Abstract
Establishing pharmacological parameters, such as efficacy, routes of administration, and toxicity, for recombinant adeno-associated virus (rAAV) vectors is a prerequisite for gaining acceptance for clinical applications. In fact, even a therapeutic window, that is, the dose range between therapeutic efficacy and toxicity, has yet to be determined for rAAV in vivo. Multiphase clinical trials investigating the safety and efficacy of recombinant AAV-based therapeutics will require unprecedented vector production capacity to meet the needs of preclinical toxicology studies, and the progressive clinical protocol phases of safety/dose escalation (phase I), efficacy (phase II), and high-enrollment, multicenter evaluations (phase III). Methods of rAAV production capable of supporting such trials must be scalable, robust, and efficient. We have taken advantage of the ease of scalability of nonadherent cell culture techniques coupled with the inherent efficiency of viral infection to develop an rAAV production method based on recombinant baculovirus-mediated expression of AAV components in insect-derived suspension cells.
Introduction
Since originally described (Smith et al., 1983), recombinant Autographa californica multiple nucleopolyhedrosis virus (AcMNPV), a baculovirus, and cell lines derived from the larvae of Spodoptera frugiperda, for example, Sf9 and Sf21, have proven to be an economical and versatile platform for recombinant protein production. In more recent years, the baculovirus expression vector (BEV) system has been used for the expression and assembly of virus-like particles for vaccine development (Lopez de Turiso et al., 1992; Roy et al., 1992; Redmond et al., 1993; Rosen et al., 1993; Brown et al., 1994; Conner et al., 1996; Li et al., 1997; Berkower et al., 2004; Galarza et al., 2005; Renoux et al., 2008) and structural analyses (Agbandje et al., 1993; Modis et al., 2002; Bishop et al., 2007), culminating in the first clinically relevant, regulatory agency-approved application of the baculovirus expression vector system, the human papillomavirus vaccine Cervarix produced by GlaxoSmithKline.
To extend the utility of the BEV system, we have sought to use insect cells for the production of not just virus-like particles, but fully functional recombinant adeno-associated virus (rAAV). Stow reported that Sf9 cells expressing a defined set of herpes simplex virus type 1 (HSV-1) genes support replication of an HSV-1 amplicon, thus suggesting that Sf9 cells are competent for mammalian DNA virus genomic replication (Stow, 1992). Moreover, we noted that members of the autonomous parvovirus subfamily Densovirinae (which are closely related to AAV) infect numerous invertebrate hosts (including certain Lepidoptera species), suggesting that the AAV origins of replication may function efficiently in insect cells. This hypothesis was tested in a set of critical experiments using Sf9 cells transfected with an AAV vector plasmid and subsequently infected with a recombinant baculovirus expressing an AAV replication initiator protein. In the presence of the AAV replication initiator, vector provirus was rescued from the plasmid DNA and converted into amplified replicative-form intermediates. Thus, Sf9 cells proved suitable for rAAV DNA replication. Building on these observations, Urabe and colleagues (2002) demonstrated that when rAAV genomes were replicated within Sf9 cells in the presence of the full complement of AAV structural and nonstructural proteins expressed from BEVs, rAAV particles were produced that were indistinguishable physically, biochemically, and biologically from mammalian cell line-produced rAAV. To produce rAAV with the BEV–insect cell system, Sf9 cells are infected with three different BEVs (Fig. 1).
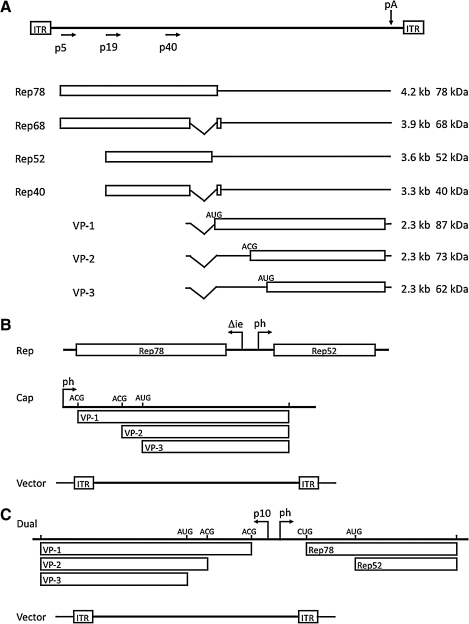
FIG. 1.
Transcription representations of wild-type AAV and baculoviral vectors. (A) The top line represents the 4.7-kb linear AAV genomic DNA. The open rectangles indicate the position of the terminal palindromic elements, or inverted terminal repeats (ITRs). The arrows indicate the positions of each of the three promoters at map positions 5, 19, and 40. The p5 rep transcripts encoding Rep78 and Rep68 differ by a 3′ splice that is common to the p19 rep transcripts encoding Rep52 and Rep40. The three structural proteins, VP1, VP2, and VP3, are encoded by the cap gene and transcriptionally regulated by the p40 promoter. The common 2.6-kb transcript is alternatively spliced, resulting in either the VP1 mRNA or the VP2/VP3 mRNA. Ribosome read-through produces both VP2 and VP3 in stoichiometric amounts. (B) The original set of baculovirus expression vectors (BEVs) used for rAAV production in Sf9 cells. Three BEVs were required to provide two Rep proteins, Rep78 and Rep52, regulated by the weakened IE promoter and the strong late p10 promoter, respectively. A second BEV provided the capsid proteins, which were expressed from a single open reading frame under the control of the strong late polyhedrin promoter. The rAAV virion DNA was introduced by a third BEV. (C) Consolidated system using two BEVs for rAAV production. Rep78 and Rep52 are expressed from a single open reading frame transcriptionally regulated by the polyhedrin promoter. Translational regulation results in similar levels of the two Rep proteins. Three capsid proteins, expressed from the p10 promoter, are regulated translationally by using an atypical initiation codon (ACG) for both VP1 and VP2 and the methionine codon for VP3.
The following sections are intended to outline some of the more recent developments, improvements, and important issues affecting the production of rAAV using the BEV system.
Regulated Expression of AAV Structural Proteins
The AAV capsid, composed of 60 subunit proteins, is assembled from three polypeptides, VP1, VP2, and VP3, derived from a single open reading frame (Fig. 1A). The average capsid is estimated to contain 5 VP1 subunits, 5 VP2 subunits, and 50 VP3 subunits (Xie et al., 2002). The stoichiometry of VP1, VP2, and VP3 within the assembled virion likely reflects the expression levels of the three capsid proteins. The AAV cap gene promoter, p40, produces a single primary 2.6-kb transcript (Marcus et al., 1981). Using alternate splice acceptors results in mRNAs lacking the AUG initiation codon for VP1. Ribosomes scanning the spliced cap mRNA lacking the VP1 AUG start codon occasionally initiate translation of VP2 at an ACG codon (Becerra et al., 1985, 1988). More frequently, scanning ribosomes proceed until encountering the VP3 AUG initiation codon. Thus, levels of the individual AAV capsid proteins are regulated at the translational level.
