Abstract
PTSD has been associated with HPA axis and immune function alterations; however few studies have simultaneously investigated these systems in participants with PTSD. In this study, HPA axis and immune function in 26 women with PTSD with and without major depressive disorder was compared to 24 traumatized controls and also to 21 non-traumatized controls. PTSD was associated with low cortisol and higher levels of DHEA and greater production of TNF-α, and IL-6 compared to traumatized and healthy controls. Women with PTSD and depression exhibited greater production of IL-6 and higher levels of DHEA than those with PTSD but without depression. These findings suggest dysregulated HPA axis and immune function in women with PTSD, and that co-morbid depression may contribute to these abnormalities.
Posttraumatic stress disorder (PTSD) is a chronic anxiety disorder that occurs following a traumatic event, and is characterized by symptoms of avoidance/numbing, hyper-arousal and re-experiencing. Depression occurs in half of individuals who develop PTSD and can result in additional psychiatric impairment (Breslau, et al., 1998; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Kinder et al., 2008), and biologic alterations (Maes et al., 1999; Young & Breslau, 2004). The hypothalamic-pituitary-adrenal (HPA) axis and the immune system exert a well described bi-directional relationship, however, excessive or prolonged biologic activation following a stressor including the experience of a traumatic event can result in disruptions in the function and communication of these two systems (Charmandari, Tsigos, & Chrousos, 2005; McEwen, 2004; Sternberg, 2006). Although abnormalities have been reported in both HPA axis function and the immune system in individuals with PTSD, very few studies have simultaneously evaluated the relationships between these abnormalities in PTSD participants, and even fewer have evaluated the impact of co-morbid major depressive disorder (MDD), or compared PTSD participants to traumatized controls.
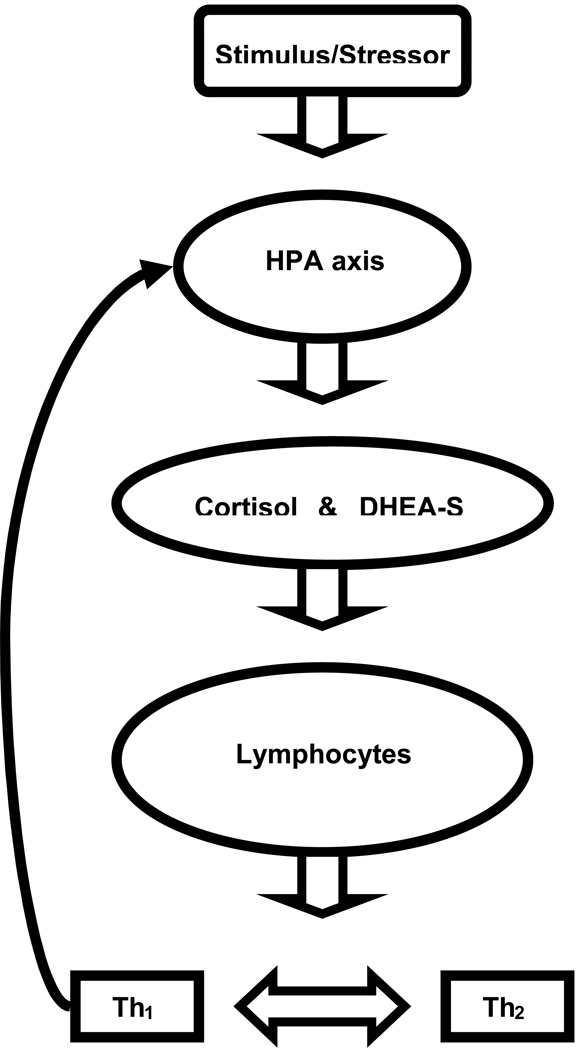
The HPA axis and immune system communicate in a complex feedback system that can be disrupted following the experience of a traumatic event, resulting in risks to physical and psychological well being (See Figure 1). In a healthy individual there is a balance between the lymphocyte production of inflammatory/cell mediated (Th1) and humoral (Th2) cytokines. Following a stressor, cortisol increases and results in suppression of Th1 cytokines by binding to glucocorticoid (GC) receptors in lymphocytes, resulting in down regulation of inflammatory activities (Raison & Miller, 2003; Sternberg, 2006). The immune system also affects HPA axis function in an effort to protect the individual. Th1 cytokines stimulate the HPA axis resulting in increased cortisol, and a reduction in Th1 immune activities (Raison & Miller, 2003). Improved understanding of relations between the HPA axis and immune system in PTSD participants is essential, as disruptions may be related to increased morbidity and mortality in PTSD participants. Chronic inflammation has been shown to exert adverse effects on many bodily systems and may contribute to chronic pain, arthritis, diabetes, and cardiovascular disease (Davis et al., 2008; Elenkov, 2004; Graves & Kayal, 2008; Koch & Distler, 2007), all of which have been associated with PTSD (Boscarino & Hoffman, 2007; Dobie et al., 2004; Kimerling, 2004;).
Figure 1.
