SUMMARY
The activation of the blastocyst, a process by which it gains competency to attach with the receptive uterus, is a prerequisite for successful implantation. However, the molecular basis of blastocyst activation remains largely unexplored. Combining molecular, pharmacological and physiological approaches, we show here that silencing of Wnt-β-catenin signaling does not adversely affect the development of preimplantation embryos to blastocysts and uterine preparation for receptivity, but remarkably blocks blastocyst competency to implantation. Using the physiologically relevant delayed implantation model and trophoblast stem cells in culture, we further demonstrate that a coordinated activation of canonical Wnt-β-catenin signaling with attenuation of the noncanonical Wnt-RhoA signaling pathway ensures blastocyst competency to implantation. These findings constitute novel evidence that Wnt signaling is at least one pathway that determines blastocyst competency for implantation.
Keywords: blastocyst activation, implantation, Wnt, β-catenin
INTRODUCTION
Synchronization of blastocyst activation with uterine receptivity is essential to implantation. While a range of signaling molecules helps in specifying uterine receptivity for implantation, the signaling network that governs blastocyst activation is not clearly understood (Wang and Dey, 2006). Since an intricate interplay between the embryo and uterus during implantation shares similar features of reciprocal cell-cell communications during organogenesis, signaling pathways driven by Wnt proteins are likely to participate in this process.
Wnt proteins function through different cell-surface and intracellular components to execute distinctive functions (Wnt home page: http://www.stanford.edu/~rnusse/wntwindow.html). For example, the canonical Wnt signaling pathway involving both Frizzled (Fzd) and low density lipoprotein receptor-related protein (LRP) 5/LRP6 receptors leads to nuclear translocation and stabilization of β-catenin, which then interacts with T cell-specific transcription factor/lymphoid enhancer-binding factor 1/ (TCF/LEF) to influence transcription of target genes (Gordon and Nusse, 2006; Willert and Jones, 2006). However, Wnt proteins can also signal through β-catenin-independent (noncanonical) pathways solely via Fzd receptors, regulating Ca++/planar cell polarity (PCP) and Rho signaling (Barrow, 2006; Veeman et al., 2003). Genetic and biochemical evidence demonstrates that bioactivities of Wnt proteins can be inhibited by direct binding to secreted frizzled-related proteins (sFRP) with similar sequence signatures as Fzd receptors in the cysteine-rich domain (Rattner et al., 1997). Alternatively, Wnt signaling can be antagonized by Dickkopf proteins (DKK) which bind to the Wnt coreceptors LRP5/6 (Bafico et al., 2001; Glinka et al., 1998; Mao et al., 2001; Semenov et al., 2001) and interact with Kremen for downregulating cell surface LRP receptors (Mao and Niehrs, 2003; Mao et al., 2002). The complexity and redundancy of Wnt family of proteins, receptors, extracellular antagonists and intracellular signaling components suggest that the nature of the cellular Wnt machinery determines whether the signaling cascade is driven by canonical or noncanonical pathways.
Previous studies have shown unique expression profiles of multiple Wnt genes and their pathway members in early embryos and uteri during the periimplantation period in mice (Hamatani et al., 2004a; Kemp et al., 2005; Mohamed et al., 2005; Paria et al., 2001; Wang et al., 2004), suggesting that Wnt signaling is operative during early pregnancy. However, it is not clearly understood how canonical and noncanonical Wnt pathways are temporally coordinated in synchronizing blastocyst activation and uterine receptivity for implantation. Exploiting multiple approaches, we here explored the physiological significance of Wnt signaling in blastocyst functions and implantation.
MATERIALS AND METHODS
Mice
Adult CD1 (Charles Rivers Laboratory) mice were housed in Vanderbilt Animal Care Facility according to National Institutes of Health and institutional guidelines for laboratory animals. Females were mated with fertile or vasectomized males to induce pregnancy or pseudopregnancy (D1 = vaginal plug), respectively.
Adenoviral vectors and drug delivery
To achieve conditional Wnt inactivation in mice, we applied an adenoviral vector carrying murine DKK1 cDNA with C-terminal His6 epitope tags (DKK1 ADV), which has been proven to silence canonical Wnt signaling in adult mouse intestine (Kuhnert et al., 2004). Intact pregnant mice received intravenous injections of control adenoviral vectors (empty ADV) or DKK1 ADV (2.5×109 pfu in 200 μl saline) through tail veins on day 1, and implantation were analyzed by the blue dye method on day 5 (Paria et al., 1993). DKK1 overexpression status was monitored by Western blot analysis of 1 μl of plasma obtained by retroorbital phlebotomy from pregnant females receiving empty or DKK1 ADV using anti-His probe antibody (Santa Cruz Biotechnology) as previously described (Kuhnert et al., 2004). To further explore the consequence of Wnt inhibition on blastocyst implantation, we applied small molecule inhibitors of β-catenin/Tcf complex, PKF115-584 and CGP049090 (Novartis) (Lepourcelet et al., 2004). Mice received subcutaneous injections of each drug at doses of 10 mg/Kg·BW on days 3 and 4 and implantation were examined on day 5 morning.
Reciprocal embryo transfer
To explore differential impacts of Wnt silencing on blastocyst activation and uterine receptivity, we performed embryo transfer experiments. Blastocysts were collected from normal pregnant mice and transferred into day 4 pseudopregnant recipients receiving an intravenous injection of empty ADV or DKK1 ADV (2.5×109 pfu in 200 μl saline) on day 1. Conversely, blastocysts retrieved from pregnant females receiving the same amount of empty ADV or DKK1 ADV were transferred into normal day 4 pseudopregnant recipients. Recipients were sacrificed 24 hours after transfer to examine the implantation status.
Delayed implantation, dormant blastocyst culture and transfer
The conditions of delayed implantation were induced by ovariectomizing pregnant or pseudopregnant mice on the morning of day 4 (0830-0900 h) and maintained by daily injections of P4 (2 mg/mouse) from day 5 until sacrifice. Activation of dormant blastocysts in P4-primed delayed implanting pregnant mice was induced by giving a single injection of E2.