The BEV system, designed for maximal heterologous protein production in Sf9 cells, is not optimized for expressing stoichiometric levels of coexpressed heterologous proteins. Varying the multiplicities of infection (MOIs) for individual BEVs may alter the relative levels of each protein; however, this approach is neither practical nor robust, especially for the simultaneous expression of multiple proteins in large-scale production. For rAAV production in Sf9 cells, requiring simultaneous infections with different multiple recombinant baculoviruses, the Poisson distribution indicates that unrealistically high MOIs are required to guarantee that a significant number of cells receive at least 1 infectious unit of each required BEV.
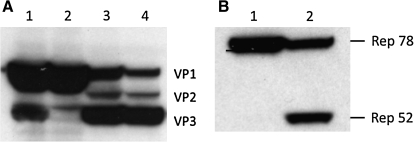
To achieve stoichiometric levels of the AAV capsid proteins, posttranscriptional regulation mechanisms were developed. Urabe and colleagues (2002) engineered BEV for translationally regulating expression of each of the three AAV capsid proteins from a single open reading frame (ORF). The VP1 AUG initiation codon was replaced with an ACG codon and the upstream nonanucleotide sequences preceding the wild-type AAV VP2 initiation codon, ACG. Substituting alternative non-AUG start codons for VP1 modulates the level of VP1 while still allowing scanning ribosomes to read through to the downstream VP2 and VP3 initiation codons (ACG and AUG, respectively). Using rabbit reticulocyte lysates, Peabody demonstrated that single base-substituted AUG codons initiate translation at various levels (Peabody, 1989). Depending on the context, initiation at non-AUG codons may generate suboptimal levels of VP1 (or VP2). Using alternative codons for VP1 initiation, capsids with a range of VP1:VP2:VP3 were generated (Fig. 2A). The cap ORF that contains AUG as the VP1 initiation codon produced primarily VP1 and, to a lesser extent, VP3 (Fig. 2A, lane 1). Altering the −3 position from a consensus Kozak “G” to a “T” had little effect on the relative amounts of the VP capsid proteins expressed (Fig. 2A, lane 2). However, VP1 constructed with non-AUG initiation codons produced dramatically different levels of capsid proteins: two leucine codons, CTG and TTG, differentially produced VP1 in excess of VP2 (Fig. 2A, lanes 3 and 4). Although not rigorously investigated in the context of AAV capsid protein expression, the initiation codon proximal sequences influence the ribosome recognition for translation initiation and single-nucleotide substitutions can substantially affect the relative and absolute levels of the capsid proteins.
FIG. 2.
(A) AAV capsid protein levels modulated by translational initiation efficiency. Western blot and silver-stained gel analysis shows the relative levels of three AAV capsid proteins expressed in BEV-infected Sf9 cells. The single open reading frame produces three polypeptides by ribosome scanning and inefficient initiation of translation. Using non-AUG codons, the rate of VP1 initiation is affected. The wild-type AAV2 initiation codon for VP2, ACG, is retained in each example shown. The VP1 initiation codons are as follows: lane 1, AUG with U at –3; lane 2, AUG; lane 3, CUG (Leu); lane 4, UUG (Leu). (B) Western blot of engineered bicistronic Rep transcription cassette products. Shown are the levels of Rep78 and Rep52 in Sf9 cell extracts infected with baculoviruses containing the bicistronic Rep78 and Rep52 open reading frame under the polyhedrin promoter. Retaining the in-frame and out-of-frame AUG codons resulted in Rep78 expression (lane 1), whereas removal of the AUGs by engineering the Rep78 unique open reading frame resulted in both Rep78 and Rep52 expression (lane 2) (see Fig. 1C).
Regulated Expression of AAV Nonstructural Proteins
In the BEV system, rep gene expression is uncoupled from both cis- and trans-acting AAV regulatory factors native to the wild-type virus infection of mammalian cells. The AAV p5 promoter (see Fig. 1A) is replaced with a baculovirus late promoter, such as the p10 or polyhedrin promoter. Although the AAV p19 promoter (located within the rep coding sequences) is unmodified, the transcriptional activity of this promoter is low in BEV-infected insect cells (Kohlbrenner et al., 2005). In mammalian cells coinfected with adenovirus and wild-type AAV2, Rep78 and Rep52 genes are expressed at similar rates, although the steady state amount of Rep52 is typically greater than that of Rep78 (Redemann et al., 1989). Substituting the mouse mammary tumor virus long terminal repeat (LTR) for the AAV p5 promoter diminishes the amount of Rep78 without affecting p19-expressed Rep52, and dramatically increased rAAV yield from transfected HEK293 cells (Grimm et al., 1998). Thus, recombinant AAV production in mammalian cells requires relatively little Rep78 compared with Rep52. On the basis of this paradigm, Urabe and colleagues (2002) designed a rep-expressing BEV that contained separate expression cassettes for Rep78 and Rep52. A weakened immediate-early promoter from the baculovirus Orgyia pseudotsugata (ΔIE) resulted in low levels of Rep78, whereas the Rep52 cassette, regulated by the baculovirus late polyhedrin promoter, was superexpressed.
Amplification of AAV Vector Genome
In transient production strategies, the AAV vector genome is introduced into permissive cells as duplex DNA, in the form of a plasmid (Maxwell et al., 1997; Grimm et al., 1998; Allen et al., 2000; Cao et al., 2000; Wright, 2009) or heterologous virus DNA (Conway et al., 1997, 1999; Zhang et al., 1999; Booth et al., 2004; Knop and Harrell, 2007). Stable “producer” cell lines maintaining the vector genome have also been described (Clark et al., 1995, 1996; Liu et al., 1999; Farson et al., 2004; Nakamura et al., 2004). In each case, the vector DNA exists as a “provirus” and is converted to the AAV replicative form under permissive conditions.
