Abstract
Gene therapy for hemophilia B has been shown to result in long-term expression and immune tolerance to factor IX (F.IX) after in vivo transduction of hepatocytes with adeno-associated viral (AAV-2) vectors in experimental animals. An optimized protocol was effective in several strains of mice with a factor 9 gene deletion (F9−/−). However, immune responses against F.IX were repeatedly observed in C3H/HeJ F9−/− mice. We sought to establish a gene transfer protocol that results in sustained expression without a requirement for additional manipulation of the immune system. Compared with AAV-2, AAV-8 was more efficient in transgene expression and induction of tolerance to F.IX in three different strains of wild-type mice. At equal vector doses, AAV-8 induced transgene product-specific regulatory CD4+CD25+FoxP3+ T cells at significantly higher frequency. Moreover, sustained correction of hemophilia B in C3H/HeJ F9−/− mice without antibody formation was documented in all animals treated with ≥4 × 1011 vector genomes (VG)/kg and in 80% of mice treated with 8 × 1010 VG/kg. Therefore, it is possible to develop a gene transfer protocol that reliably induces tolerance to F.IX largely independent of genetic factors. A comparison with other studies suggests that additional parameters besides plateau levels of F.IX expression contributed to the improved success rate of tolerance induction.
Introduction
Adeno-associated viral (AAV) vector-mediated coagulation factor IX (F.IX) gene therapy for treatment of the X-linked bleeding disorder hemophilia B has resulted in long-term therapy in animal models. However, concerns about the potential for immune responses to the vector and to the F.IX transgene product have slowed translational research (Herzog and Dobrzynski, 2004; Mingozzi and High, 2007). Studies in animals have shown that the risk for inhibitory antibody (“inhibitor”) formation to F.IX in gene transfer is influenced by vector design, dose, and route of administration, and is elevated in animals that lack endogenous F.IX expression, for example, as a result of a gene deletion (Herzog and Dobrzynski, 2004). High-titer inhibitors have been documented in muscle-directed gene transfer in animals with F.IX null mutations (Fields et al., 2001). Interestingly, hepatic gene transfer often induces immune tolerance to F.IX (Herzog, 2005). Portal vein administration of an AAV serotype 2 vector containing a strong hepatocyte-specific expression cassette efficiently induces tolerance to F.IX in mice of several genetic backgrounds (Mingozzi et al., 2003). Injection of 4 × 1012–1.2 × 1013 vector genomes (VG)/kg induced tolerance in 70–100% of F.IX knockout (F9−/−) mice on the C57BL/6, BALB/c, or CD-1 background (lower doses were sufficient in F9−/− C57BL/6 mice or in hemophilia B dogs) (Mount et al., 2002; Mingozzi et al., 2003).
Subsequent mechanistic studies provided evidence of CD4+ T cell tolerance to the transgene product, including deletion and anergy of conventional transgene product-specific CD4+ T cells. Furthermore, hepatic gene transfer induced transgene product-specific CD4+CD25+ regulatory T cells (Tregs), which increased in frequency during the first 2 months after vector delivery (Dobrzynski et al., 2004; Cao et al., 2007a,b). Induced Tregs were capable of suppressing cytotoxic T lymphocyte and antibody responses against F.IX, and were required for tolerance induction (Dobrzynski et al., 2006; Cao et al., 2007a). Once tolerance has been established, supplementary gene transfer to other organs or systemic therapeutic protein delivery can be safely applied (Hoffman et al., 2007; Passini et al., 2007; Sun et al., 2007; Luth et al., 2008). Tolerance induction by liver-restricted transgene expression has been documented for several different transgene products and vectors (Herzog, 2005; Brown et al., 2007; Cerullo et al., 2007). Because of low innate immunity and inefficient transduction of antigen-presenting cells, AAV vectors may be ideal for this purpose (Zaiss and Muruve, 2005, 2008). Nonetheless, further improvements of this approach may be required to reliably obtain tolerance.
Our new study addresses the question of whether hepatic gene transfer can be further optimized to obtain tolerance in multiple strains of mice without a requirement for additional immune modulation. To achieve this goal, we tested gene transfer with an alternative AAV serotype (AAV-8) originally described by Gao, Wilson, and colleagues to direct more efficient in vivo gene transfer to murine hepatocytes (Gao et al., 2002).
Materials and Methods
Viral vectors
AAV-ApoE/hAAT-hF.IX carries the hepatocyte-specific expression cassette for human factor IX (hF.IX) (Manno et al., 2006). This cassette includes an apolipoprotein E (ApoE) enhancer/hepatocyte control region, a human α1-antitrypsin promoter, hF.IX cDNA, a 1.4-kb portion of intron I of the F9 gene, and the bovine growth hormone poly(A) signal. AAV vector harboring the ovalbumin (OVA) transgene under the control of the human elongation factor-1α (EF-1α) promoter was as described (Dobrzynski et al., 2004). Expression cassettes are flanked by AAV-2 inverted terminal repeats. AAV vectors (serotype 2 and 8) were produced by triple transfection of HEK-293 cells, purified by CsCl gradient centrifugation, filter sterilized, and quantified by slot-blot hybridization as described (Liu et al., 2003).
