Abstract
Alcohol use disorders are a major public health concern. Despite the demonstrated efficacy of a number of different treatments for alcohol dependence, relapse remains a major problem. Healthy lifestyle changes may contribute to long-term maintenance of recovery and interventions targeting physical activity, in particular, may be especially valuable as an adjunct to alcohol treatment. In this paper, we discuss the rationale and review potential mechanisms of action whereby exercise might benefit alcohol dependent patients in recovery. We then describe the development of a 12-week moderate-intensity aerobic exercise program as an adjunctive intervention for alcohol dependent patients in recovery. Preliminary data from a pilot study (n=19) are presented and the overall significance of this research effort is discussed.
Rationale
Alcohol use disorders are a major public health concern (Gmel & Rehm, 2003; Rehm, Gmel, Sempos, & Trevisan, 2003; USDHHS, 2000). The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) characterizes an alcohol use disorder (either abuse or dependence) as a maladaptive pattern of drinking and symptoms that result in clinical impairment or distress (APA, 2000). Approximately 17.6 million Americans are estimated to meet diagnostic criteria for alcohol abuse or dependence annually (B. F. Grant et al., 2004), and associated costs number in excess of $184.6 billion per year (Harwood, 2000). Alcohol use disorders are among the most common of the psychiatric disorders in the United States, affecting as many as 18% of the population at some point in their lifetime (Kessler et al., 2005). Negative consequences of alcohol misuse include interpersonal violence (Pernanen, 1991; Stuart, 2005; Thompson & Kingree, 2006), risky sexual behavior (Cooper, 2002; C. Donovan & McEwan, 1995; Justus, Finn, & Steinmetz, 2000), drunk driving (Pickrell, 2006), and suicide (B.F. Grant & Hasin, 1999; Wilcox, Conner, & Caine, 2004).
The Problem of Relapse in Alcohol Use Disorders
Despite the demonstrated efficacy of a number of different treatments for alcohol dependence (W. R. Miller & Wilbourne, 2002; Read, Kahler, & Stevenson, 2001), relapse remains a major problem, with relapse rates in the first year following treatment ranging from 60 – 90% (Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Maisto, Connors, & Zywiak, 2000; W.R. Miller, Walters, & Bennett, 2001). Attention to the problem of relapse in alcohol dependence and other addictive disorders has intensified over the years (Marlatt & Donovan, 2005; Moos & Moos, 2006), and the significant work of Marlatt and colleagues (Marlatt & Donovan, 2005) provide a social learning model of the relapse process in addictive disorders and suggested treatment approaches to prevent relapse. In articulating their social learning-based (Albert Bandura, 1977; A. Bandura, 1986) relapse prevention model, Marlatt and Gordon's (Marlatt & Gordon, 1985) primary focus was on the individual's ability to cope with situational factors that may precipitate relapse, and the need to deal with the negative cognitive-affective reactions following an initial lapse. Although empirical support for Marlatt's relapse taxonomy was found to be lacking (c.f., (D. M. Donovan, 1996; Stout, Longabaugh, & Rubin, 1996), relapse prevention strategies (derived from Marlatt's model) for the treatment of alcohol dependence have shown promise (Carroll, 1996; Irvin, Bowers, Dunn, & Wang, 1999; Witkiewitz & Marlatt, 2004).
Although lifestyle modification was one of the main components in Marlatt's relapse prevention model (Marlatt, 1985; Marlatt & Witkiewitz, 2005), the treatment outcome literature suggests that this component has received the least emphasis in relapse prevention programs for alcoholism. Despite this lack of attention in the empirical literature, methods that attempt to foster healthy lifestyle changes may contribute to long-term maintenance of recovery and interventions targeting physical activity, in particular, may be especially valuable as an adjunct to alcohol treatment.
Aerobic Exercise as a Relapse Prevention Strategy
Physical activity is defined as any bodily movement that results in energy expenditure (Caspersen, Powell, & Christenson, 1985). Exercise is a subset of physical activity and is defined as “planned, structured, and repetitive bodily movement done to improve or maintain one or more components of physical fitness” (Caspersen et al., 1985) and can be categorized as being either aerobic, referring to activities that require oxygen uptake, or anaerobic, referring to activities that do not rely on an oxidative process (Boutcher, 1993). In recent years, a growing body of empirical research has examined the numerous physical and psychological benefits of exercise, and has begun to explore potential treatment applications of exercise (USDHHS, 1996).
Exercise may represent a potentially useful and relatively unexplored alternative behavior for alcoholics working toward long-term recovery. Indeed, in his chapter on “Lifestyle Modification,” Marlatt (Marlatt, 1985) cites exercise as “a highly recommended lifestyle change activity” (p. 309) and discusses the advantages of exercise as a relapse prevention strategy. Other writers have agreed that lifestyle-enhancing factors such as exercise and fitness may play an important role in the prevention and treatment of addictive disorders (Agne & Paolucci, 1982; C.B. Taylor, Sallis, & Needle, 1985; Tkachuk & Martin, 1999).
The adoption of exercise behavior in alcohol recovery could provide some definite advantages. Exercise offers the potential for improved health and wellness, as the physiological health benefits of exercise have been well documented in both general populations (Bovens et al., 1993; King, Frey-Hewitt, Dreon, & Wood, 1989; Kohl, LaPorte, & Blair, 1988; Oberman, 1985; Pinto & Marcus, 1994; USDHHS, 1996) and in alcoholic samples (Frankel & Murphy, 1974; Peterson & Johnstone, 1995; Sinyor, Brown, Rostant, & Seraganian, 1982; Tsukue & Shohoji, 1981). Exercise also has the potential to be cost-effective, flexible and accessible; many forms of exercise (e.g., running, fitness videotapes, swimming) may be conducted independently, either at home or outdoors, and associated costs are likely to be minimal. Finally, exercise has minimal side effects compared to pharmacological treatment (cf., (Broocks et al., 1998). With the use of proper precautions for prevention of injuries (American College of Sports Medicine, 2000), exercise carries with it far less risk of adverse events than does the use of psychotropic medication.
Thus it appears that exercise participation may offer decided advantages as a maintenance strategy for alcohol dependent individuals. Below, we review potential mechanisms of action whereby exercise might benefit alcoholics in recovery. We then present evidence for the potential efficacy of exercise as a relapse prevention strategy. Following this, we describe the development of an aerobic exercise program for alcoholics as well as presenting preliminary findings. Finally, we discuss the overall significance of this research effort.
Potential Mechanisms of Action for Exercise in Alcohol Recovery
Exercise may benefit alcoholics attempting recovery from alcohol problems through a number of different mechanisms of action. Exercise may (a) provide pleasurable states without the use of alcohol, (b) reduce depressive symptoms and negative mood, (c) increase self-efficacy, (d) provide positive alternatives to drinking, (e) decrease stress reactivity and improve coping and (f) decrease urges to drink.
Provide Pleasurable States Without Use of Alcohol
Similar to findings regarding alcohol and drug use (Froelich, 1997; O'Malley et al., 1996), exercise has been shown to have positive reinforcing properties that may be mediated by its effects on the endogenous opioid system and on dopaminergic reinforcement mechanisms (Carlson, 1991; Cronan & Howley, 1974; Thoren, Floras, Hoffmann, & Seals, 1990). Therefore, alcoholics may find exercise to be a viable means of experiencing pleasure. Indeed, Murphy and colleagues (Murphy, Pagano, & Marlatt, 1986) observed that heavy alcohol drinkers in their study experienced a “high” associated with exercise. Thus, alcoholics may find exercise to be a positively reinforcing alternative to drinking.
Provide Positive Alternatives to Drinking
Optimally, alcohol recovery involves positive lifestyle change to develop rewarding and enjoyable social and recreational activities that do not revolve around drinking (Marlatt & Gordon, 1985; Smith & Meyers, 1995). Exercise may serve as just such an activity. Moreover, many persons early in recovery may find themselves with a good deal of unstructured time that was once spent using substances. In such cases, exercise can also serve as a healthy, positive means of filling unstructured time [see(Marlatt, 1985)]. Involvement in exercise may help recovering alcoholics achieve “high-level wellness” (Bartha & Davis, 1982), allowing the patient to embrace a better quality of health and fitness, and consequently, a better quality of life.