To obtain further insight into the physiological significance of canonical Wnt pathway in blastocyst activation, we examined whether silencing β-catenin signaling would interfere with blastocyst activation in delayed implanting mice. Delayed implanting pregnant mice received an intravenous injection of empty ADV or DKK1 ADV (2.5×109 pfu in 200 μl saline) on day 5, or a subcutaneous administration of PKF115-584 (10 mg/Kg·BW) on day 7, followed by an injection of E2 (3 ng/mouse) on day 7. Implantation was examined on day 8 by the blue dye method.
To study the effect of Wnt3a and GW501516 in conferring blastocyst implantation competence, dormant blastocysts were cultured in M16 medium containing vehicle, recombinant Wnt3a protein (R&D), and/or GW501516 for 24 hours. To examine the selectivity of Wnt ligands in activating nuclear β-catenin signaling, blastocysts were pre-incubated with recombinant DKK1 protein (R&D) or PKF115-584, respectively for 1 h prior to the addition of Wnt3a protein. Blastocysts exposed to vehicle, Wnt3a protein, and/or GW501516 were transferred into the receptive uterus 4 h after P4-primed ovariectomized pseudopregnant recipients receiving an E2 injection (3 ng/mouse) as described earlier (Paria et al., 1993). All recipients were sacrificed 24 h after blastocyst transfers and the number of implantation sites was recorded by blue dye method.
Trophoblast stem cell culture
The TS cell line was developed from day 4 mouse blastocysts as previously described (Tanaka et al., 1998). Cells were maintained in a proliferative state in media containing 70% embryonic fibroblast conditioned media, 30% TS cell medium, FGF4 (25 ng/ml) and heparin (1 μg/ml). To study Wnt-β-catenin signaling, TS cells were precultured in serum-free TS medium, FGF-4 and heparin for 2 h and then challenged with recombinant Wnt3a protein and/or antagonists. After termination of culture, TS cells were lysed and lysates of membrane, cytosolic and nuclear fraction (30 μg per sample) were analyzed by immunoblotting for different Wnt family components. Targeted protein bands were visualized using an ECL kit (Amersham).
Whole mount immunofluorescence
Immunofluorescence staining in embryos was performed as described by us (Wang et al., 2003). In brief, embryos were fixed in 10% neutral buffered formalin solution at room temperature for 30 minutes, permeabilized in 2.5% Tween 20 in PBS for 5 minutes and then incubated overnight at 4°C with primary antibody (Table S1). After several washes with PBS containing 0.5% Triton X-100 and 0.5% BSA, embryos were incubated with Cy3-labeled secondary antibody and SYTO-13 green nuclear dye for 1 hour at room temperature. Fluorescence signals were viewed under a Zeiss LSM 510 confocal laser microscope.
Rho-GTP affinity assay
Analysis of active Rho protein was conducted as previously described (Berdeaux et al., 2004). Briefly, blastocysts were fixed with freshly prepared 2% PFA in PBS for 5 min at room temperature. After washing, blastocysts were permeabilized in PBS containing 3% BSA, 0.1 M glycine and 0.05% Triton X-100, followed with blocking in the same buffer containing 10% donkey serum, then incubated with 50 μg/ml of soluble GST-RBD for 2 h at 4°C. Anti-GST 1st and subsequent Cy3 conjugated 2nd antibodies were used to visualize the GST-GTP-bounded active Rho proteins. Images were obtained with a Zeiss510 LSM.
In situ hybridization
In situ hybridization was performed as previously described (Das et al., 1994). Frozen sections (10 μm) were mounted onto poly-L-lysine-coated slides and fixed in 4% paraformaldehyde solution in PBS at 4°C. After prehybridization, sections were hybridized at 45°C for 4 h in 50% formamide buffer containing 35S-labeled sense or antisense cRNA probes. After hybridization, sections were incubated with RNase A (20 μg/ml) at 37°C for 20 min, and RNase A-resistant hybrids were detected by autoradiography using Kodak NTB-2 liquid emulsion. Sections hybridized with the sense probes served as negative controls. Sections were poststained with hematoxylin and eosin.
RESULTS
Silencing nuclear β-catenin signaling has no adverse effects on preimplantation embryo development
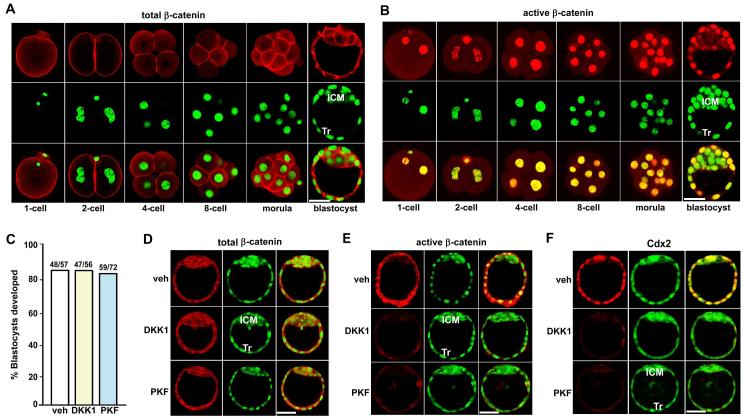
Since multiple Wnt family members are expressed in early embryos (Hamatani et al., 2004a; Kemp et al., 2005; Wang et al., 2004), we first explored the intracellular distribution of β-catenin in preimplantation embryos. Using immunofluorescence, we observed that total and dephospho (active) β-catenin was expressed at all stages of embryos spanning fertilized 1-cell embryos to blastocysts (Fig. 1A, B). It was worth noting that active β-catenin was mostly localized in the nuclei of all embryonic cells before morulae, and primarily the trophectoderm (Tr) of blastocysts.
Fig. 1. Consequence of silencing nuclear β-catenin on mouse preimplantation embryo development.
