Abstract
Background
The incidence of hypertension and progression of renal disease are greater in men than women. Data suggests that there is a dimorphic response to angiotensin II (Ang II) in rats, with male rats exhibiting a greater increase in mean arterial pressure (MAP) than females. However, during endogenous renin angiotensin systemblockade with angiotensin-converting enzyme (ACE) inhibition, female rats have a greater MAP response to Ang II. We tested weather female mice exhibit a greater MAP response to chronic Ang II during ACE inhibtion.
Methods
Twenty-week old male and female C57BL/6J mice (n≥ 6/group), treated with enalapril (40 mg/kg/day in drinking water), were assigned to groups receiving either Ang II (800 ng/kg/min) or saline for 2 weeks. Enalapril treatment began 4 days prior to and continued throughout the experiment.
Results
MAP was higher in males than females given enalapril and Ang II (Male: 144±3 vs. Female: 121±6 mmHg, p<0.05) and was not different between mice treated with enalapril alone (Male: 99±3 vs. female: 100±3 mmHg). F2-isoprostanes were not increased by Ang II; however, female mice had significantly higher levels than males. Renal cortical expression of catalase and Cu/Zn SOD was not different between experimental groups. Urinary protein was higher in male mice when compared to females, but was not changed after treatment with Ang II in either group.
Conclusions
These data suggest that there are species and sex specific differences in the mechanism of the blood pressure response to Ang II, even during ACE inhibition.
Keywords: renin-angiotensin system, sexual dimorphism, blood pressure
Introduction
Clinical and experimental data show that males have higher blood pressure (BP) than females. This sexual dimorphism is also present, and favors men, in the risk for developing cardiovascular diseases and progression to end-stage renal disease compared to age-matched pre-menopausal women. The renin-angiotensin system (RAS) is a key regulator of BP and evidence suggests that there are differences in the endogenous RAS between men and women [21]. For example, boys have higher serum angiotensin-converting enzyme (ACE) than girls and there is a direct correlation between serum ACE and blood pressure [3]. In addition, blockade of angiotensin receptors with irbesartan has a greater blood pressure lowering effect in women than in men [7].
There also appears to be a sexual dimorphism in the blood pressure response to angiotensin II (Ang II) in rodents, making them potentially useful to examine the mechanisms that lead to differential blood pressure responses in males and females. For example, male Sprague-Dawley rats chronically infused with Ang II become hypertensive with altered vascular function where as female SD rats are protected [17]. In mice, males have a greater pressure increase after chronic infusion with Ang II when compared to females, a response that is abrogated after gonadectomy [20]. Because these studies were conducted in animals with an intact RAS, and because there is evidence for the differential expression of endogenous RAS components in males and females, we recently tested whether the dimorphic Ang II responses would persist in male and female SD rats after chronic blockade of the endogenous RAS with enalapril [15]. Our data revealed that enalapril treatment reduced blood pressure to a greater extent in female rats, and that the blood pressure response to Ang II during ACE inhibition was actually greater in female rats than males when animals are fed a normal salt diet. These data suggested that during conditions where Ang II is increased, women may be more responsive to therapies involving inhibition of the endogenous ACE. Given the wide use of mice as experimental models to study mechanisms of hypertension, we asked whether underlying differences in the endogenous ACE activity may contribute to the observed dimorphic blood pressure response to Ang II in male and female mice. Therefore we tested the hypothesis that female mice will have a greater blood pressure response than males after chronic Ang II infusion on the background of ACE inhibition.
