Abstract
The possibility of permanent genetic changes to the germline is central to the bioethics of in utero gene therapy (IUGT) because of the concern of inadvertent potentially deleterious alterations to the gene pool. Despite presumed protection of the male germline due to early germ cell (GC) compartmentalization, we reported that GCs within the developing ovine testes are transduced at low levels after retrovirus-mediated IUGT, thus underscoring the need for a thorough understanding of GC development in clinically predictive models to determine the optimal time to perform IUGT and avoid germline modification. In the present studies, we used the fetal sheep model to analyze GCs for phenotype, location, proliferation, and incidence of transduction after IUGT at various fetal ages to learn when during development the nascent germline is likely to be at greatest risk of retrovirus-mediated alteration. Our studies show that although GCs were transduced at all injection ages, the levels of transduction varied by nearly 700-fold as a function of the age at transfer. After remaining largely quiescent as they migrated to/settled within nascent sex cords, GCs began active cycling before cord closure was complete, suggesting this is likely the point at which they would be most susceptible to retroviral transduction. Furthermore, we observed that compartmentalization of GCs continued into early postnatal life, suggesting the male germline may be vulnerable to low-level inadvertent retroviral vector modification throughout fetal life, but that this risk can be minimized by performing IUGT later in gestation.
Introduction
The ability to perform early prenatal diagnosis of many diseases that are candidates for treatment by gene therapy, coupled with the advantages of performing gene therapy before birth, has persuaded us and others to explore the feasibility and efficacy of gene transfer during fetal development (Themis et al., 1999; Walsh, 1999; Zanjani and Anderson, 1999; Coutelle et al., 2001, 2003; Coutelle and Rodeck, 2002; David et al., 2003, 2006; Lipshutz et al., 2003; Porada et al., 2004, 2005a,b; Jimenez et al., 2005; Lee et al., 2005; Waddington et al., 2005, 2007). Results of these studies have emphasized the need for developmental data from large animal models that would enable an accurate assessment of the risks associated with this procedure, specifically, the risk of genetic modification of the recipient's germ cells after in utero gene transfer (Wadman, 1998; Zanjani and Anderson, 1999). The possibility of permanent genetic changes to the germline is a concern that is central to the bioethics of in utero gene transfer, both because of the concern of inadvertent alterations to the gene pool in potentially deleterious ways (Gordon, 1998), and the remote implications of germline alteration being misused for the purpose of eugenics (Billings, 1999; Billings et al., 1999; King et al., 1999). Although numerous studies have now provided evidence that the germline of several species can be modified by both murine retrovirus- and lentivirus-based vectors if subjected to high multiplicities of infection in vitro (Nagano et al., 2001; Orwig et al., 2002; Kanatsu-Shinohara et al., 2004; Ryu et al., 2007), it has been presumed that vector modification of the germline in the male was unlikely to occur in vivo because of recognized early compartmentalization of germ cells (GCs) by Sertoli cells in the developing fetal testes of mammals (Coutelle and Rodeck, 2002; Coutelle et al., 2003). Contrary to this paradigm, we provided definitive evidence that fetal male GCs are in fact transduced at low levels when a murine retroviral vector is injected intraperitoneally into the developing ovine fetus (Porada et al., 2005b). This important and unexpected finding suggests that our understanding of GC behavior within fetal testes as pertains to risk of genetic modification is incomplete, and forms the basis of the present studies.
Whereas the early formation and migration of primordial germ cells (PGCs) have been well characterized in embryos of numerous species (Blandau et al., 1963; Pelliniemi, 1975; Fujimoto et al., 1977; O'Rahilly, 1983; Paranko et al., 1983; Urven et al., 1989; Satoh, 1991; Wei and Mahowald, 1994; Jezek et al., 1996; Buehr, 1997; Petitte et al., 1997; Braat et al., 1999; Molyneaux and Wylie, 2004; Raz, 2004; Stebler et al., 2004), relatively few studies have examined the behavior and maturation of GCs within the gonads during the fetal period (Baker, 1963; Pelliniemi, 1975; Motta et al., 1997a,b; Pauls et al., 2006). More importantly, no studies have yet been undertaken with the intent of assessing when during development the GCs would likely be at greatest risk of modification by the retroviral vectors currently in widespread clinical use.
In the present work, we performed studies to better understand the development of the male germline in the well-established sheep model, in the hopes that this will allow us to delineate when during development GCs would most likely be at greatest risk of modification with murine retroviral vectors, which require cell division for genomic integration. In the first set of studies, we used Ki67 to trace the proliferative state of the developing GCs, and characterized the migration and compartmentalization of GCs, examining their expression of the primitive pluripotent stem cell markers stage-specific embryonic antigen (SSEA)-1, SSEA-3, and SSEA-4. We examined these markers in the testes of normal control fetal sheep at intervals between gestational days 54 and 114 (term, 145 days) to establish the kinetics of GC movement and differentiation. We selected this period of gestation because it, in our opinion, represents the period during which in utero therapeutic intervention would likely be feasible, based on currently available diagnostic tests and technical practicality due to the larger size of the fetus. Concomitantly, in a second set of studies, we performed in utero gene transfer by intraperitoneal injection of retroviral vector into fetal sheep of various ages encompassing the same period of gestation employed for the studies on GC movement and differentiation. We then examined their testes at 30–60 days postinjection for the presence of transgene-expressing GCs to ascertain what effect recipient age had on the risk of germline alteration. The results of these two sets of studies provide some of the first detailed information regarding the characteristics and behavior of GCs during fetal testicular development in this well-established large animal model, and show that although male GCs are modified regardless of recipient age at the time of in utero gene therapy (IUGT), the rate of transduction varies by nearly 700-fold as a function of recipient age, with the greatest risk to the germline occurring if IUGT is performed early in gestation, before day 65 (term, 145 days). On the basis of our results, we propose that male fetal GCs are most likely to be vulnerable to genetic modification by murine retroviruses while they commence cell cycling within partially developed sex cords located near the rete testes, and that the risk of inadvertent male germline transduction can be minimized by performing IUGT at later points in gestation.
Materials and Methods
Gene transfer protocol
For the studies detailed in this paper assessing germ cell transduction, fetal sheep at 54–65 (n = 8) and 70–114 (n = 7) days of gestation (term, 145 days) received the MSCV-NeoR-RFP retroviral vector as described (Porada et al., 1998, 2005a,b). In short, once a surgical plane had been achieved through anesthesia, the fetal peritoneal cavity was localized by palpation, and an intraperitoneal injection of retroviral supernatant at a titer of 1 × 106 plaque-forming units/ml (1 ml for 54- to 59-day-old fetuses, 2 ml for 60- to 65-day-old fetuses, and 3 ml for 70- to 74-day-old fetuses) was given to each fetus, and the incisions were closed. As in our prior studies, these vector doses were taken from literature suggesting that, on the basis of fetal growth kinetics, we would obtain a relatively constant ratio of vector particle number to gram weight, at least from 54 to 74 days of gestation (Cock et al., 2001; Osgerby et al., 2002; Vonnahme et al., 2003; Kutzler et al., 2004). Because of limitations in the volume of vector that could safely be injected, animals at 100–114 days of gestation also received 3 ml of vector supernatant. Also, because of risks and difficulties in attempting to perform a hysterotomy at greater than 70 days of gestation, these pregnant ewes were prepared and anesthetized as described previously, but the vector was injected percutaneously under ultrasound guidance, similar to other previously published studies in sheep, rabbits, and monkeys (Baumgartner et al., 1999; Tarantal et al., 2001a,b; Peebles et al., 2004). In ongoing in utero gene transfer studies and our studies on in utero hematopoietic stem cell (HSC) transplantation, we have alternatively employed injection by palpation and injection under ultrasound guidance. In our hands, these two approaches produce essentially identical results as far as accuracy of injection into the peritoneal cavity, and also with respect to the success of the procedure, be it gene transfer or HSC engraftment. In the present studies we employed primarily the surgical/palpation method, because we are more efficient with this approach. Animals were killed at 30–60 days postinjection and the testes collected for analysis of transduction. This study was approved by the University of Nevada, Reno Institutional Animal Care and Use Committee (IACUC). Surgical procedures were performed at the surgical facility at the University of Nevada's Agriculture Experiment Station, in accordance with the facility's standard operating procedures (IACUC protocol). The animals were cared for according to the standards of the U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals (PHS Manual, pp. 116–118).