(A, B) Immunofluorescence localization of total and dephospho (active) β-catenin in mouse preimplantation embryos. (C-F) Recombinant DKK1 protein (5 μg/ml) and PKF115-584 (0.1 μM) block nuclear import of active dephospho β-catenin and Cdx2 expression in preimplantation embryos, without interfering with the cellular level of total β-catenin and the development of 2-cell embryos to blastocysts in culture. Two-cell embryos recovered by flushing day 2 pregnant oviducts were cultured in groups of 5-10 in 25 μl of M16 medium under silicon oil in an atmosphere of 5% CO2 and 95% air at 37□ for 72 h and the number of blastocysts developed was recorded. Experiments were repeated 3-5 times. The number above the bar in panel C indicates the number of blastocysts developed per the number of cultured 2-cell embryos. Images shown depict Cy3-labeled active β-catenin as red, SYTO-13-labeled nuclei as green and merge as yellow. Bar, 50 μm. ICM, inner cell mass; Tr, trophectoderm; veh: vehicle; PKF: PKF115-584.
To explore the significance of nuclear β-catenin signaling on preimplantation embryo development, we employed recombinant DKK1 protein and PKF115-584, a small molecule inhibitor of TCF/β-catenin complexes (Lepourcelet et al., 2004), in 2-cell embryo culture experiments. We noted that recombinant DKK1 protein and PKF115-584 have no apparent adverse effects on the development of 2-cell embryos to blastocysts in culture (Fig. 1C). Immunofluorescence analysis further revealed while the recombinant DKK1 protein or PKF115-584 did not alter cellular levels of total β-catenin that are primarily associated with adherens junctions (Fig. 1D), these treatments significantly blocked nuclear accumulation of β-catenin and reduced the expression of Cdx2, a key transcription factor involved in Tr lineage specification (Meissner and Jaenisch, 2006; Niwa et al., 2005), in blastocysts developed from 2-cell stage (Fig. 1E and F). These findings suggest that canonical Wnt-β-catenin signaling is not required for preimplantation embryo development, but may regulate blastocyst functions during the periimplantation period.
Inactivation of canonical Wnt signaling impairs normal implantation
Studies to explore definitive roles of Wnt proteins in blastocyst implantation are limited due to embryonic lethality resulting from targeting of Wnt genes. A recent report showed that systemic overexpression of DKK1 via adenoviral vectors (DKK1 ADV) inhibits intestinal cell proliferation due to antagonism of nuclear β-catenin signaling, providing an alternative strategy for conditional silencing of Wnt functions (Kuhnert et al., 2004). Using this strategy, we examined the consequence of conditional inactivation of Wnt-β-catenin signaling in embryo implantation in mice.
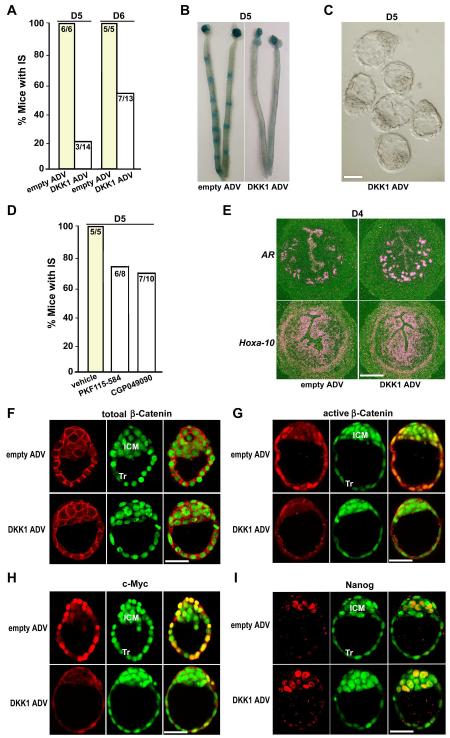
Pregnant females receiving intravenous injection of DKK1 ADV on day 1 of pregnancy had elevated circulating, oviductal and uterine levels of DKK1 (see Fig. S1A-C in the supplementary material), which was accompanied by compromised implantation. For example, only 21% (3/14) of pregnant mice treated with DKK1 ADV showed implantation when examined on day 5 morning by the blue dye method (Fig. 2A, B). Even when examined on day 6, only 53% (7/13) of DKK1 ADV-treated females showed blue reaction (Fig. 2A). Unimplanted blastocysts with morphologically normal appearance were recovered from uteri lacking blue bands (Fig. 2C), indicating that silencing of conical Wnt pathway interferes with normal implantation without any apparent detrimental effects on the development of preimplantation embryos to blastocysts. Furthermore, PKF115-584 and CGP049090, small molecule inhibitors of TCF/β-catenin complexes (Lepourcelet et al., 2004), substantially reduced implantation success in plug-positive mice (Fig. 2D).
Fig. 2. Inactivation of Wnt-β-catenin signaling derails on-time implantation.
(A) Implantation in mice receiving empty or DKK1 ADV on days 5 and 6 of pregnancy. Implantation sites (IS) were visualized by the blue dye method. Numbers within the bar indicate the number of mice with IS/total number of mice examined. (B, C) Representative photomicrograph of uteri with or without IS (blue bands) and recovered unimplanted morphologically normal blastocysts from those without blue reaction (Bar, 50 μm). (D) Implantation in mice receiving vehicle, PKF115-584 or CGP049090 (each 10 mg/Kg·BW) on day 5. Numbers within the bar indicate the number of mice with IS/total number of mice examined. (E) In situ hybridization showing comparable expressions of amphiregulin (AR) and Hoxa-10 in day 4 uteri of mice receiving empty or DKK1 ADV (Bar, 200 μm). (F-I) Overexpression of DKK1 via DKK1 ADV exerts no effects on the cellular level of total β-catenin, but remarkably attenuates nuclear stabilization of active dephospho β-catenin and c-Myc expression in blastocyst trophectoderms (Tr) when examined on day 4 midnight (day 4.5). In contrast, Nanog, an inner cell mass (ICM) marker gene, is normally expressed in ICM cells of blastocysts recovered from pregnant females receiving either DKK1 ADV or empty vectors on day 4.5. Represented immunofluorescence staining images depict Cy3-labeled antigens as red, SYTO-13-labeled nuclei as green and merge as yellow. Bar, 50 μm.