Methods
Animals
Male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were maintained on tap water and standard chow (Teklad; Harlan Sprague-Dawley, sodium chloride content 0.4%) ad libitum in an environment of 12-hour:12-hour light/dark cycle. C57BL/6J mice were studied because they are among the most common inbred strains of mice used in the study of hypertension. In addition, C57BL/6J represents the genetic background on which most genetic models are bred. Nevertheless, it is possible that different inbred strains may yield varying results. The protocols complied with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Experimental protocol
At 20 weeks of age, male and female C57BL/6J mice were treated with the ACE inhibitor enalapril (40 mg/kg/day) in the drinking water starting 4 days prior to Ang II administration. We selected this high dose of enalapril based on previous studies demonstrating that a dose 30 mg/kg/day effectively protected the kidney, as evidenced by reduced proteinuria, and lowered blood pressure in both male and female mice [5]. Enalapril was administered for the duration of the experiment. On day 5 mice were randomly assigned into experimental groups (n≥6/group) receiving either Ang II (800 ng/kg/min) or vehicle (saline) subcutaneously via osmotic minipumps (Alzet 1002) for two weeks. There were a total of 4 experimental groups: Male-Enalapril, Male-Enalapril+Ang II, Female-Enalapril, Female- Enalapril+Ang II. Metabolic cages were used to collect 24 h urine samples at the end of the treatment period. Kidneys were collected and weighed at the end of the protocol.
Measurement of blood pressure
Mean arterial pressure (MAP) was recorded at the end of the 2-week treatment period using indwelling carotid catheters as we previously described [13, 18]. Data are presented as the mean value over two consecutive days.
Urinary protein excretion
Total urinary protein excretion was measured using a commercially available reagent (Bio-Rad, Richmond, CA), as we previously described [4]; and were normalized to urinary creatinine (CR 01 Oxford Biomedical Research, Oxford, MI). Data are expressed as ng protein/mg urinary creatinine.
Urinary F2-isoprostanes excretion
At the end of the treatment period urinary 8-iso-PGF2α (F2-isoprostanes) excretion was measured by ELISA (EA 85 Oxford Biomedical Research), as we previously published [15]. Individual samples were normalized to urinary creatinine and are expressed as ng F2-isoprostsnes/mg urinary creatinine.
Kidney cortex protein expression of Cu/Zn superoxide dismutase (SOD) and catalase
At the end of the studies, kidneys were harvested, and the renal cortex was dissected. Protein expression of Cu/Zn SOD (1:4000) and catalase (1:4000) were determined by western blot in renal cortical tissue homogenates, as we have previously described [15]. The positive control for Cu/Zn SOD and catalase was rat liver extract. The antibodies used were from Biodesign International (Saco, ME). Anti-GAPDH antibody was used as the loading control. Data are presented in arbitrary units of protein optical density band/GAPDH.
Statistical Analyses
Data are expressed as mean ± SEM. Statistical analysis was performed using a one way ANOVA and the Student-Newman-Keuls method for multiple comparison procedures. Significance was defined at P<0.05.
Results
Body and kidney weights
Body weight was significantly higher in males (Male-Enalapril: 28.4±1.3 and Male-Enalapril+Ang II: 29.4±0.5 g) than females (Female-Enalapril: 19.6±0.8 and Female-Enalapril + Ang II: 21.0±0.6 g, p<0.05) and was not affected by treatment with Ang II. Renal weight was not different among the experimental groups (Male-Enalapril: 6.3±0.3, Male-Enalapril+Ang II: 5.7±0.1, Female-Enalapril: 6.4±0.1 and Female-Enalapril+Ang II: 6.4±0.2 g/Body wt*10−3, p=NS).
Blood Pressure
MAP was not different between male and female mice given only enalapril (Figure 1). Chronic Ang II infusion increased MAP in both males and females treated with enalapril, but the increase was significantly greater in male mice treated with Ang II compared to females.
Figure 1.
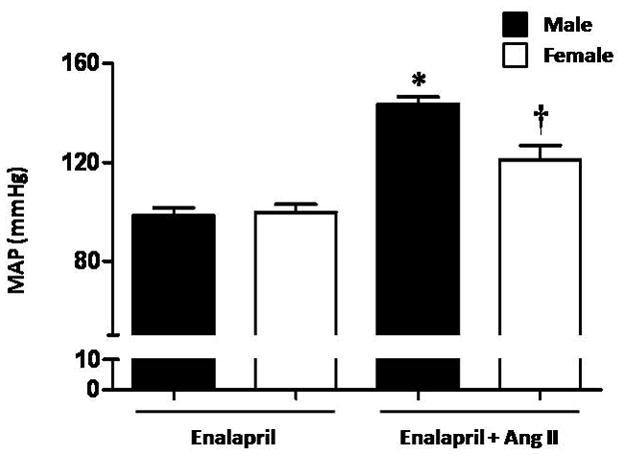
Chronic Ang II infusion causes a greater increase in mean arterial pressure (MAP) in male mice treated with enalapril when compared to female mice treated with enalapril. MAP was not different between male and female mice treated with enalapril and infused with saline. * p<0.05 vs. Male-Enalapril, Female-Enalapril and Female-Enalapril+Ang II. † p<0.05 vs. Male- Enalapril, Female-Enalapriland Male -Enalapril+Ang II (n≥ 6/group).