Reduce Depressive Symptoms and Negative Mood
Although the relationship between alcohol use and depression is complex (Brown & Ramsey, 2000; Hodgins, el Guebaly, Armstrong, & Dufour, 1999), a number of studies in recent years have found an association between depressive symptomatology and poor outcome in recovery from alcohol dependence (Greenfield et al., 1998; Hodgins et al., 1999; Rounsaville, Dolinsky, Babor, & Meyer, 1987). Exercise has been shown to have a positive impact on depressive symptoms (e.g., (Byrne & Bryne, 1993; E.J. Doyne, Chambless, & Beutler, 1983; Elizabeth J. Doyne et al., 1987; McCann & Holmes, 1984) and to result in acute improvements in mood (e.g., (Berger & Owen, 1992; Bock, Marcus, King, Borrelli, & Roberts, 1999). Furthermore, a number of controlled studies have shown aerobic exercise to be associated with positive outcomes for depression when compared to both no-treatment control conditions (E.J. Doyne et al., 1983; Elizabeth J. Doyne et al., 1987; McCann & Holmes, 1984) and to more traditional treatments for depression, such as cognitive behavioral therapy (Craft & Landers, 1998; Lawlor & Hopker, 2001) and antidepressant medication (Babyak et al., 2000). Thus, exercise may serve to alter mood and depressive symptoms and to provide an alternative for the treatment of depression.
Positive effects of exercise on psychological health have also been shown in individuals with substance use disorders. Both aerobic and strength training exercise programs during the course of alcohol treatment have resulted in decreased depressive and anxiety symptoms (Frankel & Murphy, 1974; J. Palmer, Vacc, & Epstein, 1988; J. A. Palmer, Palmer, Michiels, & Thigpen, 1995). Similar findings have emerged with smokers attempting cessation (Bock et al., 1999; Kawatchi, Troisi, Rotnitzky, Coakley, & Codlitz, 1996), and generally suggest that positive psychological health consequences of exercise extend to individuals in treatment for substance use disorders. Just as we found that improved drinking outcomes in alcohol dependent patients receiving cognitive-behavioral treatment for depression were mediated by reduction of depressive symptoms (Brown, Evans, Miller, Burgess, & Mueller, 1997), so may exercise lead to improved drinking outcomes as a result of reductions in depressive and anxiety symptoms.
Decrease Stress Reactivity and Improve Coping
Some cognitive social learning theorists have posited that alcohol-involved individuals drink at least in part because they lack certain basic coping skills necessary for dealing with stressors associated with daily living (Abrams & Niaura, 1987; William R. Miller et al., 1995; P.M. Monti, Rohsenow, Colby, & Abrams, 1995). Consistent with this theoretical formulation, exercise can serve to reduce stress reactivity and to supplant drinking as a primary coping mechanism (Hobson & Rejeski, 1993; Keller, 1980). Several studies have provided empirical support for this viewpoint (Calvo, Szabo, & Capafons, 1996; Rejeski, Gregg, Thompson, & Berry, 1991; Sinyor et al., 1982). In a meta-analytic review, (Crews & Landers, 1987) found a significant association between aerobic fitness and reactivity to stressors. Aerobically fit individuals displayed a reduced psychosocial stress response when compared to controls. Therefore, exercise may produce a buffering effect that decreases the likelihood that an individual will feel the need to use drinking as a way of coping with stress (Rejeski et al., 1991).
Increase Self-Efficacy
Bandura (Albert Bandura, 1977; A. Bandura, 1986) viewed self-efficacy (the belief in one's ability to master particular skills) as a cognitive mechanism that affects behavior. Enhancing one's self-efficacy is likely to result in positive behavior change. The “mastery hypothesis” (Tuson & Sinyor, 1993) suggests that enhanced self-efficacy for exercise (McAuley, Courneya, & Lettunich, 1991; Williams & Cash, 1999) gained from the acquisition of exercise skills may generalize to increased self-efficacy for implementing coping strategies necessary for the maintenance of long-term sobriety (see (Peterson & Johnstone, 1995).
Decrease urges to drink
Urges to drink alcohol have been identified as an important relapse trigger for abstinent alcoholics (Marlatt & Gordon, 1985; P. M. Monti, Rohsenow, & Hutchison, 2000). More recently, it has been proposed that moderate-intensity exercise may provide short-term relief from urges to drink alcohol (M. Ussher, Sampuran, Doshi, West, & Drummond, 2004). Therefore, decreases in urges to drink may mediate the effect of aerobic exercise on decreased likelihood for relapse.
Evidence for the Efficacy of Exercise in Treatment for Substance Use Disorders
Tobacco
Among addictive disorders, nicotine dependence has received the most attention with respect to the role of physical activity. Findings from early correlational studies indicated that increased fitness levels were significantly associated with decreases in smoking behaviors (Cheraskin & Ringsdorf, 1971; Hickey, Mulcahy, Bourke, Graham, & Wilson-Davis, 1975). More recently, research in smoking cessation has demonstrated the effects of exercise on decreased craving and nicotine withdrawal (Bock et al., 1999) (see also (Pomerleau et al., 1987) as well as maintenance of smoking cessation (M. H. Ussher, Taylor, West, & McEwen, 2000).
Ten controlled published studies have examined exercise interventions for smokers. Most of these studies (J. S. Hill, 1985; R. D. Hill, Rigdon, & Johnson, 1993; Russell, Epstein, Johnston, Block, & Blair, 1988; C. B. Taylor, Houston-Miller, Haskell, & Debusk, 1988) were conducted over a decade ago and did not demonstrate promising results. However, in a more recent study, Martin and colleagues (Martin et al., 1997) compared three smoking interventions (standard treatment plus nicotine anonymous meetings, behavioral counseling plus physical exercise, and behavioral counseling plus nicotine gum) in 205 recovering alcoholics. They found a significant effect for exercise on quit rates at the end of the 12-week treatment program. A series of studies by Marcus and colleagues (B. H. Marcus et al., 1999; B.H. Marcus, Albrecht, Niaura, Abrams, & Thompson, 1991; B. H. Marcus et al., 1995; B. H. Marcus et al., 2005) have also shown promising results regarding the potential influence of exercise on smoking behavior. In a large, well-controlled study, Marcus and colleagues (B. H. Marcus et al., 1999) examined the efficacy of a 12-week vigorous-intensity exercise (Commit to Quit I; CTQ I) as an aid for smoking cessation among 281 healthy, sedentary female smokers. Compared with participants in the control condition, those in the exercise group achieved significantly higher levels of abstinence at the end of treatment and at 3-month (16.4% vs 8.2%; p=.03) and 12-month (11.9% vs. 5.4%; p=.05) follow-ups. Most recently, Marcus and colleagues tested an 8-week moderate-intensity exercise intervention, Commit to Quit II (CTQ II), as an aid to smoking cessation in women (B. H. Marcus et al., 2003). While the exercise intervention did not result in significant differences between groups, those participants that did adhere to the intervention demonstrated significantly increased odds of 7-day point prevalence smoking abstinence at the end of treatment (B. H. Marcus et al., 2005).
Although more work is needed in this area (see (M. H. Ussher et al., 2000), it now appears that exercise may be a useful component of smoking cessation interventions. In light of overlapping etiologies and treatment approaches for both tobacco and alcohol use disorders (Gulliver et al., 1995; Sher, Gotham, Erickson, & Wood, 1996), we believe that research efforts in exercise and smoking cessation may provide a template for extending this work to intervention efforts with alcohol use disorders.
Polysubstance Use
While we could find no studies examining the efficacy of exercise in adults with drug abuse or dependence, exercise interventions have been applied with adolescent substance abusers. Collingwood et al. (Collingwood, Reynolds, Kohl, Smith, & Sloan, 1991) conducted a clinical trial of an 8-9 week structured fitness program with adolescent substance abusers. Participants were recruited from either a school-based “at risk” prevention program, a community substance abuse treatment program, or an inpatient hospital for substance abuse. The physical fitness program involved 1-2 meetings per week with an assignment to engage in two exercise sessions outside of the meetings. Overall, participants showed improved physical fitness (i.e., reduced time to run one mile, increased number of sit-ups in one minute, increased number of push-ups in one minute, increased flexibility, and reduced percentage of body fat), reduced polysubstance use and increased abstinence rates. Adolescents who improved on fitness outcomes also had greater self-concept and reduced anxiety and depressive symptoms relative to those whose fitness did not improve.
