Abstract
Human gene therapy with rAAV2-vector was performed for the RPE65 form of childhood blindness called Leber congenital amaurosis. In three contemporaneous studies by independent groups, the procedure was deemed safe and there was evidence of visual gain in the short term. At 12 months after treatment, our young adult subjects remained healthy and without vector-related serious adverse events. Results of immunological assays to identify reaction to AAV serotype 2 capsid were unchanged from baseline measurements. Results of clinical eye examinations of study and control eyes, including visual acuities and central retinal structure by in vivo microscopy, were not different from those at the 3-month time point. The remarkable improvements in visual sensitivity we reported by 3 months were unchanged at 12 months. The retinal extent and magnitude of rod and cone components of the visual sensitivity between 3 and 12 months were also the same. The safety and efficacy of human retinal gene transfer with rAAV2-RPE65 vector extends to at least 1 year posttreatment.
Introduction
Mutations in the RPE65 (retinal pigment epithelium-specific 65-kDa) gene cause Leber congenital amaurosis (LCA), a severe form of inherited retinal blindness in infants and children (den Hollander et al., 2008). More than 15 years of basic research on RPE65 and the visual cycle, and applied research into the pathophysiology of RPE65-LCA and relevant animal models (reviewed in Cai et al., 2009), led to clinical trials of human gene therapy for RPE65-LCA. Three independent trials provided preliminary evidence of short-term safety and efficacy (Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008). Testimonials by patients of improved vision were reported in all studies, and quantitative data indicated increased light sensitivity localized to the retinal regions of treatment in the injected eye (Bainbridge et al., 2008; Cideciyan et al., 2008). These forms of visual improvement could be dramatic and were reproducible on repeated testing over the first 3 months after treatment (Cideciyan et al., 2008), providing strong evidence of successful gene transfer to the human eye with the rAAV2/2-based vector.
A new set of questions arise 1 year after the treatment. Is it still as safe as initially reported? What is the longevity of the restored vision in the treated eye? The current report extends the safety and efficacy data to 12 months for the patients taking part in our clinical trial.
Materials and Methods
Clinical trial
Conduct, regulatory approvals, and details of oversight for the phase I clinical trial (registered at clinicaltrials.gov, NCT00481546) have been published (Cideciyan et al., 2008; Hauswirth et al., 2008). Informed consent was obtained for all procedures. There were three young adult subjects (P1, P2, and P3) with a clinical diagnosis of LCA. RPE65 mutations were determined by the Carver Nonprofit Genetic Testing Laboratory at the University of Iowa (Iowa City, IA). Inclusion and exclusion criteria for the clinical trial have been published, as has a summary of the protocol study visits (Hauswirth et al., 2008). The rAAV vector, AAV2-CBSB-hRPE65 (IND number, BB-IND 12824), and the method of administration to the retina have previously been described (Hauswirth et al., 2008).
Safety parameters
Ocular safety was assessed by standard eye examinations at baseline visits; in the immediate postoperative period; and 1, 2, 3, 6, 9, and 12 months after treatment. Systemic safety was evaluated by physical examinations (performed at baseline; in the immediate postoperative period; and 1, 3, and 12 months after treatment), routine hematology, serum chemistries, coagulation parameters, and urinalysis (performed at baseline; immediately posttreatment; and 1, 3, and 12 months after vector administration) (Hauswirth et al., 2008). Serum samples were assayed for circulating antibodies to the AAV2 capsid proteins at baseline, day 14, and at 3 and 12 months (Hauswirth et al., 2008). Anti-AAV2 antigen-specific lymphocyte proliferation responses were assessed as previously described (Hernandez et al., 1999; Hauswirth et al., 2008).
Visual function and retinal structure
Visual acuity was measured by ETDRS methodology (Ferris et al., 1982); visual field testing was performed with kinetic perimetry as published (Jacobson et al., 1989) and statistical differences between measures on different visits were determined (Ross et al., 1984). Retinal structure was assessed by cross-sectional imaging, using optical coherence tomography (OCT). Data were acquired by ultrahigh-speed and high-resolution OCT imaging with a Fourier domain (FD) OCT instrument (RTVue-100; Optovue, Fremont, CA) as described (Aleman et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008). Foveal thickness measurements were performed as described and statistical comparisons made between data from different visits (Sandberg et al., 2005).