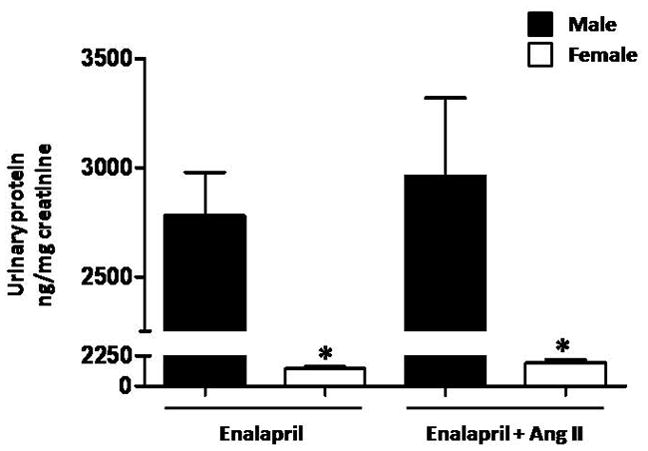
Proteinuria
Urinary protein excretion was significantly higher in males than females during ACE inhibition alone and was not affected by chronic Ang II administration in either males or females (Figure 2).
Figure 2.
Urinary protein excretion is higher in male than female mice. Ang II infusion for two weeks did not change protein excretion in males or females. * p<0.05 vs. Male- Enalapril and Male-Enalapril + Ang II(n≥ 6/group).
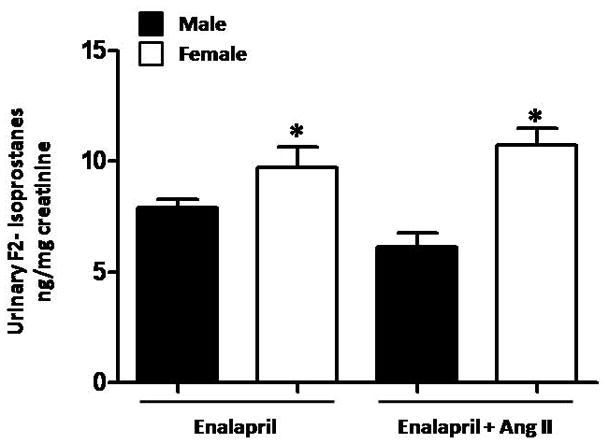
Urinary excretion of F2-isoprostanes
Urinary F2-isoprostanes were measured as a marker for oxidative stress. Female mice treated with enalapril alone had significantly higher levels of F2-isoprostane excretion compared to male mice (Figure 3). F2-isoprostane excretion was not affected by Ang II infusion in either males or females.
Figure 3.
Urinary F2-isprostanes excretion is higher in female than male mice. Ang II infusion for two weeks did not change F2-isoprostanes excretion in males or females. * p<0.05 vs. Male- Enalapril and Male -Enalapril + Ang II (n≥ 6/group).
Renal cortical protein expression of Cu/ZN SOD and catalase
Neither Cu/Zn-SOD nor catalase expression were different in male and female mice given enalapril alone (Figures 4A and B). With Ang II infusion, there was a tendency for increased expression of Cu/Zn-SOD in female mice only. This was not observed in the expression of catalase.
Figure 4.