In a larger scale study, Collingwood, Sunderlin, and Kohl (Collingwood, Sunderlin, & Kohl, 1994) evaluated the effects of an 8 – 16 week physical fitness skills training program on substance use in a sample of approximately 1500 “at-risk” adolescents. Although pre-post data were available for only a subset of the sample, outcome data revealed general improvements in fitness and self-concept, and in reduced use of cigarettes, alcohol, marijuana, uppers, cocaine, hallucinogens, steroids, and designer drugs.
Alcohol
To our knowledge, only two controlled studies have been implemented specifically to examine the effects of an exercise intervention in individuals with excessive alcohol use or alcohol use disorders. The first study by Sinyor and colleagues (Sinyor et al., 1982) reported on 58 participants receiving inpatient alcohol rehabilitation treatment. Participants engaged in six weeks of “tailored” exercise, consisting of progressively more rigorous physical exercise including stretching, calisthenics and walking/running. Results revealed that these participants demonstrated better abstinence outcomes post-treatment than did non-exercising participants from two other small comparison groups. Significant differences between exercisers and non-exercisers continued at 3-month and 18-month follow-up. Exercisers also experienced significant reductions in percent body fat and increases in maximum oxygen uptake during the course of the intervention.
A later study by Murphy, Pagano, and Marlatt (Murphy et al., 1986) randomly assigned heavy drinking college students to either running, yoga/meditation, or no treatment control. Participants tracked their exercise and drinking behavior using daily self-report journals over eight weeks. Analysis of post-intervention data indicated that participants assigned to either of the exercise conditions (running or yoga) demonstrated significant decreases in quantity of alcohol consumption that were not experienced by control participants.
Findings from both studies are consistent in supporting a positive relationship between exercise and drinking outcomes. However, both studies also suffer from methodological limitations. In the Sinyor et al. (Sinyor et al., 1982) study, participants were not randomly assigned to exercise or comparison conditions. Rather, participants in the exercise condition were compared to two small (n's = 9 and 12) comparison groups: one consisting of exercise participants from the exercise condition who did not fully participate and the other consisting of unmatched controls from another treatment facility. Thus enhanced drinking outcomes experienced by exercise participants could have been a function of factors other than exercise. Despite its overall methodological rigor, the Murphy et al. (Murphy et al., 1986) study was limited by small sample size (e.g., n = 13 in the running condition), reliance on self-report measures of exercise behavior and high dropout of participants due to dissatisfaction based on their group assignment.
These two studies in alcohol use and studies in tobacco and polysubstance use suggest the potential for positive outcomes to be achieved with exercise interventions for alcohol dependent patients. However, more work is clearly needed to develop standardized, structured, exercise-based interventions and to evaluate them in methodologically rigorous clinical trials. The current study is intended to address this need by describing the development of a moderate-intensity aerobic exercise program for alcoholics.
Program Description
Components of Intervention
There are three components to the exercise intervention: 1) moderate-intensity aerobic exercise, 2) group behavioral training component, and 3) incentive system.
Aerobic Exercise Component of the Exercise Intervention
In developing the aerobic exercise component of the intervention, considerable attention was paid to the type and structure of the exercise intervention to be developed. We considered whether to have all exercise sessions supervised, to have some sessions supervised and some take place independently, or to have all sessions take place independently. While previous studies have primarily utilized supervised exercise sessions, we were concerned that sole reliance on supervised exercise sessions might limit the generalizability of the program by serving to reduce the maintenance of exercise adherence during the follow-up period, once the intervention had ended. This is consistent with the findings of Perri and colleagues (1997), who found that adherence did not differ during the intervention phase between participants engaged in supervised versus home-based exercise programs, however higher rates of long-term exercise adherence were found in home-based exercise conditions. Therefore, we decided to have one weekly supervised exercise session and, through the behavioral group component, encourage and aid participants to develop independent exercise the other days of the week.
In determining the intensity of aerobic exercise, we considered the current public health recommendation for minimal levels of physical activity that produce health benefits (USDHHS, 1996). These current recommendations are for individuals to engage in moderate intensity physical activity for 30 minutes per day on most days of the week. Also, recent evidence suggests that adherence rates for exercise can be significantly improved with moderate intensity versus high intensity activity (Cox, Burke, Gorely, Beilin, & Puddey, 2003; Perri et al., 2002). Therefore, we decided to test the effect of a moderate-intensity exercise intervention.
In addition, we also considered whether the exercise intervention should be in an individual or group format. While previous studies varied with respect to format, we utilized a group intervention with rolling admissions, as we expected that it might be difficult to recruit an entire cohort of participants who would begin the intervention at the same time. We decided on a group format for a couple of reasons. First, there exists evidence in the exercise literature that group interventions are associated with higher adherence rates (Estabrooks & Carron, 1999). Also, group interventions, in general, can offer the added advantage of social support for behavior change – sedentary lifestyle and abstinence from alcohol, in this case.
Participants were required to attend supervised (by an exercise physiologist) aerobic exercise group sessions once a week at the study fitness facility. Prior to engaging in exercise, a breath analysis was conducted to confirm abstinence. At the beginning of the first weekly exercise group, the exercise physiologist provided information regarding the psychological and physical benefits of moderate intensity exercise. In addition, the exercise physiologist oriented the participant on all aspects of the exercise intervention which includes proper use of exercise equipment, warm-up and cool-down procedures, hours of operation of the exercise facility, and any other logistical procedures related to the exercise intervention.
At each exercise intervention session, the exercise physiologist guided participants on the intensity and duration of the exercise to be performed. Exercise sessions began at 20 minutes per session and gradually progressed to 40 minutes per session by week 12. Participants exercised at a rate that achieved 55 - 69% (moderate-intensity) of age-predicted maximal heart rate. This exercise regimen is consistent with the guidelines offered by the American College of Sports Medicine (ACSM; (American College of Sports Medicine, 2000). Heart rate and blood pressure were monitored before, during, and after exercise. Each workout session also included a 5-minute warm-up and a 10-minute cool-down to ensure safe exercise procedures. Several types of exercise equipment were available to study participants, including treadmills, recumbent bicycles, and elliptical machines. Participants in the study were also given “prescriptions” from the exercise physiologist (tailored to their level of fitness) to engage in moderate-intensity aerobic exercise on a minimum of two to three other occasions during the week for the duration of the 12- week exercise program. These other exercise occasions took place in the context of their own environment (e.g., in their home or through community resources). In addition, participants were required to self-monitor their exercise by filling out a weekly exercise log with the various exercise activities they engaged in during the week, the duration of each activity, and their self-reported rate of perceived exertion (RPE) for each activity.
Weekly Group Behavioral Training Component of the Exercise Intervention
Given the demonstrated efficacy of behavioral modification strategies in increasing physical activity (see meta-analysis by (Dishman & Buckworth, 1996), this weekly, brief (15-20 minutes) group intervention based on cognitive and behavioral techniques was incorporated as a component of the exercise intervention. Through these weekly groups, participants were guided as to how to increase overall fitness through behavioral changes in their daily lives. Each brief group session was focused on a certain topic designed to increase overall motivation resulting in improved exercise adherence and maintenance.
Decisions about which topics should be included in the behavioral group intervention were based on a review of the literature on factors influencing exercise adherence and our research team's clinical and research experience in developing and implementing interventions designed to elicit behavior changes among populations with addictive behaviors. For example, reviews of the literature on factors that predict exercise adherence reveal that time management skills, knowledge of exercise, social support, perception of barriers, perceived choice of activity, enjoyment of activity, perceived benefits of exercise are all important in predicting adherence to exercise interventions (Rhodes et al., 1999; Woodard & Berry, 2001). Each of these topics was included in the behavioral component treatment manual. Further, we utilized our familiarity and experience with the principles of relapse prevention (Marlatt & Gordon, 1985) and the Transtheoretical Model of Change (Prochaska & DiClemente, 1983) and included topics related to utilizing cognitive-behavioral approaches to overcoming barriers, getting back on track after a lapse into sedentary behavior, and motivating participants into action. On week 12 of the exercise intervention, participants received a certificate of program completion that was presented during the behavioral group component.
The behavioral training component consisted of the following modules:
1. Benefits of Exercise. This component focused on the psychological and physical/health benefits of exercise. In addition, the discussion addressed exercise's benefits on self-confidence and preventing relapse.