Animal studies
C3H/HeJ and BALB/c mice were from Jackson Laboratories (Bar Harbor, ME), CD-1 mice were from Charles River Laboratories (Wilmington, MA), and DO11-10-transgenic Rag-2 knockout (DO11.10-tg Rag-2−/−) mice were purchased from Taconic (Hudson, NY). F9−/− mice lack the promoter and exons 1–3 of F9 as a result of targeted deletion (Lin et al., 1997), and have been bred on a C3H/HeJ background for >10 generations. AAV vector (200 μl per injection) was delivered into the portal vein or the tail vein as published (Mingozzi et al., 2003). Immunizations were done by subcutaneous injection into the back at four sites, using 5 μg of hF.IX protein formulated in complete Freund's adjuvant (cFA; Sigma, St. Louis, MO). Blood samples from wild-type mice were collected via the retro-orbital plexus, using heparinized capillary tubes, whereas blood samples from hemophilia B mice were obtained by tail vein bleed into sodium citrate buffer (final concentration, 0.4%) (Fields et al., 2001). All mice were male and 4–6 weeks old at the time of gene transfer (8–10 weeks for hemophilia B mice).
Analyses of mouse plasma samples
Levels of hF.IX in plasma samples were determined by enzyme-linked immunosorbent assay (ELISA), and antibody concentrations to hF.IX were measured by immunoglobulin subclass-specific immunocapture assay (Fields et al., 2000). Activated partial thromboplastin times (aPTTs) of plasma samples were determined with a fibrometer (BBL Fibrosystem; BD Diagnostic Systems, Cockeysville, MD). Inhibitory antibodies were measured by Bethesda assay. Briefly, samples were serially diluted in imidazole buffer and mixed with an equal volume of normal human plasma (MDA Verify 1; Trinity Biotech, Bray, Ireland) and incubated at 37°C for 2 hr. One Bethesda unit (BU) is equivalent to 50% residual coagulation activity (as determined by aPTT of serially diluted MDA Verify 1).
Adoptive T cell transfer
CD4+ T cells were purified from pooled C3H/HeJ splenocytes (naive or 2 months after hepatic gene transfer with AAV vector) by magnetic cell sorting using positive selection (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The purity of CD4+ cells (90–95%) was verified by flow cytometry. CD4+ cells (1 × 107 per recipient mouse) were adoptively transferred to naive wild-type C3H/HeJ mice by tail vein injection (Mingozzi et al., 2003; Cao et al., 2007a). Recipient mice were immunized with 5 μg of hF.IX in cFA 24 hr after adoptive transfer. Anti-hF.IX IgG titers were measured 3 weeks after immunization.
Immunostaining
Of each AAV-2- or AAV-8-transduced cohort of C3H/HeJ mice, two liver lobes were collected from at least two different animals and frozen in O.C.T. compound (Sakura Finetek USA, Torrance, CA), using a dry ice–methylbutane bath. Cryosections (at least four per lobe) were analyzed for the presence of hF.IX by immunofluorescence staining, using a goat anti-hF.IX and a tetramethylrhodamine isothiocyanate (TRITC)-labeled secondary antibody (Herzog et al., 1997). Images were captured with a CoolSNAP-Pro camera (Micro-Optics, Davie, FL) and analyzed with Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
Flow cytometry
Spleens were harvested into 2-MLC medium (Dulbecco's modified Eagle's medium with 2% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 10 mM HEPES, 0.1 mM nonessential amino acids, 10−6 M 2-mercaptoethanol, and antibiotics) at room temperature, homogenized, and filtered through a 70-μm cell strainer. Cells were centrifuged for 10 min at 300 × g at room temperature. Cells were incubated with BD PharmLyse buffer (BD Biosciences, San Jose, CA) for 5 min and washed twice with 2-MLC medium. Viable splenocytes were counted with a hemacytometer and trypan blue. Nuclear stain for transcription factor FoxP3 was performed with an eBiosciences (San Diego, CA) kit, which uses anti-murine/rat FoxP3 conjugated to fluorescein isothiocyanate (FITC) (Cao et al., 2007a). Antibodies to murine CD4 (conjugated to allophycocyanin) and CD25 (conjugated to phycoerythrin) were from BD Biosciences. Controls for all stains included isotype controls and unstained cells. Flow cytometry was performed with the LSR II system (BD Biosciences), and data were analyzed with CellQuest software.
Statistics
Statistical comparisons between experimental groups were performed by two-tailed Student t test. Values were considered to be statistically significant for p < 0.05.