Preparation of frozen tissue sections
Control fetal sheep were killed at gestational ages 58, 65, 68, 74, and 103 days for studies on germ cell development/migration, and sheep that had undergone IUGT at various stages of gestation were killed 30–60 days postinjection. In all cases, death was achieved with injections of Euthasol (pentobarbital and phenytoin; Delmarva Laboratories/Virbac, Forth Worth, TX), and fetal testes were collected in ice-cold Dulbecco's phosphate-buffered saline (D-PBS) and fixed in 4% paraformaldehyde in D-PBS for 2 hr at 4°C. The samples were washed in D-PBS three times for 5 min each and then cryoprotected in 20% sucrose in D-PBS overnight at 4°C. Before freezing, the tissues were incubated for 1 hr in freezing medium consisting of 1 part Tissue-Tek O.C.T. compound (Sakura Finetek U.S.A., Torrance, CA) and 2 parts 20% sucrose in D-PBS. Fetal testes were flash frozen with the freezing medium in Peel-A-Way base molds (Thermo Fisher Scientific, Waltham, MA) by immersion in 2-methylbutane (Thermo Fisher Scientific)-containing dry ice. A microtome (Minotome; Triangle Biomedical Sciences, Durham, NC) was used to section each tissue at −20°C, and 7- to 10-μm-thick cryosections were adhered to SuperFrost slides (Thermo Fisher Scientific). The slides were allowed to dry at room temperature for at least 1 hr and then stored at −80°C until use. Multiple sections from each testis of three normal control fetuses and from the IUGT recipients were examined at each gestational age.
Immunohistochemical labeling of stage-specific embryonic antigens
Frozen sections were stained with antibodies to SSEA-1, SSEA-3, and SSEA-4 (Developmental Studies Hybridoma Bank, Iowa City, IA). After removal of freezing medium with two 5-min washes in Tris-buffered saline (TBS, pH 7.6), the tissues were outlined with a wax pen and placed in 0.3% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) in TBS (pH 7.6) for 30 min at room temperature to block endogenous peroxidase activity. Residual hydrogen peroxide was removed by three 1-min washes in TBS (pH 7.6), and the sections were then permeabilized in 0.005% Triton X-100 (Sigma-Aldrich) in TBS (pH 7.6) for 15 min. After two rinses in TBS (pH 7.6), sections were blocked for nonspecific antibody binding with 5% dry milk (Nestlé USA, Glendale, CA) in TBS (pH 7.6) for 15 min before addition of the primary antibody at a concentration of 10 μg/ml in 5% dry milk in TBS (pH 7.6). After an overnight incubation at room temperature with the primary antibody, sections were rinsed three times for 1 min each in TBS (pH 7.6) before addition, for 1 hr at room temperature, of a biotinylated secondary antibody diluted 1:500 in 5% dry milk in TBS (pH 7.6). For detection of reactive anti-SSEA-1 antibody, biotin-conjugated goat anti-mouse IgG+IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) was used, whereas anti-SSEA-3 and anti-SSEA-4 antibodies were detected with biotin-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories) and biotin-conjugated goat anti-rat IgG+IgM (Jackson ImmunoResearch Laboratories), respectively. After the secondary antibody incubation, sections were rinsed three times in TBS (pH 7.6) for 1 min each before incubation with horseradish peroxidase-conjugated streptavidin (2 μg/ml; Jackson ImmunoResearch Laboratories) in TBS (pH 7.6) for 30 min. Sections were washed three times in TBS (pH 7.6) for 3 min each followed by color development with 3,3′-diaminobenzidine (DAB) for 10 min. Finally, sections were counterstained with Mayer's hematoxylin (Sigma-Aldrich) and 0.5% ammonia water before dehydration through a graded ethanol series and xylene (Thermo Fisher Scientific). Glass coverslips were fixed over the sections with Permount (Thermo Fisher Scientific) before viewing with a BX-60 microscope (Olympus America, Center Valley, PA). For all staining experiments, sections of testes were processed through the staining protocol without primary antibody to serve as controls for specificity of the secondary antibodies and detection reagents.
Immunohistochemical labeling of proliferating cells
Frozen sections were prepared as detailed previously and then incubated at room temperature for 1 hr with rabbit anti-Ki67 antibody (Ab-4; Lab Vision/Thermo Fisher Scientific, Fremont, CA) diluted 1:50 in 4% dry milk–0.01% Tween 20–TBS (pH 7.6) in a humidified chamber. Residual unbound primary antibody was removed by three 1-min washes in TBS (pH 7.6). Sections were then incubated for 1 hr at room temperature with a biotin-SP-conjugated AffiniPure donkey anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Laboratories), washed, incubated with peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories), rinsed, and detected with DAB as described previously. Sections were then counterstained, dehydrated, and coverslipped with Permount. As in all other experiments, sections processed in the absence of primary antibody served as controls.
Immunohistochemical labeling of transduced cells
Frozen sections were prepared and processed as described previously, and then incubated overnight at 4°C in a humidified chamber with rabbit anti-neomycin phosphotransferase II (NPT) antibody (Cortex Biochem, San Leandro, CA) diluted 1:500. After three washes in TBS, sections were then incubated with biotin-SP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) washed, incubated with peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories), rinsed, and detected with DAB. Sections were then counterstained, dehydrated, and coverslipped with Permount. As in all other experiments, sections processed in the absence of primary antibody served as controls. Three sections from each of the testes from each animal were examined by light microscopy, 15,000–20,000 germ cells were identified in each testis morphologically, and expression of the transgene product (NPT) was assessed visually. The percentage of germ cells exhibiting transgene expression was then calculated by dividing the number of NPT-positive germ cells by the total number of germ cells present within each tissue section.
Immunofluorescence analysis of germ cell transduction
Mounted tissue sections were immersed in PBS, and then blocked in PBS containing 10% normal goat serum (NGS); blocked sections were incubated in primary antibody diluted in PBS with 2% NGS overnight at 4°C. Primary antibodies used were reactive to c-Kit (CD117) (Lab Vision/Thermo Fisher Scientific) and neomycin phosphotransferase II (Cortex Biochem). Slides were washed in PBS with 2% NGS and then incubated with secondary antibody in PBS with 2% NGS for 1 hr at 4°C. Secondary antibodies were conjugated to Alexa 488, 594, or 633. Sections were then labeled with 4′,6-diamidino-2-phenylindole (DAPI; BioGenex, San Ramon, CA). A FluoView 1000 confocal microscope system (Olympus America) was used to visually analyze tissue sections and capture images. Images of 10 to 12 sections from each of the testes from each animal were captured, 1500–2000 germ cells were identified in each testis morphologically and phenotypically by c-Kit expression, and the expression of the transgene product (NPT) was assessed. The percentage of germ cells exhibiting transgene expression was then calculated by dividing the number of NPT-positive germ cells by the total number of germ cells present within each tissue section.
Digital image acquisition
All images were captured with a DP70 charge-coupled device (CCD) camera attached to a BX-60 microscope, using Olympus DP Controller software version 2.1.1.183 (Olympus America). Images were then subjected to minimal global processing, such as brightness and contrast adjustment and color balance, in Adobe Photoshop CS (Adobe, San Jose, CA). For experiments involving confocal microscopy, a FluoView 1000 confocal microscope system and software were employed. Both × 40 and × 60 objectives (× 400 and × 600 visual magnification, respectively) were used to obtain fluorescence images; argon lasers emitting at 405 and 488 nm were used to excite DAPI and fluorescein isothiocyanate (FITC) fluorescence. A combinational diode laser was used to excite fluorophores in the Texas red (568 nm) spectrum. All images were manipulated with Adobe Photoshop CS to crop to specific regions and adjust the individual fluorescent channel signal-to-noise ratio.