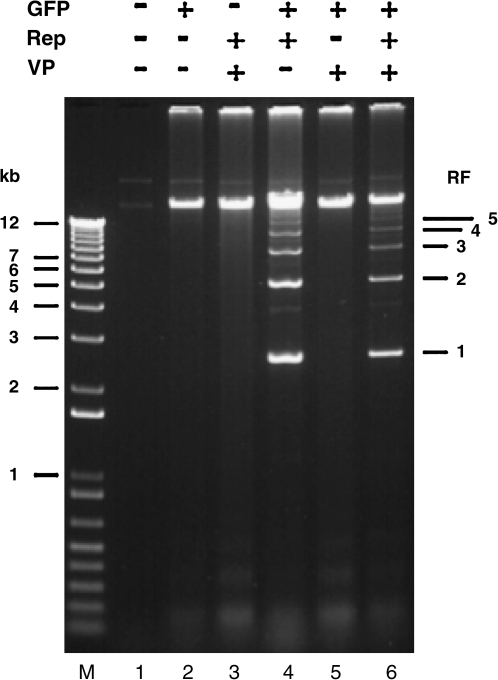
In the insect cell production strategy, a separate BEV introduces the AAV vector genome, which, in the presence of Rep78, is rescued and replicates independently of baculovirus DNA replication. The quantity of de novo AAV vector DNA synthesis is substantially greater than in transiently transfected HEK-293 cells (Urabe et al., 2002). The replication products, including duplex monomer, duplex dimer, and larger multimers, are evident in the Hirt extracts from Sf9 cells coinfected with a vector genome-baculovirus and a Rep-expressing baculovirus (Fig. 3). The large amount of replicated vector DNA serves as the pool for encapsidation. The similarities in rescued AAV genomic products and dependence of rescue and replication on Rep proteins suggest that mammalian cells and insect cells use similar rescue pathways. However, the rescue mechanism has not been adequately described in either system.
FIG. 3.
Rep-dependent rescue and replication of AAV vector DNA. Extrachromosomal DNA was extracted from Sf9 cells either uninfected (lane 1) or baculovirus infected (lanes 2 through 6, as indicated) and electrophoretically separated on an agarose–ethidium bromide gel. Headings: GFP, baculovirus bearing the rAAV virion DNA with the GFP expression cassette; Rep, baculovirus that expresses both Rep78 and Rep52 (see Fig. 1B); VP, baculovirus that expresses three AAV capsid proteins; RF, replicative forms.
Recent Developments
The original BEV configuration functioned well for producing adequate amounts of rAAV, but was suited primarily for small-scale Sf9 cell culture for two reasons. First, rAAV production required three different BEVs, necessitating high MOIs to ensure a simultaneous primary infection. Alternative strategies using low MOIs were developed, but require precise determinations of cell growth rate and baculovirus MOI (Negrete et al., 2007). Second, the presence of two Rep ORFs in a single BEV created a long inverted repeat corresponding to the Rep52 gene and the collinear ORF in Rep78. Loss of Rep expression was evidenced on BEV amplification, limiting the expansion to passage 3 or 4 (Kohlbrenner et al., 2005; Negrete et al., 2007). Expression of the VP- and vector genome-baculoviruses, however, remained stable through passage 6. Engineering the Rep ORF so that both Rep78 and Rep52 were expressed from a single transcript, and placing the Cap ORF into the same BEV as the Rep expression cassette, remedied these deficiencies (Fig. 1B) (Smith et al., 2009). Substituting the Rep78 AUG initiation codon with a non-AUG triplet is not sufficient to allow efficient translational initiation of Rep52 (Fig. 2B, lane 1) because the unique coding sequence of the AAV type 2 Rep78 ORF contains five out-of-frame and four in-frame ATG triplets. When these ATG triplets were altered to non-ATG codons, both Rep78 and Rep52 proteins were detectable by Western blots of infected Sf9 cell extracts (Fig. 2B, lane 2). As with the engineered cap gene expression, translational initiation at CTG is a relatively low-efficiency event and a portion of the scanning ribosomes bypass the Rep78 CTG initiation codon and initiate translation at the ATG start codon of Rep52.
In the consolidated Rep/Cap BEV, the cap gene was placed under the transcriptional control of the baculovirus p10 promoter in the opposite transcriptional orientation to the Rep cassette. Using the strategy of posttranscriptional regulation previously described, all three capsid proteins were expressed from one primary transcript. The resulting Rep/Cap baculovirus was shown to maintain expression of the VP and Rep proteins through at least six amplification passages (Smith et al., 2009).
With the new baculovirus–AAV reagents, two different baculoviruses, rather than three, are needed to produce rAAV. This represents a significant improvement for the following reasons. In the original configuration, a producer cell must receive at least one of each of three separate BEVs for successful rAAV production. Using an MOI of 3 (or an MOI of 1 for each baculovirus), approximately 58% of the cells are initially infected with three or more viral particles. Of 10 possible combinations of 3 different viruses, only 1 combination is productive for rAAV, and therefore the population of cells that are competent to produce rAAV by triple infection is about 6% under these conditions. The reengineered two-baculovirus system improves the chances considerably. Using the same MOI of 3, the Poisson distribution predicts that approximately 80% of cells receive at least two or more particles. Because there are three combinations of the two different viruses, approximately 27% of the cells (compared with 6% for the triple-BEV strategy) are initially competent to produce rAAV. The percentages of rAAV producer cells increase at higher MOIs.
Production
Small scale
Sf9 cells grow well in ambient atmosphere incubators at 27–28°C. Agitation of shaker flask cultures provides sufficient dissolved O2 in the medium to maintain cells in log-phase growth conditions ideal for rAAV production. Small culture-volume rAAV preparations are routinely performed by either triple infection (Rep-baculovirus, Cap-baculovirus, and vector genome-baculovirus) or double infection (Rep-Cap baculovirus plus the vector genome-baculovirus). MOIs of at least 1 per baculovirus construct are readily achieved for small-volume cell cultures. At these MOIs, baculovirus-induced cell cycle arrest typically occurs within 24 hr. AAV vector may be harvested when cell viability decreases to <30%. One simple method for recovering both the intra- and extracellular vector is by freeze–thaw lysis of the culture directly in the flask. The cell lysate may be nuclease-treated to reduce viscosity and digest extravirion DNA. Centrifugation or filtration may be used to remove debris. To concentrate the rAAV, polyethylene glycol (PEG, 8000 MW) is added to the clarified lysate (2%, w/v) to precipitate the rAAV particles. The precipitated rAAV can be resuspended in the appropriate medium for subsequent processing.
Large scale
The baculovirus–insect cell expression system is amenable to large-scale production, limited primarily by the capacity of the upstream and downstream processing systems. Insect cells grow well at 27–28°C in commercially available media, including serum-free formulations available in bulk volumes from several manufacturers. Consistently regulated temperature, agitation, and dissolved oxygen are the most critical parameters for promoting cell growth and rAAV productivity. A variety of available systems provide regulation of these parameters, from simple controllers that maintain a set value for oxygen:air mixture, to sophisticated systems that respond to changing levels of dissolved oxygen based on culture conditions. Although pH is routinely monitored, the buffering capacity of the growth media adequately maintains the acidity of the culture within levels acceptable for the growth of Sf9 cells. Dramatic shifts in pH usually indicate bacterial contamination or other problems with the culture.