Results
Superior hF.IX expression and tolerance induction in C3H/HeJ mice with AAV-8 vector
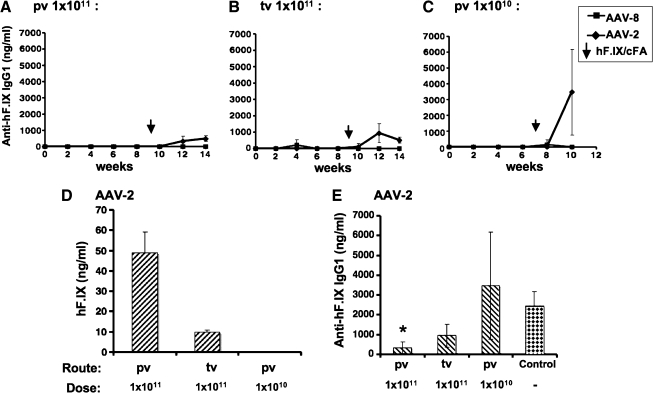
Previously, we had demonstrated efficient induction of immune tolerance to F.IX by AAV-2-mediated hepatic gene transfer in several strains of F9−/− mice (Mingozzi et al., 2003). However, C3H/HeJ F9−/− mice formed inhibitors even with our optimized protocol (Mingozzi et al., 2003; Cao et al., 2006). Therefore, we decided to evaluate a vector with enhanced gene transfer to murine liver (Gao et al., 2002). Initially, AAV-2 and AAV-8 vectors were compared in wild-type C3H/HeJ mice. Vectors were administered via the portal vein at 1 × 1010 or 1 × 1011 VG/mouse, or via the tail vein at 1010 VG/mouse. Consistent with previous findings, portal vein injection of AAV-2 yielded approximately 5-fold higher hF.IX levels compared with tail vein injection (Fig. 1A, B, and G) (Mingozzi et al., 2002). Low-dose AAV-2 injection gave no detectable hF.IX (<3 ng/ml; Fig. 1C). In contrast, AAV-8 was equally efficient by both routes and directed ≥100-fold higher levels of expression. In fact, hF.IX levels were equivalent to 100% (at the high dose) and to 8% of normal hF.IX levels at the lower dose (Fig. 1A–F and H).
FIG. 1.
Comparison of systemic human factor IX (hF.IX) levels in C3H/HeJ mice as a function of time after administration of AAV vector (serotype 2 or 8). The mice were injected via the portal vein (pv; A and C) or the tail vein (tv; B) at vector doses of 1 × 1011 VG/mouse (A, B) or 1 × 1010 VG/mouse (C). Data represent average hF.IX plasma levels ± SD for n = 4 mice per serotype (AAV-2, diamonds; AAV-8, squares). Vertical arrows indicate the time point of challenge by subcutaneous administration of hF.IX in complete Freund's adjuvant (cFA). (D–F) Comparison of hF.IX plateau levels for each cohort of AAV-2- and AAV-8-injected mice. Fold difference between AAV-2- and AAV-8-derived expression and percentage of normal hF.IX levels (in humans) are indicated. (G and H) Comparison between hF.IX plateau levels for AAV-2 (G) and AAV-8 (H) for the two routes of administration (pv and tv).
After challenge with hF.IX in cFA, all AAV-2- and AAV-8-transduced animals that had detectable hF.IX levels continued to express (Fig. 1A–C). However, AAV-2-transduced mice formed IgG1 against hF.IX (Fig. 2A–C; some animals also formed comparatively low-titer IgG2a [data not shown]). Antibody titers against hF.IX inversely correlated with hF.IX expression levels. Higher expressing AAV-2-transduced mice had lower antibody titers, reaching statistically significant suppression of antibody formation in the high-dose portal vein group when compared with immunization of naive controls (Fig. 2D and E). In contrast, none of the AAV-8-transduced mice formed antibodies to hF.IX (Fig. 2A–C).
FIG. 2.
Comparison of IgG1 antibody formation to hF.IX in C3H/HeJ mice as a function of time after administration of AAV vector (serotype 2 or 8). The mice were injected via the portal vein (pv; A and C) or the tail vein (tv; B) at vector doses of 1 × 1011 VG/mouse (A and B) or 1 × 1010 VG/mouse (C). Data represent average IgG1 anti-hF.IX titers ± SD for n = 4 mice per serotype (AAV-2, diamonds; AAV-8, squares). Vertical arrows indicate the time point of challenge by subcutaneous administration of hF.IX in cFA. (D and E) Comparison of hF.IX antigen (D) and antibody (E) plateau levels for AAV-2-injected cohorts. Antibody titers after immunization of naive control mice are also shown in (E), and statistically significant suppression of antibodies compared with these controls is indicated (*p < 0.05).