Lower levels of cortisol and altered HPA axis functioning have been observed in traumatized women without PTSD (Heim, et al., 2002; Ganzel et al., 2007). In PTSD patients, women gender was associated with lower cortisol levels in a meta-analysis (Meewisse, Reitsma, de Vries, Gerson, & Olff, 2007); however individual studies of women with PTSD report mixed findings, with reports of higher levels of salivary (Garcia-Linares, Celda-Navarro, Herbert, & Martinez, 2004; Pico-Alfonso et al., 2004; Young & Breslau, 2004) and higher plasma levels (Griffin, Resick, & Yehuda, 2005), and no differences in levels of plasma (Altemus, 2003) or salivary cortisol levels (Woods et al., 2005). In addition, lower waking salivary cortisol levels have been reported in samples of men and women with PTSD (Neylan et al., 2005; Rohleder, Joksimovic, Wolf, & Kirschbaum, 2004; Wessa, Rohleder, Kirschbaum, & Flor, 2006;).
Comorbid MDD may further alter cortisol levels in PTSD subjects. PTSD participants with comorbid MDD exhibited lower plasma cortisol levels compared to participants with only MDD (Oquendo et al., 2003), and lower continuous plasma levels compared to healthy subjects (Yehdua, Teicher, Trestman, Levengood, Siever, 1996), and also lower urinary levels in those women with lifetime PTSD and comorbid MDD compared to controls (Young & Breslau, 2004). Following stimulation with dexamethasone and corticotropin releasing hormone (CRH), PTSD+MDD subjects exhibited a lower level of adrenocorticotropin hormone compared to PTSD-MDD subjects (De Kloet et al., 2008).
DHEA and DHEA sulphate ester (DHEA-S) are produced by the adrenal cortex and under normal conditions DHEA levels are closely correlated with cortisol; however, an imbalance of cortisol/DHEA secretion may occur when an individual experiences chronic stress (Kroboth, Salek, Pittenger, Fabian, & Frye, 1999; Raison & Miller, 2003). DHEA-S is more abundant than DHEA in plasma and saliva and exerts effects at glutamate and GABA receptors that may contribute to PTSD symptoms. DHEA also modulates actions of the immune system, resulting in reduced Th1 immune activities, similar to cortisol (Schuld et al., 2000). Higher DHEA levels among participants with PTSD have been reported (Laudenslager et al., 1998; Olff, Guzelcan, de Vries, Assies, & Gersons, 2007; Spivak et al., 2000), as well as higher DHEA-S (Sondergaard & Theorell, 2003; Sondergaard, Hansson, & Theorell, 2002); however a lower level was reported in traumatized individuals that were highly comorbid with MDD and PTSD (Kanter et al., 2001).
Indicators of immune activation have been observed in studies of PTSD participants. The majority of studies in adults with PTSD have reported increased pro-inflammatory cytokine levels in plasma including; TNF-α (von Kandel et al., 2007), IL-1β (Spivak et al., 1997; von Kandel et al., 2007), IL-6 (Maes et al., 1999), IL-8 (Song, Zhou, Guan, & Wang, 2007) and stimulated levels of IL-6 (Rohleder et al., 2004) and INFγ (Woods et al., 2005). Maes et al (1999) also reported that IL-6 receptor levels were higher in PTSD participants with comorbid MDD compared to PTSD participants without MDD. In addition, salivary secretory IgA (sIgA) an immunoglobulin that protects mucosal surfaces from upper respiratory infection was lower in chronically stressed persons (Ng et al., 2004) including women with PTSD (Woods et al., 2005), possibly resulting in exposure to additional antigens that require an inflammatory immune response.
Few studies that have investigated relationships between HPA axis and immune function in PTSD participants, and inconsistent findings have been reported. In a study of male and women refugees with PTSD low levels of cortisol and high IL-6 production were reported, indicating and insufficient immune regulation (Rohleder et al., 2004); yet in a sample of all male combat veterans with more recent onset of PTSD, cortisol levels were similar to controls and lower levels of TNF-α were observed (De Kloet et al., 2007). Also in a study of male combat veterans with PTSD, no differences in cortisol levels were observed; however, higher number of total lymphocytes were reported, indicating additional immune activity, but neither a Th1 or Th2 dominant immune response (Vidovic, et al. 2007). Lastly, lower cortisol and higher numbers of lymphocytes were reported in a study of combat men with PTSD, suggesting increased immune activity (Gotovac, Sabioncello, Rabatic, Berki, & Dekaris, 2003). These studies provide evidence of links between HPA axis and immune function and suggest that when cortisol is low, there is increased Th1 immune activity. Only De Kloet et al. (2007) differentiated trauma controls from PTSD participants and investigated the possible impact of MDD, which was found not to influence any immune or HPA axis function measures.
In order to expand what is known about HPA axis and immune system interactions in PTSD participants, and specifically women, this study had three primary aims: (1) to compare salivary measures of cortisol, DHEA and sIgA, and measures of stimulated whole blood production of TNFα, IL-1β, and IL-6 among traumatized controls, non-traumatized controls and PTSD participants, (2) compare participants with PTSD with and without MDD in HPA axis and immune function measures, and (3) to investigate relationships between PTSD symptom characteristics and HPA axis and immune function measures. We expected increased production of acute phase reaction cytokines, and that these elevations would be related to low cortisol, and would be more pronounced in PTSD participants with MDD compared to PTSD participants without MDD.
