Summary
The primary cilium, a hair-like extension from a cell’s surface, acts as a sensory organelle to receive signals that regulate cellular behavior and physiology. Like most mammalian cells, neural progenitors and neurons have primary cilia. Recent studies show that this tiny projection plays important roles in brain development and diseases. Ciliary mutant mice show defects in brain patterning, progenitor proliferation, and specification of adult neural stem cells. Primary cilia also have dual opposing functions in the development of brain tumors. Ciliary defects are associated with genetic syndromes that frequently have neurological symptoms. Understanding the multifaceted roles that primary cilia have in brain development will provide important insights into the mechanism of brain development and diseases.
Introduction
The mammalian brain consists of billions of neuronal and glial cells and trillions of neuronal connections. The construction of this complex organ begins as a single layer of neuroepithelial cells and requires the proliferation, differentiation and migration of neural precursors. This remarkable transformation is precisely regulated by chemical (growth factors and morphogens) and physical cues (cell-cell and cell-matrix interactions) from the microenvironment. Defective regulation of this process results in developmental abnormalities and cancer.
An important set of clues on how neural progenitors and mature neurons function comes from a tiny cell surface protrusion called the primary cilium. The primary cilium grows from the basal body, a modified centriole that organizes the mitotic spindle during cell division. Thus, the primary cilium is resorbed before mitosis. This intrinsic relationship between cell cycle and ciliogenesis suggests that primary cilia could play key roles in cell proliferation and differentiation during development [1–3]. Furthermore, recent work has shown that the primary cilium functions as a “cellular antenna” for extracellular cues that regulate brain development [4,5]. The motile cilium is also a sensory organelle [6,7]. In the brain, motile cilia (9+2 microtubule structure) are present only in a specific set of cells: ependymal cells (E1) lining the ventricle in the adult brain and some choroid plexus cells. Polarized beating of ependymal cilia propels cerebrospinal fluid (CSF) flow, which is important for migration of young neurons produced in adult subventricular zone [8]. Ependymal cilia may monitor CSF to regulate CSF homeostasis and adult neurogenesis in the subventricular zone. Interestingly, ependymal (E2) cells with two long cilia and unique large basal bodies have been recently described in the walls of the lateral ventricle [9]. These cilia have 9+2 microtubule structure and are likely motile. Yet, their 2 cilia are unlikely to contribute significantly to CSF flow in the lateral ventricles where the majority of ependymal cells have 50 or more cilia. E2 cells are likely to have important sensory functions that remain to be identified. Ependymal cells with 1–3 cilia have also been described in the central canal where they may also have a sensory function and possibly help propel CSF through this narrow canal [10,11]. In contrast to motile cilia, primary cilia are present in most cells in the brain: neural stem and some choroid plexus cells touching the brain ventricles as well as in neurons and astrocytes within the brain parenchyma [12–16]. Here, we highlight some of the critical roles of primary cilia in the brain development.
The primary cilium: form and function
Primary cilia, typically 200 – 300 nm wide and 2 – 10 microns long, are present in most vertebrate cells (http://www.bowserlab.org/primarycilia/cilialist.html). This elongated organelle lacks protein synthesis and imports all of its components used for assembly, maintenance, and function from the cell body. Intraflagellar transport (IFT) is a dedicated mechanism, in which Kinesin-II motors move ciliary components from the cell body into the cilia (anterograde transport) and cytoplasmic dyneins move them back from the cilia to the cell body (retrograde transport) [17]. Mutations disrupting IFT cause defective ciliogenesis. Important insights into the function of primary cilia have come from studies of ciliary mutants often defective for IFT.