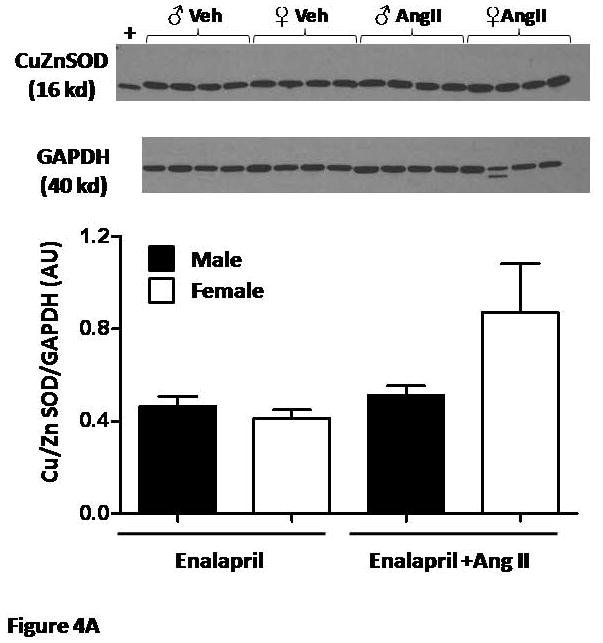
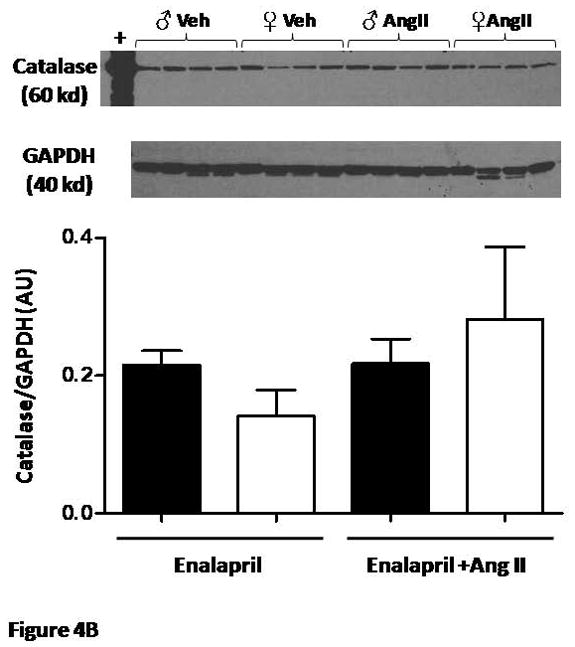
(A) Renal cortical protein expression of Cu/Zn SOD and (B) Catalase were not different between male and female mice and were not affected by Ang II (n=4/group).
Discussion
The major findings in the present study are as follows: 1) the sexual dimorphism in the blood pressure response to Ang II is maintained even during blockade of the endogenous ACE, with males exhibiting higher BP than females; 2) urinary protein excretion was higher in males than females and was not affected by Ang II in either group; 3) Urinary F2-isoprostanes are higher in female mice compared to males and were not increased by Ang II in either males or females.
Ambulatory BP has been shown to be higher in normotensive men than normotensive premenopausal women [2, 9]. We recently showed that BP is also higher in normotensive male rats than females [15]. In addition, several models of hypertension exhibit sex dependent differences in BP with males having higher BP than females [9, 10, 17]. The mechanisms responsible for the sex difference in BP are not yet clear; however, they appear to be closely associated with physiological changes that are known to promote hypertension. For example, in spontaneously hypertensive rats (SHR), ACE contributes to the dimorphic BP response given that ACE inhibition eliminates the blood pressure difference between male and female rats [11]. In addition, oxidative stress appears to have a more prominent role in the hypertension of male SHR compared with females. This is based on evidence that female SHR have increased renal NADPH oxidase activity and urinary F2-isoprostanes compared to male SHR, and that anti-oxidant therapies are more effective for reducing blood pressure and proteinuria in male SHR compared with females[14].