Method
Participants
Potential participants were recruited from a primary care clinic that served uninsured women in Baltimore, MD. Women were excluded if they were non-English speaking, over the age of 50 years, post-or peri menopausal, reported using psychotropic medication, hormonal medication other than birth control pills, stimulants, or narcotic pain medication in the last 4 weeks, had a thyroid disorder, cancer, HIV, were employed during night hours, reported physical injury or a cold or flu in the last week, reported illicit drug use in the past month, or drank three or more alcoholic drinks per day or more than 10 alcoholic drinks per week. Of those screened (250), 52.8% qualified for participation, and 63% (83) of those who qualified participated. This study was approved by the Institutional Review Board of Johns Hopkins University.
Measures
A researcher (J.G.) trained in administration of study instruments interviewed all participants. The experience of traumatic events was assessed with the Trauma Life Events Questionnaire (TLEQ), a 23 item instrument for determining the occurrence of traumatic events. Previous studies using the TLEQ have reported: Cronbach α of .94, test re-test reliability of .89, and convergent correlation with clinician interview of .86 and Distressing Life Event Questionnaires of .72 (Kubany, Leisen, Kaplan, & Kelly, 2000).
DSM-IV diagnoses of PTSD and major depressive disorder (MDD) were accomplished using the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995) and Diagnostic Inventory of Depression (DID) (Zimmerman, Chelminski, & Young 2004). Studies using the DID reported: Cronbach α of .93, test re-test reliability of .86, and convergent correlation of 0.86 with the Hamilton Depression Scale (Zimmerman, Chelminski, & Young 2004); and the CAPS: Cronbach α of .88, inter-rater reliability of .92, and convergent correlation of .83 with the PTSD Symptom Scale Interview and .73 with the Structured Clinical Interview for DSM-IV (Foa and Tollin, 2000). The primary investigator administered the CAPS and DID to all participants and received training in CAPS administration prior to beginning the study.
Procedures
At the conclusion of the diagnostic interview, participants were given oral and written directions for saliva collection, passive drool, on the night before the second appointment at 10:00 PM and at 8 AM on the morning of the second appointment. Briefly, participants were told not to brush teeth, eat or drink or smoke 30 minutes prior to collecting the saliva samples at both time points. The primary investigator was available by telephone to answer questions regarding saliva collection.
The second appointment took place between the hours of 9:30 and 10:30 AM within one week of the first appointment. Saliva was collected the night prior and the morning of the second appointment. Participants brought their saliva samples and underwent venipuncture at the clinic. Following the blood sampling, participants were asked if any notable events occurred between the first and second appointments.
Within one hour of venipuncture, blood was diluted 1:10 with media and was either co-incubated with phytohemagglutinin (PHA, 50µg/ml) plus lipopolysaccharide (LPS, 50µg/ml) or not for 48 hours, at which time the supernate was harvested, aliquoted and frozen at −80° C for batch assay. TNF-α, IL-1β and IL-6 levels in both stimulated and unstimulated supernate were then assessed in duplicate using enzyme-linked immunosorbent assay (ELISA, R and D Systems, Minneapolis, MN). When detectable, levels of unstimulated cytokines were subtracted from stimulated cytokine levels for statistical analysis. Saliva samples were aliquoted and frozen at −80° C on collection day for batch assay of cortisol, DHEA and sIgA in duplicate using ELISAs (Salimetrics, State College, PA). Both the inter- intra-assay coefficient of variation was ≤ 9% for all of the ELISA assays.
Data Analysis
Group differences were assessed using two different ANCOVA models, with both models controlling for the variables of age, body mass index (BMI) and smoking. The first ANCOVA model tested for differences between the PTSD, trauma control, and control groups. Subsequently, group differences were examined between those with PTSD with MDD versus PTSD without MDD. This analysis plan was used first to isolate the impact of PTSD in this small sample that displayed great variability in biologic measures, and subsequently to investigate possible differences in PTSD participants with MDD and PTSD participants without MDD. No interactions among the control variables of age, BMI and smoking were detected and all control variables were included in the analyses of each outcome variable. A log transformation was performed on the variables that demonstrated unequal variances among groups (by the Kruskal-Wallis test) including IL-1β, evening cortisol, DHEA, and DHEA/cortisol. ANCOVA global testing was then performed for all 8 biologic variables, and if the ANCOVA model yielded significant group differences, Scheffé post-comparisons were used to identify specific differences. Pearson’s product moment correlation coefficients were used to assess the strength of relationships between variables of interest, using Bonferroni adjustment to reduce possible type I error. Log transformed values were used for all correlations. All correlations demonstrated a linear relationship between the dependent and independent variable. The DHEA/cortisol ratio was computed for each group using the AM value, as there were more observed group differences at this time point.
Lastly, to examine group differences in diurnal rhythms of cortisol and DHEA a regression line of best fit with 95% confidence intervals was used, and a regression model was used test the diurnal pattern and group membership interaction of both control groups and the PTSD group. In all analyses, only those participants who provided a blood or saliva sample were included in the analyses of group differences in that measure.
