Abstract
Greater than 70% of the world's amphibian species are in decline. We propose that there is probably not a single cause for global amphibian declines and present a three-tiered hierarchical approach that addresses interactions among and between ultimate and proximate factors that contribute to amphibian declines. There are two immediate (proximate) causes of amphibian declines: death and decreased recruitment (reproductive failure). Although much attention has focused on death, few studies have addressed factors that contribute to declines as a result of failed recruitment. Further, a great deal of attention has focused on the role of pathogens in inducing diseases that cause death, but we suggest that pathogen success is profoundly affected by four other ultimate factors: atmospheric change, environmental pollutants, habitat modification and invasive species. Environmental pollutants arise as likely important factors in amphibian declines because they have realized potential to affect recruitment. Further, many studies have documented immunosuppressive effects of pesticides, suggesting a role for environmental contaminants in increased pathogen virulence and disease rates. Increased attention to recruitment and ultimate factors that interact with pathogens is important in addressing this global crisis.
Keywords: amphibian, decline, stress
Background
Amphibian population declines were first recognized as a global phenomenon in the early 1990s (Wake, 1991). We now know that current extinction rates for amphibians may be as much as 200 times higher than background (Roelants et al., 2007). With 32% of the world's nearly 6600 amphibian species threatened with extinction, 43% experiencing declines and another 22% with insufficient data (Stuart et al., 2004), this phenomenon represents the Earth's sixth mass extinction (Wake and Vredenburg, 2008).
A number of causes have been suggested to explain global amphibian declines. Habitat modification (loss) has played a significant role in many declines and is likely the single most important cause of local declines (Adams, 1999; Becker et al., 2007; Davidson et al., 2002; Davidson et al., 2001; Delis et al., 1996; Eigenbrod et al., 2008; Harper et al., 2008; Smith et al., 2009). Other factors may explain many local declines including the use of road salt, catastrophic events, etc. The global phenomenon, particularly declines in apparently physically undisturbed habitats [especially so-called ‘enigmatic declines’ which represent 48% of threatened amphibian species according to Stuart and colleagues (Stuart et al., 2004)], begs other explanations, however. Identifying the most urgent issues and those factors that are truly global is vital to developing plans to halt declines or to provide whatever remediation is possible.
A hierarchical approach to defining the causes
Here, we propose that ultimately there is no single cause of global amphibian declines, but rather interactions between several factors are important. Some local declines may primarily have single causes, but we propose that even local declines and extinctions are likely (and most often) caused by interactions between two or more factors. Individual factors that interact likely differ from species to species and even population to population, within a species. In addition, several factors may be more detrimental when combined, and result in emergent properties that render individual factors more severe when combined with other factors.
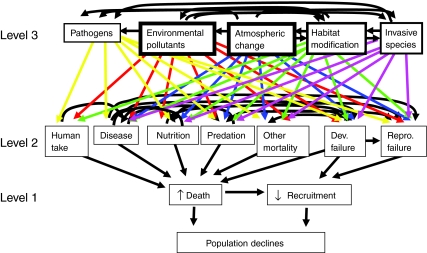
Robert May recently addressed the ‘likely causes and consequences of the manifest acceleration in extinction rates over the past few centuries’ (May, 2010), with a focus on current extinction rates in general. According to May, ‘the ultimate causes are habitat destruction, alien introductions, overexploitation and climate change’. Young and colleagues reported that declines in amphibian populations due to overexploitation are concentrated in East and South East Asia (Stuart et al., 2004); thus, overexploitation is seemingly not a global concern for amphibians. This leaves habitat destruction, alien introductions and climate change from May's list. Two additional factors are important in global amphibian declines, however: environmental pollutants and pathogens. We propose that none of these factors act alone, but that interactions between five ultimate factors (atmospheric change, environmental pollutants, habitat modification, invasive species and pathogens) are the cause of amphibian declines. We are not the first to propose that there is more than one cause of amphibian declines (Blaustein and Kiesecker, 2002; Blaustein and Wake, 1990; Kiesecker et al., 2001; Wake and Vredenburg, 2008), nor are we the first to suggest that causative factors interact (Boone and James, 2003; Boone and Semlitsch, 2002; Boone et al., 2007; Davidson et al., 2007; Davidson et al., 2002; Davidson et al., 2001; Garcia et al., 2003; Jancovich et al., 2005; Kiesecker and Blaustein, 1995b; Kiesecker and Blaustein, 1999; Marcogliese et al., 2009; Pounds et al., 2006; Pounds and Crump, 1994; Relyea, 2003; Relyea, 2004b; Relyea and Hoverman, 2008; Sodhi et al., 2008; Wake and Vredenburg, 2008). As such, our goal here is not to present an exhaustive review of all of these factors and interactions [an entire book has been written on the disease chytridiomycosis, alone (Collins et al., 2009)]. Rather, our aim is to focus on research areas that have received less attention in the published literature. We present a scheme with three hierarchical levels that depicts the immediate causes of amphibian declines (proximate causes; Level 1), followed by multiple specific causes (intermediate causes; Level 2), followed by these proposed five ultimate causes of amphibian declines (Level 3) (Fig. 1).