Cell growth rate is important for BEV propagation and rAAV production, and is likely to differ depending on culture configuration, for example, shaker flask, Wave bag (GE Healthcare Life Sciences, Piscataway, NJ), or stirred tank. Although cell densities in excess of 107/ml are easily attainable, the rAAV specific yield, that is, the rAAV particle number produced per cell, drops as the medium components deplete and noxious metabolite by-products build up in the culture.
Using MOIs ≥1 becomes impractical for large-scale bioreactor preparations, for example, ≥100 liters, requiring large volumes of titered baculoviral stocks. Moreover, the addition of spent medium in the baculoviral stock is detrimental to cell growth. Unfortunately, concentrated or diafiltered baculovirus typically results in loss of activity. The low-MOI strategy used for heterologous protein expression in the baculovirus–insect cell system has been adapted for rAAV production. Negrete and colleagues determined that using MOIs much less than 1 was effective for rAAV production if the cell density at the time of infection (TOI) was appropriately adjusted to accommodate the increased time until baculovirus-induced cell cycle arrest (Negrete et al., 2007). Initially, few cells are infected, and the probability that different viruses coinfect a cell is near zero. During the course of the baculovirus infection, each cell releases approximately 100 infectious baculoviral particles, infecting the remainder of the cells. Thus, using a primary MOI of 0.05, the MOI of the secondary round is about 5, and so on. Because progeny baculoviruses are not released synchronously from the infected cell, predicting the kinetics for the secondary (and tertiary) infections is complicated, but many of these complications are ameliorated by the two-BEV system. Cell cycle arrest, increased cell diameter, and ultimately cell death, are reliable indicators of the progress of infection.
Downstream Processing
Several strategies have been described for recovering rAAV from up to 40-liter bioreactor cultures (Chahal et al., 2007; Negrete and Kotin, 2007, 2008; Negrete et al., 2007). The biomass and extracellular rAAV can be concentrated with 100-kDa molecular weight cutoff (MWCO) tangential flow filter (TFF) cassettes, and the cell slurry may be lysed mechanically with a bench-top homogenizer. Higher flow rate homogenizers are available for processing the entire bioreactor culture without prior concentration. Alternatively, surfactants such as deoxycholate or Triton X-100 are used for cell disruption and rAAV recovery. Factors influencing rAAV extraction conditions include cell density, culture volume, compatibility with subsequent downstream processes, and, interestingly, AAV vector serotype and possibly the vector transgene. Whether a given process is compliant with current good manufacturing practice (cGMP) standards should be considered when establishing a downstream processing protocol.
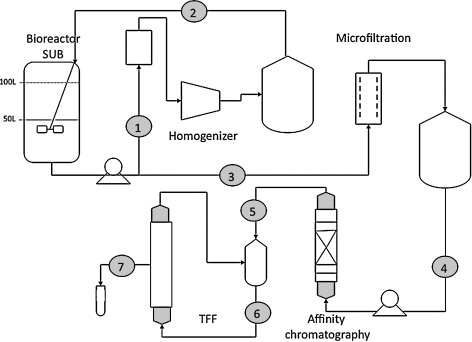
Recovering rAAV from large volumes and biomass requires developing new processing protocols (Fig. 4). The processing methods developed for small culture volume rAAV production, for example, ≤1 liter, are not directly scalable for cultures of tens or hundreds of liters. For example, cell lysis using multiple freeze–thaw cycles is not an option; however, other approaches are scalable, such as chemical lysis using surfactants or mechanical lysis using high flow rate homogenizers. Nuclease treatment and subsequent filtration steps may be proportionally scaled. Recovering vector from large-volume, clarified cell lysate presents additional challenges. Two options are either to reduce the liquid volume or process the original culture. Tangential flow filtration, using either hollow fiber or flat cassettes, effectively reduces the volume and removes a large amount of low molecular mass cell proteins, especially when coupled with diafiltration. Many high molecular mass proteins, however, remain in the retentate and may interfere with subsequent processing operations. Processing the original unconcentrated culture volume provides an alternative to TFF, but the large volumes require investing in expensive, proportionally scaled equipment.
FIG. 4.
Schematic diagram illustrating the downstream process described in the text. The entire culture volume is processed first by mechanical homogenization (1) and then returned to the stirred-tank bioreactor for nuclease treatment (2). Microfiltration (3) removes particulates and clarifies the supernatant. Immunoaffinity chromatography (4) is used for rAAV particle recovery and purification. Gel-exclusion chromatography (5) provides polishing and buffer exchange. Tangential flow filtration (TFF) is performed to concentrate the vector and diafiltrate into the final formulation buffer (6). Finally, the concentrated vector is sterilized by filtration and filled into vials (7).
Chromatography using an AAV capsid-specific immunoaffinity medium, for example, AVB Sepharose HP (GE Healthcare Life Sciences, Piscataway, NJ), allows more than 1000-fold purification of rAAV (Smith et al., 2009). Processing large volumes of rAAV-containing supernatant in a reasonable time frame at the recommended linear flow rate (2.5 cm/min) requires a relatively large-diameter column for processing large volumes. To produce a bed height of approximately 7 cm takes approximately 2 liters of AVB affinity medium in a 20-cm-diameter column. However, this bed volume may cost about $50,000. Therefore, cost is a major drawback of the AVB immunoaffinity medium. Size-exclusion chromatography using column dimensions appropriate for the loading volume may be used for final polishing and buffer exchange.