Results
GCs migrating within the rete testes and interstitial space express SSEA-1
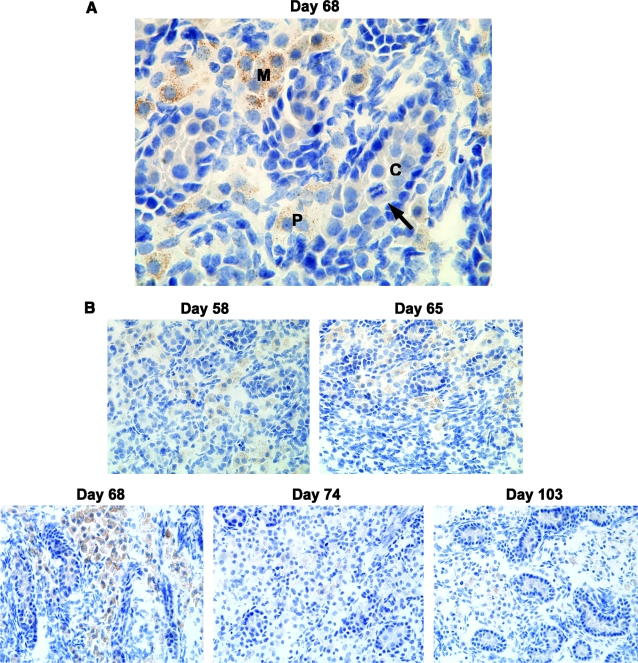
To characterize the distribution of GCs within ovine fetal testes, we detected endogenous alkaline phosphatase (AP) activity, a hallmark of early GCs, by the addition of nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP), an AP substrate that turns blue on catalysis. Surprisingly, cells with the highest AP activity localized to extracordal areas, with little AP activity in the Sertoli cells and GCs comprising the sex cords (Table 1A). Although the morphology and widespread distribution of AP-positive cells suggested that some of these were indeed migrating GCs, it was unclear whether immature somatic cells were also contributing to the AP signal. For this reason, we immunohistochemically labeled putative GCs, using monoclonal antibodies to SSEAs, which are markers of pluripotent stem cells. Immunostaining of fetal testes for expression of SSEA-1 revealed an abundance of weakly but specifically labeled GCs within the rete testes and in the interstitial areas between the sex cords. Interestingly, no anti-SSEA-1-reactive GCs were seen within well-defined sex cords, and all SSEA-1-labeled cells displayed large diffuse nuclei with high cytoplasmic content, which are characteristics of primitive GCs (Fig. 1A). Moderate amounts of GCs expressing SSEA-1 were located within the internal rete testes, where active cord formation and compartmentalization of GCs were most abundant as evidenced by partial and complete circular arrangements of densely nucleated spindle-shaped cells around GCs. However, the highest relative concentrations of SSEA-1-reactive cells were located in the extracordal areas within the cortex of the testes at all gestational ages examined (Fig. 1B), indicating that most early GCs are not passively compartmentalized by new cord formation but rather by active migration into preformed sex cords. Also of significance was the arrangement of SSEA-1-expressing GCs within fetal testes. Large numbers of extracordal SSEA-1-positive GCs appeared in clustered groups, sometimes in linear formation, indicating their state of active migration from the rete testes to the cortex. The number of SSEA-1-positive cells within the cortex of the testes peaked on day 68 with a gradual decline in the overall quantity of SSEA-1-reactive cells on day 74, and only minimal amounts of SSEA-1-labeled cells were detected within fetal testes on gestational day 103 (Fig. 1B and Table 1B).
Table 1.
Phenotypic and Proliferative Characteristics of Male GC During Development
| |
Location within fetal testes |
||
|---|---|---|---|
| Gestational day | Internal rete testes | Cortex (outside sex cords) | Cortex (inside sex cords) |
| A. Relative Abundance of Cells Exhibiting High Alkaline Phosphatase Activity | |||
| 58 | ++ | ++++ | + |
| 65 | ++ | ++++ | + |
| 68 | ++ | ++++ | + |
| 74 | ++ | +++ | — |
| 103 | + | +++ | — |
| B. Relative Abundance of Anti-SSEA-1-Reactive Cells | |||
| 58 | +++ | + | — |
| 65 | +++ | ++ | — |
| 68 | ++++ | +++ | — |
| 74 | + | ++ | — |
| 103 | + | + | — |
| C. Relative Abundance of Anti-SSEA-3-Reactive Cells | |||
| 58 | +++ | ++ | + |
| 65 | ++ | +++ | + |
| 68 | ++ | ++++ | ++ |
| 74 | ++ | ++ | +++ |
| 103 | ++ | ++ | ++++ |
| D. Relative Abundance of Anti-SSEA-4-Reactive Cells | |||
| 58 | + | + | + |
| 65 | + | + | +++ |
| 68 | + | ++ | ++++ |
| 74 | + | + | ++++ |
| 103 | + | + | ++++ |
| E. Relative Abundance of Anti-Ki67-Reactive Cells | |||
| 58 | + | + | +++ |
| 65 | + | + | ++++ |
| 68 | + | + | ++++ |
| 74 | + | + | ++++ |
| 103 | + | + | +++ |
—, no cells in region stain with marker; +, 1–24% of cells in region are positive; ++, 25–49% of cells in region are positive; +++, 50–74% of cells in region are positive; ++++, 75–100% of cells in region are positive.
FIG. 1.
Migrating GCs in the fetal testes express SSEA-1. (A) High-power (× 60 objective) magnification showing high levels of SSEA-1 expression in migrating GCs (M), weak SSEA-1 expression in partially compartmentalized GCs (P), and undetectable levels of SSEA-1 expression in GCs located within well-defined sex cords (C). Note mitotic figure (indicated by arrow) demonstrating proliferative activity of GCs within sex cord. (B) Medium-power (× 40 objective) magnification showing localization of SSEA-1 to GCs migrating within the extracordal space. Representative photomicrographs are shown.
SSEA-3 is a marker of GC transition from migration to sex cord compartmentalization
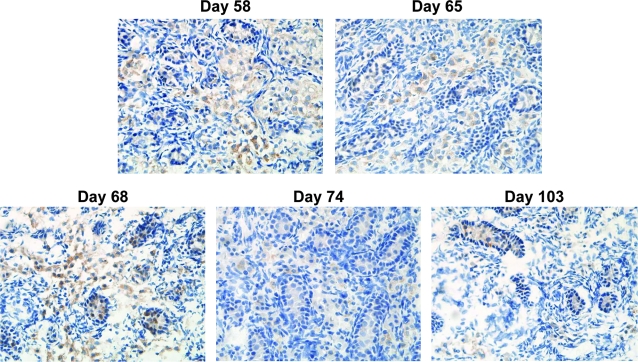
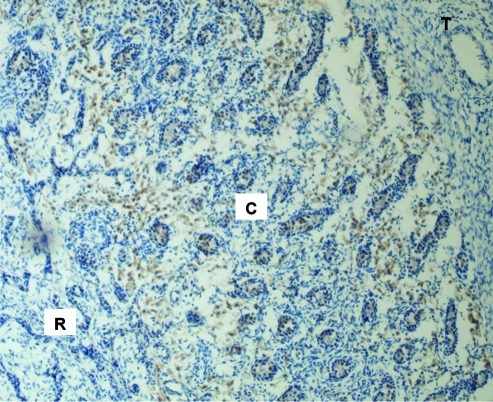
The distribution of anti-SSEA-3-reactive GCs within testes of various gestational ages closely overlapped with that of SSEA-1, especially within the internal rete testes and extracordal space of the cortex (Figs. 1B and 2; Table 1B and C). In contrast to SSEA-1, however, the reactivity of anti-SSEA-3 was higher within the cortex rather than the rete testes (Fig. 3, day 68), indicating that GCs begin to express SSEA-3 as they advance toward compartmentalization. Furthermore, throughout all gestational ages, a heterogeneous population of SSEA-3-expressing and nonexpressing GCs was found within the sex cords, which were often surrounded by numerous outlying SSEA-3-positive GCs (Fig. 2). This arrangement suggests that SSEA-3 expression within the sex cords marks GCs that have recently migrated into preformed sex cords. The relative quantity of SSEA-3-positive GCs within the extracordal space of the cortex began to decline on day 74, whereas the number of GCs within the sex cords continued to increase throughout day 103 (Fig. 2; Table 1C). The fact that both extracordal and intracordal GCs stained with anti-SSEA-3 indicates that SSEA-3 may serve as a useful marker for GCs that are undergoing the transition from migration to compartmentalization within fetal testes. As with the anti-SSEA-1 antibody, anti-SSEA-3 specifically marked only morphologically distinct GCs without any apparent labeling of somatic cells (Figs. 2 and 3).
FIG. 2.
SSEA-3 is a marker of GC transition from migratory state to sex cord compartmentalization within the fetal testes. Photomicrographs taken at medium-power magnification (× 40 objective) show the distribution of SSEA-3-labeled GCs. The anti-SSEA-3 antibody labeled migrating GCs in the extracordal area as well as GCs within well-defined sex cords at all gestational ages, indicating that it is a marker of transition to compartmentalization. Representative photomicrographs are shown.
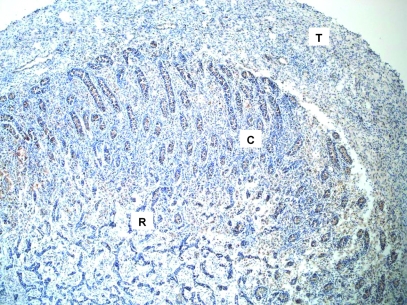
FIG. 3.
Distribution of SSEA-3-labeled GCs of fetal testes on gestational day 68. Representative low-power (×10 objective) photomicrograph shows SSEA-3-labeled GCs within the fetal testis. Note migrating GCs within the internal rete testes (R) and the presence of SSEA-3-expressing GCs both outside and within sex cords in the cortex (C). No SSEA-3 expression was detected within the tunica albuginea (T).
