Abstract
Oncolytic adenoviruses are anticancer agents that replicate within tumors and spread to uninfected tumor cells, amplifying the anticancer effect of initial transduction. We tested whether coating the viral particle with polyethylene glycol (PEG) could reduce transduction of hepatocytes and hepatotoxicity after systemic (intravenous) administration of oncolytic adenovirus serotype 5 (Ad5). Conjugating Ad5 with high molecular weight 20-kDa PEG but not with 5-kDa PEG reduced hepatocyte transduction and hepatotoxicity after intravenous injection. PEGylation with 20-kDa PEG was as efficient at detargeting adenovirus from Kupffer cells and hepatocytes as virus predosing and warfarin. Bioluminescence imaging of virus distribution in two xenograft tumor models in nude mice demonstrated that PEGylation with 20-kDa PEG reduced liver infection 19- to 90-fold. Tumor transduction levels were similar for vectors PEGylated with 20-kDa PEG and unPEGylated vectors. Anticancer efficacy after a single intravenous injection was retained at the level of unmodified vector in large established prostate carcinoma xenografts, resulting in complete elimination of tumors in all animals and long-term tumor-free survival. Anticancer efficacy after a single intravenous injection was increased in large established hepatocellular carcinoma xenografts, resulting in significant prolongation of survival as compared with unmodified vector. The increase in efficacy was comparable to that obtained with predosing and warfarin pretreatment, significantly extending the median of survival. Shielding adenovirus with 20-kDa PEG may be a useful approach to improve the therapeutic window of oncolytic adenovirus after systemic delivery to primary and metastatic tumor sites.
Introduction
Adenoviral vectors are used as an experimental approach for the treatment of cancer. Oncolytic adenoviral vectors are designed to replicate within tumor cells, with the progeny virions spreading to initially uninfected tumor cells, thus amplifying efficacy (Liu et al., 2007, 2008). In clinical trials the first oncolytic adenovirus, ONYX-015, replication of which was rendered specific or preferential to p53-negative cells as opposed to normal cells by a mutation in E1B 55K, demonstrated an acceptable level of safety, but antitumor efficacy was not observed when it was used in single-agent regimens (Kirn, 2001). An oncolytic adenovirus, H101 (Oncorine, similar to ONYX-015 but carrying a deletion of E3 region), was approved for clinical use in China to treat head-and-neck cancer patients as intratumoral injections in combination with cisplatin (Garber, 2006; Yu and Fang, 2007).
At present, oncolytic adenoviruses are used as intratumoral injections to treat accessible lesions. Theoretically, these self-amplifying anticancer agents would be most useful to treat metastatic disease. However, the innate hepatic tropism of human adenovirus type 5 (Ad5) after intravenous injection makes its use as a systemic therapeutic impractical. More than 90% of systemically injected adenovirus is taken up and destroyed by liver macrophages (Kupffer cells) (Worgall et al., 1997; Alemany et al., 2000), thus reducing the dose delivered to the target tumor site(s). Viral particles that escape Kupffer cells are taken up by hepatocytes, where unspecific viral replication results in dose-limiting hepatotoxicity that prevents the use of high doses of oncolytic adenovirus for systemic treatment (Nemunaitis et al., 2001; Reid et al., 2002; Small et al., 2006). If oncolytic adenovirus could be detargeted from hepatocytes and Kupffer cells, this should reduce liver toxicity and increase the ability of the virus to reach distant tumor sites after intravenous injection. Liver tropism is also a problem after intratumoral injection. Intratumoral injection of oncolytic adenovirus results in liver infection due to the leak of virus into the blood from the injection site (Lohr et al., 2001; Sauthoff et al., 2003; Wang et al., 2006; Dhar et al., 2009). Therefore, liver detargeting should also benefit intratumoral approaches, by reducing untoward liver toxicity.
Discovery of the hexon-mediated pathway of liver transduction through interaction of adenoviral capsid with blood-coagulation factor X (Kalyuzhniy et al., 2008; Waddington et al., 2008) opens an opportunity to interfere with this pathway to achieve a wider therapeutic window of the treatment with oncolytic adenovirus. We have demonstrated that replication-competent adenovirus can be successfully detargeted from liver by a combination of macrophage depletion achieved with adenoviral capsid predosing and anticoagulation treatment with warfarin (Shashkova et al., 2008a). Importantly, these liver-detargeting strategies resulted in improved systemic anticancer activity of a single intravenous injection of adenovirus (Shashkova et al., 2008a). As warfarin has a narrow therapeutic window and Kupffer cell depletion results in a cytokine storm, alternative strategies to detarget liver cells are required to improve the efficacy of systemically delivered oncolytic adenovirus.
Covalent linking of adenoviral particles with polymers of polyethylene glycol (PEG) or poly-N-(2-hydroxypropyl)methacrylamide (HPMA) was previously reported to have a beneficial effect on pharmacological properties of adenoviral vectors (Kreppel and Kochanek, 2008; Morrison et al., 2008; Carlisle et al., 2009). PEG is approved for clinical use as a component of various drug formulations and is used to modify the pharmacological properties of protein therapeutics. Covalent attachment of PEG (PEGylation) has been shown to extend the half-life of recombinant proteins in humans and to improve their efficacy (Harris and Chess, 2003; Fishburn, 2008; Ryan et al., 2008). This approach was also used in preclinical studies to improve pharmacological properties of recombinant adenoviruses (Croyle et al., 2001, 2005; Mok et al., 2005; Hofherr et al., 2008; Weaver and Barry, 2008; Wortmann et al., 2008). PEGylation reduces innate and adaptive immune responses to adenovirus and reduces viral binding to platelets, red blood cells, and Kupffer cells. Comparison of low and high molecular weight (MW) PEGs has shown that the larger polymers have better properties for both recombinant proteins and adenoviruses (Hofherr et al., 2008; Kreppel and Kochanek, 2008; Weaver and Barry, 2008; Wortmann et al., 2008). In particular, it was observed that conjugation of large 20-kDa PEG to replication-defective Ad5 markedly reduced liver infection after intravenous injection (Hofherr et al., 2008). We tested whether PEGylation of oncolytic adenovirus with small 5-kDa PEG and large 20-kDa PEG would improve its efficacy–toxicity profile after systemic administration in tumor-bearing animals.
Materials and Methods
Viral vectors and cell lines
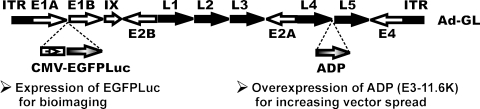
Ad-GL is a replication-competent adenovirus expressing an enhanced green fluorescent protein–firefly luciferase fusion protein (EGFPLuc) (Shashkova et al., 2008a) (Fig. 1). Ad-DsRed is a replication-defective first-generation adenoviral vector with deletions in the E1 and E3 regions that expresses the DsRed red fluorescent protein. Viruses were grown in spinner cultures of KB cells (Ad-GL) or N3S cells (Ad-DsRed). KB spinner cells were kindly provided by William S.M. Wold (St. Louis University School of Medicine, St. Louis, MO) and N3S cells were from Microbix (Toronto, ON, Canada). Vectors were extracted and purified by CsCl banding as described previously (Tollefson et al., 2007). Human hepatocellular carcinoma Hep3B, human prostate carcinoma LNCaP, and human lung adenocarcinoma A549 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS; HyClone, Logan, UT).
FIG. 1.