Visual sensitivities to transient (duration, 200 msec) stimuli presented at the extrafoveal retina were determined while subjects fixated a red target with a variable intensity that was adjusted to be easily visible. Most sensitivity measures were performed under dark-adapted conditions with a modified computerized perimeter (Humphrey field analyzer; Zeiss Meditec, Dublin, CA) as described (Jacobson et al., 1986; Cideciyan et al., 2008). The achromatic (white) stimulus (1.7° diameter; maximum luminance, 3180 cd·m−2) was presented along the vertical or horizontal meridians crossing fixation. Tests were performed at several pretreatment time points ranging from 3 to 24 months before surgery and at six posttreatment time points (1, 2, 3, 6, 9, and 12 months). Retinal loci were typically sampled at 0.6-mm intervals up to 9 mm (vertical) or 18 mm (horizontal) eccentricity from fixation. In addition, foveal sensitivities were determined while gazing at the center of four red lights forming a diamond. Extrafoveal sensitivity values were spatially smoothed with the use of a three-point moving average; foveal sensitivities were reported without spatial averaging. Locus-by-locus differences were calculated between posttreatment and pretreatment results. The statistical significance of the difference calculated at each locus was defined by comparison with the maximal expected test–retest variability (3 SD) in RPE65-LCA patients of 0.8 log units (Cideciyan et al., 2008). To obtain the most conservative estimates, the best pretreatment sensitivity was used for defining loci with significant improvement and the worst pretreatment sensitivity was used for defining loci with significant deterioration. Two-color perimetry (blue, 500 nm and red, 650 nm) was performed under standard (1–2 hr) and extended (3–8 hr) dark adaptation conditions to understand the retinal distribution of cone- and rod-mediated vision across the treated areas. In addition, testing was performed in the dark during the cone plateau period following an adapting light. Pretreatment values for cone- and rod-mediated vision were estimated mostly from achromatic sensitivities, using the most conservative assumption that both rods and cones were contributing to this low level of vision (Cideciyan et al., 2008).
Results and Discussion
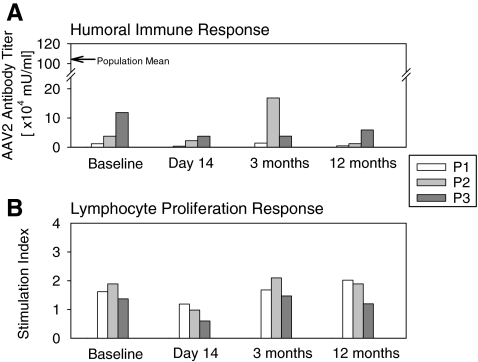
One year after gene therapy, the three young adults with RPE65-LCA (P1, age 25; P2, age 24; and P3, age 22) (Cideciyan et al., 2008; Hauswirth et al., 2008) remained healthy and without vector-related serious adverse events. Humoral immune response was monitored by measuring levels of circulating antibody to AAV serotype 2 capsid. Antibody titers at the 12-month time point (P1, 4860; P2, 12,583; P3, 59,141 mU/ml) were well below the population mean titer (1,042,089 mU/ml) and within the range of measurements obtained in each patient between baseline and 3 months posttreatment (Fig. 1A). The AAV2 capsid antigen-specific lymphocyte proliferation response at 12 months showed no significant rise in stimulation index (Fig. 1B). Physical examinations remained normal and there were no clinically significant abnormalities in routine blood and urine tests in all subjects through 12 months.
FIG. 1.
Immunological assays before surgery (baseline) and at 14-, 90-, and 365-day time points after retinal gene therapy. (A) Humoral immune response to AAV serotype 2 (AAV2) assayed by circulating serum antibody titers against AAV2 capsid in P1, P2, and P3 before and after surgery. Arrow along the vertical axis indicates mean levels from a normal reference population (n = 79) (Hauswirth et al., 2008). (B) Antigen-specific lymphocyte proliferation response (ASR) assayed in peripheral blood lymphocytes incubated in the presence versus in the absence of AAV2 capsid antigen. The stimulation index (the ratio of [3H]thymidine uptake in the presence of antigen to the uptake in its absence) in each subject after surgery is unchanged from baseline values.
