Abstract
The three-way interaction between oncolytic viruses, the tumor microenvironment, and the immune system is critical to the outcome of antitumor therapy. Classically, the immune system is thought to limit the efficacy of therapy, leading to viral clearance. However, preclinical and clinical data suggest that in some cases virotherapy may in fact act as cancer immunotherapy. In this review we discuss the ability of oncolytic viruses to alter the immunogenic milieu of the tumor microenvironment, and the role of innate and adaptive immunity in both restricting and augmenting therapy. Strategies to improve virotherapy by immunomodulation, including suppression or enhancement of the innate and adaptive responses, are discussed.
Oncolytic Viruses, the Immune System, and Splitting the Baby
In a cunning ploy to discover the truth when presented with a single baby boy claimed by two different mothers, King Solomon's response was to threaten to cut the baby in half. In this way, both parties would, in theory, receive half of what they desired most. Traditionally, viro-oncologists have perceived the immune system as nothing more than an inhibitor of viral replication through tumors. More recently, immuno-oncologists have suggested that the very same “baby” may be central to the ability of the virus to generate antitumor therapy. This immune-mediated therapy is induced, they would argue, by viral triggering of potent antitumoral immune effectors that destroy both infected (the minority) as well as uninfected (the majority) tumor cells. Moreover, this therapy may extend beyond what could realistically be expected from simple viral spread/oncolysis in an immune environment bristling with cells and molecules highly evolved to quench viral replication. Unlike the tricky situation over which Solomon had to adjudicate, there is ample scope in this case for compromise, with merit on both sides of the argument. Moreover, the technology now exists, in preclinical models at least, to wield the sword of Solomon—which he never actually needed to use—allowing the immune system to be cut in half (or even smaller bits). In this way, it will be possible to dissect out the immune responses to replication-competent viruses and to tumors undergoing oncolytic infection. These studies will show whether the responses that control increased viral replication can be selectively inhibited, while the responses that lead to immune-mediated tumor cell killing can be selectively augmented. Only when these intricate experiments have been done will it become clear which of the two plaintiffs should claim the immune system as their own.
Background
Viral infections before cases of cancer remissions have been noted throughout the last century (DePace, 1912; Bluming and Ziegler, 1971; Hansen and Libnoch, 1978). Advances in virus manufacture, genetic engineering, and cell biology have led to a surge of interest in the use of viruses for cancer therapy. Oncolytic viruses are self-replicating, theoretically tumor selective, and possess an ability to directly lyse cancer cells. On the basis of the concept that tumor-selective replication will amplify viral load enhancing antitumor efficacy, while sparing normal tissues, oncolytic viruses represent a very attractive novel approach to cancer therapy. Over the last 20 years the oncolytic activity of a wide range of viruses has been characterized, and several have entered clinical trials (Aghi and Martuza, 2005).
Despite the multitude of studies investigating direct viral effects, until more recently relatively little attention has been paid to the role of the immune system in oncolytic virotherapy. The immune system is adept at controlling viral infections, but does not have the sophistication required to distinguish between malevolent pathogens and therapeutic viruses. Therefore, its actions will potentially severely limit the efficacy of virotherapy. In contrast, evidence has accumulated that virus-induced immune activation can generate innate and adaptive immune responses that are critical to mediating tumor responses, such that oncolytic virotherapy can be considered as immunotherapy. In this review we examine the role that the immune system plays as, simultaneously, both an inhibitor and mediator of the efficacy of oncolytic virotherapy.
The Immune Response to Oncolytic Virotherapy: Can't Live with It, Can't Live without It
The majority of preclinical work involving oncolytic viruses has involved in vitro assays and immunocompromised xenograft models, focusing on the direct cytotoxic effect of the viral agent (Stojdl et al., 2000; Grote et al., 2001). These experiments have, inevitably, formed the perception that efficacy of oncolytic virotherapy is attributable solely to the ability of the virus to replicate in, and spread through, the tumor mass (Kirn et al., 2001). However, an extrapolation from immunocompromised models that an oncolytic virus will rapidly replicate, disseminate through, and destroy the tumor in immunocompetent patients is too simplistic. It is certainly true that the tumor microenvironment is usually locally immunosuppressed due to tumor mechanisms of immunosubversion (Zitvogel et al., 2006), and therefore likely to be permissive of viral replication. However, tumors still commonly contain a host of immune and stromal cells that are capable of slowing tumor progression (Prestwich et al., 2008a) and that are still highly competent at controlling viral infections. Therefore, there is little rationale to support a view that the immune system will not limit viral replication and spread within a tumor. Hence the baseline expectation of introducing an immunogenic, replication-competent vector into a tumor would be the activation of a potent, rapid innate immune response, limiting viral replication to at most a few cycles. Seen in this light, the role of the immune system would be expected to be heavily inhibitory, and immunosuppression should enhance therapeutic efficacy of oncolytic virotherapy.
Despite these arguments, the efficacy of oncolytic virotherapy has been well documented in multiple immunocompetent models in the absence of concurrent immunosuppression (Parato et al., 2005). These observations are contrary to the expectation that the immune system would limit therapy. For this there are three possible explanations: (1) the innate immune response is inefficient in the context of the tumor microenvironment, (2) viral replication proceeds faster than immune-mediated clearance, or (3) virotherapy is, at least partly, immunotherapy (Fig. 1). According to the third possibility, the immune system is responsible for antitumor activity, with the activation of immune effectors leading to bystander killing of tumor cells, while the intratumoral innate immune reactivity would limit viral replication and spread. If this concept is correct, the immune system is critical to mediating the efficacy of oncolytic virotherapy, and immunosuppression would actually reduce tumor therapy.
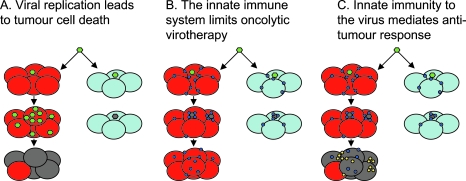
FIG. 1.
Different views on the mechanisms of oncolytic virotherapy. (A) Therapy from viral replication, spread, and lysis. Oncolytic virus (green) can infect either tumor (red) or normal (light blue) cells. The virus is either engineered to replicate only in tumor cells, or exploits defects in the antiviral innate immune response of tumor cells that allows its progressive spread through tumor but not normal cells. The antitumor effect relies solely on viral replication (dying tumor cells are gray). (B) Innate immunity to the virus is the enemy of oncolytic virotherapy. Viral infection of a tumor must also take into account the infiltrating immune cells that sense and respond to viral infection (blue circles). On exposure to virus, these cells secrete potent antiviral cytokines/interferons and other effector molecules. Many tumors are not completely defective in their responses to type I interferons and this innate activation within the tumor environment prevents viral replication and spread—even in tumor cells. This leads to clearance of the virus, extinction of the infection, and vastly reduced oncolysis and therapy. (C) Innate immunity to the virus is oncolytic virotherapy. In this view, the antiviral cytokines and interferons released by tumor-infiltrating immune cells in response to virus have significant antitumor activity themselves. These molecules act to shut down viral replication but also have directly antitumor effects by (1) killing tumor cells directly and/or (2) recruiting further antitumor immune effectors into the tumor (yellow circles), which finish off the job themselves. In this model, antitumor efficacy is mediated by the bystander killing of tumor cells induced by the immune response to viral infection.
To rationalize these contrasting influences of the immune system on oncolytic virotherapy, it is necessary to consider how viral infection of tumors will influence the immune response, along with examining data regarding the impact of innate and adaptive responses on therapeutic efficacy.
Mechanisms of Immune Activation: Danger and Pathogen-Activated Molecular Patterns
In 1989, Janeway proposed that resting antigen-presenting cells (APCs) are inactive, but could be activated via pattern recognition receptors (PRRs) binding to specific classes of pathogen-associated molecular patterns (PAMPs), allowing the APCs to recognize “infectious nonself” (INS) (Janeway, 1989). This concept has found support in the discovery of several classes of pathogen sensors, including the Toll-like receptors (TLRs), retinoic acid-inducible gene-1 (RIG-1)-like receptors (RLRs), nucleotide oligodimerization domain (NOD)-like receptors (NLRs), and some members of the group of C-type lectin receptors (Joffre et al., 2009). Dendritic cells (DCs), now identified as the professional APCs of the immune system, express a wide repertoire of these PRRs (Joffre et al., 2009).
In 1994 Matzinger formulated the “danger” theory of immune activation. This model suggests that the prime role of the immune system is to react to cellular or tissue distress, as opposed to nonself (Matzinger, 1994). According to this hypothesis, endogenous danger signals from stressed or dying cells rather than exogenous PAMPs activate APCs, providing the required costimulation to activate lymphocytes.