In Vitro Properties of Adenovirus Conjugated to 5-kDa and 20-kDa Polyethylene Glycol
PEGylation of adenovirus with 5-kDa or 20-kDa PEG
PEGylation of viruses was performed as described previously (Mok et al., 2005). After CsCl centrifugation, the CsCl was removed from adenovirus preparations by passage through Econo-Pac 10DG desalting columns (Bio-Rad, Hercules, CA), replacing the CsCl buffer with KPBS (136 mM NaCl, 2.6 mM KCl, 1.7 mM KH2PO4, 10 mM K2HPO4). The 5-kDa succinimide-activated PEG (Sunbright ME-050HS; NOF America, White Plains, NY) and 20-kDa succinimide-activated PEG (m-SCM-20K PEG; JenKem Technology, Allen, TX) were equilibrated to room temperature. Each type of PEG was added to the respective virus sample at a concentration of 10 mg per 1 × 1012 viral particles per ml of reaction mixture. Each virus–PEG solution was rotated at room temperature for 1 hr. After this incubation, the unbound PEG was removed by passage through a Sephadex G-100 (GE Healthcare, Piscataway, NJ) size-exclusion column. The mock-treated adenovirus was treated in the same manner, but no PEG was added. Vector concentration was measured by quantitative real-time polymerase chain reaction (qPCR) and expressed as viral genomes (VG) per milliliter (Hofherr et al., 2008). We use qPCR as the method of choice for assessing concentration of PEGylated adenoviral vectors because OD260 assay results and infectious titers are affected by PEGylation.
In vitro characterization of PEGylated vectors
Particle sizing was performed with a 90Plus/BI-MAS particle sizer (Brookhaven Instruments, Holtsville, NY) in accordance with the manufacturer's instructions. Particle size and polydispersity are shown as means and standard error (SE) between triplicate runs and were calculated by using the instrument's software. Molecular weights were calculated from Mark–Houwink–Sakurada equation.
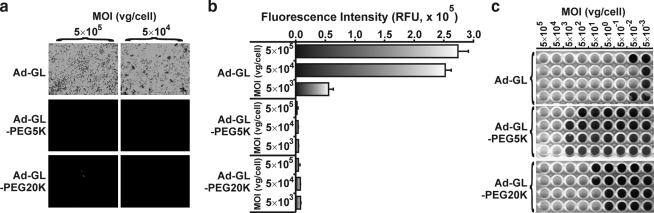
To characterize in vitro transduction properties of PEGylated vectors, A549 cells were plated at semiconfluency in 96-well plates. Twenty-four hours later, PEGylated or mock-PEGylated vectors were serially diluted 10-fold and added to monolayers of A549 cells at the indicated multiplicity of infection (MOI). Twenty-four hours later, green fluorescence resulting from EGFPLuc expression was assessed by fluorescence microscopy and microphotographs were taken. At the same time point, fluorescence in monolayers was quantified in quadruplicate by scanning the plates with a microplate reader (DTX 800 multimode detector; Beckman Coulter, Fullerton, CA). To assess the ratio of viral genomes to infectious units, A549 monolayers infected with serial dilutions of vectors were stained with crystal violet 19 days after infection and infectious titers were determined by the Spearman–Karber method.
Animal tumor models
Female nu/nu mice (4 weeks old; Harlan Sprague Dawley, Indianapolis, IN) were injected subcutaneously with 1 × 107 Hep3B cells, or with 1 × 107 LNCaP cells in 100 μl of DMEM–50% Matrigel (BD Biosciences, San Jose, CA). Thirty days later (LNCaP model) or 21 days later (Hep3B model) animals with established tumors (average tumor volume, 202 mm3 for LNCaP tumors and 545 mm3 for Hep3B tumors) were randomized and assigned to treatment groups. Mice were injected intravenously via the tail vein with a single dose of 100 μl of phosphate-buffered saline (PBS), 5 × 1010 VG of Ad-GL, or 5 × 1010 VG of PEGylated Ad-GL. Adenovirus predosing and warfarinization were performed in a subset of mice in the Hep3B tumor model before Ad-GL administration as described previously (Shashkova et al., 2008a). For predosing, mice were injected intravenously with 3 × 1010 VG of Ad-DsRed in 100 μl of PBS 4 hr before Ad-GL injection. For warfarinization, mice were injected subcutaneously with 133 μg of warfarin (Sigma-Aldrich, St. Louis, MO) in 100 μl of peanut oil 3 and 1 days before Ad-GL injection (Parker et al., 2006). Noninvasive whole body bioluminescence imaging of luciferase expression was performed at the indicated time points as in Shashkova and colleagues (2008a). Tumor dimensions were taken with calipers and tumor volumes were calculated as width2 × length × 1/2. Mice were euthanized when the tumor volume reached 2000 mm3.
Bioluminescence imaging
Animals were anesthetized with ketamine and xylazine and injected intraperitoneally with 100 μl of d-luciferin (20 mg/ml; Molecular Imaging Products, Bend, OR), and were imaged with a Kodak In-Vivo Imaging System F (Carestream Health, Rochester, NY) for 5 min. Images were processed and analyzed with Kodak imaging software (Carestream Health).
Real-time PCR for quantification of vector biodistribution
Nude mice bearing subcutaneous Hep3B tumors (average volume, 500 mm3) were injected intravenously via the tail vein with a single dose of 100 μl of PBS, 5 × 1010 VG of Ad-GL, or 5 × 1010 VG of PEGylated Ad-GL. At 24 hr after injection, animals (n = 3 per group) were euthanized and tissues (blood, tumor, liver, and spleen) were collected. DNA from tissue samples was prepared with DNeasy blood and tissue kits (Qiagen, Valencia, CA). qPCR was performed with QuantiTect SYBR green PCR master mix and primers specific for firefly luciferase (GGATTCTAAAACGGATTACCAGGG and CAGTTCTATGAGGCAGAGCGACAC). Standard curves were generated with pEGFPLuc (Invitrogen, Carlsbad, CA) diluted in purified DNA from mock-injected animals. qPCR was carried out on an ABI 7900HT (Applied Biosystems, Foster City, CA) in absolute quantification mode. The results were analyzed with ABI analysis software.
Hepatotoxity and Kupffer cell uptake
To measure the hepatotoxicity levels, female C57BL/6 mice (4–6 weeks old; Jackson Laboratory, Bar Harbor, ME) were injected intravenously via the tail vein with 1011 VG of Ad-GL or PEGylated Ad-GL. On day 3 after injection, serum samples were collected and alanine aminotransferase (ALT) levels were measured with an ALT kit as described by the manufacturer (BioTron Diagnostics, Hemet, CA).
To study the effect of PEGylation on uptake of the adenovirus by Kupffer cells, outbred female ICR mice (6 weeks old; National Cancer Institute, Rockville, MD) were injected with 100 μl of PBS, 3 × 1010 VG of Ad-DsRed, or 3 × 1010 VG of PEGylated Ad-DsRed 4 hr before injection with 3 × 1010 VG of Ad-GL. On day 1 after Ad-GL injection, expression of luciferase was measured by bioluminescence imaging.
Statistical analysis
The statistical significance was estimated with repeated measures analysis of variance (ANOVA) or one-way ANOVA followed by Tukey's HSD (honestly significantly different) test for pair-wise comparisons between multiple groups. Kaplan–Meier survival curves were plotted and analyzed with the log-rank test. p < 0.05 was considered significant.