In order to examine the mechanistic role of RAS in the dimorphic BP response, we previously used a model of Ang II hypertension in rats during blockade of the endogenous production of Ang II by ACE inhibition. We found that the sex differences in response to Ang II in this model were modulated by salt intake. Contrary to the Ang II pressor response without ACE inhibition, female rats fed a low salt diet during ACE inhibition actually exhibited a greater BP response to Ang II than males. However, when the same rats were fed a high salt diet during ACE inhibition, the BP response to Ang II was greater in male rats compared with females. We utilized a high dose of enalapril in this study to ensure blockade inhibition of endogenous ACE. Doses lower than the one currently used have been shown to effectively in both protecting the kidney, with reductions in proteinuria, glomerular extracellular matrix deposition and mesangial cell activation, and reducing blood pressure in both male and female mice [5]. Although we cannot rule out the possibility of some residual activity of ACE, it should be noted that male and female mice had similar BP after treatment with enalapril. In contrast to the study in rats, male mice fed a normal salt diet and treated with enalapril exhibited a greater blood pressure response to chronic Ang II when compared to female mice.
The present findings are consistent with data from Xue et al. showing that male mice have a greater blood pressure response to Ang II in the absence of ACE inhibition [20]. Because the endogenous ACE is blocked with enalapril in this study, our data suggest that differences in the endogenous production of Ang II may not be the mitigating factor in the dimorphic BP response to Ang II in mice. One explanation for the dimorphic pressor response during chronic ACE blockade could be related to either differential expression or differential regulation of AT1 receptors. For example, sex steroids may play an important role in the modulation of AT1 receptor function. Studies have shown that estrogens decrease AT1 receptor expression and that ovariectomy increases AT1 receptor expression [8]. A relatively recent study also alludes to the differential regulation of AT1 receptors in male and female mice [5]. In this study, Le et al. generated mice with gene duplications for the AT1a receptor. Despite the same increase in renal AT1a receptor expression between male and female mice, blood pressure only correlated with AT1areceptor copy number in females.
The data in the present study highlight not only that there are sex differences in response to Ang II, but also that there are species specific differences. For example, in our previous study, we found that Ang II increased urinary albumin excretion in both male and female rats [15]. We attributed this to a pressor effect. However, in the present study, chronic Ang II infusion did not result in a further increase in proteinuria in either sex. Thus there was no apparent pressor effect on proteinuria in the mice. This species differences may be the result of an increased glomerular permeability in mice compared to rats since mice typically excrete higher levels of urinary protein under control conditions [1]. There is also evidence to support the notion that female C57BL/6 mice may be resistant to renal damage during Ang II infusion. Wesseling et al. demonstrated that a 28 day infusion of AngII caused an increase in pressure without increasing urinary protein in female C57BL/6 mice [19]. In a separate study, Liao et al. showed that a 28 day infusion of AngII in male C57BL6 mice increased pressure and urinary protein levels [6]. Chronic ACE inhibition, which has potent renal protective effects, was not utilized in these studies. With regard to the sex difference in proteinuria, male rats have been shown to excrete more protein than females [16]. It is possible that a similar situation occurs in mice, thus explaining the higher urinary protein in male mice.
One similarity between the present study in mice and our previous one in rats is that the increase in BP caused by Ang II in both species is independent of increases in the excretion of F2-isoprostanes. In addition, chronic Ang II administration did not increase isoprostanes in rats or mice of either sex. In rats, high salt diet increased urinary F2-isoprostanes in both males and females, although the increase was slower to develop in males than females [15]. In the present study we found that urinary F2-isoprostanes were higher in female mice than males and these levels were unchanged after Ang II infusion in either sex. These data are consistent with our previous studies in SHR in which urinary excretion of F2-isoprostanes was higher in females than males [14]. We showed previously in normotensive rats that oxidative stress in males is associated with increased expression of antioxidant enzymes [4]. The lack of an increase in the antioxidant enzymes Cu/Zn-SOD and catalase in mice after Ang II infusion is consistent with this previous work and a limited role for oxidative stress in this model.
Our previous study in rats and present findings in mice that Ang II infusion is not associated with increased markers of oxidative stress or antioxidant enzymes contrasts with earlier studies showing that oxidative stress is increased after Ang II infusion. This discrepancy may be explained by the different does of Ang II used between our studies in rats (150 ng/kg/min Ang II) and those of others (0.7 mg/day approximately 485 ng/kg/min Ang II) [17]. In mice much higher doses of Ang II are required to maintain a pressor response. Alternatively, it is possible that treatment with enalapril may have prevented an increase in oxidative stress. Studies in cell culture have shown that ACE inhibition can inhibit the NADPH oxidase mediated production of superoxide, independently of Ang II [12]. Finally, it is important to recognize that F2-isoprostanes are a measure of in vivo lipid peroxidation. Therefore, it is possible that Ang II infusion increased generation of reactive oxygen species in the vasculature or kidney specifically, and this may not be reflected by this whole body measure. Importantly, the data in the present study are consistent with our previous report that Ang II hypertension in females is not associated with an increase in F2-isoprostanes[4].
