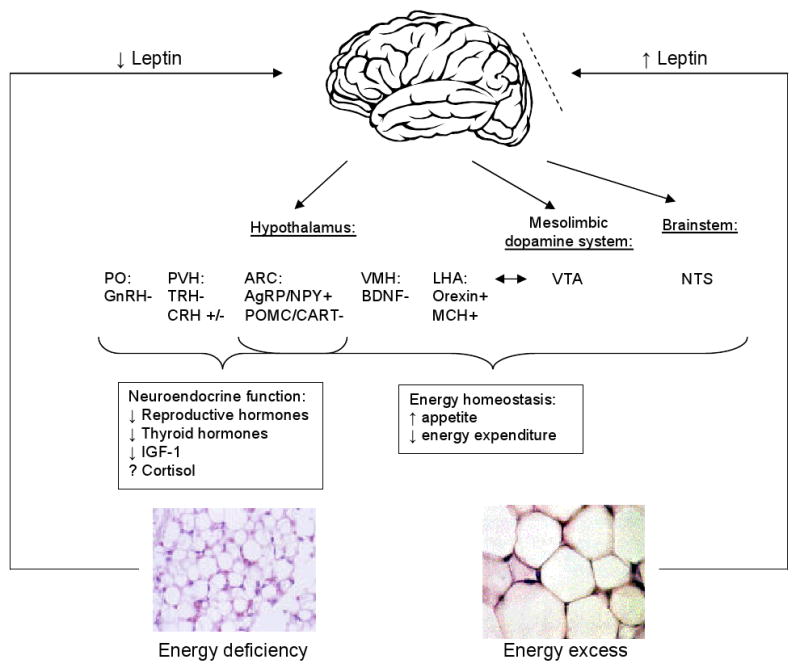
The central effects of leptin in states of energy excess and deficiency. States of energy excess are associated with hyperleptinemia but the hypothalamus is resistant or tolerant to the effects of increased leptin, represented by the dashed line. Energy deficiency results in hypoleptinemia. As a result, a complex neural circuit comprising of orexigenic and anorexigenic signals is activated to increase food intake (17). There is increased expression of orexigenic neuropeptides: AgRP and NPY in the ARC (23) and orexin and MCH in the LHA. Furthermore, there is decreased expression of anorexigenic neuropeptides: POMC and CART in the ARC (23) and BDNF in the VMH. In addition to neurons that project from the LH to the VTA, leptin also acts at the VTA of the mesolimbic dopamine system to regulate motivation for and reward of feeding. Leptin activation of the NTS of the brainstem also contributes to satiety. In addition, leptin has direct and/or downstream effects on the PVN and PO that are important for neuroendocrine responses to energy deprivation, including decreasing reproductive and thyroid hormones. While leptin only acts indirectly on the GnRH-secreting neurons in the hypothalamus, it can act directly and indirectly on TRH-secreting neurons (17). The effect of leptin on cortisol levels during starvation differs in mice and humans. Unlike in normal mice (24), leptin administration does not reverse the elevated ACTH levels associated with starvation in humans (7). The mechanism of leptin's effect on the growth hormone axis is unclear.
Abbreviations: AgRP, Agouti-related Protein; NPY, Neuropeptide Y; ARC, arcuate nucleus; MCH, Melanin-concentrating Hormone; LHA, lateral hypothalamic area; POMC, Proopiomelanocortin; CART, Cocaine- and Amphetamine-regulated Transcript; BDNF, Brain-derived Neurotrophic Factor; VMH, ventromedial hypothalamic nucleus; VTA, ventral tegmental area; PVN, paraventricular nucleus; PO, preoptic area; CRH, corticotropin-releasing hormone; GnRH, gonadotropin-releasing hormone; TRH, thyrotropin-releasing hormone; ACTH, adrenocorticotropin.
In addition to immediate effects, long-term leptin administration may result in the rewiring of the connections among hypothalamic neurons (i.e. promote synaptic plasticity) (25, 26). Specifically, when administered in leptin-deficient mice, leptin has been shown to increase the number of synapses on neurons that secrete the anorexigenic neuropeptide Proopiomelanocortin (POMC) and decrease the number of synapses on neurons that secrete the orexigenic neuropeptide Neuropeptide Y (NPY) (26).
Not only does leptin signal the central nervous system to decrease food intake, it may also increase energy expenditure. In mice, leptin increases sympathetic nerve activity (27) and activates brown adipose tissue thermogenesis (28, 29), but these effects have not been confirmed in humans (30).