The INS and danger models of immune recognition are fundamentally different. The initiator of the immune response in the INS model is microbial nonself, in contrast to endogenous alarm signals within the danger model. In terms of tumor recognition, both theories predict the common phenomenon of a failure of the immune system to reject a tumor despite the presence of tumor-associated antigens (TAAs). According to the INS concept, tumors will lack the required microbial signals to activate APCs via PRRs. The danger theory predicts that, if tumors are growing “healthily,” insufficient alarm signals will be produced to activate APCs.
Oncolytic Viruses: Provision of PAMPs and/or “Danger” Signals in the Tumor Microenvironment
Multiple mechanisms by which tumors can evade immune control have been identified, several of which may be present in an individual tumor (Real et al., 2001). These mechanisms include reduced immunogenicity, resistance to immune cell killing, and immune subversion (Zitvogel et al., 2006). The multitude of mechanisms identified illustrates the challenge to be overcome in generating effective antitumor immunity. According to the concepts of INS and “danger,” the provision of PAMPs or danger signals may “break” tumor tolerance. The ability of oncolytic virotherapy to stimulate an effective innate or adaptive antitumor immune response will depend on an ability to supply PAMPs and/or danger signals, and overcome preexisting tumor immunoevasion strategies. Viruses may influence the immune response by inducing immunogenic features of tumor cell death, altering the cytokine milieu, and by direct effects on infiltrating immune cells including dendritic cells (DCs).
Influence of Oncolytic Virotherapy on the Immunogenic Milieu of the Tumor Microenvironment
Immunogenic tumor cell death
Oncolytic virus-induced tumor cell death is expected to release TAAs into the tumor microenvironment. According to the INS and danger models, additional features will determine whether cell death is immunogenic. Apoptotic death appears to be a heterogeneous process, including stimulus-specific processes. Some of the molecular properties of immunogenic cell death have been elucidated, including preapoptotic calreticulin exposure, heat shock protein (HSP) expression and release of high-mobility group box-1 (HMGB1) (Tesniere et al., 2008), and the translocation of intracellular uric acid to the surface of injured cells (Shi et al., 2003). Endo and colleagues reported evidence that oncolytic adenovirus induced immunogenic cell death via the release of uric acid from human tumor cells (Endo et al., 2008). However, other molecular patterns of immunogenic cell death have yet to be studied in the context of oncolytic virotherapy.
Tumor-derived cytokines
Oncolytic viruses may alter the immune milieu of the tumor microenvironment by altering the cytokine profile. For example, reovirus infection of human melanoma cell lines reduced secretion of the immunosuppressive cytokine interleukin (IL)-10, while inducing the production of proinflammatory IL-6 and the chemokines IL-8, RANTES (regulated on activation, normal T cell expressed and secreted), and macrophage inflammatory protein (MIP)-1α/β (Errington et al., 2008b). Similarly we have observed that vesicular stomatitis virus (VSV) infection of the murine melanoma line B16ova induced a rapid proinflammatory cytokine response including IL-6, tumor necrosis factor (TNF)-α, and type I interferons (F. Galivo, unpublished data). Interestingly, most of the cytokines that we, and others, have shown to be induced in the tumor microenvironment after viral injection have been reported to have potent antitumor activity. Indeed, the gene therapy literature of the 1990s is strewn with articles reporting that tumor cell transfection with genes encoding cytokines, chemokines, or interferons leads to both aggressive immune-mediated rejection of the modified cells as well as the generation of antitumor immunity (Dranoff et al., 1993; Rosenthal et al., 1994; Forni et al., 2000; Parmiani et al., 2000; Lollini et al., 2006). In this respect, it is noteworthy that even the most potent antiviral cytokines, such as IFN-α and -β, have potent antitumor activities when expressed as transgenes from tumor-transfected cells, or even when given as recombinant proteins (Dranoff et al., 1993; Parmiani et al., 2000; Gogas et al., 2006). It seems reasonable, therefore, to view the direct intratumoral injection of potently immunogenic replication-competent viruses, and the subsequent innate immune response, as a particularly efficient way to induce multiple cytokines within the tumor microenvironment. From this perspective, oncolytic virotherapy may be seen merely as a means of pulling the trigger on induction of positive antitumor immunotherapeutic reactivities—which the immunogene transfer community has spent a couple of decades trying to optimize. Moreover, these beneficial therapeutic consequences may not only have minimal dependence on viral replication but will act swiftly, and very effectively, to prevent it.
Dendritic cells
Tumor-infiltrating DCs have been identified in many human cancers (Movassagh et al., 2004; Treilleux et al., 2004; Perrot et al., 2007), and the ability of infiltrating DCs to mature is reported to be impaired (Almand et al., 2000; Vicari et al., 2002; Perrot et al., 2007). DCs are critical to mediating the immune response to dying tumor cells (Casares et al., 2005; Apetoh et al., 2007), and the reversal of dysfunction of tumor-infiltrating DCs may be required for successful cancer immunotherapy.
A host of pathogen recognition receptors (PRRs) have now been implicated in viral recognition by DCs. The membrane-based TLR pathway is paralleled by a cytosolic system of RIG-1-like receptors (RLRs) (Pichlmair and Reis e Sousa, 2007). Both pathways appear capable of responding to DNA, single-stranded RNA, and double-stranded RNA, signaling for the induction of type I IFN (Pichlmair et al., 2006). In addition, endogenous danger signals may also mediate the DC response to oncolytic viruses. This was demonstrated by our observation that medium from reovirus-infected tumor cells, filtered to remove viral particles, was able to activate DCs (Errington et al., 2008a).
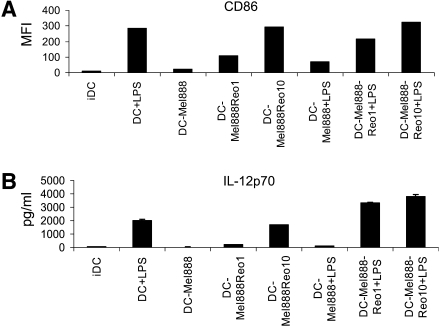
We have observed that DCs loaded with the human melanoma cell line Mel888 fail to mature in response to the Toll-like receptor (TLR)-4 ligand lipopolysaccharide (LPS). Reovirus was able to mature Mel888-loaded DCs and reverse the hyporesponsiveness to LPS, both in terms of upregulation of DC costimulatory molecules (Fig. 2A) and cytokine secretion (Fig. 2B). These data imply that reovirus has the potential to reverse preexisting DC dysfunction, overcoming at least one tumor immunoevasion strategy, and highlight the immunostimulatory potential of oncolytic viruses within the tumor microenvironment.
FIG. 2.
Reovirus activates tumor-loaded DCs, reversing their hyporesponsiveness to LPS. DCs were loaded with Mel888 cells (at a 1:3 ratio) ± reovirus (Reolysin) overnight, ±LPS (250 ng/ml) added after 8 hr. (A) After 24 hr CD86 expression on DCs was determined by flow cytometry. Median fluorescence intensity (MFI) is shown. IDC, immature dendritic cells. (B) IL-12p70 secretion was determined by ELISA of 24-hr supernatants. Error bars indicate the SE.
The immunomodulatory effects of various oncolytic viruses are, however, virus specific. Wild-type measles infects human DCs and impairs function (Grosjean et al., 1997). A wild-type Western Reserve vaccinia virus impaired DC function, whereas a modified virus Ankara strain enhanced DC allostimulatory properties (Greiner et al., 2006). An oncolytic adenovirus had a neutral effect on DC function (Schierer et al., 2008). These examples demonstrate that the impact on DCs is dependent on the type and strain of virus. Indeed, these considerations may eventually define at least one possible metric for selection of the most appropriate oncolytic virus from the host of possible candidates (Pandha et al., 2009) for any specific clinical application.
The Innate Immune Response and Oncolytic Virotherapy
Viral replication is limited in immunocompetent models
The anticipated ability of the innate immune response to limit viral replication has been demonstrated in several models (Fulci et al., 2006; Breitbach et al., 2007) and clinical studies (Pecora et al., 2002; Chiocca et al., 2004). For example, after herpes simplex virus (HSV) therapy of a rat glioma model, HSV gene expression rapidly decreased after 72 hr in association with a rapid infiltration of natural killer (NK) cells, and macrophages/microglia (Fulci et al., 2006). Similarly, after intravenous administration of an oncolytic vaccinia virus in glioma-bearing rats, levels of recoverable virus fell rapidly 72 hr after administration (Lun et al., 2009).