Fig. 1.
Tiered approach showing interactions of the many factors that contribute to amphibian declines. Effects at any given level have no impact on higher levels, but interact with other factors on the same level and impact factors at lower levels. The five ultimate factors at Level 3 affect multiple factors at Level 2, which in turn interact with each other and ultimately contribute to amphibian declines due to death and decreased recruitment (Level 1). Line weights at Level 3 reflect rankings as described in the text (‘Horizontal interactions at Level 3’).
Level 1: proximate (direct) causes of amphibian declines
There are only two proximate (direct) causes of amphibian population declines: death (or removal) of individuals from a population and reduced recruitment within a population. This observation may seem to state the obvious, but it is important to point it out. Many population declines have received attention because adults simply disappeared rapidly and/or massive die-offs were documented (Crump et al., 1992; Pounds and Crump, 1994). Just because adults are present at a locality and massive deaths are not observed in a population does not mean that a given population is not in decline. Populations can decline slowly if otherwise healthy adults are not breeding or if progeny do not develop properly.
These two causes of declines (death and recruitment failure), of course, interact in that the death of individuals will ultimately result in decreased recruitment. Although many studies have examined declines in amphibian populations due to loss of individuals (death), we are not aware of any studies in amphibians that have examined population declines resulting from failed recruitment in populations with otherwise physiologically healthy individuals (see discussion below).
Level 2: factors contributing to death and failed recruitment
There are multiple factors that contribute to the death of local populations. We include five major factors that contribute to local declines as a result of death: (1) increased disease rates can lead to individual deaths and population declines; (2) decreased nutrition (loss of food base) can result in declining numbers within a population; (3) predation can remove individuals from populations; and (4) we consider human take (exploitation) separate from predation by other animals. Finally, we have included a ‘catch all’ category, (5) ‘other mortality’, which represents everything from death due to old age, to incidental deaths not due to the aforementioned factors, to catastrophic events that may reduce or even eliminate entire populations (Fig. 1). Failed recruitment, on the other hand, can result directly from reproductive failure or as a result of some developmental failure not directly related to reproductive function. Although important in local declines, the factors presented at Level 2 are not likely to be universal factors that contribute to global amphibian declines. In other words, it is not likely that global amphibian declines are universally caused by human take, predation, incidental or catastrophic events leading to mortality, or decreased nutrition.
What is mainly important with regards to recruitment is that a number of factors (causes) can reduce recruitment in a population without otherwise causing harm (developmental or physiological) to exposed individuals. Decreased recruitment can result directly from reproductive failure or from developmental failures that lead to reproductive failure, i.e. failure of reproductive organs or structures to develop properly. Developmental failures that do not lead to death can also directly decrease recruitment (cause reproductive failure). For example, limb deformities or decreased growth are developmental effects that would limit reproductive potential without killing affected individuals. This scenario is especially true for short-lived species where failed recruitment for one or two breeding seasons may be disastrous for populations (Harper et al., 2008). Complete inhibition or delays in metamorphosis that prevent reproduction should also be considered here: although some salamander species can reproduce in the larval form, anurans cannot (Hayes, 1997).