Results
Demographics of the sample are displayed in Table 1. The sample was primarily African American, 80.5%, and 29 had current PTSD, 30 were trauma controls and 24 were non-trauma controls; both control groups did not have current MDD or PTSD. The mean age of the sample was 42.6 year, and almost half, 49.4%, reported never being married. Groups did not differ in marital status, age, race, level of education or employment status. The majority of the PTSD group, 65.5%, reported an assaultive event as the causative event for PTSD development. Thirteen of the 26 PTSD participants had co-morbid MDD. The following traumatic events were attributed to PTSD cases: 24.1% child sex abuse, 17.2% unexpected death of a family member or close friend, 13.8% rape or sexual assault, 10.3% child physical abuse, 10.3% intimate partner violence, 7.5% physical assault by a non-intimate partner, and 3.5% witnessing physical assault or murder of another person. The mean CAPS score was 88.6 (standard deviation = 8.4) in the entire PTSD group, with no difference between PTSD participants with MDD (mean = 89.4, and standard deviation = 9.3) and PTSD participants without MDD (mean =86.5, standard deviation = 7.4). Mean PTSD duration was 44.1 months (standard deviation = 12.7), with more than two thirds of the PTSD sample, 69%, diagnosed with chronic PTSD.
Table 1.
| Characteristic | Non-traumatized controls (n=21) |
Trauma controls (n=24) |
PTSD (n=26) |
|---|---|---|---|
| Race | |||
| African American % | 85.6 | 86.8 | 75.9 |
| White % | 12.2 | 10.3 | 20.7 |
| Other % | 2.2 | 3.5 | 3.5 |
| Age (M, SD) | 43.2 (7.8) | 41.2 (8.1) | 44.7 (7.5) |
| 35 years or less % | 21.0 | 24.1 | 17.2 |
| 36 to 45 years % | 33.0 | 30.9 | 31.0 |
| 46 to 50 years % | 46.0 | 45.0 | 51.7 |
| Marital status | |||
| Never married % | 63.6 | 48.6 | 50.5 |
| Married % | 24.3 | 13.8 | 22.6 |
| Separated/divorced % | 11.0 | 17.2 | 27.6 |
| Education | |||
| Less than high school % | 9.2 | 7.9 | 11.2 |
| High school graduate % | 62.6 | 55.2 | 51.7 |
| Some college or training % | 28.2 | 37.9 | 31.0 |
| Body mass index | 27.9 (SD = 4.9) | 29.6 (SD = 5.9) | 30.1 (SD = 7.8) |
| Current smokers % | 19.0 | 29 | 37.9 |
| Number of traumatic events | 0 | 4.0 (SD = 1.2) | 5.9 (SD = 2.8) |
HPA axis measures
Morning cortisol and DHEA levels differed between the groups, F(2,70) = 3.28, p < .05 and F(2,70) = 3.95, p < .05, respectively (Table 2). Scheffé post-comparisons indicated that the PTSD group exhibited lower a.m. levels of cortisol compared to the non-traumatized controls p<0.03, and the traumatized control group p<0.05. Scheffé post-comparisons indicated that the PTSD group exhibited higher a.m. levels of DHEA compared to the non-traumatized controls p <0.02, and the traumatized controls p <0.04. No differences were noted in the PM levels of cortisol or DHEA. PTSD participants with MDD exhibited lower AM DHEA levels compared PTSD participants without MDD with a mean of 223.6 versus a mean of 298.4, t(25)= 2.97, p <.05. Women with PTSD exhibited significantly higher AM DHEA/cortisol ratios compared to trauma controls and non-trauma controls, with no significant difference between the control groups F(1, 26) = 4.75, p < .05. Again, PTSD participants with and without depression differed in the DHEA/cortisol ratio, with the PTSD participants without MDD exhibiting a higher level than those with depression (1,632 versus 1,327) t(25) = 3.30, p <.05.
Table 2.