Innate antitumor activity
Manipulation of the innate immune response in favor of antitumor activity represents a potentially critical target for achieving successful tumor immunotherapy (Rosenberg et al., 2004). The innate immune response may be directly cytotoxic to tumors, while shaping the subsequent cognate immune response (Ghiringhelli et al., 2007). Among innate effectors, the strongest evidence of an anticancer role exists for NK cells (Waldhauer and Steinle, 2008). Tumor-infiltrating NK cells in humans correlate with a favorable prognosis (Coca et al., 1997; Ishigami et al., 2000; Takanami et al., 2001; Villegas et al., 2002). However, in many human tumors infiltrating NK cells are sparse (Albertsson et al., 2003; Esendagli et al., 2008). DCs, in addition to their role in adaptive T cell priming, play a pivotal role in coordinating the innate response, recruiting and reciprocally interacting with NK cells (Fernandez et al., 1999; Reschner et al., 2008). The influence of oncolytic virotherapy on the recruitment of innate immune effectors, and the reciprocal DC–NK cell axis, are likely to be fundamental to the outcome of the innate immune response.
In the context of viral infection, virus-infected DCs play a role in NK cell activation (Andoniou et al., 2005; Ebihara et al., 2007). However, only limited data are available characterizing the innate immune response after oncolytic virotherapy. Predominantly from murine model systems, these data have highlighted a central role for the innate immune system in mediating tumor responses. We have, in particular, examined the role of the innate immune response in the context of reovirus and VSV therapy.
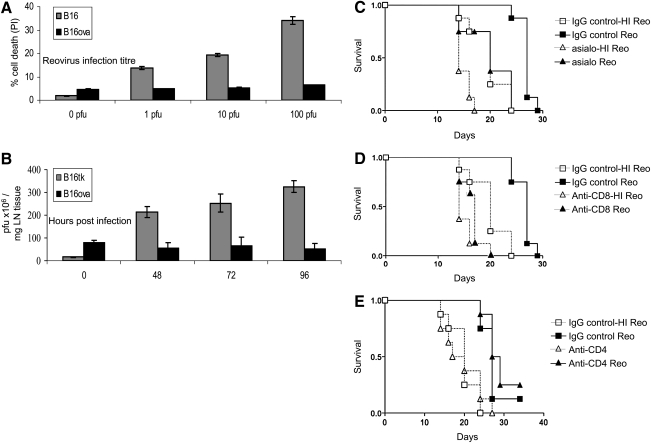
The murine melanoma line B16ova is resistant to the direct oncolytic effect of reovirus replication in vitro, in contrast to parental B16 cells (Fig. 3A); the resistance of B16ova may be due to the absence of the reovirus receptor JAM-1 (Prestwich et al., 2009a). Reovirus replication was not detected after injection of reovirus into subcutaneous B16ova tumors, in contrast to rapid replication in permissive JAM-1-positive B16 tumors (Fig. 3B). Despite in vitro resistance, and failure to support viral replication, B16ova tumors regressed in vivo after direct injection of reovirus. Antibody depletion experiments revealed that therapy was dependent on NK cells (p = 0.008) (Fig. 3C). In view of the resistance of B16ova to a direct oncolytic effect of reovirus, these data demonstrate a role for innate NK cell activity. We have extended these observations to a human in vitro system. DCs loaded with reovirus-infected Mel888 cells secreted a range of chemokines inducing NK cell migration. The secretion of IFN-β by loaded DCs induced NK cell cytotoxicity toward Mel888 cells, while NK cells reciprocally matured the DCs (Prestwich et al., 2009b). On the basis of these murine in vivo and human in vitro observations, reovirus therapy recruits NK cells into the tumor microenvironment, induces DC maturation, and activates NK cell cytotoxicity, implicating a role for NK cells in antitumor efficacy.
FIG. 3.
The B16ova cell line is resistant in vitro to reovirus, but regresses in vivo in an NK cell- and CD8+ T cell-dependent manner. (A) B16 and B16ova cell lines were infected with reovirus (Reolysin) for 48 hr, before assessment of cell death by administration of propidium iodide (PI). Error bars, SE. (B) C57BL/6 mice were seeded subcutaneously with 5 × 105 B16 or B16ova cells. After 7 days tumors were injected with a single dose of 5 × 108 plaque-forming units (PFU) of reovirus. Tumors were harvested at the indicated time points, and viral titer was determined by plaque assay. Error bars, SE. LN, lymph node. (C–E) C57BL/6 mice were seeded subcutaneously with 5 × 105 B16ova cells. A 0.1-mg amount of depleting antibody [(C) anti-NK (asialo), (D) anti-CD8, (E) anti-CD4] or control antibody was given intraperitoneally 4 days after tumor implantation (n = 8 per group), and every week for the duration of the experiment. Reovirus (5 × 108 PFU) or heat-inactivated reovirus, as a control, was injected intratumorally on days 7, 9, 11, and 13 after tumor implantation. (C) IgG control + reovirus versus NK cell depletion + reovirus; p = 0.008. (D) IgG control + reovirus versus CD8+ cell depletion + reovirus; p < 0.0001. (E) IgG control + reovirus versus CD4+ cell depletion + reovirus; p = 0.28 (by log-rank test).
Our observations with VSV have also demonstrated an absolutely critical role for innate immunity in tumor regression. We have observed that VSV replicates extremely aggressively in vitro in B16ova cells. Moreover, as expected from this in vitro correlate, intratumoral injections of VSV in B16ova led to significant tumor regressions and cures. However, more unexpectedly, this therapy was associated with a pronounced leukocyte infiltrate, and tumor regression was completely dependent on NK cells and CD8+ T cells (Diaz et al., 2007). This dependence on NK cells was also observed in a different experimental system in which VSV was administered loaded onto adoptively transferred cytotoxic T cells (Kottke et al., 2008). Consistent with these data, an oncolytic HSV, HSV-1716, induced an inflammatory infiltrate containing NK cells, monocytes, and T cells (Thomas and Fraser, 2003). Furthermore, the efficacy of intratumoral therapy of metastatic melanoma by HSV was abrogated in syngeneic models lacking NK or T cell subsets (Miller and Fraser, 2003). Similar to our reovirus data, HSV-1716 was found to induce the production of chemokines CXCL9 and CXCL10 from human DCs, and to induce NK and CD8+ T cell migration into murine tumors (Benencia et al., 2005). Therefore, innate NK cells, in addition to T cells, are required to mediate antitumor efficacy after therapy with reovirus, VSV, and HSV in different model systems.
These results dramatically highlight the emerging friction that exists between two extreme views of the immune system as it relates to the efficacy of oncolytic virotherapy. On the one hand, innate immune quenching of viral replication can be seen as a potential show stopper; on the other, there are clear experimental data indicating that without it, virotherapy may not be able to work at all. Of course, the reality, as ever, lies in between these two polarized views.
Therapeutic manipulation of the innate immune response
Attempts have been made to modulate the innate immune response, aiming either to limit the innate response in order to enhance viral replication, or to enhance innate antitumor activity. The outcomes with these conflicting strategies provide insight into the role of the innate response to oncolytic virotherapy.
a. Suppressing the innate immune response to reduce viral clearance
Cyclophosphamide
Cyclophosphamide (CPA) is an alkylating agent used in cancer treatment, and as an immunosuppressive agent in autoimmune disorders. CPA has a complex range of effects and may enhance viral replication via a reduction in neutralizing antibodies (Ikeda et al., 1999; Qiao et al., 2008c), a reduction in regulatory T cells (Treg cells) (Di Paolo et al., 2006), reduced vascular tumor permeability (Kurozumi et al., 2007), and reduced innate immune cell infiltration (Fulci et al., 2006). CPA has been combined with virotherapy on the basis that it will inhibit immune-mediated viral elimination, potentiating viral replication and hence enhancing tumor oncolysis. Immunomodulation with CPA increased viral replication and therapeutic efficacy in several models (Ikeda et al., 1999; Kambara et al., 2005; Di Paolo et al., 2006; Fulci et al., 2006; Kurozumi et al., 2007; Thomas et al., 2008; Lun et al., 2009). In rat glioma models, pretreatment with CPA inhibited an HSV-mediated increase in the infiltration of tumor-associated phagocytic cells, promoting HSV replication and improving survival (Fulci et al., 2006). CPA increased vaccinia virus replication and efficacy, also in a rat glioma model (Lun et al., 2009). Pretreatment with CPA was required to mediate the effect on viral replication (Fulci et al., 2006; Lamfers et al., 2006), and the CPA-mediated enhancement of viral replication generally correlated with antitumor activity (Kambara et al., 2005; Fulci et al., 2006; Lun et al., 2009). Similarly, another immunosuppressive agent, cyclosporine, enhanced the efficacy of a reovirus injection in a colorectal cancer murine model (Smakman et al., 2006).