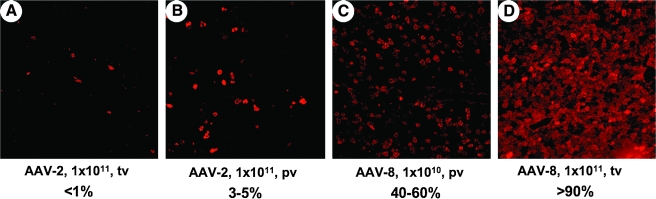
Staining of hF.IX 4 months after hepatic gene transfer showed that high-dose AAV-2 gene transfer resulted in transgene expression in 3–5% of hepatocytes (as expected) after portal vein injection, whereas few hepatocytes had been transduced after tail vein injection (<1%; Fig. 3A and B). On the other hand, AAV-8 gene transfer yielded expression in 40–60 to >90% of hepatocytes, depending on vector dose (Fig. 3C and D). Interestingly, we found that AAV-2-transduced hepatocytes were typically clustered adjacent to areas of liver tissue that had no detectable hF.IX expression (Fig. 3B and E), whereas AAV-8-transduced hepatocytes were uniformly distributed across all liver sections (Fig. 3C and D; and data not shown). This is consistent with findings by Nakai, Kay, and colleagues, who reported unrestricted transduction of murine hepatocytes with AAV-8 (Nakai et al., 2005).
FIG. 3.

Expression of hF.IX in liver tissue of C3H/HeJ mice 4 months after AAV-2 (A, B, and E) or AAV-8 (C and D) vector administration via tail vein (A and D) or portal vein (B, C, and E) at 1 × 1011 VG (A, B, D, and E) or 1 × 1010 VG (C). Immunostaining of hF.IX (red) indicates expression in hepatocytes. Original magnification was ×100 [×40 in (E)]. Range of observed percentages of hF.IX-expressing cells of total hepatocytes is indicated for each combination of serotype, route, and dose. Circles in (E) depict areas negative for hF.IX expression.
Superior tolerance induction in other strains of mice, using AAV-8 vector
AAV-2 vector was administered by tail vein injection into BALB/c and outbred CD-1 mice at a dose of 1 × 1010VG/mouse, which we expected to be insufficient for tolerance induction with this vector. Subtherapeutic hF.IX expression (20–30 ng/ml) was measured in BALB/c mice, whereas CD-1 mice had no detectable hF.IX levels (Fig. 4A and B). On challenge with hF.IX in cFA 8 weeks after gene transfer, both groups of mice formed antibodies to hF.IX (IgG1 and lower titers of IgG2a; Fig. 4C and D, and data not shown). Antibody formation correlated with a loss of systemic hF.IX levels in BALB/c mice (Fig. 4B and D). In contrast, AAV-8 directed persistent high levels of hF.IX expression in both strains (300–600 ng/ml), and no antibodies to hF.IX were found after challenge (Fig. 4A–D and data not shown).
FIG. 4.
Comparison of systemic hF.IX antigen (A and B) and IgG1 anti-hF.IX (C and D) levels in CD-1 (A and C) and BALB/c (B and D) mice as a function of time after vector administration via the tail vein (AAV-2, diamonds; AAV-8, squares; 1 × 1010 VG per serotype). Data represent averages ± SD for n = 4 mice per serotype. Vertical arrows indicate the time point of challenge by subcutaneous administration of hF.IX in cFA.
Induction of regulatory CD4+ T cells
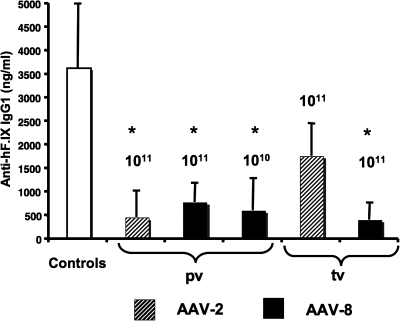
Our previous studies with AAV-2 vectors have shown that hepatic transgene expression induces Tregs that suppress antibody and cellular immune responses, and that these cells are crucial for tolerance to the transgene product (Cao et al., 2007a). To test whether AAV-8-induced immune tolerance is also associated with active T cell-mediated suppression of immune responses to hF.IX, we adoptively transferred CD4+ splenocytes from those C3H/HeJ mice that had detectable hF.IX expression (i.e., high-dose AAV-2 and all AAV-8-transduced groups shown in Fig. 1A–C) to naive mice of the same strain. Recipient mice were subsequently immunized with hF.IX–cFA. Compared with control mice, all recipients of cells from AAV-transduced mice showed suppression of antibody formation (Fig. 5). However, CD4+ cells from AAV-2 tail vein-injected mice suppressed antibody formation against hF.IX to a lesser degree compared with the other groups, which is consistent with less efficient tolerance induction by this combination of route and serotype (see Fig. 2B and E).
FIG. 5.
Evidence of induction of regulatory CD4+ T cells. CD4+ splenocytes from C3H/HeJ mice injected with AAV-2 (hatched columns) or AAV-8 (solid columns) via the portal vein (pv) or tail vein (tv) (indicated vector dose, 1 × 1010 or 1 × 1011 VG), or from naive control C3H/HeJ mice, had been adoptively transferred to naive C3H/HeJ mice. Recipient mice were immunized with hF.IX in cFA 24 hr later. Shown are IgG1 anti-hF.IX titers 3 weeks after immunization. Data represent averages ± SD for n = 6 mice per cohort of recipient mice. *Statistically significant difference compared with controls (p < 0.05).