Clinically, patients with congenital leptin deficiency due to mutations in the leptin gene or extreme leptin resistance due to mutations of the leptin receptor gene are obese due to marked hyperphagia (31, 32). For patients with leptin deficiency, administering leptin in replacement doses reduces food intake via neural circuits that diminish the perception of food reward and enhance the response to satiety signals (33) and normalizes body weight (34). However, leptin administration at pharmacologic doses to the vast majority of obese humans, who have relatively high levels of leptin and are resistant to it, induces little if any weight loss (1, 35). Thus, accumulating evidence suggests that leptin is physiologically more important as an indicator of energy deficiency and as a possible mediator of adaptation to starvation.
The role of leptin in regulating neuroendocrine function
In response to fasting, leptin levels fall rapidly before and out of proportion to any changes in fat mass (6, 7, 36), triggering the neuroendocrine response to acute energy deprivation (7, 36, 37). In mice and humans, this response includes decreasing reproductive hormone levels which prevents pregnancy (an energy-requiring process), decreasing thyroid hormone levels that slow metabolic rate, increasing growth hormone level that may mobilize energy stores, and decreasing insulin-like growth factor-1 (IGF-1) level that may slow growth-related processes (7, 37, 38). The interactions between leptin and the growth hormone and adrenal axes are apparently less important in humans than in animal models since patients with congenital leptin deficiency have normal linear growth and adrenal function, unlike mice (34, 38-40).
We originally observed neuroendocrine abnormalities when starvation-induced falls in leptin levels reached an average of 0.27 ng/mL, and leptin administration in physiologic replacement doses restored the changes in luteinizing hormone pulsatility, decreases in testosterone levels, and decreases in thyroid stimulating hormone pulsatility (7). We then ascertained whether there is a minimum leptin threshold to allow reproduction and to maintain other neuroendocrine processes. When we induced leptin deficiency in normal-weight women, who have higher baseline leptin levels, leptin levels fell to an average of 2.8 ng/mL (36). Only modest changes in LH pulsatility were observed. Our findings suggest that a leptin threshold of ∼3 ng/mL is necessary to convey to the brain the message that energy stores in adipose tissue are adequate to bring pregnancy to term. Reaching a leptin level above this threshold, as a child grows, permits the onset of puberty (41) and, in older persons, maintains reproductive and other neuroendocrine processes.
Given that women with anorexia nervosa and exercise-induced amenorrhea are chronically energy-deprived, we first hypothesized that these conditions are associated with hypoleptinemia. This was confirmed in observational studies (42-45). We then hypothesized that long-standing hypoleptinemia leads to neuroendocrine dysfunction with subsequent anovulation and osteoporosis. We conducted a proof-of-concept trial of leptin treatment in replacement doses in women with hypothalamic amenorrhea and found that it improves or fully normalizes the gonadal, thyroid, and, to a lesser degree, growth hormone axes as well as bone markers (46).
The role of leptin in insulin resistance and the metabolic syndrome
Both ob/ob mice and db/db mice, which have a leptin receptor mutation, as well as humans with congenital leptin deficiency have insulin resistance and other features of the metabolic syndrome. In the ob/ob mouse strain, leptin treatment improves hyperglycemia and hyperinsulinemia before weight loss is achieved (47). Leptin treatment in humans with congenital leptin deficiency has also been shown to improve not only hyperinsulinemia but also levels of low-density lipoprotein cholesterol, high-density liproprotein cholesterol, and triglycerides (39). These effects are mediated through central and peripheral actions, and the mechanisms are still being elucidated.
Similarly, mouse models of lipoatrophy, which lack subcutaneous adipose tissue, are hypoleptinemic due to lack of fat available to produce leptin and have metabolic abnormalities, including hyperglycemia, insulin resistance, and hyperlipidemia (48). Given the improvements in metabolic parameters in ob/ob mice after leptin administration, it was hypothesized that lipoatrophic mice may also be responsive to exogenous leptin (49). Indeed, transplantation of adipose tissue (48, 50), which produces leptin, and administration of exogenous leptin (49) in lipoatrophic mice improve hyperglycemia, insulin resistance, hypertriglyceridemia, and hepatic steatosis. This has led to trials in humans with various types of lipoatrophy and associated metabolic abnormalities (51-58), described further under Clinical Applications.
