Abstract
The influenza A virus M2 protein is a pH-gated and amantadine-inhibited proton channel important for the virus life cycle. Proton conduction by M2 is known to involve water, however direct experimental evidence of M2-water interaction is scarce. Using 1H spin diffusion solid-state NMR, we have now determined the water accessibility of the M2 transmembrane domain (M2-TM) in virus-envelope-mimetic lipid membranes and its changes with environment. Site-specific water-protein magnetization transfer indicates that, in the absence of amantadine, the initial spin diffusion rate mainly depends on the radial position of the residues from the pore: pore-lining residues along the helix have similarly high water accessibilities compared to lipid-facing residues. Upon drug binding, the spin diffusion rates become much slower for Gly34 in the middle of the helix than for the N-terminal residues, indicating that amantadine is bound to the pore lumen between Gly34 and Val27. Water-protein spin diffusion buildup curves indicate that spin diffusion is the fastest in the low-pH open state, slower in the high-pH closed state, and the slowest in the high-pH amantadine-bound state. Simulations of the buildup curves using a 3D lattice model yielded quantitative values of the water-accessible surface area and its changes by pH and drug binding. These data provide direct experimental evidence of the pH-induced change of the pore size and the drug-induced dehydration of the pore. This study demonstrates the capability of 1H spin diffusion NMR for elucidating water interactions with ion channels, water pores, and proton pumps, and for probing membrane protein conformational changes that involve significant changes of water-accessible surface areas.
Keywords: membrane proteins, influenza M2 proton channel, water accessibility, amantadine, 1H spin diffusion, solid-state NMR
Introduction
Water is essential for the folding and functions of ion channels 1,2, water pores 3, and proton pumps in biological membranes 4,5, and is important for the solvation of charged residues in lipid bilayers 6–8. Elucidating the interaction of water with membrane proteins and water dynamics in the low-dielectric core of the lipid membrane 9 is thus of fundamental interest. The influenza A M2 protein forms a pH-gated proton channel in the virus envelope that is important for the virus lifecycle 10–12. Acidification of the virus particle triggers the release of the viral RNA into the infected cell, initiating virus replication. The M2 channel activity is mediated by water molecules and by the action of a key residue, His37 13, and is inhibited by amantadine and rimantadine 14. Recent high-resolution structural studies of the M2 protein by X-ray crystallography 15 and NMR spectroscopy 16–18 provided a wealth of information about the global and site-specific conformational features important for proton conduction. However, direct experimental evidence about how water interacts with the M2 protein under different pH and drug-binding conditions is still scarce. Most proposals for the mechanism of proton conduction so far came from molecular dynamics (MD) simulations 19–21.
Solid-state NMR (SSNMR) spectroscopy provides a unique and powerful tool for studying water-protein interactions directly in native lipid bilayers 22,23. Correlation of water-protein 1H-13C signals after dipolar-driven 1H spin diffusion gives detailed information about the proximity of protein residues to water. The rate of 1H spin diffusion was initially used to determine the global topology of membrane proteins 24, and was recently shown to also give information about the water-protein surface area 25.
In this study, we use water-to-protein 1H spin diffusion NMR to investigate the water accessibilities and water dynamics of the M2 transmembrane peptide (M2-TM) in virus-envelope-mimetic lipid bilayers 26. We demonstrate that the spin diffusion buildup rates are site specific and differ between lipid-facing and pore-lining residues in the absence of drug, thus the source of water magnetization is primarily the pore water. We show that a 3D lattice model can be used to simulate the spin diffusion buildup and quantify the water-exposed protein surface area. The result indicates a close correlation between the water accessibility and the function of the M2 proton channel.
Materials and Methods
Membrane protein samples
M2(22–46) of the Udorn strain (SSDPLVVAASIIGILHLIL WILDRL) was synthesized and purified by PrimmBiotech (Cambridge, MA). Two peptide samples containing uniformly 13C, 15N-labeled residues at Leu26, Val27, Ala29, Gly34 and Ile35 were synthesized. The samples used for low- and high-pH experiments without amantadine contained labeled Leu26, Val27, Ala29 and Gly34, while the peptide used for the high-pH drug-bound experiments contained labeled Leu26, Ala29, Gly34 and Ile35. The peptide was reconstituted by detergent dialysis 27 into a lipid mixture mimicking the virus envelope lipid composition 26. The mixture contains egg sphingomyelin (SPM), DPPC, DPPE and cholesterol at molar ratios of 28%: 21%: 21%: 30%. SPM was dissolved in a chloroform/methanol (5: 1) solution before mixing with the other lipids. The lipid mixture was lyophilized, suspended in a buffer of desired pH, vortexed, and freeze-thawed several times to form large unilamellar vesicles. A phosphate buffer containing 10 mM Na2HPO4/NaH2PO4, 1 mM EDTA, and 0.1 mM NaN3 was used for the pH 7.5 samples, and a citrate buffer with 10 mM citric acid/sodium citrate, 1 mM EDTA, and 0.1 mM NaN3 was used for the pH 4.5 sample. The molar ratio of M2 monomer to lipids (not counting cholesterol) was 1:15. The proteoliposome suspensions were centrifuged at 150,000 g to obtain 40% hydrated pellets. Photometric assays showed >95% binding of M2-TM to the membrane. For the amantadine-bound sample, amantadine hydrochloride in the pH 7.5 buffer was directly titrated to the pellet to reach a M2 monomer: amantadine (Amt) molar ratio of 1: 2.