Product Characterization
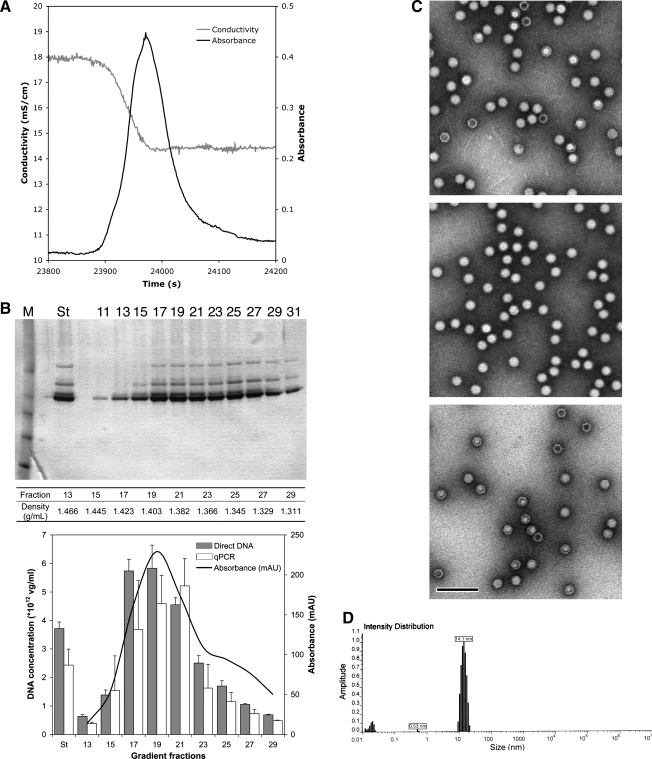
At the completion of each large-scale production run, the rAAV-containing fractions undergo analysis for a range of characteristics considered important for rAAV preclinical development. In a 100-liter preparation of rAAV6-GFP vector, the vector eluted from the immunoaffinity column in a 2-liter fraction (Fig. 5A) was concentrated to 40 ml. Analysis by CsCl isopycnic gradient centrifugation showed that approximately 50% of the capsid proteins corresponded in density to full, or DNA-containing, capsids (Fig. 5B, top). Both quantitative polymerase chain reaction (qPCR) and fluorescent dye binding (SYBR Gold; Molecular Probes Invitrogen, Carlsbad, CA) of extracted vector DNA were used to quantify the nucleic acid content in the vector fractions. With highly purified vector preparations, the values generated by the two assays were similar (e.g., Fraction 19, qPCR, 4.59 ± 0.99 × 1013 vg/ml; direct DNA measurement, 5.83 ±0.81 × 1013 vg/ml) (Fig. 5B, bottom). The extracted vector DNA, analyzed by agarose gel electrophoresis, was of the predicted size (Urabe et al., 2006). Determining the percentage of empty and filled capsids by electron microscopy (EM) in the vector preparation (Fig. 5C, top) is more convincing when used with CsCl isopycnic gradient-processed materials corresponding to a high-density fraction (for filled particles) (Fig. 5C, middle) and a low-density fraction (for empty particles) (Fig. 5C, bottom). A second orthogonal assay, such as CsCl isopycnic gradients and subsequent analyses, strengthens the filled particle content estimation. Determining particle dispersion in solution, that is, aggregation state, using dynamic light scattering, is important especially when establishing the rAAV formulation, for example, concentration and excipient composition, as well as storage conditions. The appearance of a single peak at approximately 20 nm indicates that the particles are monodisperse (Fig. 5D). Electron microscopy provides useful information about the capsid morphology and corroborates other results regarding aggregation, concentration, and empty versus filled capsids. In vitro bioassays provide convenient methods for determining the relative potency and for defining the in vivo dosage base. However, there are shortcomings because not all vectors express readily assayable products, for example, small interfering RNA (siRNA), and in vitro transduction efficiencies in commonly used cell lines, for example, HEK-293 cells, vary widely depending on vector serotype.
FIG. 5.
(A) Elution profile of rAAV6-GFP from AVB Sepharose HP. The clarified insect cell lysate (100 liters) was applied (0.3 liter/min) to the 20-cm column and washed with 10 liters of phosphate-buffered saline until the ultraviolet (UV) absorbance stabilized (black line). The elution medium (50 mM sodium citrate, pH 3.4) was applied (0.3 liter/min) and the vector-containing fraction was collected (2-liter fraction) and immediately neutralized with a 1/10 volume of 1 M Tris-HCl, pH 8.0. The vector elutes on acidification, indicated by the change in conductivity (gray line). (B) CsCl isopycnic gradients of rAAV6-GFP. Concentrated material from the AVB Sepharose HP chromatography (0.4 ml) was mixed with CsCl (density, 1.41 g/cm3) and centrifuged for 72 hr in a Beckman SW41 rotor (38,000 rpm at 20°C; Beckman Coulter, Fullerton, CA). The gradients were fractionated with a fast protein liquid chromatography system (ÄKTAfplc; GE Healthcare Life Sciences) and analyzed for protein and DNA content. After dialysis, aliquots of the CsCl gradient were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and proteins were visualized by silver staining (SilverXpress; Invitrogen) (top). The density of the fractions analyzed is indicated in between the top and bottom panels. The DNA content of the gradient fractions was determined by quantitative PCR and fluorescent dye (SYBR Gold) binding to extracted DNA (bottom). The UV absorption (280 nm) (bottom) of the CsCl gradient corresponds to the positions of the capsids. (C) Electron microscopy of particles. Samples were processed for electron microscopy (EM) as follows. The unfractionated starting material and dialyzed aliquots of gradient fractions 19 (1.403 g/cm3) and 25 (1.345 g/cm3) were applied to Formvar and carbon-coated grids and adsorbed for 1 min, washed with distilled water and then with five droplets of 2% ammonium molybdate. Excess liquid was wicked away and the samples were air dried. The grids were examined with a JEM-1200EX transmission electron microscope (JEOL, Tokyo, Japan). Original magnification, × 30,000. Top: Unfractionated vector. Middle: Fraction 19. Bottom: Fraction 25. Scale bar, 100 nm. (D) Dynamic light scattering. The effect of concentrating rAAV particles was determined by dynamic light scattering (DLS; Viscotek, Houston, TX). The aggregation state or dispersion of rAAV at 1 × 1014 VG-containing particles per milliliter, using DLS, shows that there is a major peak between 10 and 20 nm, indicating monodispersed particles.
To date, we have successfully produced rAAV with essentially wild-type capsids for serotypes 1, 2, 4, 6, and 8. The methods introduced by Urabe and colleagues for serotype 2 served as the template for generating these other AAV serotypes. So far, AAV5 remains the exception to this process, in that VP1 was not expressed at the desired level. Rather than evaluating the codon usage for the type 5 cap gene, substituting the type 5 VP1 unique region with the AAV2 homolog abrogated the problem. Also, AAV4 capsids are poorly expressed, resulting in low vector yields. However, not much effort was expended for producing AAV types 4 and 5. It is possible that the difficulties with these two serotypes result from codon usage differences between invertebrates and mammalian cells.
If the protein and nucleic acid components comprising rAAV particles produced in invertebrate and mammalian systems are indistinguishable, then the activities of the vectors are also expected to be indistinguishable. An independent laboratory tested insect cell-produced rAAV2-GFP, using C12 cells infected with adenovirus, and reported 2.17 × 1011 GCU/ml and 4 × 1012 VG/ml (GCU, green cell units; VG, vector genomes determined by qPCR). The GCU:VG ratio was 18.4, which is considered a low value for rAAV2 produced in either insect or mammalian cells (R.O. Snyder, personal communication, January 2002). In other cases, the lower infectious activities of rAAV preparations were attributable primarily to the particle composition as well as to the presence of process impurities. As described previously, the particle composition is readily assessable by polyacrylamide gel electrophoresis with silver staining and Western blotting. Expression of relatively low levels of VP1 resulted in low-infectious particles. However, as discussed, the stoichiometry of the capsid proteins is adjustable by altering the initiation codon, thereby producing fully active vector particles.