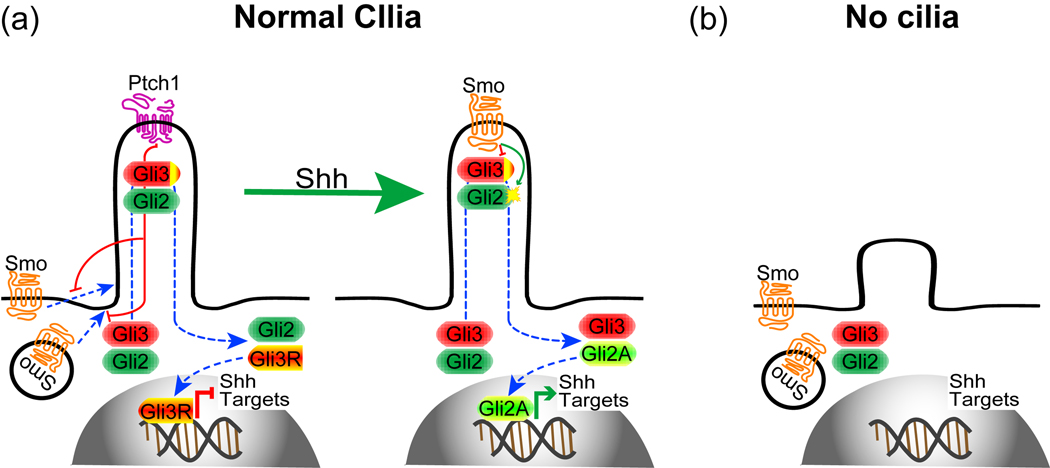
Recent studies suggest that the primary cilium is a sensory organelle. Initial clues to this sensory function came from studies in the kidney, where primary cilia have a mechanosensory role sensing flow in the renal ducts and eliciting an intracellular calcium signaling [18]. Mechanosensory function of primary cilia also regulates left-right asymmetry in the early embryo [19]. Several ion channels localize to primary cilia and patch clamp recordings on isolated cilia show prominent cation-selective channel activity [20]. As chemosensors, several G-protein coupled receptors and adenylyl cyclases localize to primary cilia, suggesting the presence of G-protein-cAMP-PKA signaling pathways in primary cilia [13••,21•,22]. Functionally, primary cilia are implicated in signaling cascades triggered by secreted proteins, sonic hedgehog (Shh), Wnt, and platelet-derived growth factor (PDGF), which play pivotal roles in animal development [4,5]. The role of primary cilia in Wnt signaling remains controversial [23,24] and it is unknown whether primary cilia have a role in PDGF signaling in vivo. However, a number of in vivo and in vitro studies have shown that primary cilia play essential roles in Shh signaling (Fig. 1). Primary cilia function is downstream of Shh receptor Patched1 (Ptch1) and Smoothened (Smo), and upstream of the Gli transcription factors (Gli1-3) [25••–32•]. Ptch1 localizes to primary cilia and inhibits the Shh signaling pathway in part by preventing Smo from entering primary cilia [33•]. Binding of Shh to Ptch1 allows Smo to enter primary cilia, where it promotes the activation of Gli2 and inhibit the formation of Gli3 repressors, resulting in the activation of target genes [34]. Notably, formation of Gli3 in the absence of Shh signaling also requires primary cilia [26,27,29,30]. Thus, primary cilia are required to both turn on and turn off Shh responses.
Figure 1.
The role of primary cilia in Shh signaling. (a) In the absence of Shh, its receptor Ptch1 localizes to primary cilia and prevents Smo from entering primary cilia. Gli3 is constitutively proteolytically cleaved to a Gli3 repressor (Gli3R) to repress Shh target genes. When Shh binds to Ptch1, Ptch1 disappears from the cilia allowing Smo to enter primary cilia, where it activates Gli2 to become transcriptional activator (Gli2A) and inhibits Gli3R formation, leading to Shh target gene expression. (b) In the absence of cilia, neither Gli2A nor Gli3R form.
The primary cilium in brain patterning
The production of distinct neuronal types is achieved during development through a progressive subdivision, along the dorsal-ventral and rostral-caudal axes, of discrete germinal domains, which express specific sets of transcription factors. Morphogens secreted by several signaling centers and interactions of cells expressing distinct transcription factors control brain patterning.
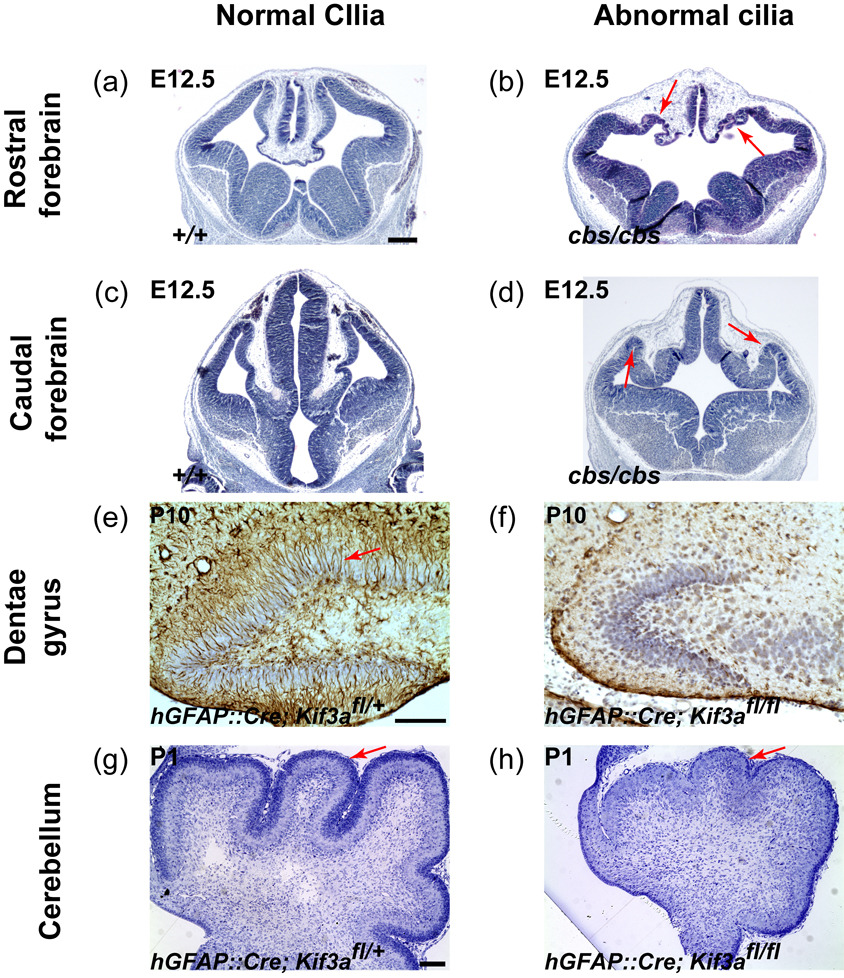
Recently, three mouse studies have shown that primary cilia play critical roles in brain patterning [35••–37••]. cobblestone (cbs) was isolated in a genetic screening for defects in neural development and turned out to be a hypomorphic allele of Ift88 that encodes an anterograde IFT component essential for ciliogenesis [36••]. Ift88cbs/cbs mutants show severe defects in the formation of dorsomedial telencephalic structures (hippocampal primordium, cortical hem, and choroid plexus) and of pallial-subpallial and telencephalic-diencephalic boundaries (Fig. 2). In Ift88cbs/cbs mutants, cells expressing dorsal or ventral markers abnormally intermingle at the boundary. A similar defect in pallial-subpallial boundary formation is observed in mice defective for Dync2h1, a cytoplasmic dynein motor subunit essential for retrograde IFT and ciliogenesis [30]. In addition, Ift88cbs/cbs mutants lack