Results
In vitro characterization of PEGylated vectors
We sought to assess whether PEGylation with high molecular weight PEG could improve the in vivo efficacy–toxicity profile of oncolytic adenoviral vectors. We used replication-competent Ad-GL, which was previously described as Ad-EGFPLuc (Shashkova et al., 2008a) (Fig. 1). This vector expresses enhanced green fluorescent protein–firefly luciferase fusion protein (EGFPLuc) at the early and late stages of adenoviral infection from the human cytomegalovirus (HCMV) immediate-early (IE) promoter and overexpresses Ad death protein (E3-11.6 K) for improved vector release and spread (Tollefson et al., 1996; Doronin et al., 2000, 2003). We hypothesized that PEGylation with high molecular weight PEG would increase adenoviral particle diameter such that transduction of hepatocytes would be prevented or reduced. At the same time, we expected that reduction of entrapment by Kupffer cells might occur with PEGylated vectors, resulting in increased efficacy as we have previously observed with depletion of Kupffer cells by adenovirus predosing in combination with anticoagulation (warfarin) treatment (Shashkova et al., 2008a).
It was previously reported that PEGylation reduces or ablates transduction of cells in vitro (Mok et al., 2005; Hofherr et al., 2008; Weaver and Barry, 2008). In accord with this observation, Ad-GL PEGylated with either 5-kDa or 20-kDa PEG had reduced ability to transduce A549 cells (Fig. 2). The ratio of viral genomes to infectious units was increased by five orders of magnitude for Ad-GL-PEG5K and by four orders of magnitude for Ad-GL-PEG20K as compared with mock-PEGylated Ad-GL (Table 1). Particle diameters and predicted molecular weight (Table 1) were found to be in accord with previously published estimates (Hofherr et al., 2008), indicating that Ad-GL was PEGylated as expected. In addition, we performed transmission electron microscopy on PEGylated and mock-PEGylated samples of vectors to control for integrity of virions in preparations and to rule out physical aggregation of particles (clumping), and found that PEGylation indeed did not cause aggregation or damage of virions (data not shown). It was previously reported that precipitation of adenovirus could take place when the concentration of PEG used in the reaction exceeded 10% (w/v); however, at the concentration of PEG we used in the PEGylation reaction (1%, w/v), precipitation of adenovirus is not expected to occur (O'Riordan et al., 1999).
FIG. 2.
In Vitro Properties of Adenovirus Conjugated to 5-kDa and 20-kDa Polyethylene Glycol
Table 1.
In Vitro Properties of Adenovirus Conjugated to 5-kDa and 20-kDa Polyethylene Glycol
| Ad-GL | Ad-GL-PEG5K | Ad-GL-PEG20K | |
|---|---|---|---|
| Viral genomesa/infectious unitsb ratio | 2.41 × 102 | 8.68 × 107 | 3.95 × 106 |
| Effective diameter (nm) | 110.4 ± 1.6 | 122.8 ± 0.9 | 138.5 ± 1.8 |
| Polydispersity | 0.063 ± 0.04 | 0.173 ± 0.008 | 0.122 ± 0.022 |
| Calculated molecular weight (×107 g/mol)c | 3.58 | 4.61 | 6.11 |
Viral genome concentration measured by qPCR.
Titer determined by limiting dilution assay in A549 cells and calculated by the Spearman–Karber method.
Determined using the Mark–Houwink–Sakurada equation.
Effect of PEGylation with 5-kDa and 20-kDa PEG on liver and tumor transduction in LNCaP xenograft tumor model
Ad-GL does not have cancer-restricting features and causes severe hepatotoxicity in mice at doses over 3 × 109 plaque-forming units per animal after intravenous administration, due to viral replication in liver (Duncan et al., 1978; Shashkova et al., 2007). We evaluated the influence of PEGylation with 5-kDa and 20-kDa PEG on liver and tumor transduction in LNCaP prostate carcinoma tumors grown as xenografts in nu/nu mice.
After a single intravenous injection, PEGylation with either 5-kDa or 20-kDa PEG markedly influenced liver transduction on days 1 and 3 (Fig. 3a). As was observed previously with replication-deficient adenoviral vector (Hofherr et al., 2008), modification with 5-kDa PEG increased expression of the reporter gene in liver, whereas modification with 20-kDa PEG reduced expression. All animals injected with mock-PEGylated Ad-GL or with Ad-GL-PEG20K survived for the duration of the experiment (250 days), whereas five of seven animals injected with Ad-GL-PEG5K died or were found moribund and were euthanized on days 3–5 after injection. This lethality/morbidity was presumably caused by hepatotoxicity mediated by adenoviral replication in mouse liver (Duncan et al., 1978; Shashkova et al., 2007).
FIG. 3.
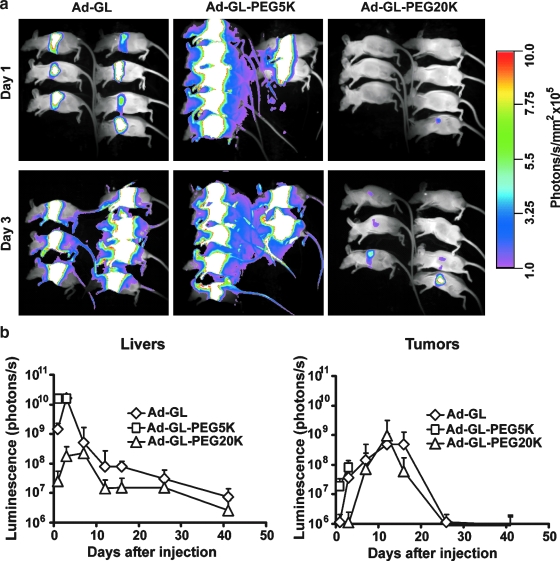
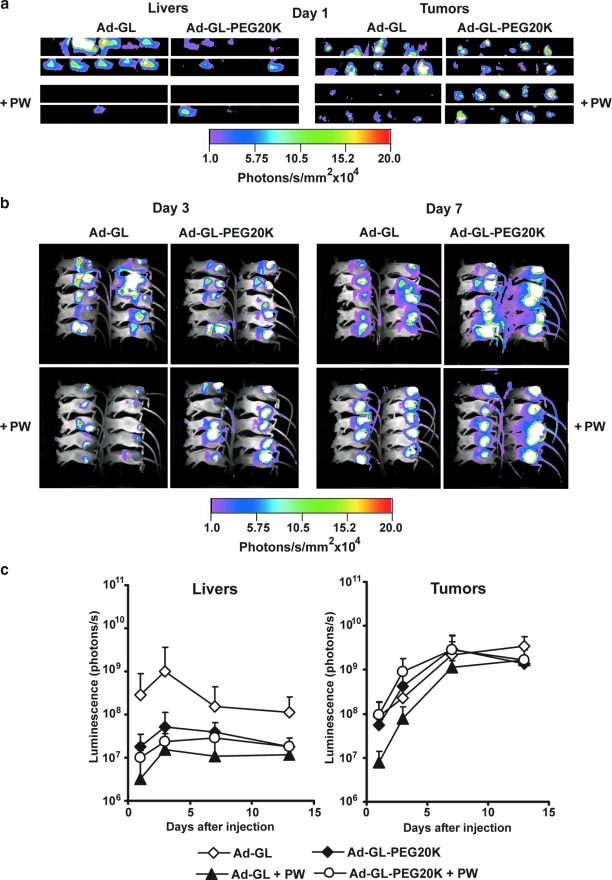
Effect of PEGylation on liver and tumor transduction in LNCaP xenograft model after systemic injection of replication-competent adenovirus. (a) PEGylation with 20-kDa PEG but not with 5-kDa PEG reduces transduction of liver in nu/nu mice bearing LNCaP tumor xenografts. Mice (n = 7 per group) with established tumors (30 days; average volume, 202 mm3) were injected intravenously with a single dose of 5 × 1010 VG of Ad-GL or PEGylated Ad-GL in 100 μl of PBS. Mice were imaged for luciferase expression on days 1 and 3 after virus injection. (b) Quantification of the effect of PEGylation on the levels and kinetics of luciferase expression in mice bearing LNCaP tumors after injection of Ad-GL. The luminescence signal in liver and tumor of mice was measured and quantified on days 1, 3, 7, 12, 16, 26, and 41 after Ad-GL injection. Data are shown as means and SD. Color images available online at www.liebertonline.com/hum.