Room for Improvement
The deficiencies of the Sf9-baculovirus system in general also apply to the rAAV production system. BEVs appear genetically stable for at least six amplification passages; however, because large-scale cGMP production requires passage 5 or higher for establishing master and working virus banks as well as stocks for production runs, the amplification through passage 6 is reasonable, but limiting.
In addition to genetic stability, storing baculoviral stocks remains a problem, especially in serum-free medium. At present, amplified and titered baculoviral stocks used for large-scale production, stored at 4°C, consistently lose infectivity. Thus, drops in titers must be taken into account for production, particularly when BEV stocks of different “vintages” are used for coinfections.
Generating baculovirus is time-consuming and realistically requires at least 6 weeks: the molecular cloning and characterization takes a certain amount of time (this step takes the same time regardless of system), and producing the first recombinant baculoviruses might consume another week depending on method. Two or three more rounds of amplification add another week, and finally, titering the baculoviral stocks requires 10 to 12 days. Titering baculoviral stocks remains idiosyncratic and if accurate MOI values are required, then laboratory-to-laboratory and user-to-user variability is likely to affect rAAV production.
Future Directions
Discussing improvements and changes to the baculovirus expression vectors per se, that is, baculovirus genetics, are outside the scope of this review. The following list represents some of the research areas in development most likely to benefit rAAV production.
Optimizing Rep78 and Rep52 expression levels has not been thoroughly analyzed. The original rep expression cassettes were modeled on the ratio of Rep78:Rep52 attained with pDG-transfected 293 cells. As with mammalian cells, a wide range of Rep78:Rep52 and rep expression levels were not evaluated for insect cell–rAAV production. These parameters may be addressed by using regulatable promoters with activity levels proportional to the amount of inducer drug added to the medium, for example, Tet-on regulation. The expression levels can then be emulated, using the suitable strength promoters.
Simplification is a pervasive objective for every aspect of the rAAV production and processing platform. Reducing the required number of baculoviruses from the original three to two substantially improved the system (Smith et al., 2009). Following this trend, Aslanidi and colleagues described an interesting and potentially useful innovation on the Sf9–baculovirus production method (Aslanidi et al., 2009). Stably transfected Sf9 cell lines express Rep78, Rep52, and VP1, VP2, and VP3, on induction by baculovirus. The rep and cap expression cassettes also incorporate a Rep-binding element (RBE) and the baculovirus cis-acting homologous region 2 (hr 2), which functions as a baculovirus origin of replication. Cells infected with the rAAV vector baculovirus induce expression of Rep78, which, acting on the RBE, rescues and episomally amplifies the rep and cap expression cassettes. Only one baculovirus is required for rAAV production in the cell lines; however, the high copy number of episomes bearing RBEs and the presence of high amounts of Rep proteins provide opportunities for encapsidating nonvector DNA. Before being widely adopted for use, the rAAV products and the cell lines used in the process require more extensive characterization.
Developing high-density cell culture is likely to be the most pronounced improvement to the system. Increasing the density between 2- and 5-fold, while maintaining similar rAAV yield per Sf9 cell, represents a cost-effective means for improving rAAV yields within the fixed volumes of a bioreactor (Elias et al., 2000). Using a fed-batch process, at 107 Sf9 cells/ml, the rAAV yield was approximately 7 times greater compared with production in cultures with 106 cells/ml. Thus, rAAV yields are increased substantially by adding nutrient supplements at various times during the culture and baculovirus infection.
Recombinant AAV production using the Sf9–baculovirus expression vector system is amenable to automation. The engineering aspects regarding partially automating the process are being developed.
Applications
Culture volumes exceeding 100 liters provide sufficient vector for preclinical studies in large animals using supratherapeutic doses. Depending on the vector genome, yields exceeding 1014 VG/liter of culture medium have been obtained; thus, even 1015 VG/dose is a feasible goal. Although many applications involve small doses of focally delivered rAAV, for example, ocular applications (1012 VG/dose), other diseases require systemic delivery of much larger doses. Developing treatments for diseases such as Duchenne muscular dystrophy (DMD) is particularly challenging. DMD is a fatal, X-linked recessive disorder affecting approximately 1 in 3500 males. The lack of the structural myocytic protein dystrophin causes inexorably progressive loss of function in skeletal muscles, followed by respiratory impairment, and finally degeneration of cardiac function. Several gene therapy-based protein replacement approaches for the treatment of DMD have been described, initially with adenoviral vectors (Ragot et al., 1993) and then with rAAV. Because of size constraints, truncated dystrophin ORFs encoding so-called minidystrophin (Wang et al., 2000; Watchko et al., 2002; Li et al., 2003) and microdystrophin (Yue et al., 2003; Athanasopoulos et al., 2004; Abmayr et al., 2005; Yuasa et al., 2007) were developed that resemble naturally occurring mutations observed in Becker muscular dystrophy, a milder form of the disease. Subsequently, elegant RNA-based strategies were developed that interfere with normal processing of the primary dystrophin transcript. “Exon-skipping,” mediated by either synthetic antisense oligonucleotides (Wilton et al., 1997; Mann et al., 2001; van Deutekom et al., 2001) or modified U7 small nuclear RNAs (snRNAs) (Gorman et al., 1998; Suter et al., 1999), efficiently prevented spliceosome recognition of cis-acting processing signals, leading to removal of the mutation-containing exon and restoration of partial dystrophin function. The impressive results obtained in the dystrophin null (mdx) mouse (Goyenvalle et al., 2004) led to optimistic expectations for a large animal trial in a clinically relevant model of Duchenne muscular dystrophy, the golden retriever X-linked muscular dystrophy (GRMD) dog. Encouraging results were obtained with GRMD dogs administered rAAV containing either microdystrophin or U7smOPT exon-skipping cassettes (L. Garcia, personal communication). Ideally, testing rAAV gene therapy for DMD would include establishing the maximal tolerated dose followed by dose de-escalation to determine the minimal effective dose. In addition to economic considerations, the quantities of rAAV required to fully support preclinical development of a preclinical DMD gene therapy trial may exceed 1017 particles. This should be obtainable from less than 10,000 liters of cell culture based on current baculovirus–AAV production values. Although this volume appears large, one large bioreactor run may achieve this yield.
Conclusions
Limited rAAV production capabilities have retarded gene therapy progress. Increased vector production capacity allows meaningful testing of rAAV vectors in animal models that are more predictive of human responses than small rodents, for example, nonhuman primates. In addition to assessing the effects of transgene expression, recombinant AAV gene therapy testing should also address the pharmacology of vector administration. Important parameters include route of administration, distribution, toxicity, and rates of clearance. In addition, establishing the therapeutic window is critical for rAAV gene therapy applications. Ideally, demonstrating lack of toxicity at doses ≥10 times the therapeutic dose would provide an additional level of confidence for human applications.