Since blastocyst activation and uterine receptivity are two distinct processes that are equally important for successful implantation (Wang and Dey, 2006), we employed reciprocal blastocyst transfer experiments to ascertain whether embryonic or uterine events or both were impaired by the conditional inactivation of canonical Wnt signaling, leading to the failure of on-time implantation. We found that normal day 4 blastocysts retrieved from untreated females showed comparable implantation rates after transferred into pseudopregnant recipients receiving either DKK1 ADV or empty vectors (empty ADV), while blastocysts recovered from pregnant females receiving an intravenous injection of DKK1 ADV exhibited considerably reduced implantation rates transferred into normal untreated pseudopregnant recipients (Table 1). This finding suggests that deficiency in blastocyst function rather than altered uterine receptivity contributes to implantation failure. This is consistent with normal uterine expression of amphiregulin and Hoxa-10 in day 4 pregnant mice receiving DKK1 ADV (Fig. 2E); these genes are associated with uterine receptivity (Das et al., 1995; Lim et al., 1999). In contrast, while no obvious changes in cellular levels of total β-catenin associated with adherens junctions were noted (Fig. 2F), the nuclear translocation of active β-catenin, which normally occurs in the Tr of day 4.5 implanting blastocysts, was greatly inhibited by increasing levels of DKK1 (Fig. 2G). Consequently, c-Myc, a target of the Wnt-β-catenin pathway (He et al., 1998) and shown to be critical for preimplantation embryo development (Paria et al., 1992), was downregulated in blastocyst Tr cells (Fig. 2H). Interestingly, Nanog, an inner cell mass (ICM) marker gene (Chambers et al., 2003; Mitsui et al., 2003), was normally expressed in ICM cells of blastocysts recovered from pregnant females receiving either DKK1 ADV or empty vectors (Fig. 2I), suggesting that canonical Wnt pathways are not essential for the development of blastocyst ICM cells during implantation. Collectively, the results show that silencing of canonical Wnt pathway does not interfere with uterine receptivity, but blocks blastocyst implantation competency, highlighting the necessity of nuclear β-catenin signaling for normal blastocyst functions during implantation.
Table 1. Reciprocal embryo transfer in mice receiving either DKK1 ADV or empty vectors.
| Treatment |
No. of blastocysts transferred |
No. of recipients |
No. of mice with IS (%) |
No. of IS (%) |
No. of blastocysts recovered from mice without IS (%) |
|
|---|---|---|---|---|---|---|
| Embryo donors | Recipients | |||||
| No treatment | Empty ADV | 104 | 7 | 7 (100%) | 60 (58%) | N/A |
| No treatment | DKK1 ADV | 135 | 9 | 9 (100%) | 73 (54%) | N/A |
| Empty ADV | No treatment | 82 | 6 | 6 (100%) | 46 (56%) | N/A |
| DKK1 ADV | No treatment | 98 | 8 | 1 (13%)* | 5 (5%)* | 28 (29%) |
Normal day 4 blastocysts retrieved from untreated mice were transferred into day 4 pseudopregnant recipients receiving treatments of either DKK1 ADV or empty vectors. Conversely, blastocysts retrieved from pregnant females receiving either DKK1 ADV or empty vectors were transferred into the uterine horn of normal untreated pseudopregnant recipients on day 4 midmorning. Implantation was examined 24 hours later by blue dye method. N/A, not applicable.
P<0.05 (Student t-test).
Wnt-β-catenin signaling in delayed implanting blastocysts
To address the significance of nuclear Wnt-β-catenin signaling in blastocyst function, we analyzed the expression profile of multiple Wnt proteins and associated signaling members in blastocysts undergoing experimentally induced dormancy and reactivation. In mice, ovariectomy prior to preimplantation ovarian estrogen secretion on day 4 of pregnancy initiates blastocyst dormancy which lasts for many days with continued progesterone (P4) treatment (Paria et al., 1993). An estrogen (E2) injection rapidly activates blastocysts with the initiation of implantation. The delayed implantation model is a powerful approach to define molecular signaling that directs blastocyst dormancy or activation.
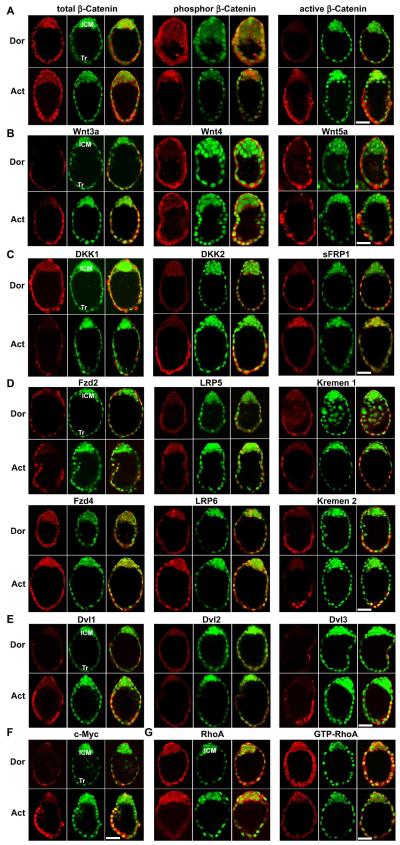
We observed by immunofluorescence staining that total β-catenin distribution in Tr and ICM cells of both dormant and activated blastocysts is comparable. In contrast, while phospho (inactive) β-catenin was clearly detected in dormant blastocysts, it was substantially downregulated in Tr cells of activated blastocysts with sustained higher levels in the ICM (Fig. 3A). Conversely, the active β-catenin accumulated in activated blastocyst Tr, but not in ICM, cells (Fig. 3A). We also found that Wnt3a was upregulated in implantation-competent Tr cells, whereas Wnt4 and Wnt5a were detected in similar intensity in both dormant and activated blastocysts (Fig. 3B). Interestingly, contrasting expression patterns of several secretory Wnt antagonists were noted in dormant and activated blastocysts. For example, DKK1 was downregulated and DKK2 was induced in Tr cells of activated blastocysts, while sFRP1 expression was restricted to the ICM of activated blastocysts (Fig. 3C). These results provide evidence that Wnt-β-catenin signaling coordinated by appropriate antagonists plays an important role in regulating blastocyst activation.