Quantification of luciferase bioluminescence from liver and tumor (Fig. 3b) demonstrated that in liver the signal peaked on day 3 after injection and declined on days 7 and 12 with consequent slow reduction of the signal on days 16, 26, and 41, consistent with the kinetics of abortive viral replication (Duncan et al., 1978). The reduction of bioluminescence signal in the PEGylated Ad-GL-PEG20K group versus the mock-PEGylated Ad-GL group was 57-fold on day 1, 90-fold on day 3, 2.3-fold on day 7, 5.5-fold on day 12, 5.1-fold on day 16, 1.9-fold on day 26, and 2.8-fold on day 41. For Ad-GL-PEG5K, on day 1 there was an 11.8-fold increase in expression signal from liver versus mock-PEGylated Ad-GL. There was no additional increase in the signal from mouse liver injected with Ad-GL-PEG5K on day 3 as compared with day 1 levels or as compared with Ad-GL-mediated levels on day 3. In tumor the signal peaked on day 12 and returned to the initial day 1 level by day 26.
Analysis by repeated measures ANOVA of bioluminescence data from liver and tumor on days 1–3 indicated a significant difference between groups (p < 0.001). Post-hoc analysis by Tukey's HSD test of the data for liver on days 1–3 has shown a significant difference between groups (p < 0.001 for all pair-wise comparisons for liver). Post-hoc analysis by Tukey's HSD test of the data for tumor on days 1–3 indicated that the Ad-GL-PEG5K group was significantly different from the other groups (p = 0.047, Ad-GL vs. Ad-GL-5K; p = 0.001, Ad-GL-20K vs. Ad-GL-5K). The difference for tumor on days 1–3 for Ad-GL versus Ad-GL-20K was not significant (p = 0.154). Analysis by repeated measures ANOVA of bioluminescence data for liver and tumor from the animals injected with mock-PEGylated Ad-GL and Ad-GL-PEG20K indicated a significant difference on days 1–41 between groups for liver (p < 0.001), whereas the signal from tumor was not significantly different (p = 0.85). Additional analysis by one-way ANOVA of bioluminescence data for tumors on separate days after injection detected significant differences on days 1 and 3 (p < 0.002); there was no significant difference on days 7, 12, 16, 26, and 41 (p > 0.2). Post-hoc analysis by Tukey's HSD test of the data on day 1 demonstrated significantly higher expression for the Ad-GL-PEG5K group versus the Ad-GL group (p = 0.006) and versus the Ad-GL-PEG20K group (p = 0.003), whereas there was no significant difference for Ad-GL versus Ad-GL-PEG20K (p = 0.97). On day 3, the expression in tumor for the Ad-GL-PEG5K group was significantly higher versus the Ad-GL-PEG20K group (p = 0.001); the difference for Ad-GL was not significant from either the Ad-GL-PEG5K group (p = 0.114) or the Ad-GL-PEG20K group (p = 0.087).
Effect of PEGylation with high molecular weight (20-kDa) PEG on antitumor efficacy in LNCaP xenograft tumor model
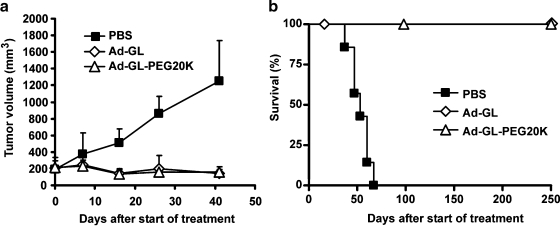
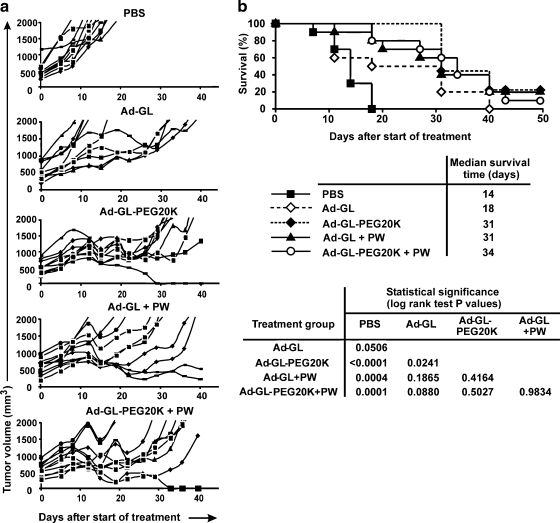
Tumor volumes for animals in the experiment shown in Fig. 3 were monitored (Fig. 4). Tumors in animals injected with buffer continued to grow. Injection with either Ad-GL or Ad-GL-PEG20K reduced tumor size (Fig. 4a). There were significant differences in tumor size between the animals injected with modified or unmodified virus versus the buffer-treated group (days 1–41, repeated measures ANOVA followed by Tukey's HSD test for pair-wise comparisons; p < 0.001). There was no significant difference in tumor size between the Ad-GL and Ad-GL-PEG20K groups (p = 0.99). On day 41 after virus injection, buffer-treated tumors started to cross the threshold of 2000 mm3 and these animals were euthanized. In contrast, tumors in both the Ad-GL and Ad-GL-PEG20K groups were completely eradicated in all animals in this experiment and did not relapse during the observation period of 250 days (Fig. 4b). Survival rates in both the Ad-GL and Ad-GL-PEG20K groups were significantly different from the buffer group (log-rank test; p < 0.0004). The Ad-GL and Ad-GL-PEG20K groups were not significantly different from each other (p value undefined as no uncensored events were detected).
FIG. 4.
Anticancer activity of PEGylated Ad-GL in LNCaP tumor xenografts in nu/nu mice after intravenous administration. (a) Effect of a single intravenous injection of Ad-GL or PEGylated Ad-GL on tumor growth. nu/nu mice (n = 7 per group) bearing established LNCaP tumors (average volume, 202 mm3) in the right hind flank were injected intravenously with a single dose of 5 × 1010 VG of unmodified Ad-GL or Ad-GL PEGylated with 20-kDa PEG. Tumor dimensions were measured with calipers twice per week and tumor volume was calculated as width2 × length × 1/2. Data are shown as means and SD. (b) Effect of a single intravenous injection of Ad-GL or PEGylated Ad-GL on survival. Animals from (a) were euthanized when the tumor volume reached 2000 mm3 (uncensored event) and Kaplan–Meier survival curves were plotted.