Solid-state NMR experiments
NMR experiments were carried out on wide-bore Bruker NMR spectrometers at 14.1 and 9.4 Tesla using 4 mm magic-angle spinning (MAS) probes. Typical radio-frequency field strengths were 50 kHz for 13C and 31P and 60–70 kHz for 1H. 13C and 31P chemical shifts were referenced to the α-Gly 13CO signal at 176.49 ppm on the TMS scale and the hydroxyapatite 31P signal at +2.73 ppm on the phosphoric acid scale, respectively. 1H chemical shifts were internally referenced to the lipid Cγ signal at 3.26 ppm on the TMS scale.
All 1D and 2D 1H spin diffusion experiments with 13C or 31P detection 28 were conducted at 293 K, where water is mobile but the protein is immobilized 26. The 2D 1H-13C and 1H-31P correlation experiments used a 1H T2 filter of 2 ms and 0.8 ms, respectively, to suppress the 1H magnetization of the rigid components. Spin diffusion mixing times (tm) were 64 ms for the 2D 1H-31P experiments and 4 to 100 ms for the 2D 1H-13C experiments. 13C double-quantum (DQ) filtered 1D spin diffusion experiments, which removed the lipid 13C signals, used the SPC-5 sequence 29 to create the DQ coherence and a 1H T2 filter of 2 ms. Most spin diffusion spectra were measured under 5 kHz MAS.
Water-protein spin diffusion intensity as a function of the square root of tm (Eq. 1) was plotted after correcting for water T1 relaxation. The water 1H T1 was measured using the standard inversion recovery sequence. Water 1H T2 relaxation times were measured using a Hahn-echo experiment and detected through the protein 13C signals.
Theoretical frameworks for determining water-protein surface areas from 1H spin diffusion
The analytical theory for determining the water-protein surface areas from spin diffusion NMR has been well developed for heterogeneous polymers 30. The protein 1H magnetization IP increases with the spin diffusion mixing time tm due to relayed magnetization transfer from water according to:
| (1) |
where Deff is the effective spin diffusion coefficient of the entire system, SWP is the water-protein surface area, and VP is the protein volume. Equation 1 indicates that the water-to-protein spin diffusion buildup with time reports SWP. The time for the protein to reach equilibrium intensity IP(∞), which can be extracted from the initial slope of the buildup curve, is inversely proportional to SWP:
| (2) |
Although this analytical approach gives useful insights into the relation between spin diffusion buildup and the water-protein surface area, it is only semi-quantitative and does not capture high-resolution structural details of the protein. Simplifying assumptions about the protein three-dimensional shape, the average diffusivity of the ternary water-protein-lipid system, and the volume fraction of water (see below) have been made to arrive at Eq. 1 30. To determine the water accessibilities of M2-TM under more realistic conditions of heterogeneous diffusion coefficients and a decidedly uneven surface, we also calculated the 1H spin diffusion buildup curves numerically using a three-dimensional lattice model 25,30. The lattice is a low-resolution model of the M2 helical bundle in a 44-Å thick lipid bilayer representing the viral membrane. Cubes with a 2-Å side (d) were used to define the positions of water, lipids, the protein, the water-protein interface, and amantadine. The time-dependent 1H magnetization at any lattice point, Mx,y,z (tm), was calculated in MATLAB as
| (3) |
Mx,y,z (tm) exchanges with the magnetization of its six neighbors at a rate determined by the spin diffusion coefficient Dij. Previous measurements have established a high water DWW of 3 nm2/ms, which reflects the fast physical diffusion of water. The water translation diffusion coefficient is about 2 μm2/ms in bulk and only 2–3 fold slower in confined environments such as ion channels 19, thus water diffuses over a distance of microns in 1 ms. Thus, for the nanometer-thick lipid bilayer, water is fully exchanged between the membrane surface and the channel within milliseconds as long as the water pathway is continuous. For the protein, a spin diffusion coefficient DPP of 0.3 nm2/ms was used based on measurements of rigid organic polymers 24,28,31,32. For the water-protein interface, we used a DWP of 0.008 nm2/ms 25, which is lower than DPP and DWW due to the inefficiency of spin diffusion across the intermolecular interface. This DWP value falls within the range of 0.0013 – 0.008 nm2/ms used before for experiments at ambient temperatures 25,28,33. The five-fold variation reflects the different viscosities of the lipid membranes due to the different lipid compositions and phase transition temperatures. The indirect pathway of water spin diffusion to lipids and then to the protein was neglected in the simulation due to the lack of lipid 1H – protein 13C cross peaks in the 2D 13C-1H spectra within the mixing times used, which indicates inefficient spin diffusion from the lipid to the protein 25.
The water magnetization of each cube was kept at 1 throughout the simulation, which represents the limit of large water reservoir. The protein magnetization was read out in 100 steps from time 0 to 625 ms to obtain the time-dependent intensity buildup curve.
Result and Discussion
Differential water accessibilities of M2-TM under different pH and drug binding conditions
We investigated the M2-water contact by measuring the protein 13C signals that originated from water by 1H spin diffusion. Using a 2D 1H-13C correlation experiment with a 1H T2 filter (2 ms) and no 1H homonuclear decoupling during the evolution period, we removed all 1H magnetization of the rigid protein 26, thus the protein 13C signals must have originated from the mobile water or lipids. The identity of the 1H magnetization source was also directly verified by the 1H chemical shifts in the 2D spectra. The use of a lipid mixture mimicking the virus-envelope membrane composition was essential for obtaining high-sensitivity spectra of M2-TM at physiological temperature, since the peptide undergoes intermediate-timescale motions in simple phosphocholine bilayers that severely broaden the NMR spectra 34,35. The M2-TM reconstituted into the cholesterol-rich viral membrane is completely tetramerized, as shown by 19F NMR spin counting experiments 36 and by thiol disulfide equilibria measurements that found increasing tetramerization from micelles to lipid bilayers, and from thin phosphocholine bilayers to thick cholesterol-containing phosphocholine bilayers 37. The five labeled residues represent different proximities to water: the N-terminal Leu26, Val27, Ala29 are closer to the bilayer surface water (1.0 – 1.5 nm) while Gly34 and Ile35 are far from the surface water (~ 2.2 nm) 38,39. On the other hand, Val27 and Gly34 line the channel pore while Ala29 faces the lipids (Fig. 1d). Thus, the labeled residues allow us to examine whether spin diffusion primarily depends on the residue proximity to the bilayer-surface water or proximity to the pore water.