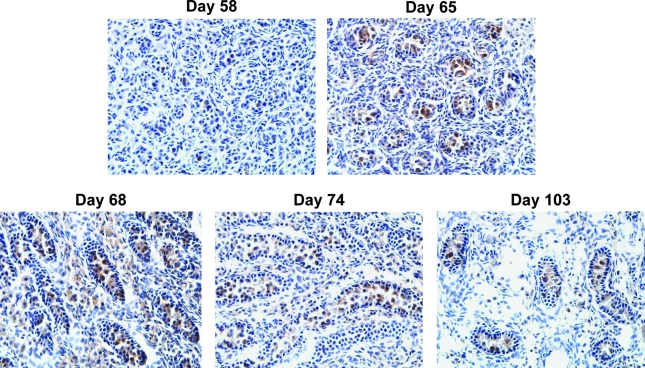
Compartmentalized GCs highly express SSEA-4
The pattern of labeling of fetal testis with anti-SSEA-4 was the most striking of the markers we investigated. Whereas only small numbers of mildly reactive GCs were found within the rete testes and extracordal space of the cortex, large numbers of robustly SSEA-4-immunoreactive GCs were almost exclusively localized to the intracordal space (Fig. 4; Table 1D), suggesting that the SSEA-4 antigen can serve as a fairly specific marker for compartmentalized GCs. The higher intensity of anti-SSEA-4 staining within the sex cords may correlate with higher expression of SSEA-4 within and on the surface of these compartmentalized GCs. However, it is also possible that the increased reactivity is due to an increased density of the SSEA-4 surface antigen concomitant with a change in the morphology and three-dimensional shape of GCs from a more diffusely structured migrating cell with extended pseudopodia to a more compact spherical cell within the sex cord.
FIG. 4.
SSEA-4 is a marker of GCs compartmentalized within fetal sex cords. Representative photomicrographs of medium-power magnification (× 40 objective) showing distribution of SSEA-4-labeled GCs within fetal testes. Robustly labeled GCs were highly localized to the intracordal area of well-defined sex cords at all gestational ages. Fetal testes of gestational day 68 showed a slight increase in anti-SSEA-4-reactive GCs in the extracordal areas compared with other gestational ages.
GCs remain quiescent during migration and proliferate within sex cords
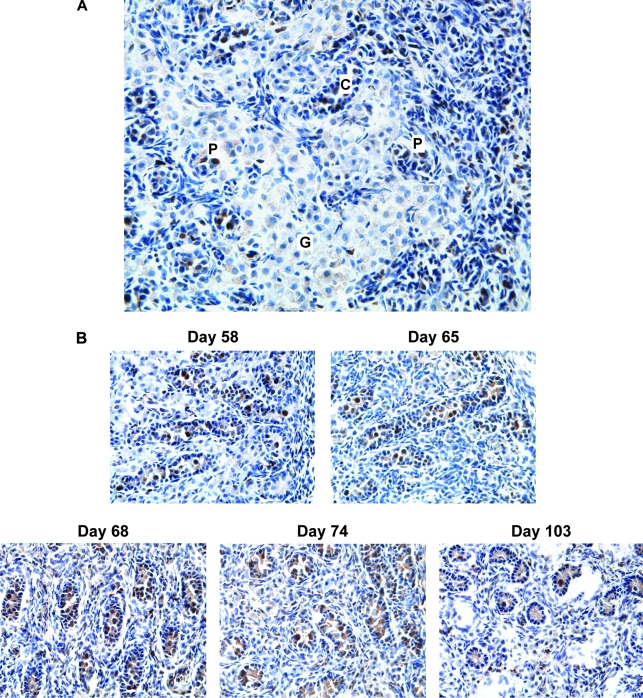
Because the murine retroviral vectors we have employed in our prior studies are able to transduce only cells that are actively cycling, because the vectors require nuclear membrane breakdown in order to achieve genomic integration, we next wished to investigate the cycling status of migrating and compartmentalized GCs within fetal testes and thus ascertain when the GCs would be most at risk of genetic alteration. To achieve this objective, we stained frozen sections of fetal sheep testes for Ki67, a nuclear protein expressed in proliferating cells during late G1, S, M, and G2 phases of the cell cycle. As can be seen in Figs. 5 and 6, the highest proliferative activity throughout all gestational ages was localized to GCs within well-defined sex cords. In addition, however, GCs within rete testes that appeared to be undergoing passive compartmentalization (as evidenced by partial cord formation with a semicircular arrangement of spindle-shaped somatic cells) also expressed Ki67, indicating that they were also actively cycling (Fig. 7A). In striking contrast to the robustly proliferating GCs within sex cords, GCs that appeared to be actively migrating within the internal rete testes and extracordal space remained predominantly quiescent (Figs. 5 and 6). Interstitial somatic cells and cells of the tunica vasculosa and albuginea also showed evidence of proliferation, but these proliferating cells were overshadowed by the degree of cycling observed in intracordal GCs (Fig. 5). As shown in Table 1 and Figs. 4 and 6B, expression of the proliferation status of GCs correlated most closely with the expression of SSEA-4.
FIG. 5.
Ki67 staining of fetal testis on day 68. Representative low-power (× 10 objective) photomicrograph shows the distribution of proliferating cells. Note highly concentrated proliferative activity within the sex cords, indicating that GCs expand in a protected environment after compartmentalization. R, internal rete testes; C, testicular cortex; T, tunica albuginea.
FIG. 6.
Compartmentalization induces cycling of GCs within fetal testes. Shown are representative photomicrographs of fetal testes after Ki67 staining. (A) Medium-power magnification (× 40 objective) shows quiescent GCs migrating through the internal rete testes (G), GCs beginning to cycle with the initiation of compartmentalization within partially formed sex cords (P), and GCs expanding within well-defined complete sex cords (C). (B) Medium-power magnification (× 40 objective) shows proliferating PGCs within sex cords and quiescent GCs within the extracordal space at all gestational ages. Note the similarity of signal distribution in comparison with Fig. 4.
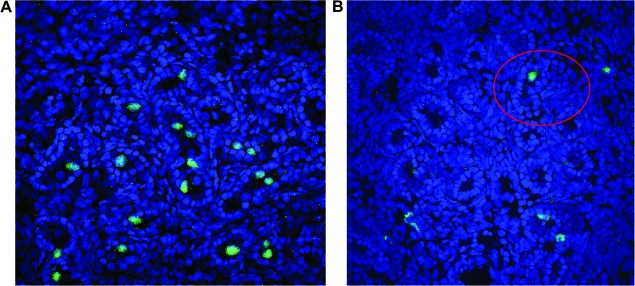
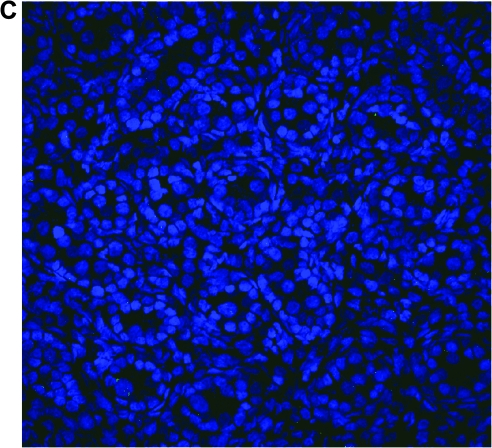
FIG. 7.
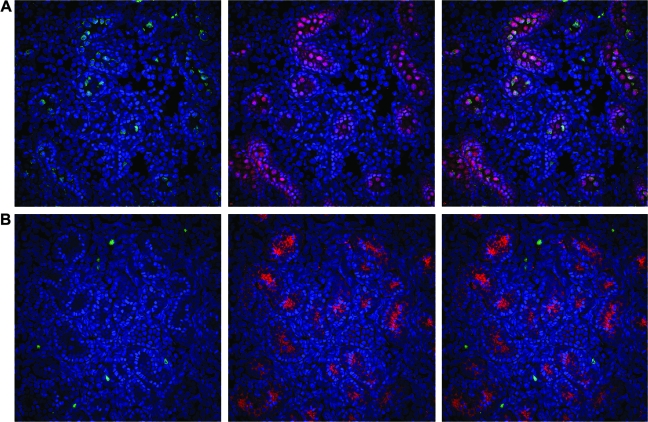
GC transduction is dependent on recipient age at time of in utero gene transfer (IUGT). (A) Representative section of testes from a sheep that underwent IUGT with the MSCVneo-DsRed retroviral vector early in gestation on day 61, showing numerous transduced GCs expressing vector-encoded neomycin phosphotransferase II (green) within well-defined sex cords. (B) Representative section of testes from a sheep that underwent IUGT with the MSCVneo-DsRed retroviral vector late in gestation on day 110, in which only a single transduced GC expressing the vector-encoded neomycin phosphotransferase II (green) can be seen (circled in red). In both images, nuclei were counterstained with DAPI (blue) to allow visualization of tissue morphology/architecture. (C) Representative section of testes from a normal control sheep stained with the antibody to NPT, demonstrating the high specificity of the antibody for the vector-encoded NeoR gene product.