There are bi-directional interactions across Level 2 (horizontal interactions), but only unidirectional (causative) interactions from this level down (Level 2 to Level 1; Fig. 1). In other words, increased death does not influence predation, nutrition, etc., and declining recruitment does not influence developmental or reproductive failures or increase any of the proximate causes of death (on the contrary, declining recruitment likely would cause a decrease in these factors). However, any of the factors that may affect death (human take, disease, nutrition, predation or other mortality) may directly or through horizontal interactions affect developmental and/or reproductive failure. For example, in many species, growth rates (and, thus, body size) determine age at sexual maturity (Hayes and Licht, 1992; Mays et al., 2006; Shine, 1979; Shine, 1989). Body size is also the basis for intrasexual and intersexual selection in many species and determines the maximum number and size of eggs that a female can produce (Mays et al., 2006; Shine, 1979; Shine, 1989). Decreased nutrition, due to loss of food base or as a result of disease, can lead to developmental failures or have direct negative impacts on reproduction. Thus, decreased nutrition could cause retarded development that could lead to reproductive failure. Decreased nutrition could also directly cause reproductive failure. Similarly, disease could cause nutritional deficiencies which could increase predation rates or lead to developmental failures that in turn lead to reproductive failure and limit recruitment, and so on.
Many studies have examined death as a cause of amphibian population declines; however, studies that examine factors that contribute to recruitment failure are lacking. Declines in populations of other animals due solely to failed recruitment have been described occasionally. For example, a local population of cotton rats (Sigmodon hispidus) in Texas appeared to decline as a result of failed reproduction, but this decline occurred after a significant die-off in younger animals in the population, leaving behind only older adult animals that did not reproduce (Chipman, 1966). It is not clear why reproduction was stalled in the cotton rat study, but in other cases external factors are involved. For example, in a study in snowshoe hares (Lepus americanus), it was proposed that increased predation raised stress hormone levels enough that reproduction was severely inhibited (Sheriff et al., 2009). Another report of freshwater turtles in the Seychelle Islands describes three species where adults are numerous, but reproduction has decreased or ceased. In the case of the Seychelles turtles, habitat suitable for adult survival is available, but suitable habitat for breeding is not. In other cases, habitat fragmentation can be an impediment to breeding, even though otherwise healthy adults are present (Gerlach, 2008).
Recognizing decreased recruitment in amphibian declines is important. In particular, many species of amphibians are rarely seen outside of breeding aggregations. For example, one of the authors (T.B.H.) studied woodfrogs (Rana sylvatica) for 3 years (1986-1989) in Massachusetts. Based on the number of egg masses produced each year, the breeding population numbered in the hundreds at most study sites (Fig. 2). Outside of the breeding season, however, adults were rarely seen (two times in 3 years, including one animal caught in a Sherman trap). At the same sites, although spotted salamanders (Ambystoma maculatum) numbered in the hundreds at breeding aggregations for a few days each year, these fossorial animals were rarely seen otherwise. Similarly, from 1995 to 1999 T.B.H. studied boreal toads (Bufo boreas). During this time period toads successfully bred each year (produced egg masses that survived to hatching) at Del Peurto Creek, Stanislaus County, CA, USA, in semi-isolated ephemeral pools along the creek (Fig. 2) with as many as 50 egg masses per 0.5 miles (0.8 km) in some years. Over the following 10 years, increased housing developments, agriculture, off-road recreational activity, mining and recovery from droughts all resulted in changes in water flow in Del Peurto Creek, which resulted in permanent standing water that allowed the successful invasion of bullfrogs. Breeding sites for B. boreas were eliminated due to the increased water flow, which filled in some breeding ponds with sand (Fig. 2E—F) and eliminated other previously isolated breeding pools by connecting them to the main stream. Reproduction has not been observed there for the last 4 years, even though adults are still present. The adults are not affected per se, but recruitment has failed. Like the examples above, in some cases it is possible that adult amphibians may survive for several years after reproduction ceases, but may not be seen by researchers. This observation is especially true for amphibians where populations are monitored by vocalizations which are typically only produced during mating. Should reproduction cease, the adults may survive for quite some time but appear to be gone.
Fig. 2.