These data support the hypothesis that innate immunosuppression will benefit virotherapy. Although we do not argue against this, we do suggest that it is important to keep alternative and or additional interpretations in mind. Hence, although cyclophosphamide is undoubtedly an immunosuppressive agent, it can also have apparently immunostimulatory activities. In particular, cyclophosphamide has been extensively shown to enhance immune-based tumor rejection regimens through an activity associated with cytokine induction and promoting homeostatic proliferation of lymphocyte populations (Bracci et al., 2007). Of particular interest, and reminiscent of the activity of oncolytic viruses (Lichty et al., 2004), CPA can induce a cytokine storm within tumors. The parallels with the immune consequences of oncolytic virus injection into tumors are marked, although a direct comparison between the cytokine storms induced in tumors by both agents has not been systematically studied. Moreover, CPA-induced cytokine storms can significantly enhance the activity of lymphocytes either endogenously present in a tumor-bearing host or adoptively transferred to treat a tumor (Brentjens et al., 2009). This is important in light of the findings discussed previously, that the efficacy of oncolytic virotherapy can be directly dependent on lymphocyte populations in vivo. It is interesting to speculate whether at least some of the reported activities of CPA in combination with oncolytic viruses may be associated with, or even attributable to, cytokine storm-like induction at the tumor site. Therefore, we believe that it is important to bear in mind that immune modifiers such as cyclophosphamide have multiple, pleiotropic effects; while the overall result may be “immunosuppressive,” other mechanisms may be operative that, if not looked for, may not be found.
Antiangiogenic agents
Immune cells traffic into tumors via the tumor vasculature. Therefore any effects of viral therapy on the tumor microvasculature can impact on the immune response. Therapy of a rat glioma with an HSV-1-derived oncolytic virus is associated with increased vascular permeability and leukocyte infiltration. Pretreatment with an angiogenesis inhibitor, cyclic RGD peptide, was found to reduce vascular permeability, inflammation, and leukocyte infiltration (Kurozumi et al., 2007). This reduction in the host immune response enhanced viral propagation and the antitumor efficacy of therapy.
Histone deacetylase inhibitors
The type I interferon response is a major component of the cellular innate antiviral response. Histone deacetylase inhibitors (HDIs) are small molecules that are under development as anticancer agents. In addition, HDIs reduce the cellular antiviral immune response, impeding the type I IFN response. HDIs have been shown to enhance the spread and anticancer efficacy of VSV in vitro and in vivo in multiple systems, in association with an abrogated type I IFN response (Nguyen et al., 2008). HDIs also enhance the oncolytic activity of vaccinia virus and HSV (Nguyen et al., 2008).
Engineering viruses to evade innate immune elimination
Engineering viruses to express immunomodulatory genes is an alternative approach to combining viruses with drug therapy. A recombinant VSV has been designed to express a protein from cytomegalovirus that downregulates the NK cell-activating ligand, CD155 (Altomonte et al., 2009). This vector inhibited the recruitment of NK cells and natural killer T (NKT) cells in vivo, and demonstrated enhanced replication and therapeutic efficacy compared with the control vector. An oncolytic measles virus strain armed to express the wild-type P gene, which inhibits type I IFN production and the antiviral type I IFN response, demonstrated increased efficacy in myeloma xenografts (Haralambieva et al., 2007).
Drawbacks of innate immunosuppression
The relationship between tumor, oncolytic virus, and innate inflammatory response is complex. If the innate immune system mediates part of the therapeutic efficacy of oncolytic viruses, inhibition of the innate immune response potentially impairs therapy. In a study using a murine colorectal tumor model, the systemic delivery of VSV or vaccinia virus resulted in infection of only a small proportion of tumor cells, yet extensive bystander death of uninfected tumor cells (Breitbach et al., 2007). The viral infection was found to trigger a dramatic reduction in tumor blood flow, leading to apoptosis of uninfected tumor cells. VSV therapy upregulated expression of genes encoding the neutrophil chemoattractants CXCL1 and CXCL5, and induced extensive tumor infiltration by neutrophils. Neutrophil depletion enhanced viral spread and replication within the tumor, yet blood flow was not reduced and bystander killing was lost. Measles virus has also been shown to promote neutrophil infiltration into tumors, which correlated with tumor regression (Grote et al., 2003). These data suggest that the inflammatory infiltrate can be required for therapy. In addition, they highlight the important concept that enhanced viral replication and spread within a tumor does not necessarily result in increased therapeutic efficacy.
One of the theoretical cornerstones for the development of many oncolytic viruses has been that an intact antiviral innate immune response is fully operational in normal cells but not in tumor cells. Therefore, a clear and present danger associated with suppressing the innate response is increased toxicity. Doses of cyclophosphamide that ablate neutralizing antibody production have been shown to lead to severe reovirus toxicity (Qiao et al., 2008c). We have also observed massive spread of VSV in normal tissues and severe toxicity in IFN-α/β knockout mice. These observations indicate the need to introduce yet further considerations on the importance of the immune system to the overall efficacy of oncolytic virotherapy. Thus, excellent antitumor efficacy will be of no value in the event of unacceptable toxicity caused by viral dissemination and uncontrolled replication in normal tissues.
b. Enhancing the innate immune response
In contrast to interventions designed to reduce the host innate type I IFN response, oncolytic viruses have been constructed to express IFN-β. The expression of IFN-β has been hypothesized to limit viral replication in normal tissues while allowing replication in tumor tissues, which are commonly resistant to the antiviral effects of type I IFNs. In addition, IFN-β may have beneficial immunological effects via the induction of tumor-specific cytotoxic lymphocytes (Brown et al., 2002) and antiangiogenic effects (Dong et al., 1999). A vaccinia virus engineered to express IFN-β (JX-795) was found to have superior tumor selectivity and efficacy, in association with the generation of antitumor immunity, when compared with a control vector lacking IFN-β (Kirn et al., 2007). Similarly, we have found that an IFN-β insert into VSV enhanced inflammatory cytokine production and NK cell activation, leading to enhanced bystander killing of tumor cells (our unpublished data).
Adaptive Antitumor Immunity and Oncolytic Viruses
Oncolytic viruses are expected to release TAAs into the tumor microenvironment, while interacting with DCs via PRRs and/or endogenous “danger” signals. Indeed, virally infected cells are more effective at delivering nonviral antigen for in vivo cross-priming of APCs than noninfected cells (Schulz et al., 2005). An extensive body of evidence has now emerged supporting the concept that oncolytic virotherapy can generate antitumor immunity. In many of these experiments it is difficult to determine the relative importance of direct viral oncolysis versus immune-mediated bystander killing of uninfected tumor cells. The most informative studies have demonstrated the potential importance of an adaptive antitumor immune response by showing activity toward noninjected disease, or responses in tumors that are resistant to direct oncolysis. Our groups have used reovirus and VSV to examine the adaptive immune response.
In human in vitro assays, reovirus infection of human Mel888 cells generated an anti-Mel888 cell immune response, and cross-primed an expansion of MART (melanoma antigen recognized by T cells)-1-reactive cytotoxic T cells (Prestwich et al., 2008b). A modified priming system precluding direct oncolysis, and the use of ultraviolet-treated replication-incompetent reovirus, has shown that direct oncolysis and viral replication are not prerequisites for priming adaptive antitumor immunity. The B16ova tumor cell line, which is not permissive of reovirus replication and is resistant to direct oncolysis, has provided useful insights into the importance of the immune response. CD8+ cytotoxic T cells (p < 0.0001) (Fig. 3D) but not CD4+ T cells (p = 0.28) (Fig. 3E) were required to mediate regression of tumors after direct injection. In addition, reovirus purged B16ova lymph node and splenic metastases in association with the generation of anti-B16 immunity. The efficacy of reovirus toward lymph node and splenic metastases was completely abrogated in severe combined immune-deficient (SCID) mice (Prestwich et al., 2009a). These findings that reovirus acts as an immune “adjuvant,” independent of direct oncolysis and viral replication are of particular importance in view of the reduced sensitivity of primary tumor samples to direct oncolysis (Errington et al., 2008b; Tumilasci et al., 2008; Van Houdt et al., 2008).
We have also demonstrated that VSV therapy generates antitumor immunity. Intratumoral injection of VSV into B16ova tumors generated tumor-reactive infiltrating lymphocytes (Diaz et al., 2007). However, the efficiency of priming was significantly greater when the tumor cells were killed by oncolysis in the lymph nodes as opposed to a subcutaneous tumor in the periphery (Qiao et al., 2008a). In addition, lymph node purging was abrogated in SCID mice, demonstrating the central role of the adaptive immune response in mediating antitumor activity.