In conclusion, leptin plays a pivotal role in the regulation of energy homeostasis, neuroendocrine function, and metabolism in not only states of energy excess but, more importantly, in states of energy deficiency. Thus, leptin deficiency results in distinct clinical phenotypes (Table 2) with associated neuroendocrine and metabolic abnormalities, for which recombinant human leptin is an emerging potential therapy.
Table 2. Leptin-deficient states.
| Syndrome | Estimated prevalence | Associated features |
|---|---|---|
| I. Congenital leptin-deficient states | ||
| A. Leptin gene mutations | ||
| Homozygous congenital leptin deficiency | Rare | Early onset morbid obesity, hyperphagia, hypogonadotropic hypogonadism, advanced bone age, hyperinsulinemia, and immune dysfunction. These manifestations are normalized by leptin treatment in replacement doses. |
| Heterozygous congenital leptin deficiency | Rare | Less severe obesity that may respond to exogenous recombinant human leptin though this remains to be studied in interventional studies (59). |
| B. Mutations leading to lipoatrophy | ||
| Congenital lipoatrophy | Rare | Lipoatrophy, diabetes, and metabolic syndrome. Metabolic abnormalities improve in response to leptin administration but no randomized, controlled trials have been performed. |
| II. Acquired leptin-deficient states | ||
| A. Generalized decrease in adipose tissue mass | ||
| Anorexia nervosa | Up to 2.2% lifetime prevalence for women (60) | Profoundly decreased body weight and fat mass, amenorrhea/infertility, osteoporosis with stress fractures, decreased thyroid hormone levels, increased growth hormone levels, and decreased IGF-1 levels. |
| Exercise-induced hypothalamic amenorrhea and/or ovulatory dysfunction | Amenorrhea has been reported in 60-69% in trained female athletes and ovulatory dysfunction in up to 78% of recreational female athletes (61) | Lower percentage of body fat with or without decreased body weight, amenorrhea/infertility, osteoporosis, and neuroendocrine abnormalities listed above. Abnormalities improved in response to leptin treatment in a proof-of-concept, controlled trial (46). Larger, randomized, placebo-controlled trials are underway. |
| Non-athletic forms of hypothalamic amenorrhea | 7.6% in women aged 15-24, 3.0% in women aged 25-34, and 3.7% in women aged 35-44 years (62) | Relatively normal or slightly decreased body weight but lower percentage of body fat, amenorrhea/infertility, and neuroendocrine abnormalities listed above. |
| B. Selective decrease in adipose tissue mass | ||
| Acquired severe lipoatrophy and insulin resistance | Rare | Lipoatrophy, insulin resistance, hypercholesterolemia, and hypertriglyceridemia. These metabolic abnormalities improved with leptin replacement in both open-label (56) and randomized, placebo-controlled, cross-over (55) clinical trials. |
| HIV lipoatrophy | 15% - 36% of all HIV-infected patients (63) | |
Abbreviations: IGF-1, insulin-like growth factor-1; HIV, Human Immunodeficiency Virus; HAART, highly-active antiretroviral therapy.
Clinical Applications: Recombinant Human Leptin as a Treatment for Leptin Deficiency Syndromes in Humans
Obesity syndromes
Leptin deficiency in obesity: mutations of the leptin gene
Patients with congenital complete leptin deficiency due to homozygous leptin gene mutations develop extreme obesity very early in life and respond to recombinant human leptin treatment, which reduces appetite and food intake leading to dramatic body fat loss (32, 34, 40). Furthermore, these patients have distinct neuroendocrine abnormalities, including hypogonadotropic hypogonadism with failure to reach puberty, which improve with leptin replacement (34). These mutations are rare, but they should be considered in young patients with severe, early-onset obesity and hyperphagia since congenital leptin deficiency is easily treated. Leptin is currently available for congenital leptin deficiency through a compassionate use program by Amylin Pharmaceuticals, Inc.
Leptin resistance in common obesity
Since mechanisms of leptin resistance remain largely unknown, strategies to address leptin resistance in common obesity have included supra-physiologic doses of leptin and coadministration with presumed leptin sensitizers. An early trial with high, pharmacologic doses of leptin resulted in no clinically significant weight loss (1). Recently, amylin, a hormone secreted by the pancreas that also contributes to the regulation of energy homeostasis, was proposed to improve leptin responsiveness in diet-induced obesity (35). A recent study conducted by Amylin Pharmaceuticals, Inc. found that overweight and obese participants lost significantly more weight on the combination of leptin and pramlintide, an analog of amylin, than treatment with either agent alone (64). Of note, the effects appear additive, suggesting that amylin may not improve sensitivity to leptin, and the drop-out rate was high at 32%.