A communal wood frog (Rana sylvatica) deposition site in Concord (Middlesex County) Massachusetts from 1988 (A). Well over 100 egg masses are shown just on the surface, indicating a local population of over 200 breeding adults. Similar numbers bred at this site in the previous 2 years, but in this same time frame only two adults were ever seen outside of the breeding season. (B—F) Breeding sites for boreal toads (Bufo boreas) along Del Peurto Creek in Stanislaus County, California. B shows a pair of copulating toads with the strings of eggs wrapped around the pair. C and D show views of one ephemeral pool where a single clutch of eggs (which can be up to 10,000 individuals) has hatched in 1996. E and F show changes in a second nearby deposition site from 1995 (E) to 1999 (F). The breeding site is on the left in both panels with Del Peurto Creek on the right. Arrow in E shows the location of the small breeding pool shown in panels C and D. Because of anthropogenic activity, water flow has changed at the site, these ephemeral breeding pools have disappeared, and breeding has not occurred here for at least 4 years.
Level 3: ultimate causes of amphibian declines
There are at least five ultimate factors that influence all seven factors at Level 2. These five (Level 3) represent the most likely factors to have truly global effects on amphibian population declines, and include atmospheric change (temperature, rainfall, UV levels, etc.), environmental pollutants, habitat loss, invasive species and pathogens. Atmospheric change is clearly a phenomenon that amphibian populations will experience globally. Also, environmental pollutants are widespread enough to have global effects. For example, aerial transport of pesticides into California's Sierra Nevada likely contributed to amphibian declines there (Davidson et al., 2002; LeNoir et al., 1999), and pesticides, like the popular herbicide atrazine, can travel over 1000 km in precipitation and contaminate otherwise pristine habitats where they are not used (Fenelon and Moore, 1998; Lode et al., 1995; Mast et al., 2007; Müller et al., 1997; Thurman and Cromwell, 2000; Vogel et al., 2008). Although direct habitat modification (loss) may not be ubiquitous, it is indeed quite widespread, but furthermore other global impacts (atmospheric changes, environmental pollutants and invasive species) all generate habitat modifications. Invasive species may be widespread, but are probably not as independently important as other factors on this level (see below). Finally, at least some pathogens may be widespread enough to have a global impact (discussed below), but in most cases this factor is likely dependent on many other factors.
Vertical interactions between Level 3 and Level 2
All five ultimate factors at Level 3 affect all factors at Level 2 and, thereby, have the potential to affect both death and recruitment. The impacts of all of the factors from Level 3 on factors at Level 2 that affect death have been well covered in the literature (Adams, 1999; Becker et al., 2007; Blaustein and Wake, 1990; Cunningham et al., 2008; D'Amen and Bombi, 2009; Davidson et al., 2001; Harper et al., 2008; Kats and Ferrer, 2003; Kiesecker et al., 2001; Kiesecker and Blaustein, 1995b; Lips et al., 2008; Puschendorf et al., 2008; Voyles et al., 2009; Vredenburg et al., 2008; Wake and Vredenburg, 2008). Therefore, we focus our discussion here on the impact of atmospheric change, environmental pollutants, habitat modification, invasive species and pathogens on factors at Level 2 that affect recruitment.
Atmospheric change affects recruitment
Atmospheric changes can have a profound impact on reproductive success in amphibians. Not only do temperature and precipitation trigger reproductive function and physiology in amphibians but also climate conditions determine the availability of suitable breeding habitat. In particular, for amphibians that depend on snow melt to fill breeding ponds or amphibians that breed in ephemeral ponds, precipitation and temperature are critically important. Furthermore, amphibians may experience temperature-induced stress when exposed to temperatures above their physiological range (Jurani et al., 1973; Pounds and Crump, 1994), which may suppress breeding activity.