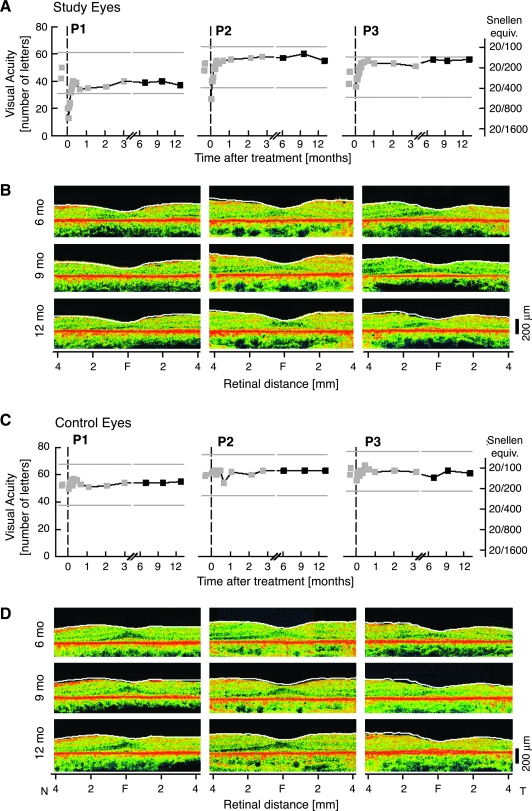
Clinical ocular examinations of study and control eyes were unchanged from 3 months. Visual acuities, central retinal structure by optical coherence tomography (Fig. 2), and standard kinetic visual fields at the 6-, 9-, and 12-month time points were not different from those measured at the 3-month time point.
FIG. 2.
Central vision and retinal structure in RPE65-LCA during the 12 months after gene therapy. (A) Visual acuity as a function of time before and after the day of surgery (0) in the study eyes of P1, P2, and P3. Gray symbols, data previously reported (Hauswirth et al., 2008). Gray lines, 15-letter gain or loss from baseline visual acuity. (B) Cross-sectional optical coherence tomography (OCT) scans of retina along the horizontal meridian 6, 9, and 12 months after surgery. Overlaid white lines represent the location of the vitreoretinal boundary 3 months posttreatment (Hauswirth et al., 2008). (C) Visual acuity and (D) OCT scans in untreated control eyes for comparison. F, fovea; N, nasal; T, temporal retina.
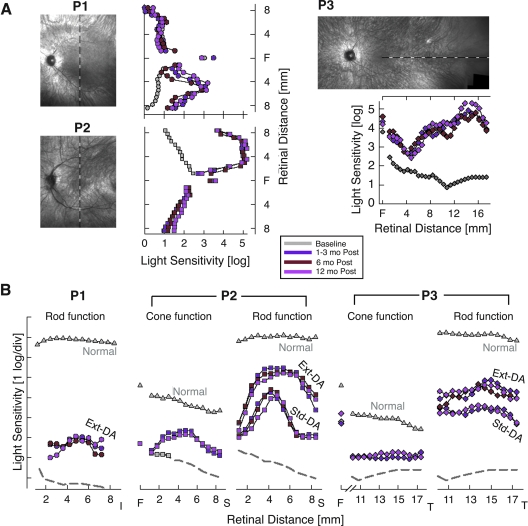
Statistically significant increases in light sensitivity were previously found in study eyes 1–3 months after treatment (Cideciyan et al., 2008). Between 3 and 12 months, there were no further changes in the magnitude or retinal extent of the visual sensitivity increase (Fig. 3A). Chromatic stimuli and light adaptation conditions were used to determine the rod and cone photoreceptor contributions to the visual sensitivity. For P1, visual sensitivity in the treated retinal region was mediated by rods. For P2 and P3, there were both rod and cone contributions depending on the testing conditions. Remarkable improvements of rod- and cone-based vision measured within the first 3 months after treatment were unchanged between 3 months and 1 year (Fig. 3B). The unexpected finding of slowed retinoid cycle kinetics (Cideciyan et al., 2008) in treated retina also remained. There were increases in rod photoreceptor sensitivity with extended dark adaptation at 1 year and these increases were comparable to those documented within the first few months after treatment (Fig. 3B).