Effect of PEGylation with high molecular weight (20-kDa) PEG on liver and tumor transduction in Hep3B xenograft tumor model in comparison with predosing–warfarin treatment
As a second model for assessing the effect of PEGylation on the efficacy and safety of oncolytic adenovirus, we used human Hep3B hepatocellular carcinoma grown as xenografts in nu/nu mice. We have previously described a predosing–warfarin treatment that allowed for reduction of liver transduction in this mouse model and led to increased treatment efficacy (Shashkova et al., 2008a). Predosing mice with an irrelevant adenovirus 4 hr before oncolytic injection kills Kupffer cells, allowing more virus to reach other sites including liver. Warfarin inhibits blood-coagulation factors involved in Ad5 uptake into hepatocytes and reduces infection and toxicity in liver (Shashkova et al., 2008a). We sought to compare PEGylation with a predosing–warfarin regimen and whether a combination of PEGylation with predosing–warfarin treatment would lead to further improvement in the vector efficacy–toxicity profile.
High molecular weight (20-kDa) PEG was chemically conjugated to the surface of Ad-GL. Ad-GL or 20-kDa PEG-modified Ad-GL was injected intravenously once into Hep3B tumor-bearing nu/nu mice with and without predosing–warfarin treatment (Fig. 5). Bioluminescence imaging on day 1, before viral replication and spread took place (Fig. 5a), revealed reduced liver transduction both in mice treated with PEGylated vector and in animals receiving predosing–warfarin pretreatment. Imaging on day 3 after injection (Fig. 5b) demonstrated increased levels of signal from both liver and tumor. On day 7 after vector injection luciferase expression was decreased in liver and continued to increase in tumor (Fig. 5b).
FIG. 5.
Effect of PEGylation with 20-kDa PEG on liver and tumor transduction in Hep3B xenograft tumor model after systemic injection of replication-competent adenovirus. (a) PEGylation with 20-kDa PEG reduces liver transduction in nu/nu mice bearing Hep3B tumor xenografts on day 1 after virus injection. Mice with established tumors (day 21; average volume, 545 mm3) were injected intravenously with a single dose of 5 × 1010 VG of Ad-GL or PEGylated Ad-GL in 100 μl of PBS. +PW, mice were pretreated by predosing (intravenous, 3 × 1010 VG of replication-deficient Ad-DsRed in 100 μl of PBS 4 hr before Ad-GL injection) and administration of warfarin (subcutaneous, 133 μg of warfarin in 100 μl of peanut oil 3 and 1 days before Ad-GL injection). Mice (n = 10 per group) were imaged for luciferase expression on day 1 after virus injection. (b) Decreased liver transduction with retained tumor transduction is maintained on days 3 and 7 after Ad-GL injection. (c) Quantification of the effect of PEGylation on the levels and kinetics of luciferase expression in mice bearing Hep3B tumors after injection of Ad-GL or PEGylated Ad-GL. +PW, mice pretreated with predosing and warfarin. Luminescence signal in liver and tumor of mice was measured and quantified on days 1, 3, 7, and 13 after Ad-GL injection. Data are shown as means and SD. Color images available online at www.liebertonline.com/hum.
Quantification of luciferase bioluminescence from liver and tumor (Fig. 5c) demonstrated that in liver the signal peaked on day 3 after injection and declined on days 7 and 13, consistent with the kinetics of abortive viral replication (Duncan et al., 1978). Predosing–warfarin treatment appeared to be slightly more efficient in reducing liver transduction than PEGylation. Tumor transduction was reduced in the predosing–warfarin group, whereas it appeared to be unaffected in the PEGylation group. Interestingly, PEGylated vector mediated a higher level of tumor transduction when animals received predosing and warfarin treatment. Bioluminescence from liver transduced with PEGylated Ad-GL-PEG20K was 15-fold lower than with Ad-GL on day 1, 19-fold on day 3, 3.8-fold on day 7, and 6-fold on day 13. In contrast, in tumor the signal increased between days 3 and 7, and reached a plateau on day 13.
Statistical analysis by repeated measures ANOVA of the data from days 1–13 indicated that there was a significant difference in bioluminescence signal from liver between groups (p < 0.001). Post-hoc analysis with Tukey's HSD test demonstrated that the signal from liver was significantly reduced in both the predosing–warfarin and PEGylation groups as compared with the unmodified Ad-GL group without predosing–warfarin treatment (Ad-GL vs. Ad-GL-PEG20K, p = 0.007; vs. Ad-GL plus predosing–warfarin, p < 0.001; vs. Ad-GL-PEG20K plus predosing–warfarin, p = 0.001). No significant difference in signal from liver was detected between PEGylated virus, predosing–warfarin treatment, and combination of PEGylation plus predosing-warfarin treatment groups (p > 0.6). Repeated measures ANOVA analysis of the data from days 1 to 13 in tumor indicated that there was no statistically significant difference between groups in terms of bioluminescence signal (p = 0.32).
Additional analysis by one-way ANOVA of bioluminescence data from tumor on separate days after injection detected significant differences on days 1 and 3 (p = 0.03 and p = 0.006); there was no significant difference on days 7 and 13 (p = 0.3 and p = 0.19). Post-hoc analysis by Tukey's HSD test of the data on day 1 demonstrated significantly lower expression for the Ad-GL plus predosing–warfarin group versus the Ad-GL-PEG20K plus predosing–warfarin group (p = 0.04); the difference versus the Ad-GL group did not reach significance (p = 0.07). There was no significant difference between other groups (p > 0.4). On day 3, expression in tumor for the Ad-GL-PEG20K plus predosing–warfarin group was significantly higher versus the Ad-GL group (p = 0.029) and versus the Ad-GL plus predosing–warfarin group (0.005); other pair-wise comparisons did not show statistical significance (p > 0.179).
Comparison of predosing–warfarin and PEGylation on antitumor efficacy in Hep3B tumor model
Tumor volumes in mice from the experiment in Fig. 5 were monitored and individual tumor volumes were plotted (Fig. 6a). Ad-GL delayed tumor growth as compared with PBS. PEGylation or predosing–warfarin treatment further delayed tumor growth and in some cases produced complete cure of the tumors. The combination of PEGylation with predosing–warfarin did not improve effects on tumor growth. When Kaplan–Meier survival curves were compared (Fig. 6b), all groups injected with virus were significantly different from the PBS-injected group (p < 0.05), except for the Ad-GL group with p = 0.506. Ad-GL-PEG20K was significantly better than Ad-GL (p = 0.02). The remaining groups were intermediate between Ad-GL and Ad-GL-PEG20K, with no significant difference detected versus either of these groups (p > 0.08 vs. Ad-GL and p > 0.4 vs. Ad-GL-PEG20K). There were two tumor-free animals surviving more than 200 days after virus injection in the predosing–warfarin group, and one animal in the Ad-GL-PEG20K group and the Ad-GL-PEG20K plus predosing–warfarin group.
FIG. 6.
Effect of PEGylation on anticancer efficacy of systemically administered replication-competent adenovirus in the Hep3B tumor model. (a) Effect of vector modification on tumor growth. nu/nu mice with established Hep3B tumors (average volume, 545 mm3) were injected intravenously with a single dose 5 × 1010 VG of Ad-GL or PEGylated Ad-GL in 100 mm3 of PBS. +PW, mice were pretreated by predosing (intravenous, 3 × 1010 VG of replication-deficient Ad-DsRed in 100 μl of PBS 4 hr before Ad-GL injection) and administration of warfarin (subcutaneous, 133 μg of warfarin in 100 μl of peanut oil 3 and 1 days before Ad-GL injection). Tumor volume (n = 10 per group) was calculated from tumor dimensions and individual tumor size was plotted. (b) Effect of vector modification on survival. Mice were euthanized when tumor volume reached 2000 mm3 (uncensored event) and Kaplan–Meier survival curves were plotted. +PW, mice were pretreated by predosing and administration of warfarin.