Environmental pollutants affect recruitment
Most studies of chemical contaminants and amphibians have focused on toxicity or developmental effects that lead to poor survivorship or death directly (Kerby et al., 2010; Relyea, 2005; Relyea, 2004a; Relyea, 2009; Relyea and Jones, 2009; Rohr and Crumrine, 2005; Rohr et al., 2003; Rohr and Palmer, 2005; Rohr et al., 2006). However, environmental pollutants can cause developmental and reproductive failures at sub-lethal doses that may otherwise have no adverse physiological effects or health consequences. In particular, atrazine has been well studied and is a potent endocrine disruptor in amphibians. Atrazine is the most common pesticide contaminant of ground, surface and drinking water and is used in agriculture throughout North America, South America, Asia and Australia (Solomon et al., 1996). Atrazine is persistent, highly mobile, and can travel up to 1000 km in rain water, contaminating otherwise pristine habitats (Thurman and Cromwell, 2000). As such, atrazine has the potential to have global effects. At sub-lethal ecologically relevant levels (0.1 p.p.b. and higher), atrazine causes a decrease in the gonadal volume and germ cells in exposed Xenopus laevis larvae (Tavera-Mendoza et al., 2002a; Tavera-Mendoza et al., 2002b). Further, atrazine induces hermaphroditism in exposed X. laevis frogs (Carr et al., 2003; Hayes et al., 2002a; Hayes et al., 2002b; Hayes et al., 2006b) and testicular oogenesis in Ranid frogs exposed as larvae (Hayes et al., 2002c; Hayes et al., 2006b; Orton et al., 2006). Sexual abnormalities are also associated with atrazine contamination in free-ranging amphibians in the wild (Hayes et al., 2002b; Hayes et al., 2002c) and where animals are exposed in mesocosm studies (Langlois et al., 2009). Furthermore, recent studies have shown that atrazine can completely feminize exposed amphibians, resulting in genetic males that breed with other males and produce viable eggs, but with skewed sex ratios (100% male offspring), a scenario that has been proposed to drive species extinction (Gutierrez and Teem, 2006). In addition, atrazine-exposed males are incapable of competing for females, have feminized laryngeal structures, decreased breeding gland size, decreased mature sperm and very low fertility. Demasculinized and feminized males have been identified in the field associated with agricultural areas where atrazine is used (McCoy et al., 2008; McDaniel et al., 2008; Mckoy et al., 2002) and where atrazine contamination occurs (Du Preez et al., 2005; Murphy et al., 2006). Other environmental pollutants may have similar endocrine-disrupting effects, but no other current-use pesticides are as widespread and as persistent as atrazine. Furthermore, data on endocrine disruption in amphibians (or any taxa for that matter) by other pesticides are not as extensive as they are for atrazine.
In addition to directly interfering with reproductive hormones, environmental pollutants can inhibit reproductive function indirectly. For example, pesticide mixtures containing atrazine can increase stress hormone (corticosterone) levels in exposed adult amphibians (Hayes et al., 2006a). Though moderate levels of corticosterone may promote reproduction by mobilizing energy stores (Moore and Jessop, 2003), chronically elevated corticosterone (and stress in general) inhibits reproductive development, behavior and fertility (Moore, 1983). Thus, environmentally induced stress may play a role in decreased reproduction in amphibians. Increased levels of corticosterone in amphibians can inhibit sex hormones (Burmeister et al., 2001) and breeding behaviors (Moore, 1983; Moore and Miller, 1984) necessary for reproductive function. Testosterone regulates many functions necessary for reproduction in male amphibians including sperm production and breeding behaviors, and acute stress can decrease testosterone levels in amphibians (Licht et al., 1983; Moore, 1983; Moore and Zoeller, 1985), thereby negatively impacting reproduction.
Field studies of southern toads (Bufo terrestris) residing in areas contaminated with coal ash effluent showed that they also exhibited elevated corticosterone levels (Hopkins et al., 1997). The stress response of male B. terrestris was characterized by significant increases in corticosterone when control animals were transplanted to enclosures at polluted sites. Male B. terrestris living in non-polluted areas had increased levels of testosterone during the months of reproductive activity (June and July), but testosterone levels declined in the non-breeding period of August. Male toads residing in coal ash-polluted sites had elevated testosterone levels during both breeding (June—July) and non-breeding periods (August). Thus, the impact of chemical-induced stress on amphibian reproductive function is complex. Many factors such as season, duration and magnitude of stress hormone secretion, behavioral influence, and species-specific differences can alter the dynamics of sex hormone suppression in reproduction (Wingfield and Sapolsky, 2003) and need to be examined in this context. Furthermore, even for well-studied compounds like atrazine, the number of species that has been examined is fairly small, and the susceptibility to endocrine disruptors will likely vary between species (Storrs and Semlitsch, 2008).