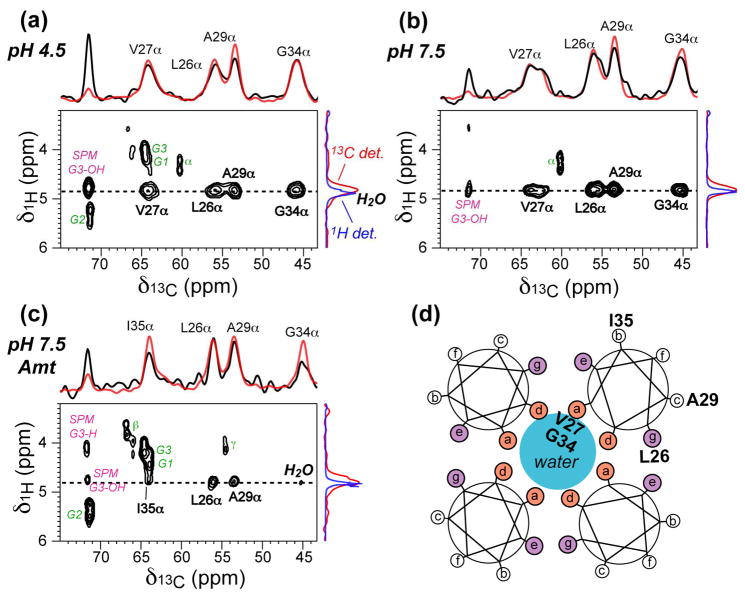
Fig. 1.
2D 13C-1H correlation spectra of M2-TM in virus-envelope-mimetic lipid membranes at 293 K. A 1H T2 filter time of 2 ms and a spin diffusion mixing time of 4 ms were used. (a) pH 4.5. (b) pH 7.5. (c) pH 7.5 with amantadine. Assignments for intermolecular water-protein cross peaks (black) as well as intramolecular phospholipid peaks (green) and sphingomyelin (SPM) peaks (magenta) are indicated. The water 1H cross section is shown at the top (black), superimposed with the water cross section from the 100 ms 2D spectra (red). The G34 Cα cross section at 100 ms is shown on the right (red), superimposed with the 1H 1D spectra (blue) to indicate the small upfield shift of the membrane-associated water from the bulk water. (d) Schematic of the M2-TM tetrameric helical bundle, where the approximate radial positions of the labeled residues are indicated.
Fig. 1 compares the 2D 1H-13C spectra of M2-TM under different pH and drug binding conditions. Spectra measured with a short mixing time of 4 ms were compared to those of 100 ms to qualitatively deduce the water accessibilities of the residues. To avoid comparing sidechains with different segmental dynamics and thus different diffusion coefficients, we mainly focused on the Cα sites. At pH 4.5, the water cross peak intensities at 4 ms relative to 100 ms are similar between the N-terminal residues (Leu26 and Val27) and the middle residue Gly34. Since Gly34 is significantly deeper in the membrane, this similarity indicates that there is a continuous water pathway in the pore from the N-terminus to the center of the helical bundle, which gives pore-facing Val27 and Gly34 similar water accessibilities. In comparison, the water cross peak of the lipid-facing Ala29 at 4 ms is weaker than the other residues, indicating that the experiment is sensitive to the water accessibility difference between lipid-facing residues and pore-lining residues, and that the radial distance to the pore water is the main determining factor for the water cross peak intensity at short mixing times.
At pH 7.5, the main features of the pH 4.5 spectrum is preserved, but now the Gly34 peak is slightly lower than the N-terminal residues at 4 ms (Fig. 1b), suggesting that the amount of water in the middle of the pore is less than at pH 4.5.
When the protein is complexed to amantadine, the relative intensities of Gly34 and Ile35 peaks at 4 ms are significantly weaker than the relative intensities of Leu26 and Ala29 peaks (Fig. 1c). Thus, in the presence of drug, the N-terminus has substantially higher water accessibilities than the middle segment of the protein, suggesting that amantadine interrupts the water pathway between Ala29 and Gly34.
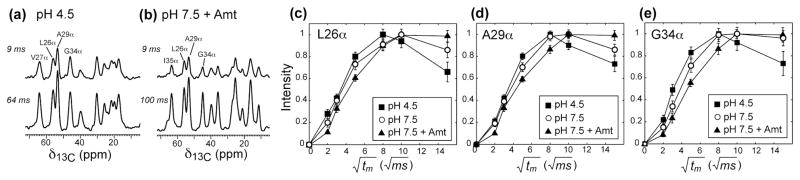
The full 2D 1H-13C spectra do not show lipid 1H – protein 13C cross peaks within the mixing times of interest (< 225 ms) (Fig. S1). Thus, a 1D version of the 2D experiment is sufficient for extracting the water-protein buildup rates. The shorter experiments allow more mixing times to be measured so that quantitative buildup curves can be obtained. To suppress the 13C signals of unlabeled lipids and cholesterol in the 1D spectra, we added a 13C DQ filter. Fig. 2 and Fig. S2 show representative 1D 13C DQ spectra and the resulting buildup curves for the three states. For all sites studied, the intensity buildup is the fastest at pH 4.5, moderately slower at pH 7.5, and more substantially slower upon amantadine binding. This trend is the most pronounced for Gly34, whose buildup rate in the drug-bound state is clearly slower than the N-terminal residues (Fig. 2e), confirming that water accessibility is lower in the middle of the TM helices than at the N-terminus in the amantadine-bound state. Quantifying the buildup rates using the initial slopes yielded values, which are inversely related to the water-accessible surface area (Fig. S3). Increasing the pH from 4.5 to 7.5 increased the average by 20% while amantadine binding increased by 56% compared to the open state (Table S1).