Rate of transduction of GCs is dependent on recipient age at time of in utero gene transfer
We next wished to examine whether the age of the recipient played a role in the susceptibility of the male germline to inadvertent transduction with retroviral vectors. Fetal sheep were injected intraperitoneally, as has been described previously in detail (Porada et al., 2005b), at ages ranging from 54 days of gestation to 114 days of gestation (term, 145 days), adjusting vector volume to account for fetal mass. These recipients were then killed at 30–60 days posttransduction and their reproductive tissues were analyzed by immunohistochemistry with an antibody to the neomycin phosphotransferase II (NPT) transgene product. Because in prior detailed studies in a relatively large number of sheep (Porada et al., 2005b), we had not ever observed transgene expression within the germ cells of females that undergone in utero gene transfer with murine retroviral vectors, in the current studies we focused our analyses on male recipients. Surprisingly, transduced GCs were seen in male recipients regardless of the age at which transfer was performed, suggesting that there may always be a risk of inadvertent genetic modification of the male germline when performing IUGT with retroviral vectors. It is critical to note, however, that although transduction of GCs was observed in animals undergoing IUGT at each of the gestational ages tested, the incidence of GC transduction was profoundly affected by the age of the recipient at the time of IUGT. While animals undergoing IUGT before day 65 of gestation (term, 145 days; n = 8) exhibited GC transduction frequencies of 8.6 ± 2.9%, only four of seven animals undergoing IUGT after 70 days of gestation exhibited GC transduction, and the rates of GC transduction in these four animals were extremely low (0.01 ± 0.01%). This low incidence of GC transduction is in agreement with our previously published work (Porada et al., 2005b), is below the upper tolerable limit set by the U.S. Food and Drug Administration (Coutelle et al., 2003), and is several orders of magnitude less than the calculated frequency of naturally occurring endogenous insertional mutations in humans (Kazazian, 1999). However, the increased sensitivity afforded by our use of frozen sections and confocal microscopy in the present studies revealed that we had underestimated the incidence of GC transduction after early IUGT in our prior studies (Porada et al., 2005b), most likely as a result of antigen masking arising from our use of paraffin-embedded, formalin-fixed tissues. Shown in Fig. 7A is a representative section of the testes from a sheep that underwent IUGT early in gestation (on day 61), revealing that numerous NPT-positive (green) GCs are present within the center of clearly defined sex cords within this sheep, whereas Fig. 7B shows a representative section of testes from a sheep that underwent IUGT late in gestation (on day 110), in which only a single NPT-positive (green) GC can be seen (circled in red). As discussed previously, only four of the seven sheep undergoing IUGT late in gestation exhibited NPT-positive GCs, and the extremely low incidence of these transduction events (1 in 5000 to 1 in 10,000) necessitated viewing numerous sections to find even a single NPT-positive GC to photograph. In each of these images, nuclei have been counterstained with DAPI to allow visualization of the testes morphology. The specificity of the anti-NPT antibody for the vector-encoded NeoR gene product was confirmed by the absence of any staining on sections from the testes of a normal control sheep (Fig. 7C).
Given the higher than expected incidence of GC transduction seen in animals undergoing IUGT early in gestation, we next confirmed that the cells we were classifying as GCs on the basis of morphology were indeed GCs, based on their expression of c-Kit, because this antigen has been previously reported to be expressed specifically on GCs within the developing human gonad (Robinson et al., 2001). As can be seen in Fig. 8A, essentially all of the transduced (green) cells that appear morphologically to be GCs also stained positively for c-Kit, confirming their identity as GCs. In agreement with our prior analyses, showing low-level transduction of GCs after IUGT late in gestation, are results shown in Fig. 8B, which is a representative section from an animal that underwent IUGT on day 110 of gestation. As can be seen in Fig. 8B, numerous cells stained positive for NPT, demonstrating that they have been transduced as a result of IUGT, but none of these cells expressed c-Kit, thus confirming that they are not male GCs, but somatic cells of the testes.
FIG. 8.
Transduced cells identified morphologically as GCs express c-Kit, confirming their identity as male germ cells. (A) Representative section of testes from a sheep that underwent IUGT early in gestation on day 61, showing that essentially all transduced cells (green) that appear morphologically to be GCs express c-Kit (red), a marker specific for male germ cells within the developing testes. (B) Representative section of testes from a sheep that underwent IUGT late in gestation on day 110, showing numerous transduced cells (green), none of which are expressing c-Kit (red), thus demonstrating that these cells are not GCs, but are of somatic origin.
GCs continue to migrate into preformed sex cords after birth
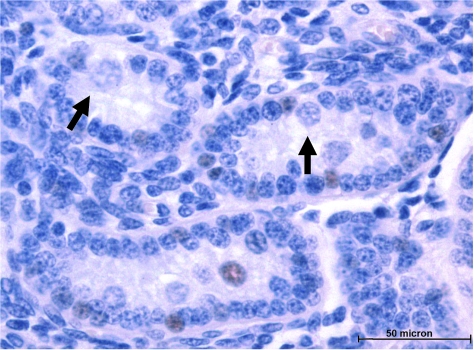
Because of the remarkable number of extracordal GCs expressing SSEAs within the fetal testes even on day 103, we reexamined closely the testes of neonates that received in utero injections of a retroviral vector (see Porada et al., 2005) for evidence of actively migrating GCs. Surprisingly, as seen in Fig. 9 (arrows), not all GCs were completely compartmentalized even after birth, as some GCs appeared to be actively migrating into well-developed sex cords. That the GCs were actually entering into the sex cord was suggested by the disruption of the juxtaposed continuous monolayer of Sertoli cells that encircled the sex cord. Although this observation could alternatively be interpreted as the GCs exiting the sex cord, this possibility seems less likely for two reasons. First, we observed that the sex cords grew in size with increasing age, rather than diminishing due to exiting of GCs over time. Second, we observed that many early sex cords did not contain GCs, which indicates that the augmentation of sex cords with increasing age was likely the result of migration of GCs into the intracordal space and/or proliferation of the germ cells after compartmentalization.
FIG. 9.
GCs migrate into the sex cords of neonatal testes. Shown is a high-power (×60 objective) magnification of neonatal testes; note the presence of germ cells entering the sex cords (arrows). DAB signal represents those cells expressing neomycin phosphotransferase II after intraperitoneal injection of the MSCVneo-DsRed retroviral vector on gestational day 61.
Discussion
The possibility of permanent genetic changes to the germline is central to the bioethics of IUGT because of concern about inadvertently introducing potentially deleterious alterations into the gene pool. Although numerous developmental studies have been performed in a range of species to examine the behavior and characteristics of germ cells (GCs), focusing primarily on their origin and migration during the embryonic and fetal periods (Baker, 1963; Blandau et al., 1963; Pelliniemi, 1975; Fujimoto et al., 1977; O'Rahilly, 1983; Paranko et al., 1983; Urven et al., 1989; Satoh, 1991; Wei and Mahowald, 1994; Jezek et al., 1996; Buehr, 1997; Motta et al., 1997a,b; Petitte et al., 1997; Braat et al., 1999; Molyneaux et al., 2001, 2003; Raz, 2002, 2003, 2004; Weidinger et al., 2002; Stebler et al., 2004; Pauls et al., 2006), none of these studies were undertaken with the goal of assessing risk of germline transduction as a function of GC development. In the present study, we performed a detailed analysis of GCs during their migration and compartmentalization in the developing fetal testes, using the clinically relevant sheep model to ascertain when during fetal development the male GCs would likely be at greatest risk of inadvertent transduction with retroviral vectors. The findings of our present investigation highlight several aspects of GC behavior within the fetal testes that may prove critical to assessing the risks of inadvertent germline modification after IUGT. First, although it has been known for some time that compartmentalization of GCs in the testes begins early in fetal development, the degree to which GCs are actually protected within the sex cords has not been examined directly. Our data provide evidence that ovine GCs sequentially express SSEA-1, SSEA-3, and SSEA-4 as they migrate to and become compartmentalized within the fetal testes, and suggest that SSEA-1 is a marker of migration and quiescence, whereas SSEA-4 is a marker of compartmentalization and proliferation. SSEA-3, on the other hand, appears to phenotypically define an intermediate stage of GC development that follows expression of SSEA-1 but precedes SSEA-4 expression. We also observed a moderate to high degree of endogenous AP activity within the internal rete testes and extracordal space of the testicular cortex throughout all gestational ages (data not shown), indicating that GC migration to and compartmentalization into sex cords is a dynamic process that progresses gradually and appears to continue after birth as evidenced by GCs migrating into well-defined sex cords within the neonatal testes. The high numbers of SSEA-1- and SSEA-3-positive GCs found within the extracordal space of fetal testes adds further support to the conclusion that the majority of GCs are in fact not compartmentalized during the early to mid-fetal period, and may thus be largely unprotected from potential modification with viral vectors.