The range of oncolytic viruses that have been reported to facilitate the generation of adaptive antitumor immunity reflects the broad applicability of the principle. Oncolytic HSV strains induce systemic antitumor protection (Toda et al., 1999; Miller and Fraser, 2003; Li et al., 2007a,b). For example, an attenuated HSV injected intratumorally into one flank of mice with established bilateral colorectal or melanoma tumors induced regression of the contralateral tumors in association with antitumor cytotoxic T cells (Toda et al., 1999). Infection of a human melanoma cell line with an attenuated vaccinia virus generated an anti-TAA response (Greiner et al., 2006). In murine models, tumor delivery of oncolytic vaccinia virus by cytokine-induced killer (CIK) cells induced antitumor immunity (Thorne and Contag, 2008). In a finding similar to our data with reovirus and B16ova, the locoregional delivery of Newcastle disease virus (NDV) induced tumor delay in liver metastases, which are resistant in vitro (Apostolidis et al., 2007). In a human assay, tumor lysates induced by oncolytic parvovirus H-1 stimulated DC maturation and cross-presented melanoma-associated antigens (Moehler et al., 2005). One of the most unexpected viruses to act in this manner was an oncolytic measles strain derived from the Edmonston vaccine strain. Measles virus is associated with a transient immunosuppressive effect, yet the oncolytic measles virus primed antitumor CD8+ T cells in an autologous human mesothelioma system (Gauvrit et al., 2008).
Improving oncolytic virotherapy as adaptive immunotherapy
Attempts have been made to enhance the immunotherapeutic potential of oncolytic viruses by incorporating immunostimulatory transgenes. Granulocyte-monocyte colony-stimulating factor (GM-CSF) promotes the differentiation of progenitor cells into dendritic cells, and has been successfully used in strategies to generate tumor-reactive cytotoxic lymphocytes (Dranoff et al., 1993). Vaccinia virus, measles, HSV, and adenoviruses have been engineered to incorporate GM-CSF with the aim of augmenting the generation of antitumor immunity (Grote et al., 2003; Liu et al., 2003; Kim et al., 2006; Lei et al., 2009). In a model of bilateral flank lymphoma, HSV expressing murine GM-CSF was injected into one side (Liu et al., 2003). HSV-GM-CSF improved tumor reduction in the injected and noninjected flanks, compared with control HSV lacking GM-CSF. This response was associated with enhanced splenocyte production of IFN-γ on tumor stimulation. Similarly, measles virus expressing murine GM-CSF showed greater efficacy compared with the vector lacking GM-CSF after intratumoral injection (Grote et al., 2003).
Chemokines have also been inserted into oncolytic viral vectors, in order to promote the recruitment of immune effectors to the tumor microenvironment. Oncolytic adenovirus expressing the chemokine RANTES recruited DCs to the tumor microenvironment, eliciting antigen-specific CTL and NK cell responses, and promoted tumor regression (Lapteva et al., 2009). Similarly, oncolytic adenovirus expressing MIP-1α and Fms-like tyrosine kinase-3 (FLT-3) ligand enhanced DC and T cell recruitment, and the generation of antitumor immunity. Interestingly, although both antiviral and antitumor responses were enhanced, the expression of chemokines improved antitumor therapy (Ramakrishna et al., 2009). In a similar immunotherapeutic approach, oncolytic viruses have been coinjected with DCs. Immature DCs injected into tumors with an oncolytic HSV-1 improved tumor control in association with antitumor immunity (Farrell et al., 2008).
Other immunotherapeutic strategies can enhance the adaptive antitumor immune response induced by virotherapy. Cytotoxic T lymphocyte antigen (CTLA)-4 transmits inhibitory signals to T cells. Anti-CTLA-4 antibody prevents normal downregulation of T cells, prolonging T cell activation. The combination of anti-CTLA-4 with VSV improved therapy of a mammary tumor model, in a CD4+ and CD8+ T cell-dependent manner (Gao et al., 2009). Treg cells suppress the generation of adaptive responses, and it has been hypothesized that Treg cell depletion would enhance antitumor immunity. However, Treg cell depletion after VSV therapy was found to have a negative therapeutic effect, relieving suppression of the antiviral immune response, leading to rapid viral clearance (Diaz et al., 2007). This highlights the important principle of investigating antiviral as well as antitumor immune responses.
The choice of viral vector is likely to have a significant impact on the nature of the immune response. We hypothesized that the ability of VSV to generate antitumor immune responses may be enhanced by expression of immunostimulatory transgenes, including CD40 ligand (CD40L), heat shock protein-70, and CCL-21. However, transgene expression demonstrated no additional benefit against established subcutaneous B16ova tumors. VSV with or without a transgene was associated with a rapid innate inflammatory response, characterized by generalized T cell activation, and priming toward viral epitopes. Viral antigens are nonself, with high precursor T cell frequencies, and are commonly immunodominant. This is in contrast to TAAs, to which precursor T cell frequencies are low and a state of T cell anergy may exist. Therefore, the immunodominance of VSV antigens over TAAs was not unexpected. The lack of benefit with immunostimulatory transgenes may be due to the already maximal nature of the immune response to this highly immunogenic vector. This is supported by the observation that a poorly immunogenic nonreplicating adenoviral vector expressing CD40L generated an adaptive antitumor response and had greater therapeutic efficacy that the VSV–CD40L construct. Highly immunogenic vectors may therefore be ineffective at priming antitumor responses in the face of a maximal innate response.
We have found that the most efficient way to prime a T cell response toward a TAA is to engineer the viral vector to express the TAA. T cell responses toward a TAA were efficiently primed in vivo after direct injection of VSV expressing the TAA (Diaz et al., 2007). We believe that this response is at least partly mediated by trafficking of the virus to lymph nodes, where priming toward virally encoded antigens will be very efficient (Diaz and Galivo unpublished data).
These observations provide important insights into future strategies for optimizing the immunotherapeutic potential of oncolytic viruses. The therapeutic and immunological consequences of virotherapy are likely to vary widely, depending on the viral platform. The genes inserted into viral vectors need to be chosen with regard to the immunogenic properties of the vector, and a combination of vectors may provide optimal immune priming while harnessing direct oncolytic mechanisms. For example, a replication-defective, poorly immunogenic vector expressing a TAA and/or costimulatory molecules may be used to initiate an immune response, which may be subsequently boosted with an oncolytic virus to kill tumor cells releasing TAA in the context of immunological “danger.” Alternatively, standard tumor vaccine approaches may be combined with an immunostimulatory oncolytic viral boost.
Clinical Evidence of the Immunotherapeutic Potential of Oncolytic Viruses in Clinical Trials
Clinical observations support the concept that oncolytic virotherapy can overcome immune tolerance, and generate immune-mediated activity toward distant tumors. For example, a targeted vaccinia virus expressing GM-CSF injected into melanoma deposits induced regression of noninjected regional dermal metastases in four of seven patients in association with an immune infiltrate (Mastrangelo et al., 1999). A phase I study investigating the injection of the vaccinia virus JX-594 into primary and secondary liver tumors in 10 evaluable patients, showed a partial response in 3 patients and stable disease in 6 patients. There was evidence of a functional response in noninjected tumors in three of seven evaluable patients (Park et al., 2008). Although there was evidence of viral dissemination to noninjected tissue, these responses may also have been immune-mediated (Prestwich et al., 2008c). Furthermore, in a phase I study investigating the injection of HSV-GM-CSF into subcutaneous metastases, posttreatment biopsies revealed a dense immune infiltrate. In addition, inflammation was observed in noninjected metastases in 4 of 30 patients (Hu et al., 2006). In a phase II study with repeated intratumoral injections of HSV-GM-CSF into melanoma lesions, a 26% response rate (RECIST) (assessment included injected and noninjected lesions) was observed (Senzer et al., 2009).
Viral Delivery and Immunological Consequences
Systemic viral delivery is limited by liver sequestration, complement, preimmune IgM, and neutralizing antibodies. Strategies designed to enhance viral delivery may in addition have immunotherapeutic consequences.
Cellular delivery of viruses has shown promise as a method of chaperoning viruses to tumors (Thorne and Contag, 2008). The cell carrier may be biologically active, providing a combination of both viroimmunotherapy strategies. For example, adoptive T cell therapy of specific cytotoxic lymphocytes may be combined with virus delivery. In this setting the proinflammatory consequences of viral therapy enhanced the persistence and proliferation of transferred T cells in the tumor microenvironment (Qiao et al., 2008b). Dendritic cells have also been found to be efficient virus carriers (Ilett et al., 2009), offering the opportunity to combine viral therapy with a dendritic cell vaccine approach. Vaccinia virus delivery by CIK cells exhibited a synergistic interaction, with viral infection sensitizing tumor targets to the killing activity of CIK cells (Thorne et al., 2006).