| PTSD (n=26) |
Trauma control (n=24) |
Non trauma control (n=21) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Saliva | ||||||
| Cortisol AM* µg/DL | 0.27 | 0.03 | 0.37 | 0.04 | 0.41 | 0.07 |
| Cortisol PM µg/DL | 0.15 | 0.09 | 0.18 | 0.12 | 0.40 | 0.07 |
| DHEA-S AM (pg/ml)** | 232.6 | 30.1 | 193.6 | 29.1 | 161.0 | 27.8 |
| DHEA-S PM (pg/ml) | 48.6 | 11.9 | 46.2 | 17.7 | 40.1 | 12.8 |
| slgA** (µg/ml) | 33.1 | 8.7 | 45.8 | 5.6 | 52.1 | 5.9 |
| DHEA-S/Cortisol** | 1,429.3 | 57.2 | 497.7 | 39.6 | 511.2 | 38.2 |
| Stimulated blood | ||||||
| IL-1β (pg/ml) | 2,048.1 | 481.6 | 1,885.3 | 531.9 | 1,759.1 | 655.7 |
| IL-6 (pg/ml)** | 14,167.3 | 776.3 | 11,997.8 | 966.1 | 9,677.8 | 313.8 |
| TNF-α (pg/ml)** | 1,787 | 377.6 | 893.7 | 256.6 | 766.9 | 244.7 |
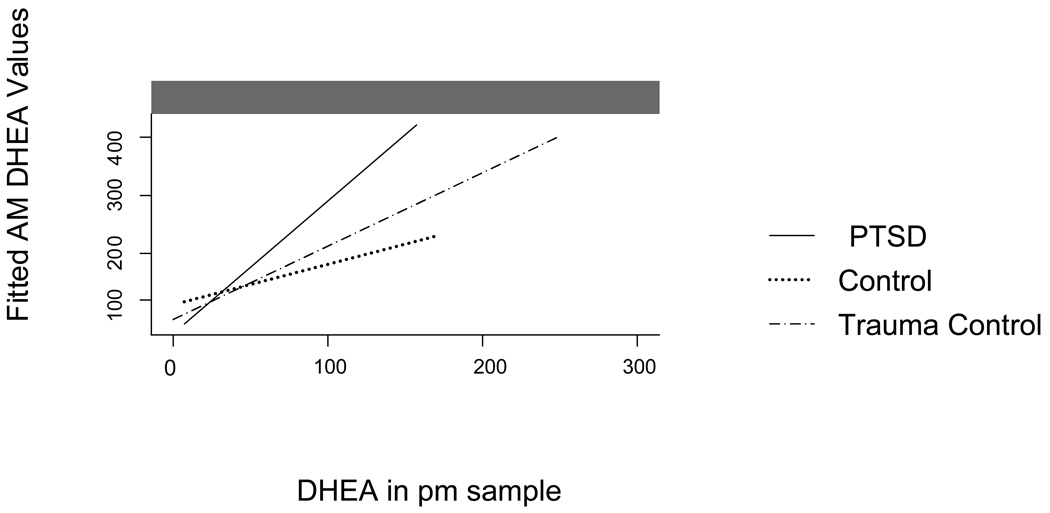
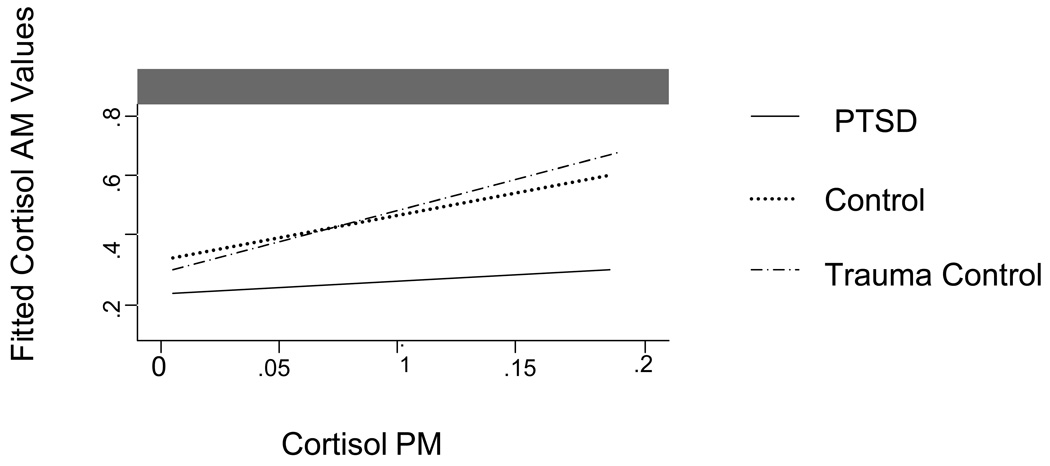
A regression fitted line of cortisol and DHEA (Figure 2 and Figure 3, respectively) in AM and PM samples, with 95% confidence intervals as well as regressed individual values. Significant group differences were detected with a regression model of cortisol DHEA p’s <.05, indicating different circadian patterns. For the PTSD group the parameter estimate was B 0.20 with a SE B 0.04. For the trauma control group the parameter estimate was 0.409 with a standard error of 0.021. For the non-traumatized control group the parameter estimate was 0.481 with a standard error of 0.018. In measures of DHEA the parameter estimate for the PTSD group was 249.32 and the standard error was 32.7. For the trauma control group the parameter estimate was 1999.6 and the standard error was 30.76. For the non-traumatized control group the parameter estimate was 202.7 and the standard error was 46.14. In addition, an interaction model with AM and PM levels and group membership indicated a significantly different pattern in those with PTSD (26) compared to both control groups (24 and 21) in the diurnal patterns of cortisol p < .01, and DHEA, p < .05. Further, among those with PTSD, 4 of the 26 participants exhibited higher PM levels than AM levels, compared to 1 of the 27 in the trauma control group and none in the control group.
Figure 2.
Figure 3.
Immune System Measures
Participants with PTSD exhibited a greater capacity for stimulated whole blood production of acute phase reaction cytokines when compared to both trauma and nontrauma control groups, including TNF-α F(2,70) = 5.91, p < .01 and IL-6 F(2,70) = 5.11, p < .01, but not IL-1β p = .07 (See Table 2). Trauma and non-trauma control groups differed only in IL-6 production, p < .05. Production of IL-6 was greater in those with PTSD and MDD compared to PTSD without MDD 18,329 versus 14,782, t(23)=3.77, p < .05. A trend of higher TNF-α production capacity was also observed in those with PTSD and MDD, compared to PTSD without MDD t(23) p < 0.08). Finally, participants with PTSD exhibited lower levels of salivary sIgA compared to both control groups F(1,70)= 5.72, p < .01].