FIG. 3.
Visual function improvement due to gene therapy is stable up to 12 months after treatment. (A) Light sensitivity to achromatic stimuli in study eyes along vertical (P1 and P2) and horizontal (P3) meridians after allowing for an extended (3–8 hr) period of dark adaptation. Sensitivities 6 and 12 months after treatment are compared with the mean value at 1, 2, and 3 months after treatment or the mean baseline value before treatment. Loci of visual function testing are shown on images of the ocular fundus of the study eyes obtained at 12 months. All images are shown as left retina for clarity and comparability. F, fovea. (B) Light sensitivity measures with chromatic stimuli support stability of rod and cone photoreceptor-based vision 12 months after treatment in retinal regions of peak response to gene therapy. Rod function measured with blue stimuli after standard (Std) or extended dark adaptation (Ext-DA) conditions. Cone function measured with red stimuli after Ext-DA conditions (at the fovea) or during the cone plateau period in the dark after light adaptation (at extrafoveal locations). The sensitivity axes are shifted vertically to match red and blue stimuli for cone-mediated detection in patients. I, inferior; S, superior; T, temporal retina.
Would maintained visual improvement have been expected 1 year after this gene therapy? Treated murine Rpe65 models have not been monitored for relatively long time periods, but durability of the human visual gains is consistent with electrophysiological data in long-term studies of treated RPE65-mutant dogs (Acland et al., 2005; Aguirre et al., 2007). A major difference between human and canine RPE65 disease, however, is the significant retinal cell death in humans at all ages (Jacobson et al., 2005, 2008a). Treated retinal areas in RPE65-LCA are not normal with a full complement of retinal cells and it is uncertain what the rate of further cell loss will be in this already degenerate, albeit better functioning, tissue. Spreading negative influences from nearby untreated degenerate retina by non–cell-autonomous mechanisms may also be a potential threat to longevity of the restored visual islands (Cronin et al., 2007).
Further reports of early and later visual consequences of ocular gene therapy in this human congenital retinal blindness will undoubtedly emerge. There will be variation in the magnitude of efficacious effect as was evident in the earliest reports (Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008). The considerable interindividual differences in severity of photoreceptor loss in the first three decades of life in RPE65-LCA patients (Jacobson et al., 2005, 2007, 2008a,b), taken together with details of injection site, vector dose and volume, and genotype should permit estimates of the basis of the variation in response and lead to refined treatment strategies in subsequent cohorts of patients in these early-phase trials.
Acknowledgments
The clinical trial was supported by National Eye Institute (National Institutes of Health, Department of Health and Human Services) grant U10 EY017280.
Author Disclosure Statement
B.J.B., W.W.H., and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. S.K. is a principal investigator of a clinical trial of AAV-RPE65 to treat LCA sponsored by AGTC. The University of Pennsylvania, University of Florida, and Cornell University hold a patent on the described gene therapy technology (U.S. Patent 20070077228, “Method for Treating or Retarding the Development of Blindness”).