Fig. 2.
Water-to-M2 1H spin diffusion buildup curves from 1D 13C DQ experiments. (a) Representative 13C DQ filtered spectra at pH 4.5. (b) Representative 13C DQ filtered spectra at pH 7.5 in the presence of amantadine. (c–e) Buildup curves of several Cα sites at pH 4.5 (filled squares), pH 7.5 (open circles), and pH 7.5 with bound amantadine (filled triangles) after correcting for water 1H T1. (c) Leu26. (d) Ala29. (e) Gly34.
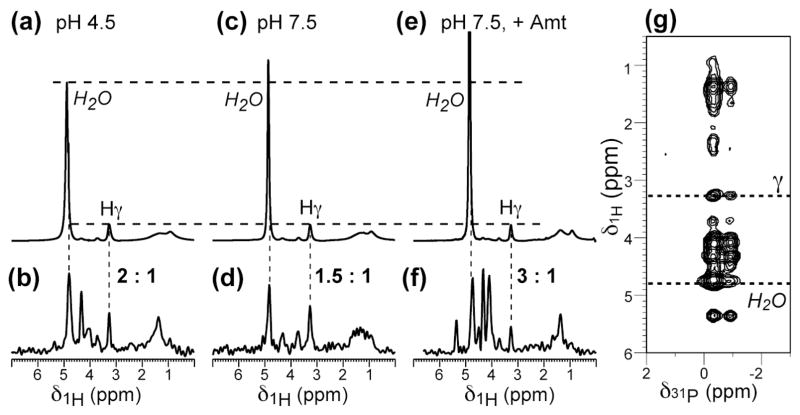
To examine whether the slower spin diffusion of the amantadine-bound M2-TM may be due to lower water content of the sample instead of obstruction of the pore, we measured the 1D 1H spectra and 2D 1H-31P correlation spectra of the three samples. The 1D 1H spectra report the total water content of each sample, including both membrane-associated water and bulk water, while the 2D 1H-31P correlation spectra report the amount of inter-bilayer water in the multilamellar vesicles. Fig. 3 shows that the total water intensity of each sample, normalized to the lipid Hγ intensity, increases in the order of pH 4.5 < pH 7.5 < pH 7.5 with amantadine. 2D 1H-31P correlation spectra further indicate that the drug-bound sample has the highest amount of membrane-associated water, since the 31P-correlated water intensity is the highest for the drug-bound sample (Fig. 3). Thus, the slow water-to-Gly34 spin diffusion in the amantadine-bound sample, despite the presence of large amounts of water on the membrane surface, must be attributed to obstruction of the water pathway in the channel.
Fig. 3.
1D 1H direct-polarization (DP) spectra and 31P-detected 1H spectra extracted from 2D 31P-1H correlation spectra of membrane-bound M2-TM. (a, c, e): 1D 1H spectra showing the full water peak. (b, d, f): Projection of the 1H cross sections of the 2D 31P-1H spectra with 64 ms spin diffusion. (a, b) pH 4.5. (c, d) pH 7.5. (e, f) pH 7.5 with amantadine. (g) A representative 2D 31P-1H spectrum for the pH 7.5 sample with amantadine.
Since the amantadine-bound sample contains 8-fold more drug than M2-TM channels, and the stoichiometry of M2 inhibition is one amantadine per channel 14, there is significant excess drug in the lipid bilayer. At the protein: lipid molar ratio of 1: 15, the amphiphilic amantadine constitutes 13 mol% of the lipid bilayer. A recent NMR relaxation analysis of the effects of amantadine on the dynamics of M2-TM and lipids 35 found that excess amantadine increases the membrane viscosity. This viscosity increase may indirectly affect water-protein spin diffusion by siphoning more water 1H magnetization to the lipids due to higher diffusion coefficients of the lipids. As a result, the protein intensity at long mixing times would be lower than if the lipid diffusion coefficients were unchanged. Thus, the 100 ms protein intensity may be moderately reduced compared to the apo samples. This indirect effect most likely accounted for the smaller increase of the Ala29 intensity from 4 ms to 100 ms in the drug-bound spectrum compared to the apo spectra (Fig. 1). It also strengthens the conclusion that the water pathway is interrupted by amantadine: if no excess drug were present in the membrane, the intensities of Gly34 and Ile35 at short mixing times would have been even lower compared to the equilibrium intensity at long mixing times.
To assess whether the water diffusion coefficient may be affected by pH and drug binding in a way that causes the observed changes in the protein buildup curves, we measured the water-to-lipid CH2 spin diffusion as a function of mixing time. The result shows that water-lipid spin diffusion is slower at low pH and is similar at high pH with or without the drug (Fig. S5), which is opposite to the trend of the water-protein spin diffusion. Therefore, the water-protein spin diffusion changes with pH and drug binding are caused by changing water accessibilities of the protein, despite small counter-directional changes of water and lipid diffusion rates.