the clear distinctions between the dorsal diencephalon and the dorsolateral telencephalon. Ift88cbs/cbs mutants also show rosette-shaped heterotopias containing ectopic mitotic cells. These suggest that IFT88, which is essential for ciliogenesis, plays a critical role in forebrain patterning. Interestingly, Ift88cbs/cbs phenotypes are strikingly similar to defects observed in Gli3 mutants [38–40]. Consistent with the requirement of primary cilia for Gli3 processing, the full-length Gli3 level is increased more than fivefold in Ift88cbs/cbs mutants compared with wild-type control, which should shift the transcriptional repression to activation. However, neither Gli3 nor Ift88cbs/cbs mutants ectopically express Shh target genes (Gli1 and Ptch1) in dorsal telencephalon [36••,39,40], suggesting that ectopic activation of Shh signaling is not responsible for defects in these mutants. Consistently, removing Shh in Gli3 mutants fail to rescue Gli3 mutant phenotype [41]. Thus, Gli3 seems to inhibit ventralization of dorsal telencephalon in a Shh-independent but cilia-dependent way.
Figure 2.
The role of primary cilia in brain development. (a–d) Ift88cbs/cbs mutants show defects in the formation of dorsomedial telencephalic structures (b) and of telenecephalic-diencephalic boundaries (d). Arrows in (b) and (d) indicate heterotopias in the mutants. Ift88cbs/cbs mutants have primary cilia, but Shh signaling within the cilium including the formation of Gli3R (see text) is probably disrupted. (e,f) At P10, control DG have many radial astrocytes (arrow, brown staining by an anti-GFAP antibody) that function as adult neural stem cells in the DG, whereas hGFAP::Cre; Kif3afl/fl mutants lacking primary cilia in GNPs fail to establish radial astrocytes. (g,h) At P1, control cerebellum has a thick external granule layer (arrow) containing many CGNPs, whereas hGFAP::Cre; Kif3afl/fl mutants lacking primary cilia in CGNPs have a small cerebellum with thin external granule layer containing a few CGNPs. (a) – (d) are reproduced from Willaredt et al with permission. Scale bar = 300 µm (a–d) and 100 µm (e–h).
Intriguingly, Ift88cbs/cbs mutants, which have 75% reduction in IFT88 protein levels compared with wild-type, show no structural abnormalities in primary cilia at the ventricular surface. Thus, the reduced level of IFT88 is sufficient to construct cilia but may not be sufficient to induce normal signal. This suggests that IFT88 is not only essential for the building of primary cilia, but also essential for signaling, possibly in the transport of signaling components.
Another genetic screening for defects in late embryogenesis identified a null mutation named alien (aln) in Ttc21b (tetratricopeptide repeat domain 21B), which encodes a retrograde IFT component IFT139. In contrast to anterograde IFT mutants, which fail to make cilia and to produce both Gli activators and Gli3 repressors, Ift139aln/aln mutants make abnormal cilia with a bulge at their distal tips filled with IFT components and constitutively produce Gli activators even in the absence of Shh activation [32•]. This suggests that retrograde IFT is required to restrict the activity of Gli activators, whereas anterograde IFT is required for the activation of Gli. Similar to Ift88cbs/cbs or Gli3 mutants Ift139aln/aln mutants show loss of dorsal cortex, dorsal-ventral patterning defects, and lack of clear distinction between telencephalon and diencephalon [37••]. Consistently, the ratio of Gli3 full length to Gli3 repressor is also increased by tenfold in Ift139aln/als mutants compared with wild-type control [32•]. However, unlike Ift88cbs/cbs or Gli3 mutants, Ift139aln/aln mutants show ectopic activation of Shh signaling, and removal of one copy of Shh partially rescues Ift139aln/aln phenotype [37••]. It is unknown how the loss of IFT139 increases Shh expression. Nevertheless, the similar phenotype in Ift139aln/aln mutants to Ift88cbs/cbs and Gli3 mutants suggests that elevated level of Gli activators have relatively minor effect in forebrain development compared with loss of Gli3 repressors.