CPA inhibits the generation of neutralizing antibodies and can enhance viral delivery (Qiao et al., 2008c). As previously discussed, immunosuppressive agents such as CPA may additionally modulate the tumor microenvironment, with consequences for the outcome of therapy. These strategies offer the possibility of combining efficient viral delivery with immunomodulation/immunotherapy.
Future Perspectives
The mechanisms of activity of oncolytic viruses are complex, involving direct oncolysis, vascular effects, and innate and adaptive immunity. The extent of viral replication does not necessarily correlate with therapeutic efficacy. Immune interactions may be either beneficial or detrimental; the reality is likely to lie between these two extremes. The variations in tumor models, anatomical location of the tumor, and properties of the different viruses are likely to explain differences in the overall impact of the immune response. It is clear that immunomodulation has the potential to improve oncolytic virotherapy, and the timing and nature of intervention are likely to be critical to its effect. One appealing possibility is that early transient immunosuppression may enhance viral replication, followed by a restoration of immune activity to harness the immunotherapeutic potential of virotherapy.
Translation of these preclinical observations into the clinic is challenging. Murine models are limited in their ability to predict outcome in trials. For this reason it is critical that preclinical assessments continue to include human assays, particularly primary tumor samples wherever possible. Clinical trials have generally conformed to the traditional phase I–III format. There is a clear need for clinical studies to be additionally designed to answer scientific questions regarding the optimal route of delivery, mechanisms of antitumor activity, immune interactions, and effects on tumor blood flow. When possible, immunological assays should include histological assessment of tumor infiltration, assays of neutralizing antibody generation, and evaluation of systemic immune responses.
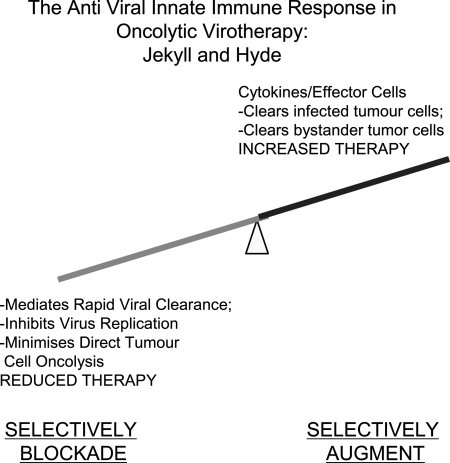
It is no longer possible to be dogmatically polarized in one's view of the role of the immune system in the efficacy of oncolytic virotherapy. Whether we like it or not, the immune system exerts multiple effects on the outcome of therapy: some positive, some negative (Fig. 4). The nature and extent of the antiviral immune response to oncolytic virus infection mediate an intricate balance between safety against systemic virus toxicity, restriction of viral replication/oncolysis, and, potentially, significant immune-mediated antitumor therapy. The challenge for the future is to understand how to accentuate the positive and how to nullify the negative. This can be achieved only by testing viruses in models that come as close as possible to the immune environment that will be encountered in the tumors of patients. It will require cross-fertilization between the disciplines of virology and immunology. We will need to appreciate how pleiotropic agents, which either negatively or positively impact therapy in preclinical models, may be having effects on the host immune system that we have not fully appreciated. We would suggest that the principle that oncolytic virotherapy may act, at least in some circumstances, as much as an immunotherapy as a virotherapy is both clearly established and rather encouraging. To date, the field has concentrated on developing viruses that replicate robustly and extensively in tumors—but with only moderate success. In retrospect, expecting extensive replication within a tumor, albeit an immunosuppressive tumor, may be asking a great deal. By viewing at least certain components of the immune system as a partner, rather than the enemy, it should now be possible to explore additional avenues of oncolytic virus design in which immune activation becomes as much a part of the solution as it has previously been viewed as the problem.
FIG. 4.
Future perspectives. The immune response to oncolytic virus comprises multiple components, some positive and some detrimental to therapy. In the future, it may be possible to identify those components that are specifically responsible for mediating viral clearance (detrimental to therapy) and those that are responsible for mediating antitumor efficacy (positive effect on therapy). Furthermore, it may be possible to block the former (such as the antiviral IFN-α/β response) while simultaneously augmenting the latter (expression of potently antitumor cytokines and interferons that are induced by IFN-α/β but that, per se, have no antiviral effects). In this way, virus design may be able to incorporate the best of both worlds—factors to reduce viral clearance (more direct oncolysis) and to increase recruitment of antitumor effectors (e.g., NK cell-recruiting cytokines).
Acknowledgments
The authors thank Toni Higgins for expert secretarial assistance. R.J.P., F.E., and A.A.M. are supported by grants from Cancer Research UK, and R.G.V. by NIH grants CA107082, CA130878, and CA132734, the Mayo Foundation, and the Richard M. Schulze Family Foundation.
Author Disclosure Statement
No competing financial interests exist.
A.A.M., K.J.H., and R.G.V. have received research grants from Oncolytics Biotech (Calgary, AB, Canada). Reovirus (Reolysin) was supplied by Oncolytics Biotech (Calgary, AB, Canada).
References
- Aghi M. Martuza R.L. Oncolytic viral therapies: The clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Albertsson P.A. Basse P.H. Hokland M. Goldfarb R.H. Nagelkerke J.F. Nannmark U. Kuppen P.J. NK cells and the tumour microenvironment: Implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Almand B. Resser J.R. Lindman B. Nadaf S. Clark J.I. Kwon E.D. Carbone D.P. Gabrilovich D.I. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- Altomonte J. Wu L. Meseck M. Chen L. Ebert O. Garcia-Sastre A. Fallon J. Mandeli J. Woo S.L. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2009;16:266–278. doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniou C.E. Van Dommelen S.L. Voigt V. Andrews D.M. Brizard G. Asselin-Paturel C. Delale T. Stacey K.J. Trinchieri G. Degli-Esposti M.A. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- Apetoh L. Ghiringhelli F. Tesniere A. Obeid M. Ortiz C. Criollo A. Mignot G. Maiuri M.C. Ullrich E. Saulnier P. Yang H. Amigorena S. Ryffel B. Barrat F. J. Saftig P. Levi F. Lidereau R. Nogues C. Mira J.P. Chompret A. Joulin V. Clavel-Chapelon F. Bourhis J. Andre F. Delaloge S. Tursz T. Kroemer G. Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Apostolidis L. Schirrmacher V. Fournier P. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int. J. Oncol. 2007;31:1009–1019. [PubMed] [Google Scholar]
- Benencia F. Courreges M.C. Conejo-Garcia J.R. Mohamed-Hadley A. Zhang L. Buckanovich R.J. Carroll R. Fraser N. Coukos G. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol. Ther. 2005;12:789–802. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bluming A.Z. Ziegler J.L. Regression of Burkitt's lymphoma in association with measles infection. Lancet. 1971;2:105–106. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- Bracci L. Moschella F. Sestili P. La Sorsa V. Valentini M. Canini I. Baccarini S. Maccari S. Ramoni C. Belardelli F. Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- Breitbach C.J. Paterson J.M. Lemay C.G. Falls T.J. Mcguire A. Parato K.A. Stojdl D.F. Daneshmand M. Speth K. Kirn D. McCart J.A. Atkins H. Bell J.C. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Brentjens R.J. Riviere I. Hollyman D. Taylor C. Nikhamin Y. Stefanski J. Lee J. Yeh R. Santos E. Sadelain M. Abstract 401. Presented at the American Society Gene Therapy Annual Meeting, May 27–May 30, 2009. San Diego, CA: 2009. Unexpected toxicity of cyclophosphamide followed by adoptively transferred CD19-targeted T cells in a patient with bulky CLL. [Google Scholar]
- Brown J.L. Barsoum J. Qin X.Q. CD4+ T helper cell-independent antitumor response mediated by murine IFN-β gene delivery in immunocompetent mice. J. Interferon Cytokine Res. 2002;22:719–728. doi: 10.1089/10799900260100222. [DOI] [PubMed] [Google Scholar]
- Casares N. Pequignot M. O. Tesniere A. Ghiringhelli F. Roux S. Chaput N. Schmitt E. Hamai A. Hervas-Stubbs S. Obeid M. Coutant F. Metivier D. Pichard E. Aucouturier P. Pierron G. Garrido C. Zitvogel L. Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca E.A. Abbed K.M. Tatter S. Louis D.N. Hochberg F.H. Barker F. Kracher J. Grossman S.A. Fisher J.D. Carson K. Rosenblum M. Mikkelsen T. Olson J. Markert J. Rosenfeld S. Nabors L.B. Brem S. Phuphanich S. Freeman S. Kaplan R. Zwiebel J. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Coca S. Perez-Piqueras J. Martinez D. Colmenarejo A. Saez M.A. Vallejo C. Martos J.A. Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- DePace N. Sulla scomparsa di un enorme cancro vegetante del callo dell'utero senza cura chirurgica. Ginecologia. 1912;9:82–88. [Google Scholar]