Fig. 3. Wnt pathways in dormant and activated blastocysts.
(A) Differential patterns of phospho (inactive) and dephospho (active) β-catenin during blastocyst activation. While total β-catenin distribution was comparable in dormant (Dor) and activated (Act) blastocysts, phospho β-catenin was primarily detected in the inner cell mass (ICM) and active β-catenin mostly in the trophectoderm (Tr) of activated blastocysts. (B) Wnt3a was induced in activated blastocyst Tr, while Wnt4 and Wnt5a were detected at high levels in the same cell-types in both dormant and activated blastocysts. (C) Dynamic expression of Wnt antagonists, DKK1, DKK2 and sFRP1 in delayed implanting blastocysts. While DKK1 was downregulated, DKK2 was induced in the Tr with blastocyst activation. In contrast, sFRP1 expression was restricted to the ICM of activated blastocysts. (D) Expression of Wnt receptor subtypes, Fzd 2, Fzd4, LRP5, LRP6, Kremen 1 and Kremen 2 in dormant and activated blastocysts. One intriguing observation is the internalization and nuclear import of Wnt receptors in activated blastocysts. (E) Expression of Dvl1-3 proteins in delayed implanting blastocysts. It was notable that Dvl1 and Dvl3 increasingly accumulated in the cytoplasm with visible nuclear localization of Dvl1 in the Tr during blastocyst activation. (F) c-Myc was induced in Tr cells of activated blastocysts. (G) Downregulation of total and GTP-bounded (active) RhoA GTPase in the Tr during blastocyst activation. Represented immunofluorescence staining images shown depict Cy3-labeled antigens as red, SYTO-13-labeled nuclei as green and merge as yellow. Bar, 50 μm.
To better understand the nuclear β-catenin signaling machinery in blastocysts, we next examined the expression patterns of Wnt receptor subtypes and intracellular scaffold proteins Dishevelled (Dvl). In dormant blastocysts, Fzd2, LRP5, Kremen 1 and Kremen 2 receptors were expressed primarily in Tr cells, and Fzd4 and LRP6 subtypes in both Tr and ICM cells with membrane and cytoplasmic localization (Fig. 3D). Surprisingly, these membrane receptors translocated into nuclei of Tr cells with blastocyst activation (Fig. 3D). Moreover, increased cytoplasmic levels of Dvl1 and Dvl3 with nuclear localization of Dvl1 were observed in Tr cells of activated blastocysts (Fig. 3E). These are interesting findings and suggest that ligand-activated Wnt receptor internalization and nuclear translocation, along with nuclear transport of Dvl proteins, participate in nuclear localization of β-catenin for transcriptional regulation of target gene expression. In fact, c-Myc, a known target of Wnt-β-catenin pathway (He et al., 1998), was markedly induced in Tr cells coinciding with the accumulation of active β-catenin in activated blastocysts (Fig. 3F), indicating that this nuclear pathway is functionally activated during blastocyst activation.
Since blastocysts also express noncanonical Wnt ligands Wnt 4 and Wnt5a, we then analyzed the activity of Wnt signaling independent of β-catenin, e.g. RhoA signaling (Veeman et al., 2003) during blastocyst activation. We observed that the total and GTP-bounded (active) RhoA GTPase were dramatically downregulated in Tr cells of activated blastocysts (Fig. 3G). It is conceivable that a coordinated activation of canonical Wnt-β-catenin signaling with concurrent inhibition of noncanonical Wnt-RhoA signaling during blastocyst activation plays an important role in regulating Tr functions during implantation.
Wnt3a activates nuclear β-catenin signaling in TS cells
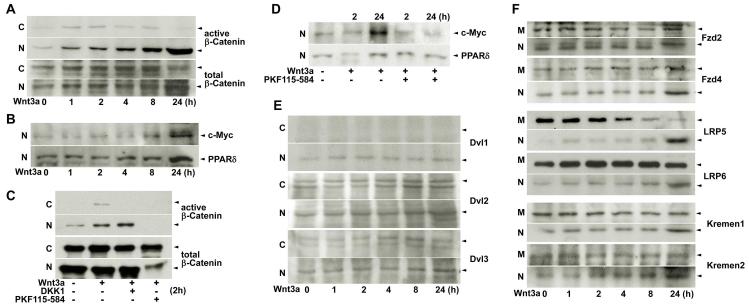
We used a mouse trophoblast stem (TS) cell line to further explore the underlying mechanism of intracellular activation and nuclear translocation of β-catenin in response to Wnt3a which is upregulated in Tr cells of activated blastocysts. We observed that recombinant Wnt3a protein rapidly induced cytoplasmic accumulation of dephospho β-catenin and its translocation into nuclei of differentiating TS cells (Fig. 4A). This progressive activation of nuclear β-catenin signaling by Wnt3a was manifested by upregulated expression of c-Myc and peroxisome proliferative activated receptor δ (PPARδ, prostacyclin nuclear receptor) (Fig. 4B), known targets of this pathway (He et al., 1999; He et al., 1998).
Fig. 4. Nuclear β-catenin signaling in TS cells in culture.
(A) Time-dependent accumulation and nuclear translocation of active β-catenin in response to recombinant Wnt3a protein (50 ng/ml) in differentiating TS cells. (B) Wnt3a induced c-Myc and PPARδ expression in differentiating TS cells. (C) DKK1 (1 μg/ml) and PKF115-584 (1 μM) blocked Wnt3a-induced cytoplasmic accumulation of active dephospho β-catenin in TS cells by 2 hour of cotreatments. Notably, even basal levels of nuclear β-catenin disappeared following PKF115-584 treatment. (D) Similar treatments with DKK1 (1 μg/ml) and PKF115-584 (1μM) downregulated Wnt3a-induced c-Myc and PPARδ expression in differentiating TS cells. (E) Dvl1-3 proteins in TS cells. While Dvl1 was only detected in the nucleus, Dvl2 and Dvl 3 were detected in both the cytoplasm and nucleus in response to Wnt3a. (F) Wnt receptors, Fzd 2, Fzd4, LRP5, LRP6, Kremen 1 and Kremen 2 in TS cells. Wnt3a facilitated nuclear import of Wnt receptor subtypes in differentiating TS cells, mimicking the finding in blastocysts during activation. Representative pictures of Western blotting analysis of Wnt family components in TS cells were presented in panels A-F. C, M or N, cytoplasmic, membrane or nuclear protein extraction.