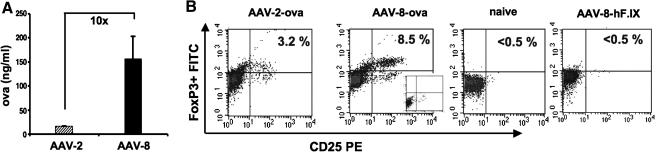
Next, we sought to measure induction of transgene product-specific Tregs in a more precise and quantitative fashion, using DO11.10-tg Rag-2−/− BALB/c mice (Cao et al., 2007a). These mice express a transgenic ovalbumin-specific T cell receptor on the surface of conventional CD4+ T cells, but are deficient in endogenous, rearranged T cell receptors and in Tregs. AAV-2 or AAV-8 vector expressing ovalbumin was injected into portal veins of these mice at 5 × 1011 VG/mouse, resulting in approximately 10-fold higher circulating OVA expression for AAV-8 compared with AAV-2 gene transfer (Fig. 6A). Consistent with our previous studies, AAV-2 gene transfer induced a splenic Treg population that was detectable by flow cytometry (Fig. 6B) (Cao et al., 2007a). The frequency of Tregs was on average 4-fold above background stain in untreated (naive) mice (Fig. 6C). AAV-8-transduced mice showed more robust induction of CD4+CD25+FoxP3+ T cells (Fig. 6B and C), with a 3.5-fold higher average frequency compared with AAV-2 (15-fold above background; Fig. 6C). Tregs were undetectable in mice transduced with AAV-8 vector expressing hF.IX (Fig. 6B and C), indicating that OVA transgene expression rather than the vector itself induced Tregs.
FIG. 6.
Induction of transgene product-specific CD4+CD25+FoxP3+ Tregs. DO11.10-tg Rag-2−/− mice were injected with AAV-2 and AAV-8 vectors expressing OVA (5 × 1011 VG/mouse via the portal vein), and systemic OVA levels were measured at week 8 (A). Subsequently, the percentage of CD25+FoxP3+ splenic CD4+ cells was determined by flow cytometry (B and C). (B) Representative examples of CD25+FoxP3+ cell frequencies (gated on CD4+ cells) for an AAV-2 or AAV-8-OVA-transduced mouse, a naive control mouse, and an AAV-8-hF.IX-transduced control mouse. Insert for AAV-8-OVA-transduced mouse represents isotype control staining. (C) Average frequencies (±SD) of CD25+FoxP3+ cells among CD4+ cells from naive, AAV-2-transduced or AAV-8-transduced naive mice (n = 5 per group), or AAV-hF.IX-transduced mice (n = 4). Fold increases compared with naive mice are indicated. Note that the difference between AAV-2 and −8 is statistically significant (p < 0.05).
Sustained correction of murine hemophilia B in C3H/HeJ mice
After having shown that AAV-8 vectors were more efficient in tolerance induction to hF.IX in wild-type mice and in induction of transgene product-specific Tregs, we performed hF.IX gene transfer experiments in C3H/HeJ F9−/− mice. As noted previously, we had repeatedly found an inhibitor response to F.IX after high-dose hepatic AAV-2 gene transfer in ≥80% of these mice, using the same hepatocyte-specific expression cassette (published and unpublished data) (Mingozzi et al., 2003; Cao et al., 2006). Here, three of three mice transduced with high-dose AAV-8 (1 × 1011 VG/mouse, tail vein injection) showed sustained and complete correction of hemophilia B for the duration of the experiment (7 months; Fig. 7A and B). At a 10-fold lower dose (1 × 1010 VG/mouse), sustained partial correction (7 months) was observed in four of four mice, with levels of expression well in the therapeutic range (400–600 ng/ml or 8–12% of normal human levels; Fig. 7A and B). At the lowest dose tested (2 × 109 VG/mouse), expression was 30–80 ng/ml in two of three mice (i.e., approximately 1% of normal human levels, the minimal therapeutic target for prophylactic treatment of hemophilia; Fig. 7A). These two animals continued to express hF.IX for at least 6 months without antibody formation even after hF.IX–cFA challenge at 5 months (Fig. 7A–C). The same observation was made in three mid-dose mice (1 × 1010 VG/mouse) that were challenged in identical fashion 7 months after gene transfer and analyzed again at 8 months (Fig. 7A–C).
FIG. 7.
Treatment of hemophilia B (C3H/HeJ F9−/−) mice by hepatic AAV-8-hF.IX gene transfer at doses of 2 × 109 (dotted lines, n = 6), 1 × 1010 (dashed lines, n = 4), or 1 × 1011 (solid lines, n = 3) VG/mouse. Shown are systemic hF.IX levels (A) and coagulation times (aPTT; B) as a function of time after vector administration via tail vein. Each line represents an individual animal. Horizontal lines in (B) mark the range of aPTTs typically measured for normal mouse plasma (25–35 sec) or hemophilia B mouse plasma (>60 sec). (C) Antibody titers (IgG1) against hF.IX as a function of time after vector administration. Note that only one animal of the low-dose cohort formed an antibody (animal depicted by solid squares and dotted line). Numbers above the symbol indicate Bethesda titers for this animal at the respective time points. Vertical arrows in each panel indicate challenge of a subset of mice with hF.IX–cFA (mid-dose-treated mice, n = 3, at 7 months; low-dose mice, n = 2, at 5 months).