2. Benefits of Exercise for Alcohol Recovery. This component focused on the potential mechanisms by which exercise may be beneficial in alcohol recovery such as achievement of pleasurable state without the use of alcohol, positive alternative behavior, group activity that provides social support, decreased stress and improved coping.
3.Goal Setting. This component focused on goal setting as a skill that is acquired through practice, how short-term goals can be developed to achieve long-term goals, specific steps toward developing effective goals (i.e., generating goals that are personal, attainable, realistic, and measurable), and generating exercise-specific goals.
4. Getting and Staying Motivated. This component focused on various techniques to get and stay motivated for exercise such as selecting activities that are enjoyable, generating and utilizing positive self-statements, visualization of success, and generating rewards for accomplished exercise goals.
5. Getting Back on Track. This component focused on how to identify situations considered high-risk for deviating from an exercise program and developing strategies on how to plan for and handle these high-risk situations.
6. Exercise and Coping with Negative Moods. This component focused on the benefits of exercise for coping with depressive and anxious moods in addition to improvements in self-concept.
7. Identifying and Overcoming Barriers. This component focused on how to identify both planned and unplanned barriers to engaging in exercise along with various cognitive and behavioral strategies to deal with them.
8. Time Management. This component focused on the key principles of time management such as prioritizing and planning activities with a special emphasis on exercise.
9. Basic Information for Exercising Wisely. This component focused on ACSM's guidelines for physical activity, how to determine whether one is engaging in moderate exercise, and the 3 components of a good workout (i.e., warm-up, moderate intensity aerobic activity, and cool-down).
10. Maintenance of Exercise. This component focused on various techniques to increase the likelihood that the exercise program will be maintained long-term such as: seeking support of others, engaging in enjoyable activities, trying new activities, setting new goals, thinking positively, reflecting on previous exercise accomplishments, and utilizing problem solving skills to address barriers to exercise.
11. Making Plans for Action. This component focused on the stages of change along with techniques to move from various stages into the action stage.
12. Social Support. This component focused on how to seek different types of support from others including general encouragement, exercise advice from knowledgeable individuals, and exercising with others.
Incentive Component of the Exercise Intervention
In order to maximize adherence to the exercise program and self-monitoring of daily exercise activities, participants earned incentives for various levels of adherence to the exercise program. The decision to include a monetary incentive as a component of the exercise intervention was based on a review of studies that have found monetary incentives to be effective in increasing adherence to exercise (Jeffery & French, 1999; Jeffery, Wing, Thorson, & Burton, 1998; Robison & Rogers, 1995; Robison et al., 1992) and work with contingency management and monetary reinforcement as related to smoking and substance use outcomes (Higgins, Heil, & Lussier, 2004; Tidey, O'Neill, & Higgins, 2002). The incentive plan consisted of providing participants with $5 for attending each weekly combined group/exercise session and an additional $5 for returning their completed exercise self-monitoring form (from the prior week) at that session. In addition, participants earned the opportunity to draw a prize from a fish bowl (value ranging from $10-$50) at each weekly session, if they had consecutive attendance (i.e., had attended the prior week's group + exercise session).
Procedure
Participants
Eligible participants: (a) were between 18 and 65 years of age, (b) met current DSM-IV criteria for alcohol dependence as assessed by the Structured Diagnosic Interview for DSM-IV (SCID-P), (c) were sedentary; i.e., have not participated regularly in aerobic physical exercise (for at least 20 minutes per day, three days per week) for the past six months, and (d) were engaged in outpatient alcohol treatment.
Exclusion criteria include: (a) current DSM-IV diagnosis of drug dependence (except nicotine dependence) as assessed by the SCID-P, (b) DSM-IV diagnosis of anorexia or bulimia nervosa as assessed by the SCID-P, (c) DSM-IV diagnosis of bipolar disorder as assessed by the SCID-P, (d) a history of psychotic disorder or current psychotic symptoms as assessed by the SCID-P, (e) current suicidality or homicidality, (f) marked organic impairment according to either the medical record or responses to the diagnostic assessments, (g) physical disabilities or medical problems (such as history of diabetes, uncontrolled hypertension, seizure disorder, coronary artery disease, valvular heart disease, and pulmonary disease) or use of medications (such as beta blockers) that would prevent or hinder participation in a program of moderate exercise, and (h) current pregnancy or intent to become pregnant during the next 12 weeks.
Recruitment
Participants were recruited from two sources. First, the medical records of all patients entering the intensive alcohol and drug treatment partial-hospitalization program at a psychiatric hospital in the Northeast were screened for the psychiatric and medical inclusion and exclusion criteria detailed above. Second, interested participants responding to study advertisements in the local newspaper called the study center. In each case, participants were screened to determine the sedentary criteria. Next participants were evaluated using the diagnostic and assessment measures detailed below to confirm eligibility. Finally, participants underwent a graded exercise test under the supervision of the study physician.
The study physician reviewed the patient's medical history. A resting electrocardiograph (ECG) was then obtained. Unless contraindicated by the resting ECG, the physician then had the patient complete a submaximal graded exercise test on a treadmill, with continuous ECG monitoring using a modified, Balke-Ware protocol (American College of Sports Medicine, 2000) that was terminated at 85% of the participant's age-predicted maximal heart rate (see below for a more detailed description of the submaximal graded treadmill test). The physician reviewed the results of the graded exercise test and made the final determination regarding study eligibility. Based on this test, if moderate to vigorous intensity exercise was contraindicated for a participant, this participant was excluded from participation in the study and referred to his/her primary care physician for a follow-up evaluation.
Follow-up Assessments
Participants completed follow-up interviews 3 and 6 months following recruitment into the study. Alcohol breathanalysis was used to confirm sobriety during all interviews. To reduce attrition, participants were paid $40 and $50 for completion of the 3- and 6-month follow-up interviews, respectively. All payments were made in gift certificates to a local mall or supermarket. Cab rides were provided for participants who were unable to transport themselves to our center for follow-up interviews. When necessary, friends or relatives listed by participants as potential contacts were asked to provide information about participants’ whereabouts. Due to concerns that relapsers’ feelings of discouragement and/or fear that they have disappointed the experimenters might serve to reduce adherence and honest reporting, all participants were informed that the information they provided was extremely important regardless of how well they might be doing regarding their drinking outcomes or exercise adherence.
Measures
Physical Activity Screen
To ensure that potential participants were blind to the nature of the screening procedure, the physical activity screen was embedded in a brief interview that assessed a variety of health behaviors such as sun safety, seatbelt use, and nutritional practices. The exercise portion of this interview determined whether patients were sedentary by asking participants the frequency and duration of at least moderate-intensity exercise (described as the equivalent of a brisk walk) over the last six months. Participants were considered sedentary if their responses suggested they were exercising less than 3 times a week for at least 20 minutes over the entire previous six months. In addition, demographic information such as date of birth, gender, ethnicity/race, marital status, and years of education were collected and re-confirmed at the diagnostic interview.
Structured Clinical Interview for DSM-IV (SCID-P; (First, Spitzer, Gibbon, & Williams, 1995)
Psychiatric disorders, both current and lifetime, for establishing inclusion/exclusion criteria were determined by the relevant sections of the SCID-P (First et al., 1995).
Time-Line-Follow-Back (TLFB; (L. C. Sobell & Sobell, 1996)
The TLFB interview (M. B. Sobell et al., 1980) was utilized to assess alcohol and drug use at baseline and during the follow-up intervals. At baseline, it was administered for the 90 days prior to admission and at each follow-up interval for the period since its last administration. The TLFB interview is a calendar-assisted structured interview that provides a way to cue memory so that accurate recall is enhanced. A structured interview of patients’ drinking behavior has been found to be the most reliable and valid method of assessing prior alcohol use (L. C. Sobell & Sobell, 1979, 1980). In particular, the TLFB interview has excellent reliability (L. C. Sobell, Maisto, Sobell, & Cooper, 1979) and validity (L. C. Sobell & Sobell, 1980). The TLFB was developed to obtain information about amount of drinking over extended periods of time.
Data from the TLFB were summarized by month to yield the primary alcohol outcome variables: drinks per possible drinking day [i.e., total number of standard drinks consumed divided by number of days not in a restricted environment where unable to drink (e.g., incarcerated or in residential treatment)] and percent days abstinent (i.e., number of abstinent days divided by number of days not in a restricted environment).