Effect of PEGylation on biodistribution, hepatotoxicity, and uptake by Kupffer cells
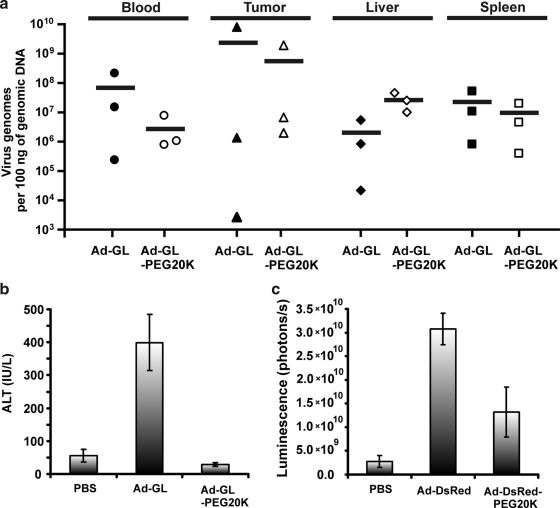
To elucidate the mechanism of increased antitumor activity of Ad-GL-PEG20K, we studied the biodistribution of Ad-GL-PEG20K in comparison with mock-PEGylated Ad-GL 24 hr after intravenous injection of vectors in nu/nu mice bearing large established Hep3B tumors (Fig. 7a). Viral genome copy number was determined by qPCR. We did not observe a significant difference in biodistribution of viral genomes in blood, tumor, or spleen (p > 0.35). Surprisingly, we found an increase in vector deposits in liver; however, this difference did not reach statistical significance (p = 0.07).
FIG. 7.
Effect of PEGylation of oncolytic adenovirus with 20K PEG on biodistribution, hepatotoxicity, and the ability of the virus to deplete Kupffer cells. (a) Biodistribution of PEGylated virus 24 hr after injection. Nude mice bearing Hep3B tumors were injected intravenously with 5 × 1010 VG of Ad-GL or Ad-GL-PEG20K. At 24 hr tissues were collected and viral genome concentration was determined by qPCR. (b) Effect of PEGylation with 20K PEG on the hepatotoxicity of Ad-GL. Four-week-old female C57BL/6 mice (n = 5 per group) were injected intravenously via the tail vein with 100 μl of PBS, 1011 VG of Ad-GL in PBS, or 1011 VG of PEGylated Ad-GL in PBS. ALT levels in serum were determined on day 3 after injection. (c) Effect of PEGylation with 20K PEG on depletion of Kupffer cells by predosing with replication-defective adenovirus. Six- to 8-week-old female ICR mice (n = 3 or 4 per group) were injected intravenously via the tail vein with 100 μl of PBS, 3 × 1010 VG of replication-deficient Ad-DsRed in PBS, or 3 × 1010 VG of PEGylated replication-deficient Ad-DsRed in PBS. Four hours later, mice were injected intravenously with 3 × 1010 VG of replication-competent Ad-GL. One day later, expression of luciferase in mouse liver was estimated by whole body bioluminescence imaging. Data are shown as means ± SE.
To confirm that Ad-GL-PEG20K was retargeted from hepatocytes and to determine whether PEGylation with 20-kDa PEG reduces hepatotoxicity of systemically injected oncolytic adenovirus, we evaluated liver enzyme ALT serum levels on day 3 after systemic administration of Ad-GL or Ad-GL-PEG20K vectors into C57BL/6 mice (Fig. 7b). Ad-GL injection produced elevated levels of ALT as compared with PBS-injected controls (p = 0.003). ALT levels in Ad-GL-PEG20K-injected mice were not significantly different from the PBS-injected group (p = 0.93) and were significantly lower as compared with the Ad-GL-injected group (p = 0.001).
We have previously shown that PEGylation reduces Ad5 uptake into macrophages and Kupffer cells (Mok et al., 2005). Because PEGylation reduces the ability of the virus to infect Kupffer cells, PEGylated Ad5 should be less effective at predosing, that is, less able to deplete Kupffer cells. To test this, mice were predosed intravenously with unPEGylated or PEGylated Ad-DsRed 4 hr before injection of Ad-GL and luciferase activity from the second virus was determined to assess how well each virus mediated the predosing effect. Predosing with unPEGylated Ad-DsRed resulted in an 11-fold increase in luciferase expression in liver compared with PBS control (p = 0.004; Fig. 7c), indicating that predosing with unmodified virus reduced Kupffer cell function in liver, resulting in a compensatory increase in hepatocyte transduction. In contrast, the PEGylated virus mediated only a partial predosing effect, producing a 4.7-fold increase in luciferase activity versus PBS control. Predosing with Ad-DsRed-PEG20K mediated significantly lower luciferase expression than predosing with Ad-DsRed (p = 0.03). These data suggest that PEGylation reduces both Kupffer cell and hepatocyte uptake of oncolytic Ad5; however, the virus can still be found in liver. It was reported previously that ablation of adenoviral transduction of hepatocytes in mice with depleted Kupffer cells did not prevent infection of endothelial cells in liver unless the vector was also ablated for transduction via the penton base RGD-integrin pathway (Liu et al., 2003; Di Paolo et al., 2009).
Discussion
After intravenous injection, a large fraction of Ad5-based vectors is depleted from the blood by at least two major mechanisms: sequestration by Kupffer cells and sequestration and infection of hepatocytes in liver. These effects result in rapid reduction of bioavailability of systemically injected adenoviral vectors and also in hepatotoxicity (Worgall et al., 1997; Alemany et al., 2000; Shashkova et al., 2007). Adenovirus is spilled into the bloodstream even during intratumoral injections or at later stages of oncolytic viral replication within tumors (Lohr et al., 2001; Sauthoff et al., 2003; Wang et al., 2006; Dhar et al., 2009); therefore, this problem is relevant not only for systemically delivered adenovirus but also for the intratumoral route of adenovirus administration.
We have reported that adenovirus can be efficiently detargeted from hepatocytes and Kupffer cells by a combination of depletion of Kupffer cells by adenovirus predosing and detargeting from hepatocytes by anticoagulant drug warfarin treatment (Shashkova et al., 2008a). Predosing–warfarin treatment allowed for improved efficacy of systemically delivered oncolytic adenovirus (Shashkova et al., 2008a).
We hypothesized that PEGylation of oncolytic adenovirus with 20-kDa PEG would reduce transduction of the liver and toxicity. We predicted that this would either not affect or might improve antitumor efficacy by liberating more virions to infect distant tumor sites. We show here that transduction of hepatocytes was significantly decreased by PEGylation in mice bearing human hepatocarcinoma and prostate carcinoma xenografts. The level of reduction of hepatocyte transduction was similar to that achieved by predosing and warfarin. PEGylation resulted in a reduction of bioluminescence signal from mouse liver of 19- to 90-fold. Whereas hepatocyte infection and damage were markedly reduced, tumor transduction and antitumor effects were notably not reduced by PEGylation. Combination of predosing–warfarin treatment with PEGylation did not result in further reduction of liver transduction or in a further increase in efficacy, implying that these interventions might operate via similar mechanisms.