Ablation of Slb (selective Lim-domain binding), encoding an anterograde IFT component (IFT172), results in complete loss of cilia and early patterning defects [35••]. Ift172Slb/Slb mutants fail to express Fgf8 in the midbrain-hindbrain boundary and in the commissural plate. These mice exhibit holoprosencephaly, exencephaly, truncation of forebrain, and a severe reduction in diencephalic structures. Interestingly, Ift172Slb/Slb mutants show decreased nodal expression in epiblast and node at E7 and E7.5 respectively. Consistently, Ift172Slb/Slb mutants show randomization of left-right asymmetry and failure to form anterior mesendoderm. Nodal signaling controls anterior mesendoderm formation, which is required for expression of Fgf8 in the midbrain-hindbrain boundary and forebrain growth [42]. Defects in Ift172Slb/Slb mutants, observed as early as E7, suggest that IFT172 and primary cilia functions in structures that predate neural tissue can critically affect brain development.
The primary cilium in hippocampal development
The majority of granule neurons in the hippocampal dentate gyrus (DG) are produced during early postnatal life. Not only postmitotic neurons but also intermediate progenitors or granule neuron precursors (GNPs) migrate away from the primary germinal zone in the VZ into the inner layer of the developing DG, where they transform into postnatal neural stem cells that continue to produce new neurons throughout life [43]. The continual production of new neurons in the DG is thought to be important for circuit plasticity, learning, and memory. Recent studies have shown that primary cilia are essential for embryonic neural progenitors’ expansion and conversion into a population of radial astrocytes that function as primary progenitors in the postnatal DG [25••,44•].
Two recent studies have investigated the function of cilia in neurogenesis by selectively removing ciliary genes in subsets of neural progenitors. In one study, Kif3a, which encodes a subunit of Kinesin-II motor, was removed using hGFAP::Cre [25••]. In the other, Stumpy, a novel gene encoding a basal body and ciliary protein [45,46], was removed using a Nestin::Cre [44•]. In both conditional mutant mice, the number of proliferating GNPs is decreased significantly, possibly due to the increase in cell cycle exit [44•], and radial astrocytes are not generated (Fig. 2). Other mutations disrupting ciliogenesis, Ift88orpk/orpk (a hypomorphic allele of Ift88) and fantom−/− (a gene encoding a basal body protein), also result in decreased GNPs proliferation [25••], substantiating that the defect in the DG are due to loss of cilia. Interestingly, the decreased proliferation of GNPs at E18.5 in ciliary mutants coincides in time with the appearance of Shh-responding cells in the developing DG, suggesting that Shh signaling is required for the proliferation of GNPs. Indeed, expression of Shh target genes (Ptch1 and Gli1) is greatly decreased in the mutant DG. Canonical Shh signaling mutants, hGFAP::Cre; Smofl/fl mice, show similar defects in GNPs proliferation and establishment of radial astrocytes [25••]. Notably, loss of Smo, which localizes to the primary cilia in radial astrocytes [44•], causes more severe defects than loss of Kif3a, which could be explained if Smo has residual activities outside of primary cilia. However, similar to Kif3a single mutants, double mutants lacking both Smo and Kif3a show less severe defects, suggesting the absence of extra-ciliary activities of Smo in these progenitor cells [25••]. The phenotypic differences in each mutant can be explained by the requirement of primary cilia to produce Gli3 repressors [26,27,29,30]; Kif3a mutants and Kif3a and Smo double mutants lack both Gli activators and Gli3 repressors, whereas Smo mutants lack Gli activators but retain Gli3 repressors.
Consistent with the mitogenic role of Shh signaling in GNPs, expression of a constitutively active form of Smo (SmoM2) in GNPs leads to increased numbers of GNPs and abnormal DG morphogenesis, but no tumor formation in the forebrain (this is in sharp contrast to the cerebellum (see below). SmoM2 localize to the primary cilia and requires primary cilia for constitutive activation of Shh signaling; concomitant removal of Kif3a in SmoM2-expressing cells results in the small DG similar to Kif3a single mutants [25••].
Importantly, loss of cilia or Smo appear not to affect the production of other astrocytes in the hippocampus, suggesting that primary cilia and Shh signaling are specifically required for the generation of the radial astrocytes that function as stem cells in this neurogenic niche throughout life. Primary cilia and Shh signaling are essential for the transition from the embryonic to adult neural stem cells in the DG.
The primary cilium in cerebellar development
Similar to DG granule neurons, the majority of cerebellar granule neurons are produced postnatally from cerebellar GNPs (CGNPs) that have migrated away from the VZ. CGNPs originate in the rhombic lip VZ and migrate rostrally on the surface of developing cerebellum to form external granule layer, where they proliferate extensively to produce cerebellar granule neurons. Shh produced by underlying Purkinje neurons functions as a mitogen for CGNPs [47–49]. Consistent with the essential roles of primary cilia in Shh signaling, CGNPs require primary cilia to proliferate. Conditional removal of Kif3a, Ift88, or Stumpy in CGNPs using hGFAP::Cre or Nestin::Cre, all result in severe hypoplasia and abnormal foliation of the cerebellum [31•,44•,50•] (Fig. 2). The expression of Shh target genes and proliferation of GNPs are greatly reduced in the ciliary mutant cerebella, and mutant CGNPs lacking primary cilia fail to respond to Shh in vitro [31•]. Furthermore, mutations in basal body proteins, Fantom or Ofd1 (Oral-facial-digital syndrome 1 gene homolog) also cause defective cerebellar development in both mice and human [51–53]. Similar to the DG, double mutant analyses show that Smo requires primary cilia to function in the cerebellum [31•].