We next asked whether recombinant DKK1 protein or PKF115-584, a small molecule inhibitor of TCF/β-catenin complex, could block Wnt3a-induced β-catenin stabilization in TS cells. Indeed, treatment with either DKK1 or PKF115-584 significantly attenuated the rapid cytoplasmic accumulation of β-catenin in TS cells in response to Wnt3a (Fig. 4C). It was remarkable to see that PKF115-584 totally prevented the nuclear accumulation of β-catenin within 2 hours. This adverse effect of PKF115-584 on Wnt3a-β-catenin signaling was also reflected by Wnt3a’s failure to induce c-Myc and PPARδ expression at 24 h of treatment (Fig. 4D). The results support our premise that Wnt-β-catenin signaling is a regulator of trophoblast differentiation.
Early evidence suggests that Dvl proteins and their subcellular localization determine Wnt downstream signaling cascades (Capelluto et al., 2002; Itoh et al., 2005; Torres and Nelson, 2000). To better understand the transducers involved in activating β-catenin signaling in TS cells, we analyzed cytoplasmic vs nuclear distribution of Dvl proteins in these cells in response to Wnt3a in culture. As illustrated in Fig. 4E, while Dvl2 and Dvl3 were detected in both the cytoplasm and nucleus, Dvl1 was primarily localized in nuclei of differentiating TS cells. We also found that Wnt3a induced internalization and nuclear import of Wnt family receptors, including Fzd2, Fzd4, LRP5, LRP6, Kremen 1 and Kremen 2 in TS cells (Fig. 4F). These results uphold the similar nature of intracellular translocation of Wnt receptors observed in blastocysts, suggesting that functional activation of nuclear β-catenin signaling in Tr/TS cells requires coordination between the cell surface and intracellular Wnt components.
Canonical Wnt signaling synergizes with that of PPARδ to confer blastocyst competency for implantation
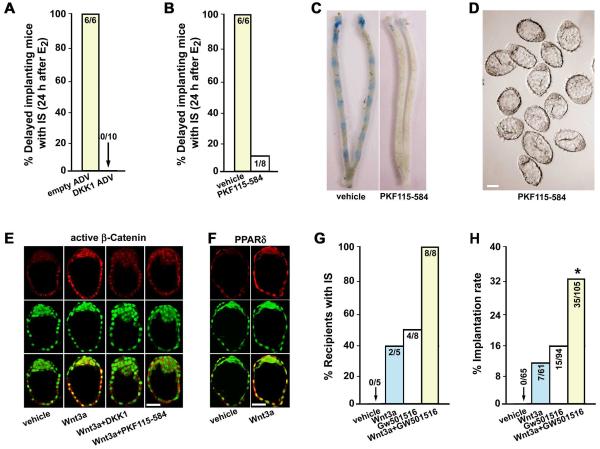
To obtain further insight into the physiological significance of canonical Wnt pathway in blastocyst activation, we again exploited delayed implantation models. For loss-of-function studies, we examined whether silencing β-catenin signaling would interfere with blastocyst activation in delayed implanting mice. Delayed implanting pregnant mice received empty ADV or DKK1 ADV on day 5, followed by an injection of E2 (3 ng/mouse) on day 7. Implantation was examined on day 8 by the blue dye method. As illustrated in Fig. 5A, overexpression of DKK1 led to complete failure of implantation initiation in response to E2. PKF115-584-treated delayed implanting mice also showed compromised implantation when examined 24 h after E2 treatment (Fig. 5B, C); morphologically dormant blastocysts were recovered from uteri not showing blue bands (Fig. 5D). These observations reinforce the concept that nuclear β-catenin signaling is essential for normal blastocyst activation for implantation.
Fig. 5. Wnt-β-catenin signaling synergizes with that of PPARδ to confer blastocyst competency for implantation.
(A, B) Overexpressing levels of DKK1 or PKF115-584 blocked activation of dormant blastocysts for implantation in response to E2 (3 ng/mouse). Numbers within the bar indicate the number of mice with implantation sites (IS)/total number of mice examined. (C, D) Representative photomicrographs of uteri without blue bands and morphologically dormant blastocysts recovered from mice treated with PKF115-584 (Bar, 50 μm). (E, F) Recombinant Wnt3a protein (200 ng/ml) induced nuclear stabilization of active dephospho β-catenin and PPARδ expression in dormant blastocysts in culture. Cotreatment of Wnt3a with DKK1 (1 μg/ml) or PKF115-584 (1 μM) antagonized Wnt3a-induced β-catenin stabilization. Images shown depict Cy3-labeled antigens as red, SYTO-13-labeled nuclei as green and merge as yellow. Bar, 50 μm. (G, H) Wnt3a and/or GW501516 conferred blastocyst implantation competency. Dormant blastocysts were cultured in the presence of vehicle, Wnt3a (200 ng/ml) and/or GW501516 (a selective PPARδ agonist, 1 μM) for 24h before transferred into pseudopregnant delayed recipients. Numbers within the bar in G indicate the number of recipients with IS/total number of mice examined, and those in H indicate the number of IS/total number of blastocysts transferred.