Comparison of the 2D correlation spectra and the 1D 1H spectra indicates that the protein- and lipid-correlated water signal resonates at a 1H chemical shift of 4.73–4.83 ppm, which is about 0.1 ppm lower than the bulk water chemical shift of 4.80–4.93 ppm (Fig. 1, 3). Thus, the 31P-correlated inter-bilayer water and the protein 13C-correlated pore water have detectably different physical properties from the bulk water outside the multilamellar vesicles. This difference is expected due to the confinement of the membrane-associated water. On the other hand, the inter-bilayer water and pore water, which are within nanometers of each other, are fully averaged on the millisecond time scale due to the fast water translational diffusion, thus their chemical shifts should be indistinguishable. Indeed, the 13C-detected and 31P-detected water chemical shifts are identical within experimental uncertainty (Fig. S1, S4).
The ability to selectively detect the pore- and inter-bilayer water but not bulk water allowed us to probe the dynamics of protein-associated water through 1H T2 relaxation times. Water molecules in the fast motional limit should exhibit long T2 relaxation times that increase with increasing temperature. Fig. 4 shows the protein-13C detected water 1H T2 at low and high pH without amantadine. The T2 increases with temperature between 253 K and 313 K for both samples, indicating fast reorientations of the water molecules, but the low-pH state has longer water T2’s than the high-pH state. Thus, water is more dynamic at low pH, again consistent with a larger pore in the open state.
Fig. 4.
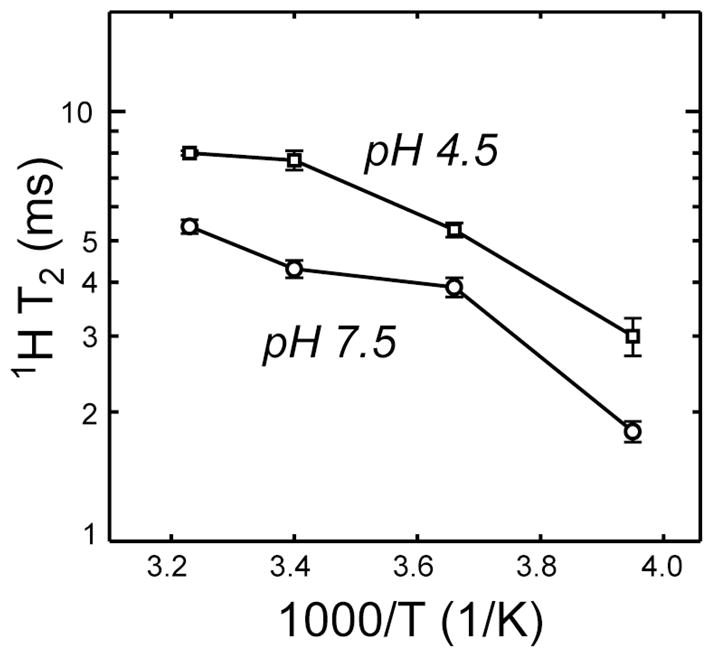
1H T2 of inter-bilayer and channel water as a function of temperature, detected through protein 13C signals after 100 ms spin diffusion. Squares: pH 4.5. Circles: pH 7.5. Error bars are indicated.
Water-M2 surface areas from 3D lattice simulations
To obtain more quantitative information about how pH and amantadine change the protein-water surface area, we calculated the water-protein 1H spin diffusion buildup curves using a 3D lattice model, where stepwise magnetization transfer among the lattice points simulates spin diffusion in real space. In the simulation, a 44-Å thick lipid bilayer 39,40 was constructed from 2-Å sized cubes, in which the four-helix bundle was represented by appropriate numbers of cubes in each plane so that the helices were tilted by about 25° from the bilayer normal (Fig. S6–S8). This tilt angle was extrapolated from the measured M2-TM orientations in bilayers of varying thicknesses, including DLPC, DMPC, and POPC bilayers 34,41–43. Additionally, amantadine causes a helix kink at Gly34 42 with a smaller tilt angle for the C-terminal segment, thus we adjusted the protein cube positions for the amantadine-bound state to create a less tilted C-terminal segment. The total volume of the protein was kept constant at ~12.7 nm3 (Table 1) based on an average protein density of 1.43 g/cm344,45. These low-resolution models do not attempt to delineate the shape and volume of the sidechains, but they are sufficient for determining the change of the protein-water surface area due to pH and drug binding. The center of the helical bundle was filled with water cubes, and one layer of interface cubes was used between the protein and water cubes. For the amantadine-bound state, the drug, whose approximate volume is 0.2 nm3, was centered near Ser31 to be consistent with the recently determined high-resolution structure of the M2-amantadine complex 38 and with the observed maximal chemical shift perturbation at Ser 31 27. The amide group of the drug was assumed to point down based on the recent crystal structure 15.
Table 1.