Breathanalysis
At baseline, each exercise session, and each follow-up assessment, expired breath was analyzed for alcohol to confirm abstinence prior to exercise participation and interview completion and to further corroborate self-report data.
Cardiorespiratory Fitness was assessed using a submaximal graded exercise protocol on a motorized treadmill at baseline and follow-up evaluations. Prior to each graded exercise test, the study physician (or masters-level exercise physiologist at follow-up) reviewed the participant's medical history (see above Health Questionnaire) and obtained a baseline 12-lead electrocardiogram and blood pressure reading. For the graded exercise test, the speed of the treadmill was constant at 3.5 mph with the initial grade set at 0% and progressed by 1% at 1-minute intervals. During testing, heart rate was assessed each minute and at the point of test termination using a 12-lead electrocardiogram. Blood pressure was measured at 2-minute intervals during the test. Further, ratings of perceived exertion (Borg 6-20 scale) were obtained at one-minute intervals during the test. This submaximal exercise test was terminated at 85% of the participant's age predicted maximal heart rate (age-predicted maximal heart rate = 220 – age of participant). In addition, test termination guidelines outlined by the American College of Sports Medicine (American College of Sports Medicine, 2000) were followed. Following test termination, each participant underwent a 3-minute active cool-down and 5-7 minute seated cool-down, during which time heart rate and blood pressure were assessed.
For the purpose of data analysis, cardiorespiratory fitness was defined in two ways. First, equations published by the American College of Sports Medicine (American College of Sports Medicine, 2000) were used to estimate the metabolic equivalents (MET) level at which the participant achieved 85% of their age-predicted maximal heart rate. In addition, the time point during the exercise test at which the participant achieved 85% of their age-predicted maximal heart rate was used for data analysis. Increases in MET level and/or duration of the exercise test are representative of improvements in cardiorespiratory fitness.
Body Composition was evaluated across multiple domains. Weight was measured using a calibrated medical scale. Following procedures outlined by the ACSM's Guidelines for Exercise Testing and Prescription (American College of Sports Medicine, 2000), body fat percentage was assessed using skinfold thickness measured on the right side at the triceps, suprailiac crest, and thigh using a Lange caliper. Also consistent with ACSM guidelines, body mass, or Quilet, index (BMI) was calculated by dividing body weight in kilograms by height in meters squared. The exercise physiologist for the study conducted these assessments.
Preliminary Outcomes
Sample Characteristics
From the alcohol and drug partial hospital treatment program, 257 charts of patients with alcohol dependence were screened. In addition, 71 calls were received in response to newspaper advertisements for the research study. Of these 328 potential participants, rule outs included: 91 (28%) for a concomitant non-alcohol substance use diagnosis, 43 (13%) for a medical problem that would prohibit safely participating in exercise, 43 (13%) were not sedentary, 37 (11%) refused participation in the study (applicable only to patients from the partial hospital treatment program), 27 (8%) for one of the psychiatric exclusion criteria, 16 (5%) were not involved in ongoing substance abuse treatment (applicable only to those calling in response to the newspaper advertisement), 10 (3%) for being older than 65 years old. In addition, during the recruitment process, 33 (10%) of potential participants either expressed loss of interest in the study, were unable to attend group nights, or we lost contact with them.
As a result, the remaining 27 participants were scheduled for baseline assessments. Of these, one was excluded because of a SCID-diagnosed psychiatric rule out (bipolar disorder), 4 did not receive medical clearance from the study physician during the submaximal graded exercise test, and 2 decided to no longer participate during the baseline assessment phase. Therefore, 20 participants were eligible to participate in the 12-week aerobic exercise intervention. Of these, one participant did not attend the intervention and was not able to be contacted further. Thus, the study sample was comprised of 19 participants who initiated the exercise intervention.
The sample of 19 participants included 11 (57.9%) females and 8 (42.1%) males. The mean age of participants was 44.4 (SD = 7.1) years. The sample was primarily Caucasian (18 of 19; 94.7%) with an average of 13.4 (SD = 2.4) years of education. Ten participants (52.6%) were either divorced or separated, while six (31.6%) were married and three (15.8)% were single or never married. In addition, nine participants (47.4%) were employed full-time, while six (31.6%) were working part-time and four (21.1%) were currently unemployed. All participants were engaged in ongoing addiction treatment for alcohol dependence. All participants reported their last drink within the last 2 months (range 0-58 days ago) with a mean of 19.4 (SD = 15.0) days elapsing since last drink.
Treatment Adherence
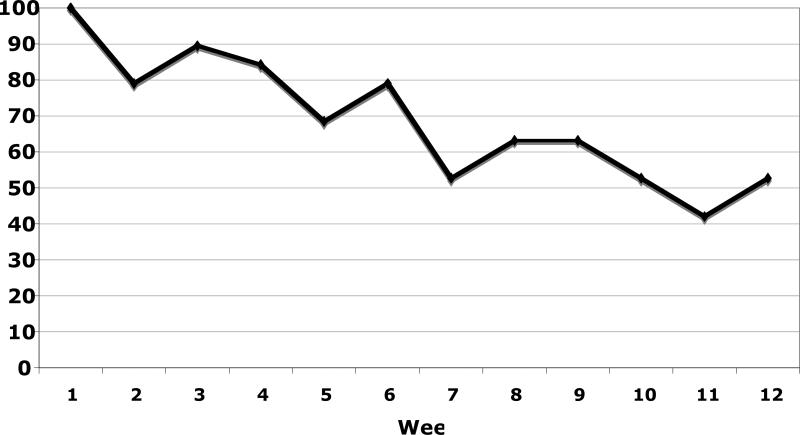
Attendance for each week of the exercise intervention is displayed in Figure 1. During the 12-week intervention, participants attended an average of 8.2 (SD = 3.8) weekly exercise sessions. Over the course of the 12-week intervention, participants exercised an average of 4.2 (SD = 1.4) days per week. In addition, participants engaged in an average of 233 (SD = 127) minutes of physical activity per week with 152 (SD = 102) of these minutes being at an exertion level of at least moderate intensity. Further, participants earned an average of $81 (SD = 37) for weekly incentives for session attendance and completion of self-monitoring forms, and $113 (SD = 71) from the fishbowl drawing for attending exercise sessions on consecutive weeks.
Figure 1.
Weekly Att
Drinking Outcomes
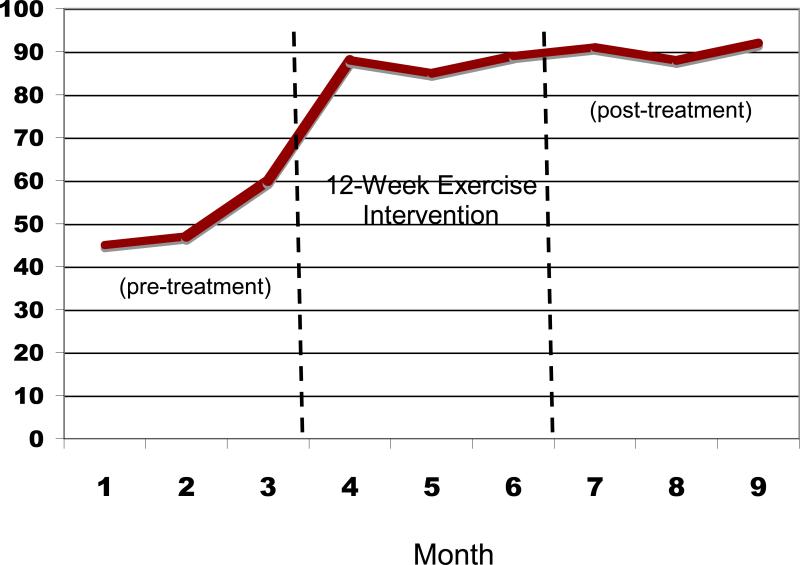
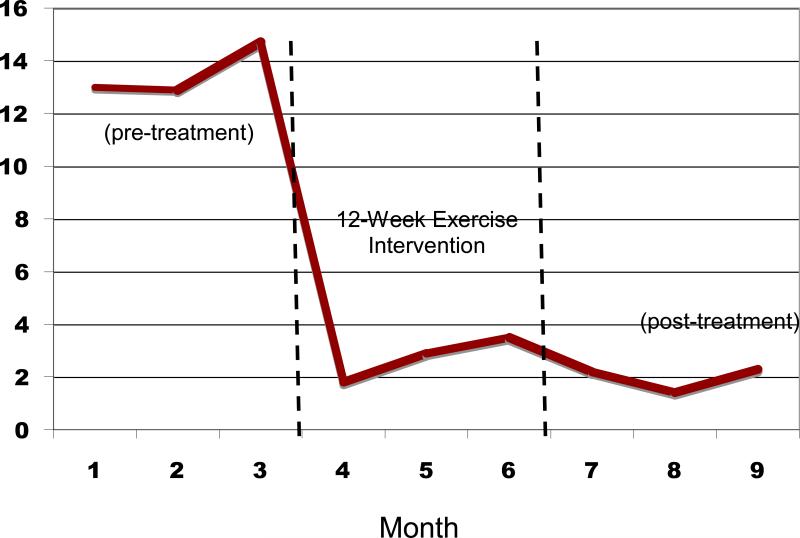
Figure 2 displays the mean percent days abstinent (PDA) for pretreatment (3 months prior to beginning the exercise intervention), during the 12-week exercise intervention, and the 3 months following the end of the intervention. Similarly, Figure 3 displays the mean number of drinks per drinking day at each of these timepoints.