References
- Acland G.M. Aguirre G.D. Bennett J. Aleman T.S. Cideciyan A.V. Bennicelli J. Dejneka N.S. Pearce-Kelling S.E. Maguire A.M. Palczewski K. Hauswirth W.W. Jacobson S.G. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre G.K. Komáromy A.M. Cideciyan A.V. Brainard D.H. Aleman T.S. Roman A.J. Avants B.B. Gee J.C. Korczykowski M. Hauswirth W.W. Acland G.M. Aguirre G.D. Jacobson S.G. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4:e230. doi: 10.1371/journal.pmed.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman T.S. Cideciyan A.V. Sumaroka A. Windsor E.A. Herrera W. White D.A. Kaushal S. Naidu A. Roman A.J. Schwartz S.B. Stone E.M. Jacobson S.G. Retinal laminar architecture in human retinitis pigmentosa caused by rhodopsin gene mutations. Invest. Ophthalmol. Vis. Sci. 2008;49:1580–1590. doi: 10.1167/iovs.07-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J.W. Smith A.J. Barker S.S. Robbie S. Henderson R. Balaggan K. Viswanathan A. Holder G.E. Stockman A. Tyler N. Petersen-Jones S. Bhattacharya S.S. Thrasher A.J. Fitzke F.W. Carter B.J. Rubin G.S. Moore A.T. Ali R.R. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Cai X. Conley S.M. Naash M.I. RPE65: Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30:57–62. doi: 10.1080/13816810802626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V. Aleman T.S. Boye S.L. Schwartz S.B. Kaushal S. Roman A.J. Pang J.J. Sumaroka A. Windsor E.A. Wilson J.M. Flotte T.R. Fishman G.A. Heon E. Stone E.M. Byrne B.J. Jacobson S.G. Hauswirth W.W. Human gene therapy for RPE65-isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T. Leveillard T. Sahel J.-A. Retinal degenerations: From cell signaling to cell therapy; pre-clinical and clinical issues. Curr. Gene Ther. 2007;7:121–129. doi: 10.2174/156652307780363143. [DOI] [PubMed] [Google Scholar]
- den Hollander A.I. Roepman R. Koenekoop R.K. Cremers F.P. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ferris F.L. Kassoff A. Bresnick G.H. Bailey I. New visual acuity charts for clinical research. Am. J. Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- Hauswirth W.W. Aleman T.S. Kaushal S. Cideciyan A.V. Schwartz S.B. Wang L. Conlon T.J. Boye S.L. Flotte T.R. Byrne B.J. Jacobson S.G. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Y.J. Wang J. Kearns W.G. Loiler S. Poirier A. Flotte T.R. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 1999;73:8549–8558. doi: 10.1128/jvi.73.10.8549-8558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G. Voigt W.J. Parel J.M. Apáthy P.P. Nghiem-Phu L. Myers S.W. Patella V.M. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93:1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- Jacobson S.G. Yagasaki K. Feuer W.J. Roman A.J. Interocular asymmetry of visual function in heterozygotes of X-linked retinitis pigmentosa. Exp. Eye Res. 1989;48:679–691. doi: 10.1016/0014-4835(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Jacobson S.G. Aleman T.S. Cideciyan A.V. Sumaroka A. Schwartz S.B. Windsor E.A. Traboulsi E.I. Heon E. Pittler S.J. Milam A.H. Maguire A.M. Palczewski K. Stone E.M. Bennett J. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G. Aleman T.S. Cideciyan A.V. Heon E. Golczak M. Beltran W.A. Sumaroka A. Schwartz S.B. Roman A.J. Windsor E.A. Wilson J.M. Aguirre G.D. Stone E.M. Palczewski K. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G. Cideciyan A.V. Aleman T.S. Sumaroka A. Windsor E.A. Schwartz S.B. Heon E. Stone E.M. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008a;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G. Aleman T.S. Cideciyan A.V. Roman A.J. Sumaroka A. Windsor E.A. Schwartz S.B. Heon E. Stone E.M. Defining the residual vision in Leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008b;50:2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A. Pugh E.N., Jr Mingozzi F. Bennicelli J. Banfi S. Marshall K.A. Testa F. Surace E.M. Rossi S. Lyubarsky A. Arruda V.R. Konkle B. Stone E. Sun J. Jacobs J. Dell'Osso L. Hertle R. Ma J.X. Redmond T.M. Zhu X. Hauck B. Zelenaia O. Shindler K.S. Maguire M.G. Wright J.F. Volpe N.J. McDonnell J.W. Auricchio A. High K.A. Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D.F. Fishman G.A. Gilbert L.D. Anderson R.J. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch. Ophthalmol. 1984;102:1004–1010. doi: 10.1001/archopht.1984.01040030806021. [DOI] [PubMed] [Google Scholar]
- Sandberg M.A. Brockhurst R.J. Gaudio A.R. Berson E.L. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2005;46:3349–3354. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]