Water-accessible surfaces and pore parameters of M2-TM in viral membranes obtained from 3D lattice simulations of water-protein 1H spin diffusion.
| Parameters | pH 4.5 | pH 7.5 | pH 7.5, Amt |
|---|---|---|---|
| Number of protein cubes | 1592 | 1584 | 1608 |
| Number of interface cubes | 472 | 355 | 249 |
| Number of drug cubes | 0 | 0 | 28 |
| VP (nm3) | 12.7 | 12.7 | 12.8 |
| SWP (nm2) | 18.9 | 14.2 | 10.0 |
| Relative SWP | 100% | 75% | 53% |
| SWP VP | 1.48 | 1.12 | 0.78 |
| Minimum pore diameter (nm) | 0.6 | 0.2 | 0 |
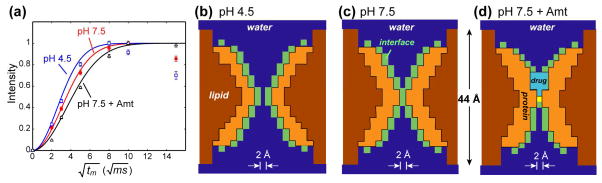
The number of water cubes and protein-water interface cubes were varied to simulate the measured buildup curves, which were taken from the integrated intensities between 64 and 16 ppm in the 13C DQ filtered spectra (Fig. S2). Fig. 5a shows the best-fit buildup curves for the three states. Side views of the structural models used to obtain the best fits are given in Fig. 5b–d. The molecular distributions in all the planes across the bilayer are shown in Fig. S6–S8. The low-pH sample exhibits the fastest buildup and was best fit by a protein-water surface area SWP of 18.9 nm2. Increasing the pH to 7.5 reduced SWP by 25%, to 14.2 nm2 (Table 1). Correspondingly, the minimum pore diameter was 0.6 nm (including the interface cubes) at pH 4.5 but decreased to 0.2 nm at pH 7.5. The requirement of keeping the protein volume constant while reducing the water-exposed area resulted in a tighter helical bundle with thicker cross sections at high pH (Fig. 5c), and a more expanded helical bundle at low pH. This change, while simple, already reproduced certain features of MD simulations, such as the significantly reduced water amount in the vicinity of Val27 at high pH 20,21.
Fig. 5.
Quantification of the water-accessible surface area of M2-TM from 1H spin diffusion buildup curves. (a) Normalized water-to-M2 spin diffusion buildup curves from the integrated intensities (64-16 ppm) of the 1D 13C DQ filtered spectra. Error bars are 1–2% on the normalized scale and are mostly smaller than the symbols. Best-fit buildup curves (lines) were obtained as described in the text. (b–d) Low-resolution structural models of the M2-TM proton channels used to obtain the best fits. (b) pH 4.5. (c) pH 7.5. (d) pH 7.5 with bound amantadine. Water: blue; protein: orange; lipid: brown; water-protein interface: green; amantadine: cyan. The bilayer thickness (44 Å) and pixel size (2 Å) of the 3D lattice are indicated.
Amantadine binding decreased SWP further to 10.0 nm2, representing a 47% reduction of the water accessibility compared to the low-pH state (Table 1). The channel is now devoid of water for about 6 planes or 12 Å along the pore axis (Fig. S8). Thus the slow buildup of Gly34 and Ile35 is the direct result of amantadine-induced dehydration of the pore and the interruption of the water pathway between Val27 and Gly34. It is worth noting that the spin diffusion experiment detects only mobile water sources and filters out the magnetization of potentially rigid water molecules. The crystal structure of M2-TM suggests that there may be rigid water molecules near Gly34 15, which would not be detectable by the current technique.
In our simulations we assumed the water reservoir to be infinitely large, which was achieved by keeping the magnetization of each water cube at 100% throughout the spin diffusion process. Alternative simulations that allowed the water magnetization to decrease indicate that a water layer of about 10 nm is necessary to reproduce the infinite-reservoir buildup behavior. The actual water amount in our samples corresponded to a water layer thickness of about 4.5 nm for each lipid bilayer. This finite water reservoir may partly account for the drop of protein intensity at long mixing times. If an water amount approaching the 100% volume fraction (fW) were used in the samples, then the equilibrium protein intensity IP (∞) would be higher than observed, and normalization as required by Eq. (1) would reduce the initial slope, leading to a smaller water-protein surface SWP. Expressed mathematically, the full equation for water-protein spin diffusion buildup includes dependence on both fW and SWP according to:
| (4) |
Thus, when fW is smaller than 1, the true SWP is smaller than the apparent surface area extracted from the buildup curves. Since the actual water amount in our samples is about two-fold lower than the 100% limit, the 3D lattice calculations over-estimate the true SWP by two-fold. On the other hand, the reduced complexity of the low-resolution 3D models compared to the true protein structure under-estimates the actual SWP. A recent study comparing SWP obtained from spin diffusion data and from the VADAR web server 46 found that the spin diffusion analysis under-estimates the water-protein surface area by about 3-fold 25. Taken together, these two systematic errors should largely cancel to make the SWP values in Table 1 quite realistic. Regardless of the absolute values of SWP, the relative changes of the protein-water surface area are unaffected by these systematic uncertainties, thus the high-pH induced decrease of pore diameter and the drug-induced dehydration of the pore remain quantitatively valid.
Fig. 6 shows a schematic of the water accessibility of the amantadine-bound M2 in lipid membranes, using the recently determined high-resolution structure of the drug-complexed M2-TM at high pH 38. The virus-envelope-mimetic lipid bilayer is drawn to scale with the tetrameric helical bundle. Water from the two bilayer surfaces permeate to the top and bottom of the channel pore, but is obstructed by the drug between Val27 and Gly34. The amantadine location in the pore, obtained from independent protein-amantadine distance measurements 38, is in excellent agreement with the observed reduction of water-Gly34 spin diffusion rate in the drug-bound state.
Figure 6.