Acknowledgments
The authors were supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) and receive additional support from the International Collaborative Effort for Duchenne Muscular Dystrophy, Duchenne Parent Project France (DPPF), and the Association Monegasque contre les Myopathies (AMM). The authors thank Mathew P. Daniels and the NHLBI Electron Microscopy Core Facility for assisting and advising on the use of the electron microscope. Dr. Masashi Urabe's contributions, including Figure 3, is greatly appreciated. The authors thank Dr. Richard H. Smith for critical reading of the manuscript.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- Abmayr S. Gregorevic P. Allen J.M. Chamberlain J.S. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol. Ther. 2005;12:441–450. doi: 10.1016/j.ymthe.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Agbandje M. McKenna R. Rossmann M.G. Strassheim M.L. Parrish C.R. Structure determination of feline panleukopenia virus empty particles. Proteins. 1993;16:155–171. doi: 10.1002/prot.340160204. [DOI] [PubMed] [Google Scholar]
- Allen J.M. Halbert C.L. Miller A.D. Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6. Mol. Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- Aslanidi G. Lamb K. Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5059–5064. doi: 10.1073/pnas.0810614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos T. Graham I.R. Foster H. Dickson G. Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD) Gene Ther. 2004;11(Suppl. 1):S109–S121. doi: 10.1038/sj.gt.3302379. [DOI] [PubMed] [Google Scholar]
- Becerra S.P. Rose J.A. Hardy M. Baroudy B.M. Anderson C.W. Direct mapping of adeno-associated virus capsid proteins B and C: A possible ACG initiation codon. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S.P. Koczot F. Fabisch P. Rose J.A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J. Virol. 1988;62:2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I. Raymond M. Muller J. Spadaccini A. Aberdeen A. Assembly, structure, and antigenic properties of virus-like particles rich in HIV-1 envelope gp120. Virology. 2004;321:75–86. doi: 10.1016/j.virol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Bishop B. Dasgupta J. Klein M. Garcea R.L. Christensen N.D. Zhao R. Chen X.S. Crystal structures of four types of human papillomavirus L1 capsid proteins: Understanding the specificity of neutralizing monoclonal antibodies. J. Biol. Chem. 2007;282:31803–31811. doi: 10.1074/jbc.M706380200. [DOI] [PubMed] [Google Scholar]
- Booth M.J. Mistry A. Li X. Thrasher A. Coffin R.S. Transfection-free and scalable recombinant AAV vector production using HSV/AAV hybrids. Gene Ther. 2004;11:829–837. doi: 10.1038/sj.gt.3302226. [DOI] [PubMed] [Google Scholar]
- Brown C.S. Welling-Wester S. Feijlbrief M. Van Lent J.W. Spaan W.J. Chimeric parvovirus B19 capsids for the presentation of foreign epitopes. Virology. 1994;198:477–488. doi: 10.1006/viro.1994.1059. [DOI] [PubMed] [Google Scholar]
- Cao L. Liu Y. During M.J. Xiao W. High-titer, wild-type free recombinant adeno-associated virus vector production using intron-containing helper plasmids. J. Virol. 2000;74:11456–11463. doi: 10.1128/jvi.74.24.11456-11463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal P.S. Aucoin M.G. Kamen A. Primary recovery and chromatographic purification of adeno-associated virus type 2 produced by baculovirus/insect cell system. J. Virol. Methods. 2007;139:61–70. doi: 10.1016/j.jviromet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Clark K.R. Voulgaropoulou F. Fraley D.M. Johnson P.R. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- Clark K.R. Voulgaropoulou F. Johnson P.R. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]
- Conner M.E. Zarley C.D. Hu B. Parsons S. Drabinski D. Greiner S. Smith R. Jiang B. Corsaro B. Barniak V. Madore H.P. Crawford S. Estes M.K. Virus-like particles as a rotavirus subunit vaccine. J. Infect. Dis. 1996;174(Suppl. 1):S88–S92. doi: 10.1093/infdis/174.supplement_1.s88. [DOI] [PubMed] [Google Scholar]
- Conway J.E. Zolotukhin S. Muzyczka N. Hayward G.S. Byrne B.J. Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap. J. Virol. 1997;71:8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J.E. Rhys C.M. Zolotukhin I. Zolotukhin S. Muzyczka N. Hayward G.S. Byrne B.J. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999;6:986–993. doi: 10.1038/sj.gt.3300937. [DOI] [PubMed] [Google Scholar]
- Elias C.B. Zeiser A. Bedard C. Kamen A.A. Enhanced growth of Sf-9 cells to a maximum density of 5.2 × 107 cells per mL and production of β-galactosidase at high cell density by fed batch culture. Biotechnol. Bioeng. 2000;68:381–388. doi: 10.1002/(sici)1097-0290(20000520)68:4<381::aid-bit3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Farson D. Harding T.C. Tao L. Liu J. Powell S. Vimal V. Yendluri S. Koprivnikar K. Ho K. Twitty C. Husak P. Lin A. Snyder R.O. Donahue B.A. Development and characterization of a cell line for large-scale, serum-free production of recombinant adeno-associated viral vectors. J. Gene Med. 2004;6:1369–1381. doi: 10.1002/jgm.622. [DOI] [PubMed] [Google Scholar]
- Galarza J.M. Latham T. Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- Gorman L. Suter D. Emerick V. Schumperli D. Kole R. Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4929–4934. doi: 10.1073/pnas.95.9.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyenvalle A. Vulin A. Fougerousse F. Leturcq F. Kaplan J.C. Garcia L. Danos O. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Grimm D. Kern A. Rittner K. Kleinschmidt J.A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Knop D.R. Harrell H. Bioreactor production of recombinant herpes simplex virus vectors. Biotechnol. Prog. 2007;23:715–721. doi: 10.1021/bp060373p. [DOI] [PubMed] [Google Scholar]
- Kohlbrenner E. Aslanidi G. Nash K. Shklyaev S. Campbell-Thompson M. Byrne B.J. Snyder R.O. Muzyczka N. Warrington K.H., Jr. Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.H. Zhang S.M. Fang S.Y. Chen C.L. Luo Y.D. Guan Y. Wang D.W. Xiao X. Adeno-associated virus vector carrying human minidystrophin gene SMCKA3999 effectively ameliorates dystrophic pathology in mdx model mice. Zhonghua Yi Xue Za Zhi. 2003;83:1513–1516. [PubMed] [Google Scholar]
- Li T.C. Yamakawa Y. Suzuki K. Tatsumi M. Razak M.A. Uchida T. Takeda N. Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.L. Clark K.R. Johnson P.R. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 1999;6:293–299. doi: 10.1038/sj.gt.3300807. [DOI] [PubMed] [Google Scholar]