In addition, there are a number of environmental pollutants that delay or, in some cases, completely inhibit metamorphosis. Perchlorate, a contaminant associated with rocket fuel production, is a well-known example (Goleman et al., 2002a; Goleman et al., 2002b; Ortiz-Santaliestra and Sparling, 2007; Theodorakis et al., 2006). A number of pesticides can inhibit or delay anuran metamorphosis as well (Distel and Boone, 2009; Howe et al., 2004; Mackey and Boone, 2009; Sparling and Fellers, 2009), including atrazine (Carr et al., 2003; Sullivan and Spence, 2003). Some pesticides accelerate metamorphosis — for example, atrazine accelerated metamorphosis in at least two species of salamanders (Forson and Storfer, 2006b; Rohr et al., 2004) — and some can even have biphasic effects (Brodeur et al., 1997). Finally, pesticide mixtures can also delay metamorphosis, probably via an increase in corticosterone (Hayes et al., 2006a). Both delaying and inhibiting metamorphosis and certainly complete inhibition of metamorphosis will negatively impact reproduction, even though the exposed larvae are not killed or otherwise harmed.
Habitat modification affects recruitment
Habitat modification (non-chemical) also directly impacts recruitment in amphibians. Habitat fragmentation, logging, drainage of wetlands and filling of wetlands that historically served as breeding habitat for amphibian populations are all examples of habitat loss that negatively impact recruitment by reducing available habitats for breeding or limiting the ability of adults to reach suitable breeding habitats (Adams, 1999; Davidson et al., 2002; Davidson et al., 2001; Delis et al., 1996; Harper et al., 2008; Semlitsch et al., 2008; Todd et al., 2009). As discussed earlier, habitat loss is the most important cause of local declines and there is a vast amount of literature addressing this issue, so we will not cover it extensively here.
Invasive species affect recruitment
In addition to directly competing for food and preying on local species (Adams, 1999; Boone et al., 2007; D'Amore et al., 2009), invasive species may also inhibit recruitment and reproductive success in native species. For example, invasive species may compete with local amphibians for breeding sites. At least one study suggests that male Rana blairi (a species that is currently expanding its range) may directly compete with Rana pipiens (leopard frogs) males for females, generating hybrids in areas where the species now overlap (Di Candia and Routman, 2007). Similarly, B. boreas males have often been observed in amplexus with invasive juvenile Rana catesbeiana in California (T.B.H., personal observation). Given the limited opportunities that some males may have to copulate, the time spent with a non-conspecific may decrease reproductive success.
Pathogens affect recruitment
Pathogens can lead to diseases that cause developmental failures that can in turn lead to reproductive failures. For example, parasites may cause nutritional losses that can lead to impaired development or reproductive failure, but this possibility has not been adequately addressed in the literature. At least one study has shown that ticks can reduce growth in adult toads (Bufo marinus) (Lampo and Bayliss, 1996). In larval amphibians, findings vary. For example, one study reported that monostome trematodes both caused malformations and reduced growth in exposed anuran larvae (Rajakaruna et al., 2008). Belden (Belden, 2006) found that echinostome trematodes did not affect larval growth, however. Furthermore, Kiesecker and Blaustein (Kiesecker and Blaustein, 1999) demonstrated how complex these interactions can be. In a study where Rana cascadae and Pseudacris regilla were exposed to the water mold (Saprolegnia), R. cascadae survived in fewer numbers, but larvae that survived were significantly larger at metamorphosis.
In addition to affecting growth, however, malformations caused by pathogens will also impact reproduction. Parasites that cause limb deformities (Johnson et al., 1999; Johnson et al., 2007; Kiesecker, 2002; Linzey et al., 2003) or other malformations (Rajakaruna et al., 2008) may not cause death of the infected host but may limit the reproductive potential of males, which need functioning limbs to copulate with females. The main point is, disease does not necessarily lead to death, but may inhibit or even totally prevent adults from reproducing.