Two of the major goals of the present studies were to ascertain when during gestation the GCs are likely to be at risk of transduction and to quantitate that risk. Because the fetal testes have extensive lymphatic networks that are directly connected with the peritoneal cavity, this seems to be the most likely route by which the intraperitoneally injected vector could reach the testes, with any migrating GCs that are not retained within the sex cords being exposed to the vector but not transduced because of their quiescent state. Once within the sex cords, GCs begin to proliferate and expand within the protected environment created by the encircling Sertoli cells and their presumed tight junctions. Because Sertoli cells comprising the sex cord also proliferate as numbers of intracordal GCs increase, they may serve as a cellular barrier by taking the hit of the virus in lieu of the GCs. This model is likely to explain the high ratio of Sertoli-to-germ cell transduction events that we observed previously (Porada et al., 2005b) and in the present studies after IUGT late in gestation, and that has been reported in mature mice after direct intratesticular injection of retroviral vectors (Ikawa et al., 2002). Thus our data suggest that the most vulnerable point of development of the fetal testes during which retroviral transduction could likely take place is during the passive compartmentalization of GCs, most notably within the rete testes, where we observed the commencement of GC cycling within partially formed sex cords. It is interesting to speculate that this point of GC vulnerability may not be limited to retroviral vectors, but may also be the point at which genetic modification takes place in response to environmental mutagens.
Our present findings have important implications for IUGT because one would presume that, in the male, the quiescent nature of the majority of the unprotected migrating GCs would render them largely refractory to transduction with murine retroviral vectors, which require cell division for transduction. In the present studies, we used highly sensitive immunofluorescence and confocal microscopy to demonstrate that significant levels of male GCs are in fact transduced if IUGT is performed early in gestation during the period when large numbers of SSEA-1- and SSEA-3-positive GCs are present within the unprotected extracordal spaces. In contrast, only extremely low levels of GCs are transduced if IUGT is performed late in gestation, presumably because of the greater percentage of GCs that are compartmentalized at this time. This extremely low risk to the male germline after direct intraperitoneal injections of murine retroviral vectors into fetal sheep is in agreement with our prior studies (Porada et al., 2005b). Importantly, however, the use of far more sensitive detection methodology and the switch to frozen sections, with the consequential elimination of potential antigen masking as a result of the paraffin embedding employed in our prior studies, enabled us to reveal higher risk to the male germline than was previously appreciated, and show that this risk is dramatically affected by age at the time of IUGT. This pronounced difference in incidence of germline alteration occurred over a fairly narrow window of gestation, with the change taking place sometime between gestational days 65 and 70 in the sheep. Although we do not have a definitive explanation for the mechanism responsible for this marked change, important anatomic alterations in the developing male gonads may provide an answer. In sheep, full term is 145 days, and therefore 65–70 days of gestation corresponds to roughly half-way through gestation, or weeks 18–20 in human pregnancy. During this period of time in the human fetus, the testicles descend from the pelvis to the scrotum, thus removing them from the immediate area of intraperitoneal vector injection, and providing a possible explanation for the dramatic decrease in germline transduction we observed over this relatively short period of time.
Interestingly, in the sheep model, we have not yet observed GC transduction within female recipients in any of our studies, which is surprising given the higher proliferative nature of migratory female GCs during fetal development. Our findings would appear to be at odds with elegant studies performed in nonhuman primates by Lee and colleagues (2005), in which the authors demonstrated that only female (not male) GCs are transduced after IUGT, and that they are transduced at substantially higher rates than we observed in the male germline in our prior studies (Porada et al., 2005b), but much more in line with the levels we observed in our present studies if IUGT was performed early in gestation. However, several key differences between our studies and those of Lee and colleagues (2005) could explain these seemingly contradictory results. First, Lee and colleagues employed nonhuman primates whereas we used sheep in our studies. We have not yet observed transduction of the female germline, which agrees with other IUGT studies performed in sheep (Themis et al., 1999; David et al., 2003), suggesting that the species may play a role in the susceptibility of the germline to transduction. Second, we employed murine retrovirus-based vectors, whereas Lee and colleagues employed vesicular stomatitis virus (VSV)-pseudotyped lentiviral vectors. The difference in both the vector itself and also the envelope used would likely have a pronounced effect on the cell types transduced after in utero intraperitoneal injection, especially because transduction with lentiviral vectors is not strictly restricted to mitotically active cells as is murine retrovirus-mediated transduction. Third, the methodology used for detection of transduced cells differs significantly between our present studies and those of Lee and colleagues. In the present studies, we employed confocal immunofluorescence microscopy to detect the vector-encoded NPT protein, whereas Lee and colleagues used laser dissection followed by polymerase chain reaction to detect vector genomes. Thus, the absence of “transduction” of female germ cells we observed could in fact be due not to lack of transduction, but to promoter silencing as a result of the relative transcriptional inactivity of germ cells (Lund et al., 1996; Koul et al., 2002). This is an area we plan to explore further as methods for isolating female ovine germ cells become available. Nevertheless, the disparate results of our studies and those of Lee and colleagues suggest that the choice of vector backbone and pseudotype will be key determinants of the risk to the germline after early IUGT.
In conclusion, we have demonstrated that GC migration and compartmentalization in the fetal testes of the clinically relevant sheep model is a gradual process that involves sequential expression of specific SSEAs at a distinct developmental stage, perhaps exerting an active physiologic or biochemical role in this complex process. We have provided evidence that the age of the recipient exerts a large impact on the risk of male GC transduction, at least in this model system, because the level of transduction of male GCs varied by nearly 700-fold as a function of fetal age at the time of IUGT. Whereas performing IUGT late in gestation appears to place the germline at extremely low risk (0.01–0.05%) of modification, performing IUGT before 65 days of gestation resulted in GC transduction rates of roughly 8%, at least within the large number of tissue sections that we examined in these studies. Thus, to maintain a risk of inadvertent modification that is below the calculated frequency of naturally occurring endogenous insertional mutations in humans and the upper tolerable limit of 1 event in 6000 sperm that was set by the U.S Food and Drug Administration (Kazazian, 1999; Coutelle et al., 2003), it may be necessary to optimize methods that allow efficient IUGT to be performed later in gestation to minimize the risk of inadvertent germline alteration. Alternatively, the use of vectors with carefully selected pseudotypes/serotypes (MacKenzie et al., 2002; Lipshutz et al., 2003; Bilbao et al., 2005; Pacak et al., 2006), or the development of novel vector systems or surgical/delivery procedures (Tarantal et al., 2001a; Henriques-Coelho et al., 2007) that allow targeting of desired tissues/cell types, could potentially eliminate this risk. In addition, we have presented here the first evidence of GCs actively migrating into sex cords in the neonatal period of a clinically relevant animal model, suggesting that male germline cells of newborns may be more vulnerable to genetic modifications than previously believed. Our findings indicate that the reproductive toxicology of both in utero and neonatal gene therapy studies involving direct in vivo administration of gene transfer vectors should be carefully examined for possible inadvertent germline modifications, because our findings and those of other groups suggest that the risk of vector integration into germ cells will likely vary with the type of viral vector, the species, the route of administration, and perhaps even the gender of the recipient (Gordon, 1998; Arruda et al., 2001; Lipshutz et al., 2003; Lee et al., 2005).
Acknowledgments
This paper is dedicated to the memory of Eileen Meredith, who is dearly missed and whose technical assistance made this work possible.
This work was supported by grant HD043038 from the National Institutes of Health. Evan Colletti is supported by CAT32 09563-19 from the National Institutes of Health. The SSEA monoclonal antibodies, developed by Davor Solter and Barbara Knowles, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences of the University of Iowa (Iowa City, IA).
Author Disclosure Statement
None of the authors has anything to disclose, as no competing financial interests exist.