The primary cilium in brain tumor
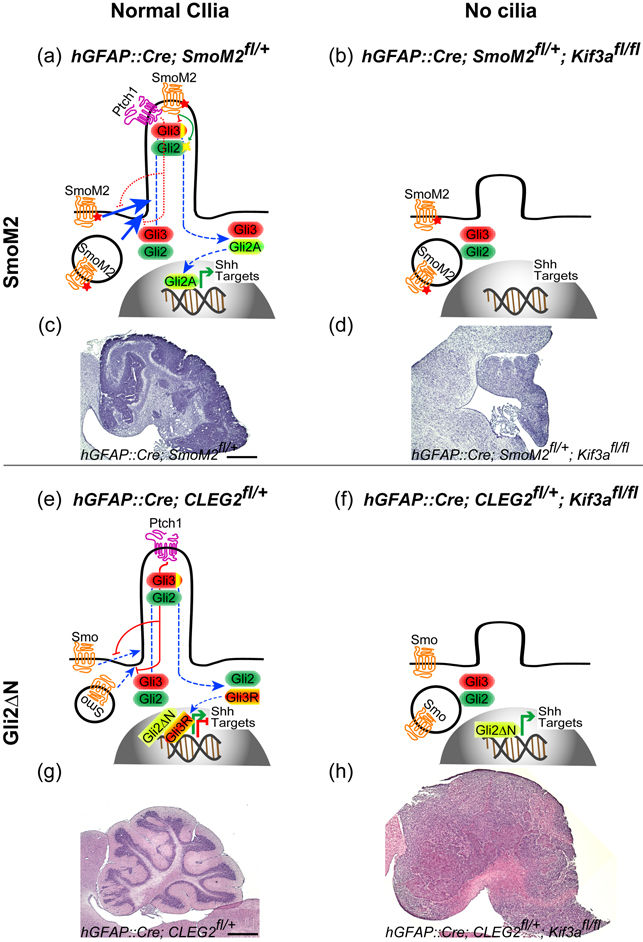
While Shh is essential for proliferation of CGNPs, abnormal activation of this signaling pathway can lead to the formation of medulloblastoma [54], the most common malignant brain tumor in children. Primary cilia also play critical roles in this Shh signaling-driven tumorigenesis [55••] (Fig. 3). Expression of SmoM2 in CGNPs leads to medulloblastoma, but it fails to induce tumor without primary cilia. Strikingly however, primary cilia suppress medulloblastoma development when CGNPs express a constitutively active form of GLI2, which lacks the N-terminal repressor domain (GLI2ΔN) [56]. Independent of primary cilia, GLI2ΔN activates downstream Shh target genes. Surprisingly, however, GLI2ΔN induces medulloblastoma only when primary cilia are removed. Removal of primary cilia disrupts Gli3 repressor production, which could possibly counteract the oncogenic activity of GLI2ΔN. Thus, the primary cilium functions as a double-edged sword in medulloblastoma development: it is required for oncogenic mutations upstream of the cilia but functions as a suppressor for those downstream of the cilia. This dual role may also apply to other tumors with alterations in the Shh pathway [57••].
Figure 3.
The role of primary cilia in medulloblastoma formation. (a–d) SmoM2 is insensitive to Ptch1 inhibition and constitutively localizes to primary cilia, where it induces Gli2A formation and inhibits Gli3R formation (a), leading to medulloblastoma development (c). Without primary cilia in hGFAP::Cre; SmoM2fl/+; Kif3afl/fl mutants, SmoM2 as well as normal Shh signaling cannot activate downstream signaling (b), resulting in hypomorphic cerebellum (d). (e,g) hGFAP::Cre; CLEG2fl/+ mutants express Gli2ΔN, which constitutively activates Shh target genes independent of Shh signaling or primary cilia (e), but alone does not induce medulloblastoma (g). The presence of Gli3R may counteract oncogenic activity of Gli2ΔN (e). (f, h) Gli2ΔN, without cilia in hGFAP::Cre; CLEG2fl/+; Kif3afl/fl mutants, induce medulloblastoma (h). Without cilia, Gli3R does not form, which might allow Gli2ΔN to induce medulloblastoma formation (f). Scale bar = 100 µm.
Interestingly, some human medulloblastomas have primary cilia, while others do not [55••]. Primary cilia are present almost exclusively in medulloblastomas with activation in SHH or WNT signaling, suggesting that this organelle plays similar dual roles in human medulloblastomas. Tumors often hijack programs used during normal development for their abnormal proliferation. Primary cilia could play important roles in tumorigenesis driven by other tumorigenic programs in addition to Shh signaling; loss of cilia in a subset of human medulloblastomas, but its presence in those driven by WNT activation, supports this hypothesis.
The primary cilium in human diseases
Recently, defective primary cilia or basal bodies have been associated with many human genetic syndromes, collectively called “ciliopathies” [5]. The causative genes for these syndromes, all seem to be involved in ciliogenesis or ciliary function. For example, recently, mutations in INPP5E gene, encoding inositol polyphosphate-5-phosphatase E, which hydrolyzes the 5-phosphate of Phosphotidylinositol (PtdIns) (3,4,5)P3 and PtdIns(4,5)P2, have been associated with Joubert and MORM syndromes [58,59]. INPP5E regulates the stability of primary cilia upon mitogenic activation of a cell. Interestingly, INPP5E localizes to primary cilia. BBS5 (a protein encoded by a gene mutated in Bardet-Biedl syndrome) and Tubby (a ciliary protein) bind PtdIns, suggesting that PtdIns signaling may in part occur inside primary cilia [60,61].