- Diaz R.M. Galivo F. Kottke T. Wongthida P. Qiao J. Thompson J. Valdes M. Barber G. Vile R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Di Paolo N.C. Tuve S. Ni S. Hellstrom K.E. Hellstrom I. Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z. Greene G. Pettaway C. Dinney C.P. Eue I. Lu W. Bucana C.D. Balbay M.D. Bielenberg D. Fidler I.J. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-β. Cancer Res. 1999;59:872–879. [PubMed] [Google Scholar]
- Dranoff G. Jaffee E. Lazenby A. Golumbek P. Levitsky H. Brose K. Jackson V. Hamada H. Pardoll D. Mulligan R.C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T. Masuda H. Akazawa T. Shingai M. Kikuta H. Ariga T. Matsumoto M. Seya T. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int. Immunol. 2007;19:1145–1155. doi: 10.1093/intimm/dxm073. [DOI] [PubMed] [Google Scholar]
- Endo Y. Sakai R. Ouchi M. Onimatsu H. Hioki M. Kagawa S. Uno F. Watanabe Y. Urata Y. Tanaka N. Fujiwara T. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene. 2008;27:2375–2381. doi: 10.1038/sj.onc.1210884. [DOI] [PubMed] [Google Scholar]
- Errington F. Steele L. Prestwich R. Harrington K.J. Pandha H.S. Vidal L. De Bono J. Selby P. Coffey M. Vile R. Melcher A. Reovirus activates human dendritic cells to promote innate antitumor immunity. J. Immunol. 2008a;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- Errington F. White C.L. Twigger K.R. Rose A. Scott K. Steele L. Ilett L.J. Prestwich R. Pandha H.S. Coffey M. Selby P. Vile R. Harrington K.J. Melcher A.A. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008b;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esendagli G. Bruderek K. Goldmann T. Busche A. Branscheid D. Vollmer E. Brandau S. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Farrell C.J. Zaupa C. Barnard Z. Maley J. Martuza R.L. Rabkin S.D. Curry W.T., Jr. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin. Cancer Res. 2008;14:7711–7716. doi: 10.1158/1078-0432.CCR-08-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C. Lozier A. Flament C. Ricciardi-Castagnoli P. Bellet D. Suter M. Perricaudet M. Tursz T. Maraskovsky E. Zitvogel L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Forni G. Lollini P.L. Musiani P. Colombo M.P. Immunoprevention of cancer: Is the time ripe? Cancer Res. 2000;60:2571–2575. [PubMed] [Google Scholar]
- Fulci G. Breymann L. Gianni D. Kurozomi K. Rhee S.S. Yu J. Kaur B. Louis D.N. Weissleder R. Caligiuri M.A. Chiocca E.A. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. Whitaker-Dowling P. Griffin J.A. Barmada M.A. Bergman I. Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors. Cancer Gene Ther. 2009;16:44–52. doi: 10.1038/cgt.2008.55. [DOI] [PubMed] [Google Scholar]
- Gauvrit A. Brandler S. Sapede-Peroz C. Boisgerault N. Tangy F. Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F. Apetoh L. Housseau F. Kroemer G. Zitvogel L. Links between innate and cognate tumor immunity. Curr. Opin. Immunol. 2007;19:224–231. doi: 10.1016/j.coi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gogas H. Ioannovich J. Dafni U. Stavropoulou-Giokas C. Frangia K. Tsoutsos D. Panagiotou P. Polyzos A. Papadopoulos O. Stratigos A. Markopoulos C. Bafaloukos D. Pectasides D. Fountzilas G. Kirkwood J.M. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N. Engl. J. Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- Greiner S. Humrich J.Y. Thuman P. Sauter B. Schuler G. Jenne L. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clin. Exp. Immunol. 2006;146:344–353. doi: 10.1111/j.1365-2249.2006.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean I. Caux C. Bella C. Berger I. Wild F. Banchereau J. Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote D. Russell S.J. Cornu T.I. Cattaneo R. Vile R. Poland G.A. Fielding A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- Grote D. Cattaneo R. Fielding A.K. Neutrophils contribute to the measles virus-induced antitumor effect: Enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- Hansen R.M. Libnoch J.A. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch. Intern. Med. 1978;138:1137–1138. [PubMed] [Google Scholar]
- Haralambieva I. Iankov I. Hasegawa K. Harvey M. Russell S. J. Peng K. W. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol. Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.C. Coffin R.S. Davis C.J. Graham N.J. Groves N. Guest P.J. Harrington K.J. James N.D. Love C.A. McNeish I. Medley L.C. Michael A. Nutting C.M. Pandha H.S. Shorrock C.A. Simpson J. Steiner J. Steven N.M. Wright D. Coombes R.C. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Ichikawa T. Wakimoto H. Silver J.S. Deisboeck T.S. Finkelstein D. Harsh G.R.T. Louis D.N. Bartus R.T. Hochberg F.H. Chiocca E.A. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- Ilett E.J. Prestwich R.J. Kottke T. Errington F. Thompson J.M. Harrington K.J. Pandha H.S. Coffey M. Selby P.J. Vile R.G. Melcher A.A. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami S. Natsugoe S. Tokuda K. Nakajo A. Che X. Iwashige H. Aridome K. Hokita S. Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- Janeway C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Joffre O. Nolte M.A. Sporri R. Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Kambara H. Saeki Y. Chiocca E. A. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Oh J.Y. Park B.H. Lee D.E. Kim J.S. Park H.E. Roh M.S. Je J.E. Yoon J.H. Thorne S.H. Kirn D. Hwang T.H. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Kirn D. Martuza R.L. Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Kirn D.H. Wang Y. Le Boeuf F. Bell J. Thorne S.H. Targeting of interferon-β to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T. Diaz R.M. Kaluza K. Pulido J. Galivo F. Wongthida P. Thompson J. Willmon C. Barber G.N. Chester J. Selby P. Strome S. Harrington K. Melcher A. Vile R.G. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol. Ther. 2008;16:1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K. Hardcastle J. Thakur R. Yang M. Christoforidis G. Fulci G. Hochberg F.H. Weissleder R. Carson W. Chiocca E.A. Kaur B. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J. Natl. Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- Lamfers M.L. Fulci G. Gianni D. Tang Y. Kurozumi K. Kaur B. Moeniralm S. Saeki Y. Carette J.E. Weissleder R. Vandertop W.P. Van Beusechem V.W. Dirven C.M. Chiocca E.A. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol. Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapteva N. Aldrich M. Weksberg D. Rollins L. Goltsova T. Chen S.Y. Huang X.F. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N. Shen F.B. Chang J.H. Wang L. Li H. Yang C. Li J. Yu D.C. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- Li H. Dutuor A. Fu X. Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J. Gene Med. 2007a;9:161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- Li H. Dutuor A. Tao L. Fu X. Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin. Cancer Res. 2007b;13:316–322. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- Lichty B.D. Power A.T. Stojdl D.F. Bell J.C. Vesicular stomatitis virus: Re-inventing the bullet. Trends Mol. Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Liu B.L. Robinson M. Han Z.Q. Branston R.H. English C. Reay P. McGrath Y. Thomas S.K. Thornton M. Bullock P. Love C.A. Coffin R.S. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- Lollini P.L. Cavallo F. Nanni P. Forni G. Vaccines for tumour prevention. Nat. Rev. Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- Lun X.Q. Jang J.H. Tang N. Deng H. Head R. Bell J.C. Stojdl D.F. Nutt C.L. Senger D.L. Forsyth P.A. McCart J.A. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- Mastrangelo M.J. Maguire H.C., Jr. Eisenlohr L.C. Laughlin C.E. Monken C.E. McCue P.A. Kovatich A.J. Lattime E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Miller C.G. Fraser N.W. Requirement of an integrated immune response for successful neuroattenuated HSV-1 therapy in an intracranial metastatic melanoma model. Mol. Ther. 2003;7:741–747. doi: 10.1016/S1525-0016(03)00120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehler M.H. Zeidler M. Wilsberg V. Cornelis J.J. Woelfel T. Rommelaere J. Galle P.R. Heike M. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum. Gene Ther. 2005;16:996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- Movassagh M. Spatz A. Davoust J. Lebecque S. Romero P. Pittet M. Rimoldi D. Lienard D. Gugerli O. Ferradini L. Robert C. Avril M.F. Zitvogel L. Angevin E. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–2198. doi: 10.1158/0008-5472.can-03-2969. [DOI] [PubMed] [Google Scholar]