None of the AAV-8-transduced C3H/HeJ F9−/− mice discussed so far had detectable antibodies to hF.IX by Bethesda or immunocapture assay (Fig. 7C; and data not shown). However, one mouse of the lowest dose cohort failed to express hF.IX (<3 ng/ml) at all time points measured (1–5 months), and had the longest coagulation times of all treated mice (Fig. 7A and B; mouse depicted with square symbols and dotted line). This mouse had formed an inhibitor (3 BU at 1 month, increasing to 18 BU at 3 months, 2–4 μg of anti-hF.IX IgG1/ml; Fig. 7C). However, when three additional C3H/HeJ F9−/− mice were treated with the lowest vector dose of 2 × 109 VG/mouse, these expressed hF.IX at levels of 30–50 ng/ml for at least 2 months, had partially corrected aPTTs (∼50 sec at 2 months), and failed to form antibodies against hF.IX (Fig. 7A–C).
Discussion
An optimal gene transfer protocol for hemophilia B should provide therapeutic levels of expression while at the same time maintaining tolerance to F.IX. Ideally, the effectiveness of such a protocol should be independent of the underlying F.IX mutation and of other genetic effects. Our previously published data showed that optimized AAV-2 gene transfer to the liver met most but not all of these criteria. Tolerance induction was successful in several strains of mice with an F.IX gene deletion, but induced inhibitors in C3H/HeJ F9−/− mice (Mingozzi et al., 2003). This strain exhibits stronger B and T cell responses to F.IX than other strains, and higher vector doses are required to achieve therapeutic hepatocyte-derived expression (Mingozzi et al., 2003; Zhang et al., 2004; Wang et al., 2005; Xu et al., 2007). Others have shown that besides the underlying F.IX mutation, several additional genetic factors influence the risk of immune responses on gene transfer. Genomic sequences implicated in modulating the immune response to transgene products include polymorphisms in or near the MHC locus and at loci containing cytokine-encoding genes such as interleukin (IL)-10, IL-22, and interferon (IFN)-γ (which affect the function of several T cell subsets, including Th1, Th2, Th17, and CD8+ T cells), chemokine receptors, and others (Lozier, 2009).
Optimal hepatic gene transfer reduces impact of genetic factors on immune responses
The data presented here provide proof-of-principle that it is possible to develop a hepatic gene transfer protocol that confers immune tolerance to F.IX largely independent of genetic effects. Sustained expression and correction of hemophilia B was achieved in animals with an F.IX gene deletion in the context of a genetically unfavorable strain background. These findings underscore the ability of robust hepatocyte-derived expression to induce tolerance to the transgene product. The substantial superiority in transduction of murine hepatocytes of the AAV-8 serotype has not always scaled up to large animals such as canines and nonhuman primates, where expression levels of coagulation factor transgenes were comparable to AAV-2 (Sarkar et al., 2006; Nathwani et al., 2007). Therefore, it is unclear that AAV-8 would be superior for immune tolerance induction in humans. Nonetheless, improvements in expression on gene transfer to livers of rhesus and cynomolgus macaques and neonatal dogs and to canine skeletal muscle have been reported for different transgenes (Gao et al., 2006; Koeberl et al., 2008; Ohshima et al., 2009).
Regardless, AAV-8 gene transfer in mice should be an ideal model with which to study mechanisms of immune tolerance induction and to define parameters for successful protocols that are less influenced by genetic effects. For example, increased transgene expression from the AAV-8 vector correlated with a higher frequency of induced transgene product-specific Tregs, supporting the hypothesis that tolerance induction is facilitated by optimal induction of Tregs. While our published data indicated a requirement for Tregs in tolerance induction by hepatic gene transfer, others have shown that these cells may not only be required but sufficient to prevent certain types of immune responses (Cao et al., 2007a; Hoffman and Herzog, 2008; Luth et al., 2008).
AAV-8 improves efficacy and tolerance induction
Our previous studies have shown that a certain minimal level of F.IX expression is required for immune tolerance (we estimated 30–50 ng/ml) (Mingozzi et al., 2003). At lower levels of expression, antibodies against F.IX may be formed. The lowest AAV-8 dose administered to hemophilia B mice here yielded levels of expression right at this threshold, which may explain why one animal developed an inhibitor. However, previous studies with AAV-2 vectors in the C3H/HeJ F9−/− strain mostly failed to induce tolerance, although administered vector doses were adequate to reach expression levels above the AAV-8 low dose in this current study (Mingozzi et al., 2003; Cao et al., 2006). On the basis of the data collected thus far on AAV-2 and AAV-8 F.IX gene transfer to hemophilia B mice of different strain backgrounds, we conclude that (1) the overall level of hepatocyte-restricted F.IX expression is the most important factor for tolerance induction; and (2) AAV-8 vectors offer additional advantages that help tip the balance toward tolerance. Hence, tolerance induction was successful over a wide range of vector doses and expression levels in 80–100% of C3H/HeJ F9−/− mice.