HPA Axis and Immune Measures Relation in PTSD Symptom Characteristics
Correlations between the neuroendocrine and immune outcomes were explored to better understand the nature of alterations associated with PTSD symptoms determined by CAPS scores (Table 3). Key findings include significant correlations of TNF-α and IL-6 production with PTSD symptom intensity, re-experiencing and hyper-arousal symptom clusters. The hyper-arousal symptom cluster was negatively correlated with salivary cortisol, and positively with DHEA and DHEA/cortisol. Avoidance symptoms did not correlate with any biologic measure.
Table 3.
| Intensity of PTSD | Duration of PTSD | Re-experiencing symptoms | Hyper-arousal symptoms | Intensity of depression | |
|---|---|---|---|---|---|
| TNF-α pg/ml | 0.52* | ns | 0.24* | 0.41* | 0.64** |
| IL-6 pg/ml | 0.50 | ns | 0.38* | 0.40* | 0.62 |
| Cortisol AM µg/ml | ns | -0.63* | ns | -0.46* | ns |
| DHEA-S AM pg/ml | 0.42* | 0.47* | ns | 0.51* | −0.36* |
| DHEA-S/Cortisol | 0.31* | 0.52* | 0.40* | 0.54* | −0.46 |
Discussion
The current study found alterations in HPA axis and immune function in women with PTSD, specifically finding high levels of DHEA and low levels of cortisol, and high levels of whole blood stimulated TNF-α and IL-6 production. Findings of this study are consistent with those of Rohleder et al. (2004), who reported lower cortisol levels and greater whole blood IL-6 production in male and women Bosnian war refugees with PTSD, as well as those of Pervanidou et al. (2007), who linked elevated evening cortisol and morning serum IL-6 with PTSD development in children following a motor vehicle accident. Together these studies provide evidence of insufficient glucocorticoid signaling in PTSD participants, such that impaired feedback regulation of stress responses and HPA axis activity may be linked to greater pro-inflammatory immune responsiveness (Raison & Miller, 2003). Our finding of lower sIgA levels among those with PTSD may also be related as lower levels of sIgA could contribute to exposure to additional antigens that would require the mounting of an immune response.
In contrast, findings from other studies of immune and HPA axis activity in individuals with PTSD participants have not indicated insufficient glucocorticoid (GC) signaling. In particular, de Kloet et al. (2007) observed lower GC receptor density and lower levels of stimulated TNF-α in men with PTSD compared to controls. De Kloet et al. (2007) used a more recently traumatized group compared to Rohleder et al (2004), as well as the current study. PTSD duration may also affect HPA axis and immune function. Consistent with this suggestion, Vidovek et al. (2007) reported a positive correlation between GC receptor expression and number of years since combat in veteran men. Sex and PTSD duration may underlie these inconsistent findings, and further study is warranted.
The higher production capacity of TNFα, and IL-6 in women with PTSD that was observed in this study is consistent with previous findings (Baker et al., 2001; Maes et al., 1999; Spivak et al., 1997; Woods et al. (a), 2005) as well as other studies exhibiting increased inflammatory activity (Altemus, Dhabhar, & Wang, 2006; Song et al., 2007; Watson et al., 1993; Woods et al., 2005 (b); Vidovic et al., 2007). In this study both LPS and PHA were used to stimulate cytokine production, testing both the capacity of monocytes and macrophages, a similar method used by de Kloet et al. (2007). Greater inflammatory immune activity observed in this study provides some understanding of the mechanisms of increased health conditions including cardiovascular disease, chronic pain, diabetes and other medical conditions which are reported more often in individuals with PTSD (Boscarino et al., 2007; Dobie, 2004; Kimerling, 2004). These findings also provide additional incentive to develop interventions to treat these immune alterations that can lead to health declines.
In the current study, PTSD and MDD was associated with significantly lower AM DHEA and greater IL-6 production compared to PTSD without MDD. PTSD and MDD often co-occur, especially in women (Breslau et al., 1998; Kessler, et al., 1995). Other studies have shown IL-6 to be correlated with depression in individuals with PTSD (Miller et al., 2001) and the level of the IL-6 receptor to be significantly higher in individuals with PTSD and MDD compared to PTSD without MDD (Maes et al., 1999). DHEA administration has been shown to reduce MDD symptoms in depressed individuals (Hsiao, 2006), indicating a possible mechanism for symptom improvement. DHEA levels following HPA axis stimulation were inversely related to negative mood among women with chronic PTSD such that higher levels of depressive symptoms were related to lower production of DHEA (Rasmusson et al., 2004). Sondergaard (2002) reported that DHEA levels were lower in participants with PTSD and MDD compared to participants with PTSD and no MDD. Therefore, PTSD when it is comorbid with MDD may result in different biologic profiles when compared to PTSD alone and these biologic differences may have ramifications to psychological and physical health.