We studied the ability of PEGylated Ad-GL-PEG20K to evade hepatocyte transduction and entrapment by Kupffer cells. We found that hepatotoxicity caused by Ad-GL-PEG20K was reduced in animals injected with PEGylated virus to levels not distinguishable from those of the buffer control group. The ability of 20-kDa PEG-modified adenovirus to deplete Kupffer cells was reduced as compared with mock-PEGylated adenovirus, suggesting that the level of uptake by Kupffer cells might have been reduced. However, we have observed increased concentrations of viral genomes in livers of mice treated with Ad-GL-PEG20 at 24 hr after injection. It was shown that endothelial cells in liver sinusoids can be transduced by adenovirus via the penton base RGD motif through secondary adenoviral receptors, αvβ3–5 integrins (Liu et al., 2003; Di Paolo et al., 2009). We have previously demonstrated that PEGylation with 5-kDa PEG does not completely ablate transduction via the RGD-integrin pathway (Mok et al., 2005), and therefore it can be anticipated that endothelial cells in the liver could still be transduced with PEGylated adenoviruses. Mutation of the RGD motif in adenoviral vectors could provide a solution to this problem (Di Paolo et al., 2009); however, this mutation might simultaneously decrease the antitumor efficacy of oncolytic adenovirus (Shayakhmetov et al., 2005).
We have observed the decreased transduction of hepatocytes by Ad-PEG20K in two different in vivo xenograft tumor models. We hypothesize that the size of adenoviral particles covalently linked with 20-kDa PEG exceeds the size of liver sinusoid fenestrae, thus providing a physical barrier for infection of hepatocytes by PEGylated adenoviruses. In support of this hypothesis, we have previously reported that PEGylation of replication-deficient adenovirus with 20-kDa and 35-kDa PEG, but not with the smaller 5-kDa PEG, resulted in decreased transduction of hepatocytes in mice (Mok et al., 2005; Hofherr et al., 2008). In this paper, we demonstrate that PEGylation with 20-kDa, but not with 5-kDa, PEG detargets oncolytic adenovirus from hepatocytes in mouse liver. The size of liver sinusoid fenestrae varies between species (Snoeys et al., 2007; Wisse et al., 2008), and therefore different sizes of PEG might be required to observe the same detargeting effect in animal models of different species or in human patients.
Simultaneously with the decreased transduction of hepatocytes, we have observed the retained transduction of tumor, and retained/increased anticancer efficacy. It was reported previously that PEGylation of adenovirus results in diminished in vitro infectivity (O'Riordan et al., 1999; Mok et al., 2005; Hofherr et al., 2008), the phenomenon that we have observed for Ad-GL PEGylated with either 5-kDa or 20-kDa PEG. It is plausible to hypothesize that the same mechanism that is postulated for improved pharmacological properties of PEGylated proteins and small drugs is operational in the case of adenoviral vector (Fishburn, 2008), that is, that PEGylated adenoviruses have an improved pharmacokinetic profile due to reduced interaction with blood cells and plasma proteins, resulting in prolonged circulation time and enhanced tumor permeability and retention effect (Alemany et al., 2000; Ogawara et al., 2004; Gao et al., 2007; Hofherr et al., 2008).
To our knowledge, this is first report on PEGylation of oncolytic adenovirus with high molecular weight PEG, demonstrating reduction of liver transduction with concomitant retention or increase in anticancer efficacy after systemic administration. We used unrestricted replication-competent adenovirus to study hepatotoxicity caused by adenoviral replication in liver. Wild-type Ad5 replicates in the liver of, and causes morbidity and mortality in, immunocompromised human patients (Kojaoghlanian et al., 2003). Ad5 was shown to replicate in the liver of and kill immunosuppressed Syrian hamsters (Toth et al., 2008), and was shown to replicate abortively in mouse liver, resulting in severe and fatal liver damage (Duncan et al., 1978; Shashkova et al., 2007). It can be anticipated that PEGylation of oncolytic adenovirus may be combined with genetic modification of adenoviral hexon, ablating binding to factor X (Kalyuzhniy et al., 2008; Waddington et al., 2008), resulting in further reduction of hepatotoxicity with a simultaneous increase in efficacy. Importantly, ablation of liver sinusoid endothelial cell transduction might be needed to further improve transductional detargeting of oncolytic adenovirus from liver (Di Paolo et al., 2009). Transcriptional control and/or deletion/modification of transcriptional activators encoded by adenovirus allow for decreased off-target adenoviral replication, providing an increase in therapeutic window independent of transductional targeting/detargeting (capsid modifications) (Doronin et al., 2001; Jakubczak et al., 2003; Cascallo et al., 2007; Shashkova et al., 2007). In addition, the oncolytic adenoviral vector toxicity/efficacy profile can be improved by expression of therapeutic transgenes (Sova et al., 2004; Heideman et al., 2005; Sarkar et al., 2005; Barton et al., 2006; Su et al., 2006; Post et al., 2007; Schepelmann et al., 2007; Luo et al., 2008; Shashkova et al., 2008b). It can be anticipated that transductional and transcriptional targeting approaches may be combined with the arming approaches, resulting in a safe and efficacious treatment for cancer (Hermiston, 2006; Alemany, 2007; Cattaneo et al., 2008).
Acknowledgments
The authors thank William S.M. Wold for KB cells and Mary Barry for excellent technical assistance. This work was supported by an NIH P50 CA91956 Prostate Cancer SPORE grant to the Mayo Clinic.
Author Disclosure Statement
No competing financial interests exist.