References
- Arruda V.R. Fields P.A. Milner R. Wainwright L. De Miguel M.P. Donovan P.J. Herzog R.W. Nichols T.C. Biegel J.A. Razavi M. Dake M. Huff D. Flake A.W. Couto L. Kay M.A. High K.A. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Baker T.G. A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. Lond. B Biol. Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Baumgartner T.L. Baumgartner B.J. Hudon L. Moise K.J., Jr. Ultrasonographically guided direct gene transfer in utero: Successful induction of β-galactosidase in a rabbit model. Am. J. Obstet. Gynecol. 1999;181:848–852. doi: 10.1016/s0002-9378(99)70312-1. [DOI] [PubMed] [Google Scholar]
- Bilbao R. Reay D.P. Li J. Xiao X. Clemens P.R. Patterns of gene expression from in utero delivery of adenoviral-associated vector serotype 1. Hum. Gene Ther. 2005;16:678–684. doi: 10.1089/hum.2005.16.678. [DOI] [PubMed] [Google Scholar]
- Billings P.R. In utero gene therapy: The case against. Nat. Med. 1999;5:255–256. doi: 10.1038/6457. [DOI] [PubMed] [Google Scholar]
- Billings P.R. Hubbard R. Newman S.A. Human germline gene modification: A dissent. Lancet. 1999;353:1873–1875. doi: 10.1016/S0140-6736(99)01173-3. [DOI] [PubMed] [Google Scholar]
- Blandau R.J. White B.J. Rumery R.E. Observations on the movements of the living primordial germ cells in the mouse. Fertil. Steril. 1963;14:482–489. doi: 10.1016/s0015-0282(16)34981-0. [DOI] [PubMed] [Google Scholar]
- Braat A.K. Speksnijder J.E. Zivkovic D. Germ line development in fishes. Int. J. Dev. Biol. 1999;43:745–760. [PubMed] [Google Scholar]
- Buehr M. The primordial germ cells of mammals: Some current perspectives. Exp. Cell Res. 1997;232:194–207. doi: 10.1006/excr.1997.3508. [DOI] [PubMed] [Google Scholar]
- Cock M.L. Albuquerque C.A. Joyce B.J. Hooper S.B. Harding R. Effects of intrauterine growth restriction on lung liquid dynamics and lung development in fetal sheep. Am. J. Obstet. Gynecol. 2001;184:209–216. doi: 10.1067/mob.2001.108858. [DOI] [PubMed] [Google Scholar]
- Coutelle C. Rodeck C. On the scientific and ethical issues of fetal somatic gene therapy. Gene Ther. 2002;9:670–673. doi: 10.1038/sj.gt.3301761. [DOI] [PubMed] [Google Scholar]
- Coutelle C. Themis M. Schneider H. Kiserud T. Cook T. Douar A.M. Hanson M. Pavirani A. Rodeck C. Fetal somatic gene therapy: A preventive approach to the treatment of genetic disease: The case for. Ernst Schering Res. Found. Workshop. 2001;33:99–114. doi: 10.1007/978-3-662-04469-8_7. [DOI] [PubMed] [Google Scholar]
- Coutelle C. Themis M. Waddington S. Gregory L. Nivsarkar M. Buckley S. Cook T. Rodeck C. Peebles D. David A. The hopes and fears of in utero gene therapy for genetic disease: A review. Placenta. 2003;24((Suppl. B)):S114–S121. doi: 10.1016/s0143-4004(03)00140-1. [DOI] [PubMed] [Google Scholar]
- David A. Cook T. Waddington S. Peebles D. Nivsarkar M. Knapton H. Miah M. Dahse T. Noakes D. Schneider H. Rodeck C. Coutelle C. Themis M. Ultrasound-guided percutaneous delivery of adenoviral vectors encoding the β-galactosidase and human factor IX genes to early gestation fetal sheep in utero. Hum. Gene Ther. 2003;14:353–364. doi: 10.1089/104303403321208952. [DOI] [PubMed] [Google Scholar]
- David A.L. Peebles D.M. Gregory L. Waddington S.N. Themis M. Weisz B. Ruthe A. Lawrence L. Cook T. Rodeck C.H. Coutelle C. Clinically applicable procedure for gene delivery to fetal gut by ultrasound-guided gastric injection: Toward prenatal prevention of early-onset intestinal diseases. Hum. Gene Ther. 2006;17:767–779. doi: 10.1089/hum.2006.17.767. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Miyayama Y. Fuyuta M. The origin, migration and fine morphology of human primordial germ cells. Anat. Rec. 1977;188:315–330. doi: 10.1002/ar.1091880305. [DOI] [PubMed] [Google Scholar]
- Gordon J.W. Germline alteration by gene therapy: Assessing and reducing the risks. Mol. Med. Today. 1998;4:468–470. doi: 10.1016/s1357-4310(98)01323-9. [DOI] [PubMed] [Google Scholar]
- Henriques-Coelho T. Gonzaga S. Endo M. Zoltick P.W. Davey M. Leite-Moreira A.F. Correia-Pinto J. Flake A.W. Targeted gene transfer to fetal rat lung interstitium by ultrasound-guided intrapulmonary injection. Mol. Ther. 2007;15:340–347. doi: 10.1038/sj.mt.6300057. [DOI] [PubMed] [Google Scholar]
- Ikawa M. Tergaonkar V. Ogura A. Ogonuki N. Inoue K. Verma I.M. Restoration of spermatogenesis by lentiviral gene transfer: Offspring from infertile mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7524–7529. doi: 10.1073/pnas.072207299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek D. Hittmair A. Rogatsch H. Kos M. Lamina propria of sex cords in human fetal testis: An immunohistological and stereological study. Anat. Embryol. 1996;193:181–190. doi: 10.1007/BF00214709. [DOI] [PubMed] [Google Scholar]
- Jimenez D.F. Lee C.I. O'Shea C.E. Kohn D.B. Tarantal A.F. HIV-1-derived lentiviral vectors and fetal route of administration on transgene biodistribution and expression in rhesus monkeys. Gene Ther. 2005;12:821–830. doi: 10.1038/sj.gt.3302464. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M. Toyokuni S. Shinohara T. Transgenic mice produced by retroviral transduction of male germ line stem cells in vivo. Biol. Reprod. 2004;71:1202–1207. doi: 10.1095/biolreprod.104.031294. [DOI] [PubMed] [Google Scholar]
- Kazazian H.H., Jr. An estimated frequency of endogenous insertional mutations in humans. Nat. Genet. 1999;22:130. doi: 10.1038/9638. [DOI] [PubMed] [Google Scholar]
- King D. Shakespeare T. Nicholson R. Clarke A. McLean S. Risks inherent in fetal gene therapy. Nature. 1999;397:383. doi: 10.1038/16998. [DOI] [PubMed] [Google Scholar]
- Koul S. Houldsworth J. Mansukhani M.M. Donadio A. McKiernan J.M. Reuter V.E. Bosl G.J. Chaganti R.S. Murty V.V. Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol. Cancer. 2002;1:8. doi: 10.1186/1476-4598-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler M.A. Ruane E.K. Coksaygan T. Vincent S.E. Nathanielsz P.W. Effects of three courses of maternally administered dexamethasone at 0.7, 0.75, and 0.8 of gestation on prenatal and postnatal growth in sheep. Pediatrics. 2004;113:313–319. doi: 10.1542/peds.113.2.313. [DOI] [PubMed] [Google Scholar]
- Lee C.C. Jimenez D.F. Kohn D.B. Tarantal A.F. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta) Hum. Gene Ther. 2005;16:417–425. doi: 10.1089/hum.2005.16.417. [DOI] [PubMed] [Google Scholar]
- Lipshutz G.S. Titre D. Brindle M. Bisconte A.R. Contag C.H. Gaensler K.M. Comparison of gene expression after intraperitoneal delivery of AAV2 or AAV5 in utero. Mol. Ther. 2003;8:90–98. doi: 10.1016/s1525-0016(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Lund A.H. Duch M. Pedersen F.S. Transcriptional silencing of retroviral vectors. J. Biomed. Sci. 1996;3:365–378. doi: 10.1007/BF02258042. [DOI] [PubMed] [Google Scholar]
- MacKenzie T.C. Kobinger G.P. Kootstra N.A. Radu A. Sena-Esteves M. Bouchard S. Wilson J.M. Verma I.M. Flake A.W. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol. Ther. 2002;6:349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- Molyneaux K. Wylie C. Primordial germ cell migration. Int. J. Dev. Biol. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- Molyneaux K.A. Stallock J. Schaible K. Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev. Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Molyneaux K.A. Zinszner H. Kunwar P.S. Schaible K. Stebler J. Sunshine M.J. O'Brien W. Raz E. Littman D. Wylie C. Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- Motta P.M. Makabe S. Nottola S.A. The ultrastructure of human reproduction. I. The natural history of the female germ cell: Origin, migration and differentiation inside the developing ovary. Hum. Reprod. Update. 1997a;3:281–295. doi: 10.1093/humupd/3.3.281. [DOI] [PubMed] [Google Scholar]