A flattened diurnal cortisol pattern was observed in PTSD participants in this study, replicating findings from studies including women with IPV and PTSD (Inslicth et al., 2006; Pico-Alfonso et al., 2004), women and men with PTSD plus MDD, but not PTSD alone (Tucker et al., 2004), adolescents with PTSD (Goenjian et al., 1996) and women with trauma and chronic pelvic pain, 69% of whom had PTSD (Heim et al., 1998).
There are limitations of the current study that should be noted. One limitation is that only two samples of saliva and one sample of blood was collected, possibly missing time points where biologic differences may have been better detected, and only 90% of participants provided saliva samples and 95% provided a blood sample. Also the use of non-stimulated basal samples for cortisol and DHEA and a cross-sectional design limited understanding of how these alterations resulted and how best to address them with an intervention. Another limitation of the study was the inclusion of only urban and primarily African American women, excluding the possibility of sex comparisons and generalizability; however few biologic studies of PTSD participants have included such a sample, making this study unique. In addition, this study differentiated traumatized participants with and without PTSD, indicating that it is primarily the development of PTSD is associated with biologic alterations.
Finally, PTSD hyper-arousal symptoms were associated with HPA axis alterations including low morning cortisol and high DHEA levels, with increased pro-inflammatory immune responses; however investigation of PTSD symptom clusters to HPA axis and immune function has been limited and further studies are needed. Insufficient glucocorticoid signaling may mediate such a profile; however investigation of PTSD symptom clusters to HPA axis and immune function has been limited. In addition, duration of PTSD symptoms was related to reduced cortisol and higher DHEA levels. Vidovic (2007) reported that GCR number was related to years since the trauma and to cortisol, suggesting that individuals with chronic PTSD may re-establish the set point for biologic functioning through the process of allostasis (McEwen, 2004).
In conclusion, the current study suggests that alterations in HPA axis and immune functions are exhibited in women with PTSD, and may be related. Some alterations may serve a protective or mitigating role, whereas others may result in physical and psychological health declines. Improved understanding of relations between HPA axis function and the immune system in PTSD participants is important, as it may result in the development of improved interventions for traumatized individuals, and may explain the mechanisms of increased medical co-morbidity observed in PTSD participants. The results of this study should therefore spur further study to understand the temporal relation of these alterations and how best to prevent biologic disruptions in traumatized individuals which may proceed PTSD development.
Funding Acknowledgments
National Institutes of Health, National Institute of Nursing Research (NINR) Postdoctoral Fellowship: 8326927, Individual National Research Service Award (NRSA) F31 NR009166 funded through NINR, Institutional Training Grant funded through NINR T32 NR 07968: Health Disparities in Underserved Populations, The Freedom from Fear Sharon Davies Memorial Grant.
Contributor Information
Jessica Gill, National Institute of Nursing Research, National Institutes of Health, 10 Center Drive, 10/CRC 2-1339, Bethesda, MD 20892-1506, Phone: (301) 451-1678, Fax: (301) 480-1413, E-mail: jgill@mail.nih.gov.
Meena Vythilingam, National Institutes of Mental Health.
Gayle G. Page, Johns Hopkins University, School of Nursing.
References
- Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. American.Journal of Psychiatry. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- Altemus M, Dhabhar FS, Yang R. Immune function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:167–183. doi: 10.1196/annals.1364.013. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney D. The development of a clinican adminsitered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Hoffman SN. Consistent association between mixed lateral preference and PTSD: Confirmation among a national study of 2490 US Army Vietnam veterans. Psychosomatic Medicine. 2007;69:365–369. doi: 10.1097/PSY.0b013e31805fe2bc. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain Behavior and Immunity. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Bikker A, Meulman E, Geuze E, Kavelaars A, et al. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Molecular Psychiatry. 2007;12:443–453. doi: 10.1038/sj.mp.4001934. [DOI] [PubMed] [Google Scholar]
- De Kloet CS, Vermetten E, Lentjes E, Geuze E, van Pelt J, Manuel R, et al. Differences in the response to the combined DEX-CRH test between PTSD patients with and without co-morbid depressive disorder. Psychoneuroendocrinology. 2008;33(3):313–320. doi: 10.1016/j.psyneuen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in women veterans: Association with self-reported health problems and functional impairment. Archives of Internal Medicine. 2004;164:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Annals of the New York Academy of Sciences. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Eckenrode JJ, Kim P, Wethington E, Horowitz E, Temple E. Salivary cortisol levels and mood vary by lifetime trauma exposure in a sample of healthy women. Journal of Traumatic Stress. 2007;20:689–699. doi: 10.1002/jts.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, et al. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. American Journal of Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Gotovac K, Sabioncello A, Rabatic S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: Lower quantity of GCR in patients with posttraumatic stress disorder (PTSD) Clinical & Experimental Immunology. 2003;131:335–339. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Frontiers in Bioscience. 2008;13:1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: Relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacology Bulletin. 1997;33:185–192. [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: A multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Hsiao CC. Difference in pre- and post-treatment plasma DHEA levels were significantly and positively correlated with difference in pre- and post-treatment Hamilton depression scores following successful therapy for major depression. Psychoneuroendocrinology. 2006;31:839–846. doi: 10.1016/j.psyneuen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, et al. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biological Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kimerling R. An investigation of sex differences in nonpsychiatric morbidity associated with posttraumatic stress disorder. Journal of the American medical Women’s Association. 2004;59:43–47. [PubMed] [Google Scholar]