- Lopez de Turiso J.A. Cortes E. Martinez C. Ruiz De Ybanez R. Simarro I. Vela C. Casal I. Recombinant vaccine for canine parvovirus in dogs. J. Virol. 1992;66:2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C.J. Honeyman K. Cheng A.J. Ly T. Lloyd F. Fletcher S. Morgan J.E. Partridge T.A. Wilton S.D. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc. Natl. Acad. Sci. U.S.A. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C.J. Laughlin C.A. Carter B.J. Adeno-associated virus RNA transcription in vivo. Eur. J. Biochem. 1981;121:147–154. doi: 10.1111/j.1432-1033.1981.tb06443.x. [DOI] [PubMed] [Google Scholar]
- Maxwell F. Harrison G.S. Maxwell I.H. Improved production of recombinant AAV by transient transfection of NB324K cells using electroporation. J. Virol. Methods. 1997;63:129–136. doi: 10.1016/s0166-0934(96)02121-0. [DOI] [PubMed] [Google Scholar]
- Modis Y. Trus B.L. Harrison S.C. Atomic model of the papillomavirus capsid. EMBO J. 2002;21:4754–4762. doi: 10.1093/emboj/cdf494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S. Nakamura R. Shibata K. Kobayashi M. Sahara N. Shigeno K. Shinjo K. Naito K. Ohnishi K. Kasahara N. Iwaki Y. Development of packaging cell lines for generation of adeno-associated virus vectors by lentiviral gene transfer of trans-complementary components. Eur. J. Haematol. 2004;73:285–294. doi: 10.1111/j.1600-0609.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- Negrete A. Kotin R.M. Production of recombinant adeno-associated vectors using two bioreactor configurations at different scales. J. Virol. Methods. 2007;145:155–161. doi: 10.1016/j.jviromet.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete A. Kotin R.M. Large-scale production of recombinant adeno-associated viral vectors. Methods Mol. Biol. 2008;433:79–96. doi: 10.1007/978-1-59745-237-3_5. [DOI] [PubMed] [Google Scholar]
- Negrete A. Yang L.C. Mendez A.F. Levy J.R. Kotin R.M. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J. Gene Med. 2007;9:938–948. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- Peabody D.S. Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 1989;264:5031–5035. [PubMed] [Google Scholar]
- Ragot T. Vincent N. Chafey P. Vigne E. Gilgenkrantz H. Couton D. Cartaud J. Briand P. Kaplan J.C. Perricaudet M. Kahn A. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Redemann B.E. Mendelson E. Carter B.J. Adeno-associated virus Rep protein synthesis during productive infection. J. Virol. 1989;63:873–882. doi: 10.1128/jvi.63.2.873-882.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond M.J. Ijaz M.K. Parker M.D. Sabara M.I. Dent D. Gibbons E. Babiuk L.A. Assembly of recombinant rotavirus proteins into virus-like particles and assessment of vaccine potential. Vaccine. 1993;11:273–281. doi: 10.1016/0264-410x(93)90029-w. [DOI] [PubMed] [Google Scholar]
- Renoux V.M. Fleury M.J. Bousarghin L. Gaitan J. Sizaret P.Y. Touze A. Coursaget P. Induction of antibody response against hepatitis E virus (HEV) with recombinant human papillomavirus pseudoviruses expressing truncated HEV capsid proteins in mice. Vaccine. 2008;26:6602–6607. doi: 10.1016/j.vaccine.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Rosen E. Stapleton J.T. McLinden J. Synthesis of immunogenic hepatitis A virus particles by recombinant baculoviruses. Vaccine. 1993;11:706–712. doi: 10.1016/0264-410x(93)90253-t. [DOI] [PubMed] [Google Scholar]
- Roy P. French T. Erasmus B.J. Protective efficacy of virus-like particles for bluetongue disease. Vaccine. 1992;10:28–32. doi: 10.1016/0264-410x(92)90415-g. [DOI] [PubMed] [Google Scholar]
- Smith G.E. Fraser M.J. Summers M.D. Molecular engineering of the Autographa californica nuclear polyhedrosis virus genome: Deletion mutations within the polyhedrin gene. J. Virol. 1983;46:584–593. doi: 10.1128/jvi.46.2.584-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.H. Levy J.R. Kotin R.M. A simplified baculovirus–AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009 doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N.D. Herpes simplex virus type 1 origin-dependent DNA replication in insect cells using recombinant baculoviruses. J. Gen. Virol. 1992;73:313–321. doi: 10.1099/0022-1317-73-2-313. [DOI] [PubMed] [Google Scholar]
- Suter D. Tomasini R. Reber U. Gorman L. Kole R. Schumperli D. Double-target antisense U7 snRNAs promote efficient skipping of an aberrant exon in three human β-thalassemic mutations. Hum. Mol. Genet. 1999;8:2415–2423. doi: 10.1093/hmg/8.13.2415. [DOI] [PubMed] [Google Scholar]
- Urabe M. Ding C. Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Urabe M. Nakakura T. Xin K.Q. Obara Y. Mizukami H. Kume A. Kotin R.M. Ozawa K. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J. Virol. 2006;80:1874–1885. doi: 10.1128/JVI.80.4.1874-1885.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deutekom J.C. Bremmer-Bout M. Janson A.A. Ginjaar I.B. Baas F. den Dunnen J.T. van Ommen G.J. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum. Mol. Genet. 2001;10:1547–1554. doi: 10.1093/hmg/10.15.1547. [DOI] [PubMed] [Google Scholar]
- Wang B. Li J. Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko J. O'Day T. Wang B. Zhou L. Tang Y. Li J. Xiao X. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum. Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- Wilton S.D. Dye D.E. Laing N.G. Dystrophin gene transcripts skipping the mdx mutation. Muscle Nerve. 1997;20:728–734. doi: 10.1002/(sici)1097-4598(199706)20:6<728::aid-mus10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wright J.F. Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene Ther. 2009;20:698–706. doi: 10.1089/hum.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. Bu W. Bhatia S. Hare J. Somasundaram T. Azzi A. Chapman M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K. Yoshimura M. Urasawa N. Ohshima S. Howell J.M. Nakamura A. Hijikata T. Miyagoe-Suzuki Y. Takeda S. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Yue Y. Li Z. Harper S.Q. Davisson R.L. Chamberlain J.S. Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin–glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. De Alwis M. Hart S.L. Fitzke F.W. Inglis S.C. Boursnell M.E. Levinsky R.J. Kinnon C. Ali R.R. Thrasher A.J. High-titer recombinant adeno-associated virus production from replicating amplicons and herpes vectors deleted for glycoprotein H. Hum. Gene Ther. 1999;10:2527–2537. doi: 10.1089/10430349950016861. [DOI] [PubMed] [Google Scholar]