- Motta P.M. Nottola S.A. Makabe S. Natural history of the female germ cell from its origin to full maturation through prenatal ovarian development. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997b;75:5–10. doi: 10.1016/s0301-2115(97)00216-9. [DOI] [PubMed] [Google Scholar]
- Nagano M. Brinster C.J. Orwig K.E. Ryu B.Y. Avarbock M.R. Brinster R.L. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R. The timing and sequence of events in the development of the human reproductive system during the embryonic period proper. Anat. Embryol. 1983;166:247–261. doi: 10.1007/BF00305086. [DOI] [PubMed] [Google Scholar]
- Orwig K.E. Avarbock M.R. Brinster R.L. Retrovirus-mediated modification of male germline stem cells in rats. Biol. Reprod. 2002;67:874–879. doi: 10.1095/biolreprod.102.005538. [DOI] [PubMed] [Google Scholar]
- Osgerby J.C. Wathes D.C. Howard D. Gadd T.S. The effect of maternal undernutrition on ovine fetal growth. J. Endocrinol. 2002;173:131–141. doi: 10.1677/joe.0.1730131. [DOI] [PubMed] [Google Scholar]
- Pacak C.A. Mah C.S. Thattaliyath B.D. Conlon T.J. Lewis M.A. Cloutier D.E. Zolotukhin I. Tarantal A.F. Byrne B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Paranko J. Pelliniemi L.J. Vaheri A. Foidart J.M. Lakkala-Paranko T. Morphogenesis and fibronectin in sexual differentiation of rat embryonic gonads. Differentiation. 1983;23((Suppl.)):S72–S81. doi: 10.1007/978-3-642-69150-8_13. [DOI] [PubMed] [Google Scholar]
- Pauls K. Schorle H. Jeske W. Brehm R. Steger K. Wernert N. Buttner R. Zhou H. Spatial expression of germ cell markers during maturation of human fetal male gonads: An immunohistochemical study. Hum. Reprod. 2006;21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- Peebles D. Gregory L.G. David A. Themis M. Waddington S.N. Knapton H.J. Miah M. Cook T. Lawrence L. Nivsarkar M. Rodeck C. Coutelle C. Widespread and efficient marker gene expression in the airway epithelia of fetal sheep after minimally invasive tracheal application of recombinant adenovirus in utero. Gene Ther. 2004;11:70–78. doi: 10.1038/sj.gt.3302130. [DOI] [PubMed] [Google Scholar]
- Pelliniemi L.J. Ultrastructure of the early ovary and testis in pig embryos. Am. J. Anat. 1975;144:89–111. doi: 10.1002/aja.1001440106. [DOI] [PubMed] [Google Scholar]
- Petitte J.N. Karagenc L. Ginsburg M. The origin of the avian germ line and transgenesis in birds. Poultry Sci. 1997;76:1084–1092. doi: 10.1093/ps/76.8.1084. [DOI] [PubMed] [Google Scholar]
- Porada C.D. Tran N. Eglitis M. Moen R.C. Troutman L. Flake A.W. Zhao Y. Anderson W.F. Zanjani E.D. In utero gene therapy: Transfer and long-term expression of the bacterial neor gene in sheep after direct injection of retroviral vectors into preimmune fetuses. Hum. Gene Ther. 1998;9:1571–1585. doi: 10.1089/hum.1998.9.11-1571. [DOI] [PubMed] [Google Scholar]
- Porada C.D. Park P. Almeida-Porada G. Zanjani E.D. The sheep model of in utero gene therapy. Fetal Diagn. Ther. 2004;19:23–30. doi: 10.1159/000074255. [DOI] [PubMed] [Google Scholar]
- Porada C.D. Park P.J. Almeida-Porada G. Liu W. Ozturk F. Glimp H.A. Zanjani E.D. Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol. Ther. 2005a;11:284–293. doi: 10.1016/j.ymthe.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Porada C.D. Park P.J. Tellez J. Ozturk F. Glimp H.A. Almeida-Porada G. Zanjani E.D. Male germ-line cells are at risk following direct-injection retroviral-mediated gene transfer in utero. Mol. Ther. 2005b;12:754–762. doi: 10.1016/j.ymthe.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Raz E. Primordial germ cell development in zebrafish. Semin. Cell Dev. Biol. 2002;13:489–495. doi: 10.1016/s1084952102001027. [DOI] [PubMed] [Google Scholar]
- Raz E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003;4:690–700. doi: 10.1038/nrg1154. [DOI] [PubMed] [Google Scholar]
- Raz E. Guidance of primordial germ cell migration. Curr. Opin. Cell Biol. 2004;16:169–173. doi: 10.1016/j.ceb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Robinson L.L. Gaskell T.L. Saunders P.T. Anderson R.A. Germ cell specific expression of c-Kit in the human fetal gonad. Mol. Hum. Reprod. 2001;7:845–852. doi: 10.1093/molehr/7.9.845. [DOI] [PubMed] [Google Scholar]
- Ryu B.Y. Orwig K.E. Oatley J.M. Lin C.C. Chang L.J. Avarbock M.R. Brinster R.L. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J. Androl. 2007;28:353–360. doi: 10.2164/jandrol.106.001511. [DOI] [PubMed] [Google Scholar]
- Satoh M. Histogenesis and organogenesis of the gonad in human embryos. J. Anat. 1991;177:85–107. [PMC free article] [PubMed] [Google Scholar]
- Stebler J. Spieler D. Slanchev K. Molyneaux K.A. Richter U. Cojocaru V. Tarabykin V. Wylie C. Kessel M. Raz E. Primordial germ cell migration in the chick and mouse embryo: The role of the chemokine SDF-1/CXCL12. Dev. Biol. 2004;272:351–361. doi: 10.1016/j.ydbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Tarantal A.F. Lee C.I. Ekert J.E. McDonald R. Kohn D.B. Plopper C.G. Case S.S. Bunnell B.A. Lentiviral vector gene transfer into fetal rhesus monkeys (Macaca mulatta): Lung-targeting approaches. Mol. Ther. 2001a;4:614–621. doi: 10.1006/mthe.2001.0497. [DOI] [PubMed] [Google Scholar]
- Tarantal A.F. O'Rourke J.P. Case S.S. Newbound G.C. Li J. Lee C.I. Baskin C.R. Kohn D.B. Bunnell B.A. Rhesus monkey model for fetal gene transfer: Studies with retroviral-based vector systems. Mol. Ther. 2001b;3:128–138. doi: 10.1006/mthe.2000.0255. [DOI] [PubMed] [Google Scholar]
- Themis M. Schneider H. Kiserud T. Cook T. Adebakin S. Jezzard S. Forbes S. Hanson M. Pavirani A. Rodeck C. Coutelle C. Successful expression of β-galactosidase and factor IX transgenes in fetal and neonatal sheep after ultrasound-guided percutaneous adenovirus vector administration into the umbilical vein. Gene Ther. 1999;6:1239–1248. doi: 10.1038/sj.gt.3300970. [DOI] [PubMed] [Google Scholar]
- Urven L.E. Abbott U.K. Erickson C.A. Distribution of extracellular matrix in the migratory pathway of avian primordial germ cells. Anat. Rec. 1989;224:14–21. doi: 10.1002/ar.1092240104. [DOI] [PubMed] [Google Scholar]
- Vonnahme K.A. Hess B.W. Hansen T.R. McCormick R.J. Rule D.C. Moss G.E. Murdoch W.J. Nijland M.J. Skinner D.C. Nathanielsz P.W. Ford S.P. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol. Reprod. 2003;69:133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- Waddington S.N. Kramer M.G. Hernandez-Alcoceba R. Buckley S.M. Themis M. Coutelle C. Prieto J. In utero gene therapy: Current challenges and perspectives. Mol. Ther. 2005;11:661–676. doi: 10.1016/j.ymthe.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Waddington S.N. Buckley S.M. David A.L. Peebles D.M. Rodeck C.H. Coutelle C. Fetal gene transfer. Curr. Opin. Mol. Ther. 2007;9:432–438. [PubMed] [Google Scholar]
- Wadman M. NIH launches discussion of in utero gene therapy…and seeks to repair “flaw” in review process. Nature. 1998;395:420. doi: 10.1038/26564. [DOI] [PubMed] [Google Scholar]
- Walsh C.E. Fetal gene therapy. Gene Ther. 1999;6:1200–1201. doi: 10.1038/sj.gt.3300991. [DOI] [PubMed] [Google Scholar]
- Wei G. Mahowald A.P. The germline: Familiar and newly uncovered properties. Annu. Rev. Genet. 1994;28:309–324. doi: 10.1146/annurev.ge.28.120194.001521. [DOI] [PubMed] [Google Scholar]
- Weidinger G. Wolke U. Koprunner M. Thisse C. Thisse B. Raz E. Regulation of zebrafish primordial germ cell migration by attraction towards an intermediate target. Development. 2002;129:25–36. doi: 10.1242/dev.129.1.25. [DOI] [PubMed] [Google Scholar]
- Zanjani E.D. Anderson W.F. Prospects for in utero human gene therapy. Science. 1999;285:2084–2088. doi: 10.1126/science.285.5436.2084. [DOI] [PubMed] [Google Scholar]