Ciliopathies are pleiotropic disorders affecting many organs and can cause neurological symptoms including obesity, mental retardation, and ataxia (Table 1). Ataxia may result from defective CGNP proliferations and cerebellar morphogenesis. Defects in cortical development as well as abnormal specification of stem cells and the development of the DG could explain some of the cognitive deficits in these patients.
Table 1.
Examples of ciliopathies showing brain malformation or neurological symptoms
| Syndrome | Known genes | Symptoms |
|---|---|---|
| Alström syndrome | ALMS1 | Cone-rod dystrophy, obesity, progressive sensorineural hearing impairment, dilated cardiomyopathy, type 2 diabetes mellitus, hepatic and renal dysfunction, and developmental delay |
| Bardet–Biedl syndrome |
BBS1, BBS2, ARL6 (BBS3), BBS4, BBS5, BBS6 (MKKS), BBS7, TTC8 (BBS8), PTHB1 (BBS9), BBS10, TRIM32 (BBS11), BBS12, MKS1 (BBS13), CEP290 (BBS14, MKS4, NPHP6) |
Cone-rod dystrophy, obesity, polydactyly, cognitive impairment, male hypogonadotrophic hypogonadism, complex female genitourinary malformations, and renal dysfunction. |
| Hydrolethalus syndrome | HYLS-1 | Hydrocephaly with absent upper midline structures of the brain, micrognathia and polydactyly |
| Joubert syndrome |
AHI1, CEP290 (BBS14, MKS4, NPHP6), TMEM67 (MKS3), NPHP1, INPP5E |
Cerebellar and brainstem malformation, hypotonia, developmental delays, and episodic hyperpnea or apnea or atypical eye movements or both |
| Meckel syndrome |
MKS1 (BBS13), TMEM67 (MKS3), CEP290 (MKS4, BBS13, NPHP6), RPGRIP1L (MKS5), CC2D2A (MKS6) |
CNS malformation (posterior encephalocele, cerebral and cerebellar hypoplasia), polycystic or hypoplastic kidneys, and polydactyly |
| MORM syndrome | INPP5E | Mental retardation, obesity, congenital nonprogressive retinal dystrophy, and micropenis in males |
| Oro-facio-digital syndrome 1 | OFD1 | Malformations of the face, oral cavity, digits, and brain (intracerebral cysts, corpus callosum agenesis, cerebellar agenesis with or without Dandy-Walker malformation), and polycystic kidney disease |
Additional evidence suggests that genes associated with other developmental disorders may be connected to ciliary function. A family with X-linked recessive mental retardation and macrocephaly was found to have a novel mutation of the OFD1 gene, which is mutated in Oral-facial-digital type 1 syndromes [62]. Pericentrin, which is associated with microcephaly in human is required for ciliogenesis in flies and hypomorphic pericentrin mutant mice fail to form olfactory cilia [63,64]. Abnormal spindle-like microcephaly-associated protein (ASPM), which is asscociated with neurogenesis and microcephaly, has an ASH domain frequently found in ciliary proteins [65].
Neurons also have primary cilia, thus, it is possible that some of neurological symptoms in ciliopathy patients could be due to defects in neuronal cilia. Primary cilia are also likely to be involved in other neurological disorders. Conditional ablation of primary cilia in the POMC-positive neurons in the hypothalamus causes hyperphagia-induced obesity with increased leptin levels, which regulate feeding behaviors [66••]. Polymorphisms of adenylyl cyclase III, which localizes to neuronal cilia is associated with obesity in human [67], and mutant mice for adenylyl cyclase III are resistant to leptin and become obese [68•]. Three of the proteins mutated in Bardet Biedl syndrome (BBS2, 4, 6) are required for leptin receptor signaling in the hypothalamus [69•]. BBS2 and 4 are also required for the localization of somatostatin receptor 3 and melanin-concentrating hormone receptor 1 (MCHR1) into neuronal cilia [21•]. MCHR1 is involved in feeding behavior and in emotional processing. Interestingly, treatment with lithium, a well-known mood stabilizer and used to treat bipolar disorder, induces elongation of cilia in the mouse brain and cultured cells, suggesting that primary cilia might be involved in the therapeutic response and/or be part of the etiology of the diseases [70,71]. BBS4 interacts with disrupted-in-schizophrenia 1 that is associated with schizophrenia and knockdown of BBS4 causes defective neuronal migration in developing cortex [72].
Conclusions and perspectives
During the past decade, primary cilia have gained center stage in understanding development and diseases. This tiny protrusion from the surface of a cell is centrally involved in sensing chemical and mechanical information, and its defects lead to diseases affecting multiple organs. As dual sensors for chemical and physical cues, primary cilia are well-placed integrating critical signals that surround cells. This integration may lead to cellular fate changes. In dividing cells, primary cilia could be the key to whether the cell reenters the cell cycle or remains quiescent.