- Nguyen T.L. Abdelbary H. Arguello M. Breitbach C. Leveille S. Diallo J.S. Yasmeen A. Bismar T.A. Kirn D. Falls T. Snoulten V.E. Vanderhyden B.C. Werier J. Atkins H. Vaha-Koskela M.J. Stojdl D.F. Bell J.C. Hiscott J. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandha H. Melcher A. Harrington K. Vile R. Oncolytic viruses: Time to compare, contrast, and combine? 5th international meeting on replicating oncolytic virus therapeutics. Mol. Ther. 2009;17:934–935. doi: 10.1038/mt.2009.86. Banff, Alberta, Canada, 18–22 March 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parato K.A. Senger D. Forsyth P.A. Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Park B.H. Hwang T. Liu T.C. Sze D.Y. Kim J.S. Kwon H.C. Oh S.Y. Han S.Y. Yoon J.H. Hong S.H. Moon A. Speth K. Park C. Ahn Y.J. Daneshmand M. Rhee B.G. Pinedo H.M. Bell J.C. Kirn D.H. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Parmiani G. Rodolfo M. Melani C. Immunological gene therapy with ex vivo gene-modified tumor cells: A critique and a reappraisal. Hum. Gene Ther. 2000;11:1269–1275. doi: 10.1089/10430340050032375. [DOI] [PubMed] [Google Scholar]
- Pecora A.L. Rizvi N. Cohen G.I. Meropol N.J. Sterman D. Marshall J.L. Goldberg S. Gross P. O'Neil J.D. Groene W.S. Roberts M.S. Rabin H. Bamat M.K. Lorence R.M. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- Perrot I. Blanchard D. Freymond N. Isaac S. Guibert B. Pacheco Y. Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- Pichlmair A. Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Pichlmair A. Schulz O. Tan C.P. Naslund T.I. Liljestrom P. Weber F. Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Prestwich R.J. Errington F. Hatfield P. Merrick A.E. Ilett E.J. Selby P.J. Melcher A.A. The immune system: Is it relevant to cancer development, progression and treatment? Clin. Oncol. (R. Coll. Radiol.) 2008a;20:101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Prestwich R.J. Errington F. Ilett E.J. Morgan R.S. Scott K.J. Kottke T. Thompson J. Morrison E.E. Harrington K.J. Pandha H.S. Selby P.J. Vile R.G. Melcher A.A. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 2008b;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich R.J. Harrington K.J. Vile R.G. Melcher A.A. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol. 2008c;9:610–612. doi: 10.1016/S1470-2045(08)70163-3. [DOI] [PubMed] [Google Scholar]
- Prestwich R.J. Ilett E.J. Errington F. Diaz R.M. Steele L.P. Kottke T. Thompson J. Galivo F. Harrington K.J. Pandha H.S. Selby P.J. Vile R.G. Melcher A.A. Immune mediated anti-tumour activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009a;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich R.J. Errington F. Steele L.P. Ilert E.J. Morgan R.S. Harrington K.J. Pandha S.H. Selby P.J. Vile R.G. Melcher A.A. Reciprocal human dendritic cell–natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J. Immunol. 2009b;183:4312–4321. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- Qiao J. Kottke T. Willmon C. Galivo F. Wongthida P. Diaz R.M. Thompson J. Ryno P. Barber G.N. Chester J. Selby P. Harrington K. Melcher A. Vile R.G. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008a;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Qiao J. Wang H. Kottke T. Diaz R.M. Willmon C. Hudacek A. Thompson J. Parato K. Bell J. Naik J. Chester J. Selby P. Harrington K. Melcher A. Vile R.G. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008b;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J. Wang H. Kottke T. White C. Twigger K. Diaz R.M. Thompson J. Selby P. De Bono J. Melcher A. Pandha H. Coffey M. Vile R. Harrington K. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008c;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna E. Woller N. Mundt B. Knocke S. Gurlevik E. Saborowski M. Malek N. Manns M.P. Wirth T. Kuhnel F. Kubicka S. Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses. Cancer Res. 2009;69:1448–1458. doi: 10.1158/0008-5472.CAN-08-1160. [DOI] [PubMed] [Google Scholar]
- Real L.M. Jimenez P. Kirkin A. Serrano A. Garcia A. Canton J. Zeuthen J. Garrido F. Ruiz-Cabello F. Multiple mechanisms of immune evasion can coexist in melanoma tumor cell lines derived from the same patient. Cancer Immunol. Immunother. 2001;49:621–628. doi: 10.1007/s002620000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschner A. Hubert P. Delvenne P. Boniver J. Jacobs N. Innate lymphocyte and dendritic cell cross-talk: A key factor in the regulation of the immune response. Clin. Exp. Immunol. 2008;152:219–226. doi: 10.1111/j.1365-2249.2008.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Yang J.C. Restifo N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004;10:909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal F.M. Zier K.S. Gansbacher B. Human tumor vaccines and genetic engineering of tumors with cytokine and histocompatibility genes to enhance immunogenicity. Curr. Opin. Oncol. 1994;6:611–615. doi: 10.1097/00001622-199411000-00014. [DOI] [PubMed] [Google Scholar]
- Schierer S. Hesse A. Muller I. Kampgen E. Curiel D.T. Schuler G. Steinkasserer A. Nettelbeck D.M. Modulation of viability and maturation of human monocyte-derived dendritic cells by oncolytic adenoviruses. Int. J. Cancer. 2008;122:219–229. doi: 10.1002/ijc.23074. [DOI] [PubMed] [Google Scholar]
- Schulz O. Diebold S.S. Chen M. Naslund T.I. Nolte M.A. Alexopoulou L. Azuma Y.-T. Flavell R.A. Liljestrom P. Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Senzer N.N. Kaufman H. Amatruda T. Nemunaitis M. Reid T. Daniels G. Gonzalez R. Glaspy J. Whitman E. Harrington K. Coffin R. Nemunaitis J. Abstract 225. Presented at the American Society Gene Therapy Annual Meeting, May 27–May 30, 2009. San Diego, CA: 2009. Updated results of a phase II clinical trial with a second generation, enhanced potency, immune-enhanced, oncolytic herpesvirus in unresectable metastatic melanoma. [DOI] [PubMed] [Google Scholar]
- Shi Y. Evans J.E. Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Smakman N. Van Der Bilt J.D. Van Den Wollenberg D.J. Hoeben R.C. Borel Rinkes I.H. Kranenburg O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–818. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B. Knowles S. Marius R. Atkins H. Sonenberg N. Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Takanami I. Takeuchi K. Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2001;121:1058–1063. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- Tesniere A. Apetoh L. Ghiringhelli F. Joza N. Panaretakis T. Kepp O. Schlemmer F. Zitvogel L. Kroemer G. Immunogenic cancer cell death: A key–lock paradigm. Curr. Opin. Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Thomas D.L. Fraser N.W. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol. Ther. 2003;8:543–551. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- Thomas M.A. Spencer J.F. Toth K. Sagartz J.E. Phillips N.J. Wold W.S. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol. Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne S.H. Contag C.H. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene Ther. 2008;15:753–758. doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- Thorne S.H. Negrin R.S. Contag C.H. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Toda M. Rabkin S.D. Kojima H. Martuza R.L. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum. Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Treilleux I. Blay J.Y. Bendriss-Vermare N. Ray-Coquard I. Bachelot T. Guastalla J.P. Bremond A. Goddard S. Pin J.J. Barthelemy-Dubois C. Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- Tumilasci V.F. Oliere S. Nguyen T.L. Shamy A. Bell J. Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J. Virol. 2008;82:8487–8499. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt W.J. Smakman N. Van Den Wollenberg D.J. Emmink B.L. Veenendaal L.M. Van Diest P.J. Hoeben R.C. Borel Rinkes I.H. Kranenburg O. Transient infection of freshly isolated human colorectal tumor cells by reovirus T3D intermediate subviral particles. Cancer Gene Ther. 2008;15:284–292. doi: 10.1038/cgt.2008.2. [DOI] [PubMed] [Google Scholar]
- Vicari A.P. Caux C. Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin. Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- Villegas F. R. Coca S. Villarrubia V. G. Jimenez R. Chillon M. J. Jareno J. Zuil M. Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Waldhauer I. Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- Zitvogel L. Tesniere A. Kroemer G. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]