References
- Alemany R. Cancer selective adenoviruses. Mol. Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Alemany R. Suzuki K. Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Barton K.N. Paielli D. Zhang Y. Koul S. Brown S.L. Lu M. Seely J. Kim J.H. Freytag S.O. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Carlisle R.C. Di Y. Cerny A.M. Sonnen A.F. Sim R.B. Green N.K. Subr V. Ulbrich K. Gilbert R.J. Fisher K.D. Finberg R.W. Seymour L.W. Human erythrocytes bind and inactivate type 5 adenovirus by presenting coxsackie virus–adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascallo M. Alonso M.M. Rojas J.J. Perez-Gimenez A. Fueyo J. Alemany R. Systemic toxicity–efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- Cattaneo R. Miest T. Shashkova E.V. Barry M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A. Chirmule N. Zhang Y. Wilson J.M. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A. Le H.T. Linse K.D. Cerullo V. Toietta G. Beaudet A. Pastore L. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- Dhar D. Spencer J.F. Toth K. Wold W.S. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J. Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo N.C. van Rooijen N. Shayakhmetov D.M. Redundant and synergistic mechanisms control the sequestration of blood-borne adenovirus in the liver. Mol. Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K. Toth K. Kuppuswamy M. Ward P. Tollefson A.E. Wold W.S. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K. Kuppuswamy M. Toth K. Tollefson A.E. Krajcsi P. Krougliak V. Wold W.S. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J. Virol. 2001;75:3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K. Toth K. Kuppuswamy M. Krajcsi P. Tollefson A.E. Wold W.S. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- Duncan S.J. Gordon F.C. Gregory D.W. McPhie J.L. Postlethwaite R. White R. Willcox H.N. Infection of mouse liver by human adenovirus type 5. J. Gen. Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- Fishburn C.S. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- Gao J.Q. Eto Y. Yoshioka Y. Sekiguchi F. Kurachi S. Morishige T. Yao X. Watanabe H. Asavatanabodee R. Sakurai F. Mizuguchi H. Okada Y. Mukai Y. Tsutsumi Y. Mayumi T. Okada N. Nakagawa S. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J. Control. Release. 2007;122:102–110. doi: 10.1016/j.jconrel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Harris J.M. Chess R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- Heideman D.A. Steenbergen R.D. van Der T.J. Scheffner M. Alemany R. Gerritsen W.R. Meijer C.J. Snijders P.J. van Beusechem V.W. Oncolytic adenovirus expressing a p53 variant resistant to degradation by HPV E6 protein exhibits potent and selective replication in cervical cancer. Mol. Ther. 2005;12:1083–1090. doi: 10.1016/j.ymthe.2005.06.443. [DOI] [PubMed] [Google Scholar]
- Hermiston T. A demand for next-generation oncolytic adenoviruses. Curr. Opin. Mol. Ther. 2006;8:322–330. [PubMed] [Google Scholar]
- Hofherr S.E. Shashkova E.V. Weaver E.A. Khare R. Barry M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008;16:1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- Jakubczak J.L. Ryan P. Gorziglia M. Clarke L. Hawkins L.K. Hay C. Huang Y. Kaloss M. Marinov A. Phipps S. Pinkstaff A. Shirley P. Skripchenko Y. Stewart D. Forry-Schaudies S. Hallenbeck P.L. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: Dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003;63:1490–1499. [PubMed] [Google Scholar]
- Kalyuzhniy O. Di Paolo N.C. Silvestry M. Hofherr S.E. Barry M.A. Stewart P.L. Shayakhmetov D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: What have we learned? Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- Kojaoghlanian T. Flomenberg P. Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- Kreppel F. Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol. Ther. 2008;16:16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- Liu Q. Zaiss A.K. Colarusso P. Patel K. Haljan G. Wickham T.J. Muruve D.A. The role of capsid–endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- Liu T.C. Galanis E. Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Liu T.C. Thorne S.H. Kirn D.H. Oncolytic adenoviruses for cancer gene therapy. Methods Mol. Biol. 2008;433:243–258. doi: 10.1007/978-1-59745-237-3_15. [DOI] [PubMed] [Google Scholar]
- Lohr F. Huang Q. Hu K. Dewhirst M.W. Li C.Y. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin. Cancer Res. 2001;7:3625–3628. [PubMed] [Google Scholar]
- Luo J. Xia Q. Zhang R. Lv C. Zhang W. Wang Y. Cui Q. Liu L. Cai R. Qian C. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin. Cancer Res. 2008;14:2450–2457. doi: 10.1158/1078-0432.CCR-07-4596. [DOI] [PubMed] [Google Scholar]
- Mok H. Palmer D.J. Ng P. Barry M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Morrison J. Briggs S.S. Green N. Fisher K. Subr V. Ulbrich K. Kehoe S. Seymour L.W. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol. Ther. 2008;16:244–251. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J. Cunningham C. Buchanan A. Blackburn A. Edelman G. Maples P. Netto G. Tong A. Randlev B. Olson S. Kirn D. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: Safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- O'Riordan C.R. Lachapelle A. Delgado C. Parkes V. Wadsworth S.C. Smith A.E. Francis G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- Ogawara K. Rots M.G. Kok R.J. Moorlag H.E. van Loenen A.M. Meijer D.K. Haisma H.J. Molema G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- Parker A.L. Waddington S.N. Nicol C.G. Shayakhmetov D.M. Buckley S.M. Denby L. Kemball-Cook G. Ni S. Lieber A. McVey J.H. Nicklin S.A. Baker A.H. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Post D.E. Sandberg E.M. Kyle M.M. Devi N.S. Brat D.J. Xu Z. Tighiouart M. van Meir E.G. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67:6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T. Warren R. Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.M. Mantovani G. Wang X. Haddleton D.M. Brayden D.J. Advances in PEGylation of important biotech molecules: Delivery aspects. Expert Opin. Drug Deliv. 2008;5:371–383. doi: 10.1517/17425247.5.4.371. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Su Z.Z. Vozhilla N. Park E.S. Gupta P. Fisher P.B. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauthoff H. Hu J. Maca C. Goldman M. Heitner S. Yee H. Pipiya T. Rom W.N. Hay J.G. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: Virus persists and spreads systemically at late time points. Hum. Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- Schepelmann S. Ogilvie L.M. Hedley D. Friedlos F. Martin J. Scanlon I. Chen P. Marais R. Springer C.J. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007;67:4949–4955. doi: 10.1158/0008-5472.CAN-07-0297. [DOI] [PubMed] [Google Scholar]
- Shashkova E.V. Spencer J.F. Wold W.S. Doronin K. Targeting interferon-α increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol. Ther. 2007;15:598–607. doi: 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- Shashkova E.V. Doronin K. Senac J.S. Barry M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008a;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Shashkova E.V. Kuppuswamy M.N. Wold W.S. Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-α and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008b;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov D.M. Eberly A.M. Li Z.Y. Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J. Virol. 2005;79:1053–1061. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E.J. Carducci M.A. Burke J.M. Rodriguez R. Fong L. Van Ummersen L. Yu D.C. Aimi J. Ando D. Working P. Kirn D. Wilding G. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Snoeys J. Lievens J. Wisse E. Jacobs F. Duimel H. Collen D. Frederik P. de Geest B. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]
- Sova P. Ren X.W. Ni S. Bernt K.M. Mi J. Kiviat N. Lieber A. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol. Ther. 2004;9:496–509. doi: 10.1016/j.ymthe.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Su C. Peng L. Sham J. Wang X. Zhang Q. Chua D. Liu C. Cui Z. Xue H. Wu H. Yang Q. Zhang B. Liu X. Wu M. Qian Q. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-γ gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol. Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Tollefson A.E. Scaria A. Hermiston T.W. Ryerse J.S. Wold L.J. Wold W.S. The adenovirus death protein (E3-11.6 K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A.E. Kuppuswamy M. Shashkova E.V. Doronin K. Wold W.S. Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol. Med. 2007;130:223–235. doi: 10.1385/1-59745-166-5:223. [DOI] [PubMed] [Google Scholar]
- Toth K. Spencer J.F. Dhar D. Sagartz J.E. Buller R.M. Painter G.R. Wold W.S. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington S.N. McVey J.H. Bhella D. Parker A.L. Barker K. Atoda H. Pink R. Buckley S.M. Greig J.A. Denby L. Custers J. Morita T. Francischetti I.M. Monteiro R.Q. Barouch D.H. van Rooijen N. Napoli C. Havenga M.J. Nicklin S.A. Baker A.H. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wang Y. Wang H. Li C.Y. Yuan F. Effects of rate, volume, and dose of intratumoral infusion on virus dissemination in local gene delivery. Mol. Cancer Ther. 2006;5:362–366. doi: 10.1158/1535-7163.MCT-05-0266. [DOI] [PubMed] [Google Scholar]
- Weaver E.A. Barry M.A. Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Hum. Gene Ther. 2008;19:1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. Jacobs F. Topal B. Frederik P. de Geest B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- Worgall S. Wolff G. Falck-Pedersen E. Crystal R.G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- Wortmann A. Vohringer S. Engler T. Corjon S. Schirmbeck R. Reimann J. Kochanek S. Kreppel F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- Yu W. Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]