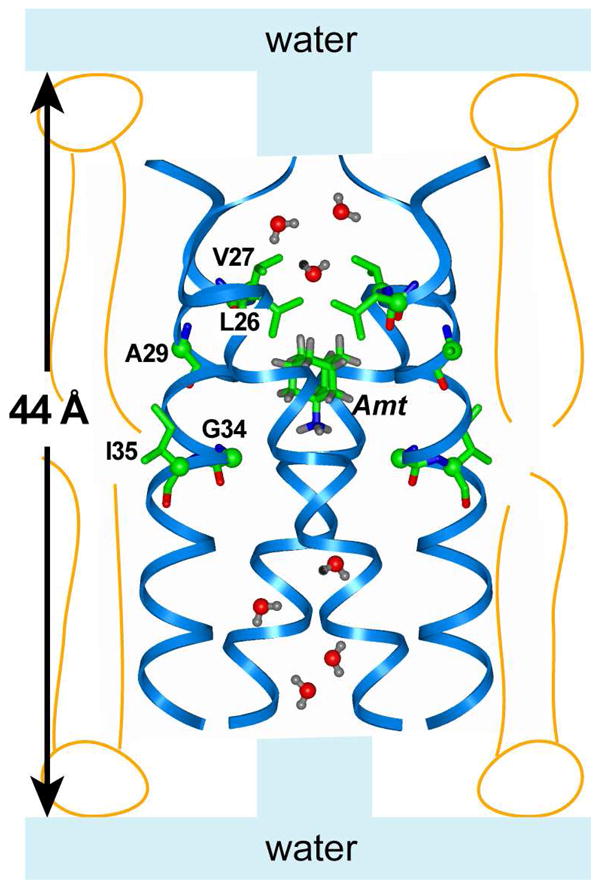
Water accessibility of amantadine-bound M2-TM at high pH in virus-envelope-mimetic lipid membranes. The water pathway through the pore is interrupted by amantadine. The five residues measured in this study are shown as sticks. The protein structure and amantadine position were determined from independent solid-state NMR distance experiments 38. The bilayer thickness is shown to scale with the helical bundle. The water molecules in the channel are for illustration only; their density, orientation, and diffusion rate are outside the scope of this study.
Since the spin diffusion NMR technique probes water magnetization transfer on the long timescale of milliseconds, even in the somewhat confined environment between two membrane surfaces and within a channel, water would have diffused over hundreds of nanometers to microns and fully equilibrated with the protein protons if it is unobstructed. Thus, the fact that Gly34 in the middle of the helix showed significantly lower relative intensity at 4 ms than N-terminal residues indicates that the channel is blocked for milliseconds over many angstroms. This blockage time scale extends by six orders of magnitude the MD simulated nanosecond interruption of the water wire 19,20, and underscores the striking ability of amantadine to dehydrate the channel.
Conclusions
Using a 1H spin diffusion NMR technique, we obtained for the first time experimental evidence of the pH- and drug-induced changes of the water accessibilities of the influenza M2 proton channel. At short mixing times, water-to-protein spin diffusion is primarily dependent to the radial position of the residues from the pore: lipid-facing residues receive less magnetization from water than pore-lining residues. Analysis of the integrated water-protein magnetization transfer indicates that the water-M2 surface area decreased by ~25% from the open state to the closed state. This change is smaller than that of the chimeric potassium channel KcsA-Kv1.3, whose SWP decreased by ~40% from the open to the closed state 25. Thus, the conformational changes associated with M2 channel activation is more modest, which is consistent with the fact that all key functions of this channel, including the selectivity filter and gating, are contained within a single TM helix, in contrast to multi-spanning potassium channels. Amantadine binding decreased the water accessibility of M2 by 47% compared to the open state, indicating that amantadine binds to the pore rather than the surface as suggested by a recent solution NMR study of M2(18–60) 18. The significant slowing-down of spin diffusion to Gly34 and Ile35 can only be explained by drug occlusion of the pore between Val27 and Gly34, which interrupts the water pathway for several milliseconds. These results are in excellent agreement with the high-resolution structure of the amantadine-complexed M2 in lipid bilayers at high pH 38. This spin diffusion NMR approach is generally applicable to membrane proteins, and allows for the investigation of site-specific water-protein interactions and functionally important changes in the water accessibility and conformations of membrane proteins in lipid bilayers.
Supplementary Material
Acknowledgments
This work is supported by an NSF grant MCB-0543473 and an NIH grant R01 GM088204 to M.H. The 600 MHz solid-state NMR instrument was funded by an NSF grant and DBI-0421374. The authors thank Prof. Klaus Schmidt-Rohr for helpful discussions and Fanghao Hu for experimental assistance.