Here, we have reviewed some of the initial glimpses on the function of primary cilia in brain development and tumor formation. Primary cilia are critical in early brain patterning, the formation of adult neural stem cells, and tumor growth. Most evidence so far indicates that primary cilia are required for Shh- or Gli3-dependent signaling in the brain for progenitor proliferation and fate specification. However, many interesting questions remain. Neuroepithelial cells, radial glia, and a subpopulation of adult neural stem cells have primary cilia sticking into the brain ventricles. Do these cilia sense chemicals in the cerebrospinal fluid (CSF) and flow of CSF to regulate the behavior of neural stem cells? Are primary cilia required for additional signaling pathways that control brain development? For example, some Fgf proteins (Fgf4, 8 and 24) and their receptors (Fgfr1 and 2) regulate ciliogenesis [73,74]. Are some of the functions of these Fgfs in brain development mediated by regulating ciliogenesis? Are primary cilia required for neuronal migration? Studies on the role of BBS proteins already suggest that primary cilia may play important roles in cell migration [72,75]. One of the key events in development is the control of symmetric versus asymmetric division of progenitor/stem cells to produce daughters with identical or different fates. Do primary cilia play a role to determine stem cell’s division modes? The orientation of the cleavage plane during mitosis is thought to play important roles in determining the symmetry of stem cell division. Loss of IFT20, another essential component for IFT and ciliogenesis, leads to abnormal orientation of cleavage plane in kidney ductile cells [76•], thus suggesting that primary cilia could influence the symmetry of stem cell division. Primary cilia could also influence the fate of two daughter cells after division. A daughter cell inheriting the older mother centriole makes cilia earlier than the other daughter inheriting the new mother centriole [77•]. This difference in time to make cilia was suggested to affect the timing of cell’s ability to respond to extracellular signal, which could influence the cell fate. As we explore these interesting questions on the role of primary cilia, we are likely to gain surprising new perspectives into the mechanism of brain development.
ACKNOWLEDGEMENTS
We thank Dr. KL Tucker at University of Heidelberg for providing us with forebrain pictures of cbs mutants and controls, which we used in Figure 2a–d. Y.-G. H. was, in part, supported by Mark Linder/American Brain Tumor Association Fellowship. The work was supported by grants from US National Institute of Health (NS28478 and HD32116) and a grant from the Goldhirsh foundation to A. A–B. We thank T. Nguyen for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuura K, Lefebvre PA, Kamiya R, Hirono M. Kinesin-II is not essential for mitosis and cell growth in Chlamydomonas. Cell Motil Cytoskeleton. 2002;52:195–201. doi: 10.1002/cm.10051. [DOI] [PubMed] [Google Scholar]
- 8.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 9.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama Y, Kohno K. Number and polarity of the ependymal cilia in the central canal of some vertebrates. J Neurocytol. 1974;3:449–458. doi: 10.1007/BF01098732. [DOI] [PubMed] [Google Scholar]
- 12.Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 13. Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. •• A thorough study showing that type III adenylyl cyclase is a powerful marker for both neuronal and glial cells throughout the adult mouse brain.
- 14.Cohen E, Meininger V. Ultrastructural analysis of primary cilium in the embryonic nervous tissue of mouse. Int J Dev Neurosci. 1987;5:43–51. doi: 10.1016/0736-5748(87)90047-5. [DOI] [PubMed] [Google Scholar]
- 15.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 18.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 19.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 20.Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 21. Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. • A study showing that BBS2 and BBS4 are required for the localization of somatostatin receptor 3 and melanin-concentrating hormone receptor 3 to neuronal cilia. This suggests that some BBS phenotypes could be due to defective signaling caused by mislocalization of G protein coupled receptors.
- 22.Raychowdhury MK, Ramos AJ, Zhang P, McLaughin M, Dai XQ, Chen XZ, Montalbetti N, Del Rocio Cantero M, Ausiello DA, Cantiello HF. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol. 2009;296:F87–F97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- 23.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. •• The first indication that hippocampal neural progenitors require primary cilia for detection of Shh signaling for the expansion and establishment of postnatal neural stem cells. Mice lacking primary cilia in embryonic neural progenitors have small dentate gyrus lacking radial astrocytes, the adult neural stem cells in the dentate gyrus.
- 26.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 29.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 30.May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 31. Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. • A study showing that primary cilia are required for cerebellar granule neuron precursors to detect Shh and proliferate.
- 32. Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. • A study showing that retrograde IFT is required to restrict hedgehog signaling. Mice mutant for IFT139, a retrograde IFT component, show overactivated Shh signaling phenotype.
- 33. Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. • A study showing that Shh receptor, Patched1, localizes to primary cilia, where it binds to Shh. Upon binding Patched1 leaves primary cilia allowing Smoothened to enter primary cilia to initiate Shh signaling cascade.
- 34.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 35. Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. •• A study showing that primary cilia functions in structures that predate neural tissue can critically affect brain development. Mice mutant for IFT172, an anterograde IFT component, show decreased nodal expression and fail to form anterior mesendoderm, resulting in brain patterning defects.
- 36. Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, Gojak CP, Gorgas K, Bradford CL, Spatz J, Wolfl S, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. •• A study showing that IFT88, an anterograde IFT component, is essential for proper brain patterning. Hypomorphic Ift88 mutants show brain patterning defects that are strikingly similar to Gli3 mutant phenotypes.