Several parameters may be responsible for improved tolerance induction by AAV-8
It is conceivable that expression levels relative to the number of injected vector particles are important, as hepatocyte-derived transgene expression is tolerogenic whereas viral particles elicit innate and adaptive responses. Interestingly, hepatic innate responses to AAV-2 vector particles are weak, and neither serotype is efficient in transduction of antigen-presenting cells in vivo (Zaiss and Muruve, 2005; Vandendriessche et al., 2007). We found that both serotypes caused equivalent IgG2a formation to vector particles, suggesting similarly strong B cell responses to the vector (data not shown). However, Wilson and colleagues showed that T cell responses to AAV-8 are substantially lower compared with T cell responses to AAV-2 in mice and nonhuman primates (Vandenberghe et al., 2006; Wang et al., 2007). There are also distinct differences between these serotypes in kinetics and distribution of hF.IX expression. AAV-8 vectors uncoat faster and express high levels early after gene transfer, whereas AAV-2-derived expression increases gradually during the first month after vector administration (Thomas et al., 2004). In addition, our data show an even distribution of transgene-expressing hepatocytes with AAV-8, whereas AAV-2-transduced hepatocytes are more clustered with large areas devoid of expression. It is conceivable that rapid onset of expression or optimal distribution of F.IX antigen in the liver facilitates antigen presentation in a tolerogenic hepatic microenvironment.
In summary, efficient gene transfer to hepatocytes can be exploited to design an optimal protocol for the treatment of hemophilia B, resulting in induction of immune tolerance largely independent of genetic factors. Future studies will determine more precisely the parameters required for tolerance in such an optimal protocol.
Acknowledgments
This work was supported by NIH grants R01 AI/HL51390 to R.W.H., P01 HL078810 to C.T. and R.W.H., and by T32DK074367 (support for S.N.). B.E.H. and O.C. are supported by a fellowship and a Scientist Development Grant from the American Heart Association.
Author Disclosure Statement
M. Cooper performed most of the experiments, compiled the data, and wrote parts of the manuscript. N. Nayak, O. Cao, and B.E. Hoffman assisted with experiments and analyses (vector administration and flow cytometry). C. Terhorst advised on Treg experiments. O. Cao helped design experiments. R.W. Herzog designed experiments, supervised the study, and wrote parts of the manuscript.
References
- Brown B.D. Cantore A. Annoni A. Sergi L.S. Lombardo A. Della Valle P. D'Angelo A. Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- Cao O. Armstrong E. Schlachterman A. Wang L. Okita D.K. Conti-Fine B. High K.A. Herzog R.W. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O. Dobrzynski E. Wang L. Nayak S. Mingle B. Terhorst C. Herzog R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007a;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O. Furlan-Freguia C. Arruda V.R. Herzog R.W. Emerging role of regulatory T cells in gene transfer. Curr. Gene Ther. 2007b;7:381–390. doi: 10.2174/156652307782151506. [DOI] [PubMed] [Google Scholar]
- Cerullo V. McCormack W. Seiler M. Mane V. Cela R. Clarke C. Rodgers J.R. Lee B. Antigen-specific tolerance of human α1-antitrypsin induced by helper-dependent adenovirus. Hum. Gene Ther. 2007;18:1215–1224. doi: 10.1089/hum.2006.036. [DOI] [PubMed] [Google Scholar]
- Dobrzynski E. Mingozzi F. Liu Y.L. Bendo E. Cao O. Wang L. Herzog R.W. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- Dobrzynski E. Fitzgerald J.C. Cao O. Mingozzi F. Wang L. Herzog R.W. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P.A. Kowalczyk D.W. Arruda V.R. McCleland M.L. Hagstrom J.N. Pasi K.J. Ertl H.C. Herzog R.W. High K.A. Choice of vector determines T cell subsets involved in immune responses against the secreted transgene product factor IX. Mol. Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- Fields P.A. Arruda V.R. Armstrong E. Chu K. Mingozzi F. Hagstrom J.N. Herzog R.W. High K.A. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol. Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Gao G. Lu Y. Calcedo R. Grant R.L. Bell P. Wang L. Figueredo J. Lock M. Wilson J.M. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L. Calcedo R. Johnston J. Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog R.W. Recent advances in hepatic gene transfer: More efficacy and less immunogenicity. Curr. Opin. Drug Discov. Dev. 2005;8:199–206. [PubMed] [Google Scholar]
- Herzog R.W. Dobrzynski E. Immune implications of gene therapy for hemophilia. Semin. Thromb. Hemost. 2004;30:215–226. doi: 10.1055/s-2004-825635. [DOI] [PubMed] [Google Scholar]