For gain-of-function studies, we asked whether Wnt3a could override blastocyst dormancy state via activating nuclear β-catenin signaling. As shown in Fig. 5E, recombinant Wnt3a protein induced β-catenin stabilization and nuclear translocation in dormant blastocysts after 24 hour of culture. However, this effect was largely blocked by cotreatment with recombinant DKK1 protein or PKF115-584. Moreover, Wnt3a upregulated the expression of PPARδ in cultured blastocysts (Fig. 5F). To see that dormant blastocysts gain implantation competency when exposed to Wnt3a, we conducted blastocyst transfer experiments in delayed pseudopregnant recipients. Dormant blastocysts cultured for 24 h in the presence or absence of Wnt3a (200 ng/ml) were transferred into uterine lumens of P4-treated pseudopregnant recipients 4 h after an injection of E2 (3 ng per mouse). Under these conditions, while dormant blastocysts fail to implant, activated blastocysts do implant (Paria et al., 1993). We observed that about 11% of Wnt3a-treated blastocysts showed implantation in 2 of 5 recipients, while those exposed to vehicle failed to implant in similarly treated uteri (Fig. 5G, H). This result suggests that blastocysts in culture remain implantation incompetent, but partially gain implantation competency when treated with Wnt3a in the medium. However, the relatively low implantation rate of dormant blastocysts in response to Wnt3a alone suggests that there are alternative pathways that synergize with Wnt-β-catenin signaling to regulate blastocyst activation.
Since Wnt3a upregulates the expression of PPARδ in blastocysts in culture, and since cyclooxygenase-2 (COX-2, one of the limiting enzyme of prostaglandin synthesis) and PPARδ are substantially upregulated in Tr cells during blastocyst activation in vivo (see Fig. S2 in the supplementary material), we next explored potential roles of PPARδ signaling in blastocyst activation. 20 We found that GW501516, a selective PPARδ agonist (Oliver et al., 2001), promoted dormant blastocysts to partially achieve implantation competency in culture. For example, about 16% of GW501516-treated blastocysts showed implantation in 4 of 8 recipients (Fig. 5G, H). Interestingly, a cotreatment of Wnt3a and GW501516 further improved the implantation rate of dormant blastocyst after culture. Approximately 33% of the transferred blastocysts exposed to Wnt3a plus GW501516 implanted in all recipients (8/8) (Fig. 5G, H). These results suggest that nuclear Wnt-β-catenin signaling which upregulates PPARδ expression synergizes its function to confer blastocyst competency for implantation.
DISCUSSION
Synchronization of blastocyst activation with uterine receptivity is essential to normal implantation. While a wide range of signaling molecules helps in specifying uterine receptivity for implantation (Wang and Dey, 2006), there is limited information regarding the signaling network that governs blastocyst activation (Hamatani et al., 2004b; Paria et al., 1998; Wang et al., 2003). Our present findings using multiple approaches demonstrate an essential role of nuclear Wnt-β-catenin signaling in ensuring blastocyst competency to implantation.
Although recent evidence suggests that preimplantation embryos possess the machinery of Wnt-β-catenin signaling (Hamatani et al., 2004a; Kemp et al., 2005; Wang et al., 2004), it remained unknown whether this signaling is critical for the development of preimplantation embryos to blastocysts and subsequent implantation. Studies with genomic β-catenin null mice showed that β-catenin null mutant embryos from heterozygous crossings developed to blastocysts and implanted normally in heterozygous mother, but showed first sign of abnormal development of embryonic ectoderm on day 7.5 of pregnancy (Haegel et al., 1995; Huelsken et al., 2000). However, the contribution of nuclear β-catenin signaling in early embryo development and blastocyst implantation cannot be assessed in this mouse model, since β-catenin null mutant blastocysts contain a large amount of maternally derived β-catenin, and cannot be distinguished from littermate heterozygous and wild-type blastocysts by immunostaining, even on days 5 and 6 in culture (Haegel et al., 1995).
A recent study using conditional elimination of β-catenin in oocytes provides evidence that zygotes, even with depletion of both maternal and zygotic β-catenin, form blastocysts in culture, suggesting that β-catenin does not play a critical role during preimplantation embryo development (De Vries et al., 2004). However, a potential role of β-catenin in blastocyst function during implantation is predicted by this study. For example, although oocytes with conditional deletion of β-catenin develop into blastocysts, female mice with maternal β-catenin depletion produce reduced number of pups when crossbred with wild-type males in comparison to those of wild-type to wild-type mating. However, this reduction in pup numbers is rescued in females with conditional deletion of both β-catenin and E-cadherin in oocytes (De Vries et al., 2004). Considering diverse roles of β-catenin in cellular functions, including its association with E-cadherin in adherens junctional complexes and functioning as an intermediate in canonical Wnt pathways, this study suspected that paternal derived β-catenin in blastocysts with maternal β-catenin depletion is primarily incorporated into adherens junctions, causing insufficiency for nuclear Wnt signaling and thereby leading to loss of blastocysts during the periimplantation period. In contrast, simultaneous depletion of β-catenin and E-cadherin restores nuclear β-catenin signaling in blastocysts, because in the presence of less E-cadherin more β-catenin is available for nuclear Wnt signaling (De Vries et al., 2004). Our present investigation using the strategy of DKK1-mediated functional inhibition of nuclear β-catenin signaling and small molecule inhibitors of Wnt signaling provides direct evidence that canonical Wnt-β-catenin signaling is unlikely required for preimplantation embryo development, but is essential for normal blastocyst functions during implantation. Our reciprocal embryo transfer experiments also reveal that silencing of Wnt-β-catenin pathway does not interfere with uterine receptivity, but primarily blocks blastocysts’ competency to implant, highlighting the necessity of nuclear β-catenin signaling in blastocyst activation for implantation.