- 37. Stottmann RW, Tran PV, Turbe-Doan A, Beier DR. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.08.023. •• A study showing that mutant mice for Ift139 show hyperactivation of Shh signaling and brain patterning defects. The patterning defect is similar to Gli3 mutant phenotype. However, unlike Gli3 mutant, the Ift139 mutant phenotype can be partially rescued by removing one copy of Shh.
- 38.Fotaki V, Yu T, Zaki PA, Mason JO, Price DJ. Abnormal positioning of diencephalic cell types in neocortical tissue in the dorsal telencephalon of mice lacking functional Gli3. J Neurosci. 2006;26:9282–9292. doi: 10.1523/JNEUROSCI.2673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- 40.Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- 41.Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camus A, Davidson BP, Billiards S, Khoo P, Rivera-Perez JA, Wakamiya M, Behringer RR, Tam PP. The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo. Development. 2000;127:1799–1813. doi: 10.1242/dev.127.9.1799. [DOI] [PubMed] [Google Scholar]
- 43.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 44. Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. • A study showing that a novel ciliogenic gene, stumpy, is required for the expansion and establishment of postnatal hippocampal progenitors.
- 45.Ponsard C, Skowron-Zwarg M, Seltzer V, Perret E, Gallinger J, Fisch C, Dupuis-Williams P, Caruso N, Middendorp S, Tournier F. Identification of ICIS-1, a new protein involved in cilia stability. Front Biosci. 2007;12:1661–1669. doi: 10.2741/2178. [DOI] [PubMed] [Google Scholar]
- 46.Town T, Breunig JJ, Sarkisian MR, Spilianakis C, Ayoub AE, Liu X, Ferrandino AF, Gallagher AR, Li MO, Rakic P, et al. The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci U S A. 2008;105:2853–2858. doi: 10.1073/pnas.0712385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 48.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 50. Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. • The first study showing that cerebellar granule neuron precursors require primary cilia for cerebellar granule neuron precursors proliferation and cerebellar development.
- 51.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 52.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 53.Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 54.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 55. Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009 doi: 10.1038/nm.2020. •• A study showing that primary cilia play critical roles in tumorigenesis. Primary cilia are either required for or suppress medulloblastoma development depending on where the mutation is in the Shh signaling pathway.
- 56.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009 doi: 10.1038/nm.2011. •• This study also shows a dual role of primary cilia in skin cancer (basal cell carcinoma.
- 58.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 60.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 61.Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 62.Budny B, Chen W, Omran H, Fliegauf M, Tzschach A, Wisniewska M, Jensen LR, Raynaud M, Shoichet SA, Badura M, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120:171–178. doi: 10.1007/s00439-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol. 2004;165:673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyoshi K, Kasahara K, Miyazaki I, Shimizu S, Taniguchi M, Matsuzaki S, Tohyama M, Asanuma M. Pericentrin, a centrosomal protein related to microcephalic primordial dwarfism, is required for olfactory cilia assembly in mice. Faseb J. 2009 doi: 10.1096/fj.08-124420. [DOI] [PubMed] [Google Scholar]
- 65.Ponting CP. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics. 2006;22:1031–1035. doi: 10.1093/bioinformatics/btl022. [DOI] [PubMed] [Google Scholar]
- 66. Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. •• The first evidence that neuronal cilia play an important role in the regulation of feeding behavior of mice. Mice lacking primary cilia in POMC neurons eat more than wild-type and become obese, suggesting that cilia on POMC neurons are involved in detecting satiety signals.
- 67.Nordman S, Abulaiti A, Hilding A, Langberg EC, Humphreys K, Ostenson CG, Efendic S, Gu HF. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int J Obes (Lond) 2008;32:407–412. doi: 10.1038/sj.ijo.0803742. [DOI] [PubMed] [Google Scholar]
- 68. Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, Storm DR. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:e6979. doi: 10.1371/journal.pone.0006979. • A study showing that loss of type 3 adenylyl cyclase causes leptin resistance and obesity.
- 69. Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. • A study showing that BBS proteins are required for leptin receptor trafficking and signaling. BBS2, 4, and 6 mutant mice, are all resistant to leptin and show defects in leptin signaling in hypothalamus.
- 70.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- 71.Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, Tsukita S, Pulver AE, Nakajima K, Cascella NG, et al. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 2008;65:996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamauchi H, Miyakawa N, Miyake A, Itoh N. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Dev Biol. 2009;332:177–185. doi: 10.1016/j.ydbio.2009.05.568. [DOI] [PubMed] [Google Scholar]
- 75.Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183:377–384. doi: 10.1083/jcb.200808137. • A study showing that primary cilia can influence the cleavage plane of mitosis. Loss of IFT20 in kidney collecting duct causes centrosome positioning defects and randomization of mitotic spindle orientation, which is parallel to the long axis of the duct in wild-type.
- 77. Anderson CT, Stearns T. Centriole Age Underlies Asynchronous Primary Cilium Growth in Mammalian Cells. Curr Biol. 2009 doi: 10.1016/j.cub.2009.07.034. • A study showing that two daughter cells after division make cilia asynchronously. This difference is suggested to differentially influence the fate of the daughters.