- Herzog R.W. Hagstrom J.N. Kung S.H. Tai S.J. Wilson J.M. Fisher K.J. High K.A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B.E. Herzog R.W. Coaxing the liver into preventing autoimmune disease in the brain. J. Clin. Invest. 2008;118:3271–3273. doi: 10.1172/JCI37079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B.E. Dobrzynski E. Wang L. Hirao L. Mingozzi F. Cao O. Herzog R.W. Muscle as a target for supplementary factor IX gene transfer. Hum. Gene Ther. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- Koeberl D.D. Pinto C. Sun B. Li S. Kozink D.M. Benjamin D.K., Jr. Demaster A.K. Kruse M.A. Vaughn V. Hillman S. Bird A. Jackson M. Brown T. Kishnani P.S. Chen Y.T. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol. Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Lin H.F. Maeda N. Smithies O. Straight D.L. Stafford D.W. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- Liu Y.L. Wagner K. Robinson N. Sabatino D. Margaritis P. Xiao W. Herzog R.W. Optimized production of high-titer recombinant adeno-associated virus in roller bottles. Biotechniques. 2003;34:184–189. doi: 10.2144/03341dd07. [DOI] [PubMed] [Google Scholar]
- Lozier J. Genetics of the immune response to proteins expressed after gene transfer. In: Herzog R.W., editor. Gene Therapy Immunology. Wiley-Blackwell; Hoboken, NJ: 2009. pp. 289–310. [Google Scholar]
- Luth S. Huber S. Schramm C. Buch T. Zander S. Stadelmann C. Bruck W. Wraith D.C. Herkel J. Lohse A.W. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J. Clin. Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R. Glader B. Ragni M. Rasko J.J. Ozelo M.C. Hoots K. Blatt P. Konkle B. Dake M. Kaye R. Razavi M. Zajko A. Zehnder J. Rustagi P.K. Nakai H. Chew A. Leonard D. Wright J.F. Lessard R.R. Sommer J.M. Tigges M. Sabatino D. Luk A. Jiang H. Mingozzi F. Couto L. Ertl H.C. High K.A. Kay M.A. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Schuttrumpf J. Arruda V.R. Liu Y. Liu Y.L. High K.A. Xiao W. Herzog R.W. Improved hepatic gene transfer by using an adeno-associated virus serotype 5 vector. J. Virol. 2002;76:10497–10502. doi: 10.1128/JVI.76.20.10497-10502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. Liu Y.L. Dobrzynski E. Kaufhold A. Liu J.H. Wang Y. Arruda V.R. High K.A. Herzog R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount J.D. Herzog R.W. Tillson D.M. Goodman S.A. Robinson N. McCleland M.L. Bellinger D. Nichols T.C. Arruda V.R. Lothrop C.D., Jr. High K.A. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Nakai H. Fuess S. Storm T.A. Muramatsu S. Nara Y. Kay M.A. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J. Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J. Ng C.Y. Zhou J. Spence Y. Cochrane M. Gray E. Tuddenham E.G. Davidoff A.M. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima S. Shin J.H. Yuasa K. Nishiyama A. Kira J. Okada T. Takeda S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol. Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini M.A. Bu J. Fidler J.A. Ziegler R.J. Foley J.W. Dodge J.C. Yang W.W. Clarke J. Taksir T.V. Griffiths D.A. Zhao M.A. O'Riordan C.R. Schuchman E.H. Shihabuddin L.S. Cheng S.H. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R. Mucci M. Addya S. Tetreault R. Bellinger D.A. Nichols T.C. Kazazian H.H., Jr. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia A dogs and mice. Hum. Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- Sun B. Bird A. Young S.P. Kishnani P.S. Chen Y.T. Koeberl D.D. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.E. Storm T.A. Huang Z. Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Wang L. Somanathan S. Zhi Y. Figueredo J. Calcedo R. Sanmiguel J. Desai R.A. Chen C.S. Johnston J. Grant R.L. Gao G. Wilson J.M. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Vandendriessche T. Thorrez L. Acosta-Sanchez A. Petrus I. Wang L. Ma L. DE Waele L. Iwasaki Y. Gillijns V. Wilson J.M. Collen D. Chuah M.K. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- Wang L. Cao O. Swalm B. Dobrzynski E. Mingozzi F. Herzog R.W. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Wang L. Figueredo J. Calcedo R. Lin J. Wilson J.M. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Xu L. Mei M. Haskins M.E. Nichols T.C. O'Donnell P. Cullen K. Dillow A. Bellinger D. Ponder K.P. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb. Res. 2007;120:269–280. doi: 10.1016/j.thromres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Zaiss A.K. Muruve D.A. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- Zaiss A.K. Muruve D.A. Immunity to adeno-associated virus vectors in animals and humans: A continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Zhang J. Xu L. Haskins M.E. Parker Ponder K. Neonatal gene transfer with a retroviral vector results in tolerance to human factor IX in mice and dogs. Blood. 2004;103:143–151. doi: 10.1182/blood-2003-06-2181. [DOI] [PubMed] [Google Scholar]