Figure 2.
Percent Days Abstinent before and after the 12-Week Exercise Intervention
Figure 3.
Drinks per Drinking Day before and after the 12-Week Exercise Intervention
These results suggest that, compared to the mean pretreatment percent days abstinent (PDA), significant increases in PDA were observed at the end of the 12-week exercise intervention (t=3.94, df=17, p=.001) and at the 3-month post-intervention follow-up (t=4.77, df=15, p<.001). In addition, there was a trend toward decreased drinks per drinking day at end of treatment (t= 2.0, df=17, p=.06) and a significant reduction in drinks per drinking day at 3-months post-intervention (t=2.43, df=15, p<.05) compared to baseline levels of drinking.
Cardiorespiratory Outcomes
Table 1 displays the means and standard deviations of each of the cardiorespiratory and body composition measures at baseline, end of treatment, and the 3-month post-intervention follow-up. Paired sample t-test comparisons were conducted to determine whether there were statistically significant differences between each baseline measure of cardiorespiratory fitness and body composition and their corresponding measure at the end of treatment and 3-month assessment timepoints. Compared to baseline, participants significantly improved on the duration of the submaximal treadmill test at end of treatment (t=2.35, df=15, p<.05). Further, participants demonstrated a decrease in body mass index from baseline to end of treatment (t=2.19, df=15, p<.05). No significant differences were observed between baseline and the 3-month post intervention follow-up on measures of cardiorespiratory fitness or body composition.
Table 1.
Changes in Cardiorespiratory Fitness and Body Composition over Time
| Baseline | End of Treatment | 3-Month Follow-up | |
|---|---|---|---|
| Metabolic Equivalents (METs) | 8.1(2.1) | 8.9(2.6) | 8.5(1.7) |
| Duration of Exercise Test (secs)* | 646.8(266.2) | 786.7(311.5) | 717.8(281.1) |
| Body Mass Index (BMI)* | 25.6(5.0) | 25.0(4.9) | 25.4(3.5) |
| % Body Fat | 26.1(7.7) | 23.9(7.3) | 26.4(7.1) |
| Waist-to-Hip Ratio | .87(.07) | .87(.07) | .89(.09) |
Significant differences between baseline and end of treatment.
Numbers in parentheses reflect standard deviations of the means.
Conclusion
Relapse continues to pose a major problem to the alcohol treatment field as a whole and to individuals attempting recovery from alcohol use disorders. Studies evaluating strategies to enhance maintenance of treatment gains have devoted relatively little attention to lifestyle modification, and research in the area of exercise and recovery from alcohol use disorders is still in its infancy. For over two decades, researchers have called for studies examining the role of exercise in recovery from alcohol and other substance use disorders, yet to date, only two controlled trials from the 1980's have specifically examined the application of exercise interventions in individuals with alcohol use problems. Further, many patients in substance abuse programs express interest in incorporating exercise into their recovery (Read et al., 2001b). In light of the promising findings from these studies as well as those from well-controlled studies in smoking cessation, more research is clearly needed in this promising area.
To address the existing gap in this literature, we developed an aerobic exercise intervention as an adjunct to addiction treatment for alcohol dependent patients. In this pilot study, sedentary alcohol dependent patients engaged in a 12-week individually-tailored moderate-intensity aerobic exercise intervention. The preliminary outcomes of this pilot study revealed good adherence to the exercise intervention with demonstrated benefits in cardiorespiratory fitness by the end of the 12-week intervention. In addition, compared to baseline, there were significant increases in percent days abstinence as well as decreases in drinks per drinking day at follow-up timepoints.
This program is notable in that it represents the development and evaluation of a structured and well-specified aerobic exercise intervention for alcohol dependent individuals. The intervention has several novel components, including cognitive-behavioral components to facilitate the adoption and maintenance of exercise behavior, an incentive system to increase program adherence and the involvement of an exercise physiologist to provide participants with individually tailored exercise prescriptions.
Limitations of this pilot study include the lack of a control group and a small sample size. These limitations prevent us from making definitive statements about the efficacy of the exercise intervention and from examining mediating mechanisms whereby participation in aerobic exercise may have led to reduced drinking outcomes. However, the current study's promising findings warrant further investigation of the efficacy of the exercise intervention in future randomized clinical trials. If the efficacy of this moderate-intensity aerobic exercise intervention can be demonstrated, alcohol dependent patients may be provided with a valuable adjunct to traditional alcohol treatment. Furthermore, future studies may contribute much-needed knowledge about the role of aerobic exercise in reducing alcohol use and increasing fitness in alcohol dependent patients.
ACKNOWLEDGMENTS
Supported in part by grant AA13418 from the National Institute on Alcoholism and Alcohol Abuse to Dr. Richard A. Brown
Contributor Information
Richard A. Brown, The Warren Alpert Medical School of Brown University/Butler Hospital.
Ana M. Abrantes, The Warren Alpert Medical School of Brown University/Butler Hospital
Jennifer P. Read, University at Buffalo, The State University of New York
Bess H. Marcus, The Warren Alpert Medical School of Brown University/The Miriam Hospital
John Jakicic, University of Pittsburgh
David R. Strong, The Warren Alpert Medical School of Brown University/Butler Hospital
Julie R. Oakley, The Westerly Hospital, Westerly, Rhode Island
Susan E. Ramsey, The Warren Alpert Medical School of Brown University/Rhode Island Hospital
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University
Gregory G. Stuart, The Warren Alpert Medical School of Brown University/Butler Hospital
Mary Ella Dubreuil, Butler Hospital.