Footnotes
Supporting Information Available: Full 2D 1H-13C and 1H-31P spin diffusion spectra with complete assignment, 1D series of 13C DQ filtered spin diffusion spectra, extraction and table, water-lipid spin diffusion curves, and cube distributions for the 3D lattice simulations of spin diffusion are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Swartz KJ. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson JM, Maupin CM, Chen H, Petersen MK, Xu J, Wu Y, Voth GA. J Phys Chem B. 2007;111:4300–4314. doi: 10.1021/jp070104x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu D, Lu M. Mol Membr Biol. 2007;24:366–374. doi: 10.1080/09687680701446965. [DOI] [PubMed] [Google Scholar]
- 4.Brzezinski P, Gennis RB. J Bioenerg Biomembr. 2008;40:521–531. doi: 10.1007/s10863-008-9181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanyi JK. Biochim Biophys Acta. 2006;1757:1012–1018. doi: 10.1016/j.bbabio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Freites JA, Tobias DJ, von Heijne G, White SH. Proc Natl Acad Sci USA. 2005;102:15059–15064. doi: 10.1073/pnas.0507618102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorairaj S, Allen TW. Proc Natl Acad Sci U S A. 2007;104:4943–4948. doi: 10.1073/pnas.0610470104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCallum JL, Bennett WF, Tieleman DP. Biophys J. 2008;94:3393–3404. doi: 10.1529/biophysj.107.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasaiah JC, Garde S, Hummer G. Annu Rev Phys Chem. 2008;59:713–740. doi: 10.1146/annurev.physchem.59.032607.093815. [DOI] [PubMed] [Google Scholar]
- 10.Pinto LH, Holsinger LJ, Lamb RA. Cell. 1992;69:517–28. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 11.Pinto LH, Lamb RA. J Biol Chem. 2006;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 12.Cady SD, Luo WB, Hu F, Hong M. Biochemistry. 2009;48:7356–7364. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Lamb RA, Pinto LH. Biophys J. 1995;69:1363–1371. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Takeuchi K, Pinto LH, Lamb RA. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Fu R, Nishimura K, Zhang L, Zhou HX, Busath DD, Vijayvergiya V, Cross TA. Proc Natl Acad Sci USA. 2006;103:6865–6870. doi: 10.1073/pnas.0601944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo W, Mani R, Hong M. J Phys Chem. 2007;111:10825–10832. doi: 10.1021/jp073823k. [DOI] [PubMed] [Google Scholar]
- 18.Schnell JR, Chou JJ. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smondyrev AM, Voth GA. Biophys J. 2002;83:1987–1996. doi: 10.1016/S0006-3495(02)73960-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi M, Cross TA, Zhou HX. J Phys Chem B. 2008;112:7977–7979. doi: 10.1021/jp800171m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana E, Dal Peraro M, DeVane R, Vemparala S, DeGrado WF, Klein ML. Proc Natl Acad Sci U S A. 2009;106:1069–1074. doi: 10.1073/pnas.0811720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong M. Structure. 2006;14:1731–1740. doi: 10.1016/j.str.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hong M. Acc Chem Res. 2006;39:176–183. doi: 10.1021/ar040037e. [DOI] [PubMed] [Google Scholar]
- 24.Kumashiro KK, Schmidt-Rohr K, Murphy OJ, Ouellette KL, Cramer WA, Thompson LK. J Am Chem Soc. 1998;120:5043–5051. [Google Scholar]
- 25.Ader C, Schneider R, Seidel K, Etzkorn M, Becker S, Baldus M. J Am Chem Soc. 2009;131:170–176. doi: 10.1021/ja806306e. [DOI] [PubMed] [Google Scholar]
- 26.Luo W, Cady SD, Hong M. Biochemistry. 2009;48:6361–6368. doi: 10.1021/bi900716s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cady SD, Mishanina TV, Hong M. J Mol Biol. 2009;385:1127–1141. doi: 10.1016/j.jmb.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huster D, Yao XL, Hong M. J Am Chem Soc. 2002;124:874–883. doi: 10.1021/ja017001r. [DOI] [PubMed] [Google Scholar]
- 29.Hohwy M, Jakobsen HJ, Eden M, Levitt MH, Nielsen NC. J Chem Phys. 1998;108:2686–2694. [Google Scholar]
- 30.Schmidt-Rohr K, Spiess HW. Multidimensional Solid-State NMR and Polymers. Academic Press; San Diego: 1994. [Google Scholar]
- 31.Clauss J, Schmidt-Rohr K, Spiess HW. Acta Polym. 1993;44:1–17. [Google Scholar]
- 32.Gallagher GJ, Hong M, Thompson LK. Biochemistry. 2004;43:7899–7906. doi: 10.1021/bi0356101. [DOI] [PubMed] [Google Scholar]
- 33.Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Proc Natl Acad Sci USA. 2006;103:16242–16247. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cady SD, Goodman C, Tatko CD, DeGrado WF, Hong M. J Am Chem Soc. 2007;129:5719–5729. doi: 10.1021/ja070305e. [DOI] [PubMed] [Google Scholar]
- 35.Cady SD, Hong M. J Biomol NMR. 2009;45:185–196. doi: 10.1007/s10858-009-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo W, Hong M. J Am Chem Soc. 2006;128:7242–7251. doi: 10.1021/ja0603406. [DOI] [PubMed] [Google Scholar]
- 37.Cristian L, Lear JD, DeGrado WF. Proc Natl Acad Sci USA. 2003;100:14772–7. doi: 10.1073/pnas.2536751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Nature. 2010 doi: 10.1038/nature08722. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niemela PS, Hyvonen MT, Vattulainen I. Biochim Biophys Acta. 2009;1788:122–135. doi: 10.1016/j.bbamem.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Duong-Ly KC, Nanda V, DeGrado WF, Howard KP. Protein Sci. 2005;14:856–861. doi: 10.1110/ps.041185805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cady SD, Hong M. Proc Natl Acad Sci USA. 2008;105:1483–1488. doi: 10.1073/pnas.0711500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Asbury T, Achuthan S, Li C, Bertram R, Quine JR, Fu R, Cross TA. Biophys J. 2007;92:4335–4343. doi: 10.1529/biophysj.106.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Kim S, Kovacs F, Cross TA. Prot Sci. 2001;10:2241–2250. doi: 10.1110/ps.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer H, Polikarpov I, Craievich AF. Protein Sci. 2004;13:2825–2828. doi: 10.1110/ps.04688204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quillin ML, Matthews BW. Acta Crystallogr D Biol Crystallogr. 2000;56:791–794. doi: 10.1107/s090744490000679x. [DOI] [PubMed] [Google Scholar]
- 46.Willard L, Ranjan A, Zhang H, Monzavi H, Boyko RF, Sykes BD, Wishart DS. Nucleic Acids Res. 2003;31:3316–3319. doi: 10.1093/nar/gkg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.