The significance of this pathway in blastocysts is further evidenced from our findings in delayed implantation models, showing that the activity of nuclear β-catenin signaling distinguishes blastocyst dormancy and activation. Coincident with blastocyst activation, Wnt antagonist DKK1 is downregulated, while canonical ligand Wnt3a is induced at higher levels, leading to intracellular accumulation of dephospho β-catenin in blastocyst Tr cells. Interestingly, DKK2 is upregulated in activated blastocysts, perhaps functioning as a negative or positive feedback regulator of β-catenin signaling depending on the presence or absence of cell-surface Kremen 2 receptors (Mao and Niehrs, 2003). In addition, sFRP1 remaining only in the ICM of activated blastocysts may help maintain the pluripotency of ICM cells by suppressing Wnt signaling during blastocyst activation. This is consistent with early observations that inhibition of endogenous Wnt signals in mouse embryonic stem cells prevents the cells from differentiating into mesoderm (Lindsley et al., 2006); whereas constitutive expression of active β-catenin protein in early embryos leads to premature epithelial-mesenchymal transition in the embryonic ectoderm layer of early postimplantation embryos (Kemler et al., 2004). This enhanced β-catenin signaling, particularly in Tr cells, is physiologically relevant to blastocyst functions, since our gain-of-function experiments demonstrate that Wnt3a is able to induce PPARδ expression in the Tr and confer blastocyst competency for implantation in cooperation with GW501516, a selective PPARδ agonist.
In parallel to activation of canonical Wnt signaling, the RhoA signaling, a potential mediator of noncanonical Wnt pathway (Veeman et al., 2003), was attenuated in Tr cells with blastocyst activation. Since Rho proteins are required for maintenance of adherens junctions (Braga et al., 1997; Sahai and Marshall, 2002), this downregulation of RhoA GTPase protein and activity perhaps causes cytoskeletal reorganization and disassembly of adherens junction, thus destabilizing the leading edge of epithelial Tr cells conducive to blastocyst-uterine attachment. However, the molecular basis of divergence of Wnt signaling during blastocyst activation remains unknown.
Dvl proteins function as intermediate transducers balancing the transduction of Wnt-Fzd receptor downstream to β-catenin dependent vs independent pathways (Capelluto et al., 2002; Itoh et al., 2005; Torres and Nelson, 2000). For example, nuclear translocation of vesicular Dvl proteins triggers the accumulation and stabilization of β-catenin, whereas actin-binding Dvl trafficking to the plasma membrane results in RhoA activation, affecting cell shape and morphology. In this respect, our findings of increased cytoplasmic accumulation of Dvl1 and Dvl3, and nuclear translocation of Dvl1 in the implantation-competent Tr are well correlated with activation of β-catenin signaling and attenuation of RhoA signaling during blastocyst activation, supporting the concept that Dvl controls the diversification of Wnt pathways. However, our observation of accumulation of Dvl1 & Dvl3 proteins in the Tr of implantation-competent blastocysts is contradictory to a recent report showing enrichment of Dvl3 in ICM cells of implanting blastocysts, although this study states similar activation of canonical Wnt pathway without showing detail cell-type distribution of active β-catenin (Na et al., 2007). To confirm our findings in blastocysts, we also examined the activation of the Wnt-β-catenin signaling in Tr-derived TS cells in culture. The results showing localization of Dvl1 primarily in the nucleus and of Dvl2 and Dvl3 in both the cytoplasm and nucleus along with nuclear stabilization of active β-catenin in response to Wnt3a uphold our initial findings in delayed and activated blastocysts.
Another intriguing finding is the internalization and nuclear import of Wnt family of receptors in Tr cells during blastocyst activation. The significance and underlying mechanism of this phenomenon during Wnt signaling transduction remain mostly unknown. Recent evidence shows that endocytosis and nuclear import of Fzd2 receptor transduce Wingless signaling critical for synapse development in Drosophila (Mathew et al., 2005). It was also worthy of noting that reduced cell-surface Kremen 2 receptors with increased levels of DKK2 in Tr cells perhaps further enhance nuclear β-catenin signaling during blastocyst activation. Therefore, it is possible that the internalization of Wnt receptors in response to Wnt ligands involves the transduction of Wnt downstream signaling rather than the simple inactivation of receptors. Nonetheless, our present study illustrates the physiological significance of canonical vs noncanonical Wnt pathways in blastocyst functions during implantation.
Supplementary Material
Acknowledgments
We thank Novartis for providing us with PKF115-584 and CGP049090. This work was supported in parts by the National Institutes of Health Grants (HD050315 to HW and HD12304, DA06668 & CA77839 to SKDe). SKDe is recipient of Method to Extend Research in Time Awards from the National Institute of Child Health and Human Development and the National Institute on Drug Abuse (NIDA). ST is supported by an NRSA individual fellowship from NIDA (F31 DA021062). We also thank the Turner Foundation for generous support (to HW).
Abbreviations
- ADV
adenoviral vector
- COX
cyclooxygenase
- DKK
Dickkopf
- Dvl
Dishevelled
- E2
estradiol-17β
- Fzd
Frizzled
- ICM
inner cell mass
- LEF/TCF
lymphoid enhancer-binding factor 1/T cell-specific transcription factor
- LRP
low density lipoprotein receptor-related protein
- P4
progesterone
- PCP
planar cell polarity
- PPAR
peroxisome proliferative activated receptor
- sFRP1
secreted frizzled related protein 1
- Tr
trophectoderm
- TS cell
trophoblast stem cell
REFERENCES
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Barrow JR. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17:185–93. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Berdeaux RL, Diaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–23. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–31. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–9. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–83. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–45. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004a;6:117–31. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 2004b;101:10326–31. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–45. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–78. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–24. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–75. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–71. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–17. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–83. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–7. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212–5. doi: 10.1038/nature04257. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–84. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Lykke-Andersen K, Torres Padilla ME, Zernicka-Goetz M. Dishevelled proteins regulate cell adhesion in mouse blastocyst and serve to monitor changes in Wnt signaling. Dev Biol. 2007;302:40–9. doi: 10.1016/j.ydbio.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–29. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr., Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–11. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Dey SK, Andrews GK. Antisense c-myc effects on preimplantation mouse embryo development. Proc Natl Acad Sci U S A. 1992;89:10051–5. doi: 10.1073/pnas.89.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–62. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Lim H, Wang XN, Liehr J, Das SK, Dey SK. Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology. 1998;139:5235–46. doi: 10.1210/endo.139.12.6386. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–52. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–63. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–15. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–5. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–42. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci U S A. 2003;100:14914–9. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–44. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.