In summary, we show that after inhibition of ACE with enalapril, the administration of Ang II raises BP in male and female mice but to a greater extent in males. This differential response is not associated with an increase in urinary levels of F2-isoprostanes. Taken together with our recent observations and the reports of others, these data illustrate the importance of carefully considering species when examining mechanisms that mimic hypertension or sex differences in the control of blood pressure in humans. In addition, this underscores the need for further studies to understand the exquisite regulation of the renin angiotensin system not only between males and females but also among species. Finally, the data in the present study highlight the continued need to develop experimental models that closely resemble human pathophysiology and to recognize that some animal models may be more representative of different human populations.
Acknowledgments
M.V-P. and J.C.S-V are recipients of American Heart Association Greater Southeast Affiliate Postdoctoral Fellowship (2260874 and #725561B, respectively). This work was supported by HL51971, HL69194, and HL66072 from the National Institutes of Health to J.F.R. and, HL085907, HL085907S3 and HL092284 to M.J.R.
Footnotes
Conflict of Interest: None
Disclosures
None
Reference List
- 1.Alt JM, Maess B, Hackbarth H. Species and strain differences in urinary protein excretion. Ren Physiol. 1985;8(6):301–309. doi: 10.1159/000173062. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25 (3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.eteau-Burnat B, Baudin B, Morgant G, Baumann FC, Giboudeau J. Serum angiotensin-converting enzyme in healthy and sarcoidotic children: comparison with the reference interval for adults. Clin Chem. 1990;36(2):344–346. [PubMed] [Google Scholar]
- 4.Fortepiani LA, Reckelhoff JF. Increasing oxidative stress with molsidomine increases blood pressure in genetically hypertensive rats but not normotensive controls. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R763–R770. doi: 10.1152/ajpregu.00526.2004. [DOI] [PubMed] [Google Scholar]
- 5.Guo S, Kowalewska J, Wietecha TA, Iyoda M, Wang L, Yi K, Spencer M, Banas M, Alexandrescu S, Hudkins KL, Alpers CE. Renin-angiotensin system blockade is renoprotective in immune complex-mediated glomerulonephritis. J Am Soc Nephrol. 2008;19(6):1168–1176. doi: 10.1681/ASN.2007050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension. 2008;52(2):256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17(9):2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 8.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97(22):2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 9.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37 (5):1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 10.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31 (1 Pt 2):435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 11.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin–angiotensin system. Hypertension. 2000;35(1 Pt 2):480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 12.Rosenkranz AC, Lob H, Breitenbach T, Berkels R, Roesen R. Endothelial antioxidant actions of dihydropyridines and angiotensin converting enzyme inhibitors. Eur J Pharmacol. 2006;529(1–3):55–62. doi: 10.1016/j.ejphar.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension. 2006;48(5):988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 14.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34(9):938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 15.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51(4):1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1573–R1579. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 17.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol. 2005;83(5):413–422. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 18.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones A, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1282–R1289. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesseling S, Ishola DA, Jr, Joles JA, Bluyssen HA, Koomans HA, Braam B. Resistance to oxidative stress by chronic infusion of angiotensin II in mouse kidney is not mediated by the AT2 receptor. Am J Physiol Renal Physiol. 2005;288(6):F1191–F1200. doi: 10.1152/ajprenal.00322.2004. [DOI] [PubMed] [Google Scholar]
- 20.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288(5):H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zapater P, Novalbos J, Gallego-Sandin S, Hernandez FT, bad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. 2004;43(5):737–744. doi: 10.1097/00005344-200405000-00018. [DOI] [PubMed] [Google Scholar]