- Kinder LS, Bradley KA, Katon WJ, Ludman E, McDonell MB, Bryson CL. Depression, posttraumatic stress disorder, and mortality. Psychosomatic Medicine. 2008;70:20–26. doi: 10.1097/PSY.0b013e31815aac93. [DOI] [PubMed] [Google Scholar]
- Koch AE, Distler O. Vasculopathy and disordered angiogenesis in selected rheumatic diseases: Rheumatoid arthritis and systemic sclerosis. Arthritis Research & Therapy. 2007;9 Suppl 2:S3. doi: 10.1186/ar2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA: A review. Journal of Clinical Pharmacology. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Leisen MB, Kaplan AS, Kelly MP. Validation of a brief measure of posttraumatic stress disorder: The Distressing Event Questionnaire (DEQ) Psychological Assessment. 2000;12:197–209. doi: 10.1037//1040-3590.12.2.197. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Aasal R, Adler L, Berger CL, Montgomery PT, Sandberg E, et al. Elevated cytotoxicity in combat veterans with long-term posttraumatic stress disorder: Preliminary observations. Brain Behavior and Immunity. 1998;12:74–79. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biological Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: A pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Ng V, Koh D, Mok B, Lim LP, Yang Y, Chia SE. Stressful life events of dental students and salivary immunoglobulin A. International Journal of Immunopathology and Pharmacology. 2004;17:49–56. doi: 10.1177/03946320040170S209. [DOI] [PubMed] [Google Scholar]
- Olff M, Guzelcan Y, de Vries GJ, Assies J, Gersons BP. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:1220–1230. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Olff M, de Vries GJ, Guzelcan Y, Assies J, Gersons BP. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology. 2007;32:619–626. doi: 10.1016/j.psyneuen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, et al. Lower cortisol levels in depressed patients with comorbid posttraumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Pico-Alfonso MA, Garcia-Linares MI, Celda-Navarro N, Herbert J, Martinez M. Changes in cortisol and dehydroepiandrosterone in women victims of physical and psychological intimate partner violence. Biological Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;9:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biological Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Schuld A, Mullington J, Friess E, Hermann DM, Galanos C, Holsboer F, et al. Changes in dehydroepiandrosterone (DHEA) and DHEA-sulfate plasma levels during experimental endotoxinemia in healthy volunteers. Journal of Clinical Endocrinology & Metabolism. 2000;85:4624–4629. doi: 10.1210/jcem.85.12.7055. [DOI] [PubMed] [Google Scholar]
- Sondergaard HP, Hansson LO, Theorell T. Elevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: Findings from a longitudinal study of refugees in Sweden. Psychotherapy and Psychosomatics. 2002;71:298–303. doi: 10.1159/000064806. [DOI] [PubMed] [Google Scholar]
- Sondergaard HP, Theorell T. A longitudinal study of hormonal reactions accompanying life events in recently resettled refugees. Psychother.Psychosom. 2003;72:49–58. doi: 10.1159/000067185. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhou D, Guan Z, Wang X. Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in Northern China. Neuroimmunomodulation. 2007;14:248–254. doi: 10.1159/000112050. [DOI] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, et al. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biological Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Spivak B, Maayan R, Kotler M, Mester R, Gil-Ad I, Shtaif B, et al. Elevated circulatory level of GABA(A)--antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychological Medicine. 2000;30:1227–1231. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nature Reviews. Immunology. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, et al. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biological Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Vidovic A, Vilibic M, Sabioncello A, Gotovac K, Rabatic S, Folnegovic-Smalc V, et al. Circulating lymphocyte subsets, natural killer cell cytotoxicity, and components of hypothalamic-pituitary-adrenal axis in Croatian war veterans with posttraumatic stress disorder: Cross-sectional study. Croatian Medical Journal. 2007;48:198–206. [PMC free article] [PubMed] [Google Scholar]
- von KR, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Watson IP, Muller HK, Jones IH, Bradley AJ. Cell-mediated immunity in combat veterans with post-traumatic stress disorder. Medical Journal of Australia. 1993;159:513–516. doi: 10.5694/j.1326-5377.1993.tb138003.x. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Woods AB, Page GG, O'lCampo P, Pugh LC, Ford D, Campbell JC. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. American Journal of Community Psychology. 2005;36:159–175. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]
- Woods SJ, Wineman NM, Page GG, Hall RJ, Alexander TS, Campbell JC. Predicting immune status in women from PTSD and childhood and adult violence. Advances in Nursing Science. 2005;28:306–319. doi: 10.1097/00012272-200510000-00003. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Archives of General Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Young D. On the threshold of disorder: A study of the impact of the DSM-IV clinical significance criterion on diagnosing depressive and anxiety disorders in clinical practice. Journal of Clinical Psychiatry. 2004;65:1400–1405. [PubMed] [Google Scholar]