Alan A. Gordon, Butler Hospital
References
- Abrams DB, Niaura RS. Social learning theory. In: Leonard HTBKE, editor. Psychological theories of drinking and alcoholism. The Guilford Press; New York: 1987. pp. 131–178. [Google Scholar]
- Agne C, Paolucci K. A holistic health approach to an alcoholic treatment program. Journal of Drug Education. 1982;12:137–145. [Google Scholar]
- American College of Sports Medicine . Guidelines for exercise testing and prescription. Lippincott, Williams and Wilkins; New York: 2000. [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. American Psychiatric Association; Arlington, VA: 2000. Text Revision. [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, et al. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychomomatic Medicine. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A cognitive theory. Prentice Hall; Englewood Cliffs: 1986. [Google Scholar]
- Bartha R, Davis T. Holism and high level wellness. Journal of Alcohol and Drug Education. 1982;28:28–31. [Google Scholar]
- Berger B, Owen D. Mood alteration with yoga and swimming: Aerobic exercise may not be necessary. Perceptual and Motor Skills. 1992;75:1331–1343. doi: 10.2466/pms.1992.75.3f.1331. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24(3):399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Boutcher SH. Conceptualization and quantification of aerobic fitness and physical activity. In: Seraganian P, editor. Exercise psychology: The influence of physical exercise on psychological processes. John Wiley & Sons; New York, N.Y.: 1993. [Google Scholar]
- Bovens AM, Van Baak MA, Vrencken JG, Wijnen JA, Saris WH, Verstappen FT. Physical activity, fitness, and selected risk factors for CHD in active men and women. Medicine and Science in Sports and Exercise. 1993;25:572–576. [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155(5):603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive behavioral treatment for depression in alcoholism. Journal of Consulting and Clinical Psychology. 1997;65(5):715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Ramsey SE. Addressing comorbid depressive symptomatology in alcohol treatment. Professional Psychology: Research and Practice. 2000;31(4):418–422. [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. American Psychologist. 1986;41(7):765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Byrne A, Bryne DG. The effect of exercise on depression, anxiety and other mood states: A review. Journal of Psychosomatic Research. 1993;37(6):565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Szabo A, Capafons J. Anxiety and heart rate under psychological stress: The effects of exercise training. Anxiety, Stress, and Coping: An International Journal. 1996;9(321337) doi: 10.1080/10615809608249409. [DOI] [PubMed] [Google Scholar]
- Carlson NR. Physiology of behavior. Allyn & Bacon; Boston: 1991. [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4(1):46–54. [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Cheraskin E, Ringsdorf WM. Predictive medicine: Physical activity. Journal of the American Geriatric Society. 1971;19:969–973. doi: 10.1111/j.1532-5415.1971.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. Journal of Drug Education. 1991;21(1):73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Sunderlin J, Kohl HW. The use of a staff training model for implementing fitness programming to prevent substance abuse with at-risk youth. American Journal of Health Promotion. 1994;9:20–23. 33. doi: 10.4278/0890-1171-9.1.20. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Alcohol use and risky sexual behavior among college students and youth: Evaluating the evidence. Journal of Studies on Alcohol. 2002;(Suppl14):101–117. doi: 10.15288/jsas.2002.s14.101. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40-65 years: The S.W.E.A.T. Study (Sedentary Women Exercise Adherence Trial). Preventive Medicine. 2003;36(1):17–29. doi: 10.1006/pmed.2002.1134. [DOI] [PubMed] [Google Scholar]
- Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. Journal of Sport and Exercise Psychology. 1998;20:339–357. [Google Scholar]
- Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Medicine & Science in Sports and Exercise. 1987;19(5 Suppl):S114–120. [PubMed] [Google Scholar]
- Cronan TL, Howley ET. The effect of training on epinephrine and norepinephrine excretion. Medicine in Science and Sports. 1974;5:122–125. [PubMed] [Google Scholar]
- Dishman RK, Buckworth J. Increasing physical activity: A quantitative synthesis. Medicine and Science in Sports and Exercise. 1996;28:706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Donovan C, McEwan R. A review of the literature examining the relationship between alcohol use and HIV-related sexual risk-taking in young people. Addiction. 1995;90(3):319–328. doi: 10.1046/j.1360-0443.1995.9033192.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM. Marlatt's classification of relapse precipitants: is the Emperor still wearing clothes. Addiction. 1996;91(Supplement):S131–S137. [PubMed] [Google Scholar]
- Doyne EJ, Chambless DL, Beutler LE. Aerobic exercise as a treatment for depression in women. Behavior Therapy. 1983;14:434–440. [Google Scholar]
- Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, Neimeyer RA. Running versus weight lifting in the treatment of depression. Journal of Consulting and Clinical Psychology. 1987;55(5):748–754. doi: 10.1037//0022-006x.55.5.748. [DOI] [PubMed] [Google Scholar]
- Estabrooks PA, Carron AV. Group cohesion in older adult exercisers: prediction and intervention effects. Journal of Behavioral Medicine. 1999;22(6):575–588. doi: 10.1023/a:1018741712755. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Frankel A, Murphy J. Physical fitness and personality in alcoholism. Quarterly Journal of Studies on Alcohol. 1974;35:1272–1278. [PubMed] [Google Scholar]
- Froelich JC. Opioid peptides. Alcohol Health and Research World. 1997;21:132–135. [PMC free article] [PubMed] [Google Scholar]
- Gmel G, Rehm J. Harmful alcohol use. Alcohol Research and Health. 2003;27(1):52–62. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Dependence. 2004;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS. Suicidal ideation among the United States drinking population: Results of the National Longitudinal Epidemiological Survey. Journal of Studies on Alcohol. 1999;60:422–429. doi: 10.15288/jsa.1999.60.422. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, et al. The effect of depression on return to drinking: A prospective study. Archives of General Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Gulliver SB, Rohsenow DJ, Colby SM, Dey AN, Abrams DB, Niaura RS, et al. Interrelationship of smoking and alcohol dependence, use and urges to use. Journal of Studies on Alcohol. 1995;56(2):202–206. doi: 10.15288/jsa.1995.56.202. [DOI] [PubMed] [Google Scholar]
- Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Report prepared by the Lewin Group for the National Institute on Alcohol Abuse and Alcoholism. 2000.
- Hickey N, Mulcahy R, Bourke T, Graham I, Wilson-Davis K. Study of coronary risk factors related to physical activity in 15,171 men. British Medical Journal. 1975;3:507–509. doi: 10.1136/bmj.3.5982.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review in Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Hill JS. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Canadian Journal of Public Health. 1985;76:183–186. [PubMed] [Google Scholar]
- Hill RD, Rigdon M, Johnson S. Behavioral smoking cessation treatment for older chronic smokers. Behavior Therapy. 1993;24:321–329. [Google Scholar]
- Hobson ML, Rejeski WJ. Does the dose of acute exercise mediate psychophysiological responses to mental stress? Journal of Sport Psychology. 1993;15:77–87. [Google Scholar]
- Hodgins DC, el Guebaly N, Armstrong S, Dufour M. Implications of depression on outcome from alcohol dependence: A 3-year prospective follow-up. Alcoholism: Clinical and Experimental Research. 1999;23:151–157. [PubMed] [Google Scholar]
- Irvin JE, Bowers CA, Dunn ME, Wang MC. Efficacy of relapse prevention: A meta-analytic review. Journal of Consulting and Clinical Psychology. 1999;67:563–570. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, French SA. Preventing weight gain in adults: the pound of prevention study. American Journal of Public Health. 1999;89(5):747–751. doi: 10.2105/ajph.89.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. Journal of Consulting and Clinical Psychology. 1998;66(5):777–783. doi: 10.1037//0022-006x.66.5.777. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. The influence of traits of disinhibition on the association between alcohol use and risky sexual behavior. Alcoholism: Clinical and Experimental Research. 2000;24(7):1028–1035. [PubMed] [Google Scholar]
- Kawatchi I, Troisi RJ, Rotnitzky AG, Coakley EH, Codlitz MD. Can physical activity minimize weight gain in women after smoking cessation? American Journal of Public Health. 1996;86:999–1004. doi: 10.2105/ajph.86.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SM. Physical fitness hastens recovery from psychological stress. Medicine and Science in Sports and Exercise. 1980;12:118–119. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs. exercise in weight maintenance: The effects of minimal intervention strategies on long-term outcomes in men. Archives of Internal Medicine. 1989;149:2741–2746. doi: 10.1001/archinte.149.12.2741. [DOI] [PubMed] [Google Scholar]
- Kohl HW, LaPorte RE, Blair SN. Physical activity and cancer. Sports Medicine. 1988;6:222–237. doi: 10.2165/00007256-198806040-00004. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. British Medical Journal. 2001;322(7289):763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Zywiak WH. Alcohol treatment, changes in coping skills, self-efficacy, and levels of alcohol use and related problems 1 year following treatment initiation. Psychol Addict Behav. 2000;14(3):257–266. doi: 10.1037//0893-164x.14.3.257. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Internal Medicine. 1999;159(11):1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, Niaura RS, Abrams DB, Thompson PD. Usefulness of physical exercise for maintaining smoking cessation in women. American Journal of Cardiology. 1991;68(406407) doi: 10.1016/0002-9149(91)90843-a. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, Niaura RS, Taylor ER, Simkin LR, Feder SI, et al. Exercise enhances the maintenance of smoking cessation in women. Addictive Behaviors. 1995;20(1):87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine and Tobacco Research. 2005;7(6):871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, King TK, Albrecht AE, Hogan J, Bock B, et al. Rationale, design, and baseline data for Commit to Quit II: an evaluation of the efficacy of moderate-intensity physical activity as an aid to smoking cessation in women. Preventive Medicine. 2003;36(4):479–492. doi: 10.1016/s0091-7435(02)00051-8. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Lifestyle modification. In: Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. Second Edition Guildford Press; New York: 2005. [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. [Google Scholar]
- Marlatt GA, Witkiewitz K. Relapse prevention for alcohol and drug problems. In: Marlatt GA, Donovan DM, editors. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd Edition Guildford Press; New York: 2005. pp. 1–44. [Google Scholar]
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. Jouranal of Consulting and Clinical Psychology. 1997;65(1):190–194. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- McAuley E, Courneya KS, Lettunich J. Effects of acute and long-term exercise on self-efficacy responses in sedentary, middle-aged males and females. The Gerontologist. 1991;31:534–542. doi: 10.1093/geront/31.4.534. [DOI] [PubMed] [Google Scholar]
- McCann IL, Holmes DS. Influence of aerobic exercise on depression. Journal of Personality and Social Psychology. 1984;46:1142–1147. doi: 10.1037//0022-3514.46.5.1142. [DOI] [PubMed] [Google Scholar]
- Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, Luckie LF, et al. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. Second ed. Allyn & Bacon; Needham Heights: 1995. pp. 12–44. [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States. Jounal of Studies on Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97(3):265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Coping and social skills training. In: Hester RJ, Miller WR, editors. Handbook of alcoholism treatment approaches:Effective alternatives. Allyn and Bacon; Boston: 1995. pp. 221–241. [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–236. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101(2):212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: Effects of aerobic exercise and meditation. Addictive Behaviors. 1986;11:175–186. doi: 10.1016/0306-4603(86)90043-2. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- Oberman A. Exercise and the primary prevention of cardiovascular disease. American Journal of Cardiology. 1985;55:10D–20D. doi: 10.1016/0002-9149(85)91049-5. [DOI] [PubMed] [Google Scholar]
- Palmer J, Vacc N, Epstein J. Adult inpatient alcoholics: Physical exercise as a treatment intervention. Journal of Studies on Alcohol. 1988;49(5):418–421. doi: 10.15288/jsa.1988.49.418. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Palmer LK, Michiels K, Thigpen B. Effects of type of exercise on depression in recovering substance abusers. Perceptual and Motor Skills. 1995;80:523–530. doi: 10.2466/pms.1995.80.2.523. [DOI] [PubMed] [Google Scholar]
- Pernanen K. Alcohol in human violence. Guilford Press; New York: 1991. [Google Scholar]
- Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychology. 2002;21(5):452–458. [PubMed] [Google Scholar]
- Peterson M, Johnstone BM. The Atwood Hall Health Promotion Program Federal Medical Center, Lexington, KY: Effects on drug-involved federal offenders. Journal of Substance Abuse Treatment. 1995;12:43–48. [PubMed] [Google Scholar]
- Pickrell TM. Driver alcohol involvement in fatal crashes by age group and vehicle type. 2006. NHTSA Research Note, DOT HS 810 598.