A more promising area of clinical interest is the potential role of leptin treatment in weight loss maintenance. It has been proposed that falling leptin levels due to weight loss activate neuroendocrine mechanisms which may drive patients to regain weight. These mechanisms may include increasing energy intake, by increasing hunger, and decreasing energy expenditure, by decreasing thyroid hormone levels and subsequently slowing metabolism (65). Thus, replacing leptin may restore these neuroendocrine abnormalities and prevent “yo-yo” dieting commonly seen in clinical practice. This is currently being investigated and, if successful, may have major implications in weight loss management.
States of energy deficiency
Leptin deficiency with generalized decrease in adipose tissue mass: Exercise- and diet-induced hypothalamic amenorrhea
Hypothalamic amenorrhea is defined as the cessation of menstrual cycles due to dysfunction of the hypothalamic-pituitary-gonadal axis in the absence of organic disease. It is associated with strenuous exercise, stress, and reduced food intake and includes patients with anorexia nervosa, female athletes with the well-recognized triad (low energy availability with or without disordered eating, amenorrhea/neuroendocrine dysfunction, and osteoporosis), and normal-weight patients with ovulatory dysfunction.
Following our observational studies showing that women with hypothalamic amenorrhea are hypoleptinemic (42-44), our proof-of-concept study demonstrated that leptin replacement in these women not only normalizes the levels of estrogen, thyroid hormones, and IGF-1 but more importantly restores ovulatory menstruation (46). Leptin also increased markers of bone formation but did not alter bone resorption. Further randomized, placebo-controlled studies are currently elucidating the effects of longer-term recombinant human leptin treatment on neuroendocrine function, immune function, and bone metabolism in these women.
Leptin deficiency with selective decrease in adipose tissue mass: Lipoatrophy
Persons with rare syndromes of congenital lipoatrophy have severe insulin resistance, hypercholesterolemia, and hypertriglyceridemia. Observational studies have shown that these subjects have hypoleptinemia (66, 67), and several uncontrolled studies have demonstrated that treatment with recombinant human leptin improves insulin resistance, suppresses hepatic gluconeogenesis, decreases hemoglobin A1c by ∼3.5%, improves hyperlipidemia (57, 58), and reverses hepatic steatosis (53, 54). Studies have also shown that leptin treatment restores menstrual cycles in women with lipoatrophy and features of polycystic ovarian syndrome (68). Currently, leptin is available for congenital lipoatrophy through a FDA-approved, expanded access program by Amylin Pharmaceuticals, Inc.
Although congenital lipoatrophy is rare, Human Immunodeficiency Virus (HIV) lipoatrophy associated with HIV and/or highly-active antiretroviral therapy (HAART) has recently become more prevalent, currently estimated between 15% and 36% of all HIV-infected patients (63). We have shown that these patients, who also have increased cardiovascular risk (69), have relative leptin deficiency (51). Subsequently, we demonstrated in our proof-of-concept trial that treatment with recombinant human leptin in individuals with HAART-induced metabolic syndrome and hypoleptinemia improves insulin resistance, improves hyperlipidemia, and decreases central fat mass within two months (55). An independent study of six months duration confirmed these results (56). Once further clinical trials define the treatment protocols for optimal efficacy and safety, human recombinant leptin alone or in combination with thiazolidinediones, which also improves glucose homeostasis possibly through another adipocyte-secreted hormone adiponectin (70), may be able to serve this growing population.
Conclusion
Leptin regulates energy homeostasis, neuroendocrine function, and metabolism. Leptin deficiency is a clinical syndrome associated with distinct phenotypes, which encompass a very small subset of obesity (i.e. those from leptin-related gene mutations), hypothalamic amenorrhea, and lipoatrophy. Recombinant human leptin is an emerging potential therapy for these leptin-deficient conditions, since in replacement doses it normalizes neuroendocrine and metabolic functions in recent proof-of-concept clinical trials. Randomized, placebo-controlled clinical trials are currently evaluating leptin as a potential treatment for weight loss maintenance, and the development of leptin sensitizers for common obesity is greatly anticipated in the near future. Hopefully, recombinant human leptin will soon find its place in our therapeutic armamentarium.
Summary box (take home points).
The circulating leptin level mainly reflects the amount of energy stores in adipose tissue and directs the central nervous system in regulating energy homeostasis, neuroendocrine function, and metabolism.
Leptin deficiency results in neuroendocrine deficits, including infertility, as well as metabolic abnormalities.
States of complete or severe leptin deficiency include rare cases of congenital leptin deficiency (due to mutations of leptin-related genes) and congenital lipoatrophy (due to lack of fat available to produce leptin).
States of relative, acquired leptin deficiency include more prevalent conditions such as anorexia nervosa, exercise-induced hypothalamic amenorrhea, and HIV lipoatrophy.
Recombinant human leptin treatment, in physiologic replacement doses, normalizes neuroendocrine and metabolic abnormalities in states of complete and relative leptin deficiency.
Acknowledgments
Grant support: NIDDK DK58785, DK 79929, DK 81913
Dr. Mantzoros has received grant support from Amgen Inc. through Beth Israel Deaconess Medical Center and has been utilizing leptin provided by Amylin Pharmaceuticals, Inc. for investigator-initiated studies.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
References
- 1.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 4.Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3(5):575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 5.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81(9):3419–23. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 7.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366(9479):74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81(9):3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 10.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82(2):579–84. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 11.Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139(8):3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–47. [PubMed] [Google Scholar]
- 13.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94(13):7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 16.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 17.Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008;94(5):637–42. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274(42):30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 20.Munzberg H. Differential leptin access into the brain--a hierarchical organization of hypothalamic leptin target sites? Physiol Behav. 2008;94(5):664–9. doi: 10.1016/j.physbeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90(3):1618–24. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- 22.Chan JL, Moschos SJ, Bullen J, Heist K, Li X, Kim YB, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90(3):1625–31. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 23.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 24.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–63. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 25.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 26.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 27.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100(2):270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380(6576):677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 29.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273(1 Pt 1):E226–30. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 30.Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 2007;66(1):49–57. doi: 10.1111/j.1365-2265.2006.02684.x. [DOI] [PubMed] [Google Scholar]
- 31.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 33.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 35.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105(20):7257–62. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 38.Chan JL, Williams CJ, Raciti P, Blakeman J, Kelesidis T, Kelesidis I, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab. 2008;93(7):2819–27. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 41.Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab. 1997;82(4):1066–70. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- 42.Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry. 1998;3(6):544–7. doi: 10.1038/sj.mp.4000418. [DOI] [PubMed] [Google Scholar]
- 43.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85(12):4511–4. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 44.Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82(6):1845–51. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- 45.Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83(7):2309–12. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 46.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 47.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139(1):8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 48.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105(3):271–8. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 50.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275(12):8456–60. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 51.Brennan AM, Lee JH, Tsiodras S, Chan JL, Doweiko J, Chimienti SN, et al. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160(2):173–6. doi: 10.1530/EJE-08-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebihara K, Masuzaki H, Nakao K. Long-term leptin-replacement therapy for lipoatrophic diabetes. N Engl J Med. 2004;351(6):615–6. doi: 10.1056/NEJM200408053510623. [DOI] [PubMed] [Google Scholar]
- 53.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 54.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753–60. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91(7):2605–11. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 56.Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94(4):1137–44. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 58.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414(6859):34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 60.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164(8):1259–65. doi: 10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]
- 61.Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 62.Warren MP. Clinical review 77: evaluation of secondary amenorrhea. J Clin Endocrinol Metab. 1996;81(2):437–42. doi: 10.1210/jcem.81.2.8636244. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40(12):1837–45. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 64.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17(9):1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardini VC, Victoria IM, Rocha SM, Andrade DG, Rocha AM, Pieroni FB, et al. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatropic diabetes. J Clin Endocrinol Metab. 1998;83(2):503–8. doi: 10.1210/jcem.83.2.4567. [DOI] [PubMed] [Google Scholar]
- 67.Nagy GS, Tsiodras S, Martin LD, Avihingsanon A, Gavrila A, Hsu WC, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis. 2003;36(6):795–802. doi: 10.1086/367859. [DOI] [PubMed] [Google Scholar]
- 68.Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255–63. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 70.Mantzoros CS. Whither recombinant human leptin treatment for HIV-associated lipoatrophy and the metabolic syndrome? J Clin Endocrinol Metab. 2009;94(4):1089–91. doi: 10.1210/jc.2009-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]