- Pinto BM, Marcus BH. Physical activity, exercise and cancer in women. Medicine, Exercise, Nutrition, and Health. 1994;3:102–111. [Google Scholar]
- Pomerleau OF, Scherzler HH, Grunberg NE, Pomerleau CS, Judge J, Fertig JB, et al. The effects of acute exercise on subsequent cigarette smoking. Journal of Behavioral Medicine. 1987;10(2):117–127. doi: 10.1007/BF00846420. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Read JP, Kahler CW, Stevenson JF. Bridging the gap between alcoholism treatment and practice: What works and why. Professional Psychology: Research and Practice. 2001;32:227–238. [Google Scholar]
- Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol Research and Health. 2003;27(1):39–51. [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Gregg E, Thompson A, Berry M. The effects of varying doses of acute aerobic exercise on psychophysiological stress responses in highly trained cyclists. Journal of Sport and Exercise Psychology. 1991;13:188–199. [Google Scholar]
- Rhodes RE, Martin AD, Taunton JE, Rhodes EC, Donnelly M, Elliot J. Factors associated with exercise adherence among older adults. An individual perspective. Sports Medicine. 1999;28(6):397–411. doi: 10.2165/00007256-199928060-00003. [DOI] [PubMed] [Google Scholar]
- Robison JI, Rogers MA. Impact of behavior management programs on exercise adherence. American Journal of Health Promotion. 1995;9(5):379–382. doi: 10.4278/0890-1171-9.5.379. [DOI] [PubMed] [Google Scholar]
- Robison JI, Rogers MA, Carlson JJ, Mavis BE, Stachnik T, Stoffelmayr B, et al. Effects of a 6-month incentive-based exercise program on adherence and work capacity. Medicine and Science in Sports and Exercise. 1992;24(1):85–93. [PubMed] [Google Scholar]
- Rounsaville BJ, Dolinsky ZS, Babor TF, Meyer RE. Psychopathology as a predictor of treatment outcome in alcoholics. Archives of General Psychiatry. 1987;44:505–513. doi: 10.1001/archpsyc.1987.01800180015002. [DOI] [PubMed] [Google Scholar]
- Russell PO, Epstein LH, Johnston JJ, Block DR, Blair E. The effects of physical activity as maintenance for smoking cessation. Addictive Behaviors. 1988;13(2):215–218. doi: 10.1016/0306-4603(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ, Erickson DJ, Wood PK. A propspective, high-risk study of the relationship between tobacco dependence and alcohol use disorders. Alcoholism: Clinical and Experiemental Research. 1996;20:485–492. doi: 10.1111/j.1530-0277.1996.tb01079.x. 3, May. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. Journal of Studies on Alcohol. 1982;43(3):380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Smith JE, Meyers RJ. The community reinforcement approach. In: Hester RJ, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. Allyn and Bacon; Boston: 1995. pp. 251–266. [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behavior Research & Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Validity of self-reports in three populations of alcoholics. Journal of Consulting and Clinical Psychology. 1979;46:901–907. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: An approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Pergamon Press; New York: 1980. pp. 177–183. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Canada: 1996. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper TC, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Pergamon Press; New York: 1980. [Google Scholar]
- Stout RL, Longabaugh R, Rubin A. Predictive validity of Marlatt's relapse taxonomy versus a more general relapse code. Addiction. 1996;91(Supplement):S99–S110. [PubMed] [Google Scholar]
- Stuart GL. Improving violence intervention outcomes by integrating alcohol treatment. Journal of Interpersonal Violence. 2005;20(4):388–393. doi: 10.1177/0886260504267881. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Houston-Miller N, Haskell WL, Debusk RF. Smoking cessation after acute myocardial infarction: the effects of exercise training. Addictive Behaviors. 1988;13(4):331–335. doi: 10.1016/0306-4603(88)90039-1. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Sallis JF, Needle R. The relation of physical activity and exercise to mental health. Public Health Reports. 1985;100(195201) [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Kingree JB. The roles of victim and perpetrator alcohol use in intimate partner violence outcomes. Journal of Interpersonal Violence. 2006;21(2):163–177. doi: 10.1177/0886260505282283. [DOI] [PubMed] [Google Scholar]
- Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: Physiological mechanisms and clinical implications. Medicine and Science in Sports and Exercise. 1990;22:417–428. [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10(3):241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: Research and clinical implications. Professional Psychology: Research and Practice. 1999;30(3):275–282. [Google Scholar]
- Tsukue I, Shohoji T. Movement therapy for alcoholic patients. Journal of Studies on Alcohol. 1981;42(1):144–149. doi: 10.15288/jsa.1981.42.144. [DOI] [PubMed] [Google Scholar]
- Tuson KM, Sinyor D. On the affective benefits of acute aerobic exercise: Taking stock after twenty years of research. In: Serganian P, editor. Exercise psychology: The influence of physical exercise on psychological processes. John Wiley and Sons; New York: 1993. pp. 80–121. [Google Scholar]
- USDHHS . Physical activity and health: A report of the Surgeon General. Centers for Disease Control; Atlanta, GA: 1996. [Google Scholar]
- USDHHS . 10th Special Report to the U.S. Congress on Alcohol and Health. U.S. Department of Health and Human Services; Rockville, MD: 2000. [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction. 2000;95:199–208. doi: 10.1046/j.1360-0443.2000.9521996.x. [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug and Alcohol Dependence. 2004;76(Suppl):S11–19. doi: 10.1016/j.drugalcdep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Williams PA, Cash TF. A controlled study of the effects of circuit weight training on body image.. Paper presented at the Annual meeting of the Association for the Advancement of Behavior Therapy; Toronto, Ontario. 1999. [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. American Psychologist. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Woodard CM, Berry MJ. Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. Journal of Cardiopulmonary Rehabilitation. 2001;21(4):201–209. doi: 10.1097/00008483-200107000-00002. [DOI] [PubMed] [Google Scholar]