Horizontal interactions at Level 3
There are multiple horizontal interactions between the factors listed at Level 3. In examining which factors at Level 3 have greater global influences we considered the ratio of the number of other factors that are influenced by a given factor to the number of factors that impact that given factor. We used evidence from the literature (not weighted in any way) to determine this number. The highest ratios were obtained for atmospheric change and environmental pollutants. Atmospheric change affects pathogen prevalence, environmental pollutant levels experienced by amphibians, habitat modification and the success of invasive species, whereas only habitat modification and environmental pollutants influence the impact of atmospheric change (an affector:affected ratio of 4:2=2). Environmental pollutants (score 4:2=2) similarly affect all other factors at Level 3, but this factor is impacted by atmospheric change and habitat modification only. Using this approach, these two factors are followed in significance by habitat modification (score 4:3=1.33), invasive species (score 2:4=0.5), and pathogens (0/5=0). Pathogens become the least independently significant factor, because their impact is determined or affected by environmental pollutants, atmospheric change, habitat modification and invasive species, but pathogens impact none of the other four factors at Level 3. Even if we consider the possibility that local pathogens might limit the success of an invasive species (for which there is no evidence, but rather the contrary is supported by the literature), the score for pathogens becomes 1/5 or 0.2.
These ‘rankings’ do not imply that any one factor is independently more important or significant than another, but rather reflect a given factor's potential to interact with other factors in a way that amplifies one or both factors. For example, as stated earlier, habitat modification is likely the single most important factor affecting local amphibian populations. However, halting deforestation at any given locality will not slow amphibian declines globally, even though it would have an important local effect. On the other hand, slowing atmospheric change (e.g. slowing warming trends), will ameliorate the impact of climate change on all seven factors at Level 2 (which affect both death and recruitment). In addition, because atmospheric change impacts all other factors at Level 3, slowing atmospheric change would lessen the impact of habitat modification, invasive species, environmental pollutants and pathogens on all seven factors at Level 2; thus the higher ‘ranking’ of atmospheric change. Below, we discuss in detail evidence for the impact (horizontal interactions) of the five factors assigned to Level 3.
Atmospheric change
Atmospheric change affects all four other factors at Level 3 (see below). Atmospheric change is affected, in turn, by habitat modification and environmental pollution only. Environmental pollutants (such as greenhouse gases) increase temperature and industrial chemicals can result in acidic rainfall, both of which can negatively impact amphibians (Ling et al., 1986). As for the effects of habitat modification on atmospheric change, deforestation, as an example, contributes to the prevalence of greenhouse gases because plants reduce CO2 in the atmosphere (Turner et al., 2009). In addition, removal of trees increases exposure of ground and aquatic habitats, increasing the temperature extremes and fluctuations in available water to which amphibians will be exposed. Habitat modification can similarly affect drainage, and rainfall patterns. We could find no references indicating that pathogens or invasive species affect atmospheric change directly. Below we discuss the impact of atmospheric change on the other factors at Level 3.
Atmospheric change affects environmental pollutants
Atmospheric change impacts environmental pollutants. The metabolism and, thus, half-lives of chemical pollutants are temperature dependent. Thus, increases in temperature might reduce the impact of pesticides by decreasing their half-life in some cases. Alternatively, in cases where the resulting metabolites are more hazardous than the parent compounds, increased temperatures could enhance the adverse effects of a chemical. High temperatures and the lack of precipitation can also increase the vaporization of volatile pesticides into the atmosphere. Increased UV exposure may also affect environmental pollutants because some chemicals are sensitive to UV light and might be broken down into less harmful products (Chen et al., 2009). It is possible that some chemicals may be modified into more harmful ones, however. In addition, atmospheric moisture, such as fog, scavenges contaminants and re-deposits air-borne chemicals back to land at greater concentrations (Davidson et al., 2002; LeNoir et al., 1999; Sparling et al., 2001) which amphibians can take up through their skin. In areas where rainfall is increased, the transport of environmental pollutants may be enhanced and chemicals may travel further from the point of application (in the case of pesticides) (Hatfield et al., 1996; Lode et al., 1995; Mast et al., 2007; Vogel et al., 2008). Exposure to pesticides in the aquatic environment, especially during larval stages, is one way that amphibians are exposed. Adult amphibians also take up pesticides from the soil even during adult terrestrial life phases. Absorption of atrazine from the soil, for example, has been demonstrated in Bufo americanus (Mendez et al., 2009). Further, amphibians can absorb pesticides at greater rates relative to other vertebrates. For example, one study showed that frogs absorbed atrazine across their skin at rates 300 times higher than mammals, in part explaining the greater sensitivity of amphibians to environmental pollutants relative to mammals (Quaranta et al., 2009).
In addition, there are indirect biological effects of atmospheric changes on the impact of environmental pollutants. For example, temperature affects amphibian larval developmental rates directly (Hayes et al., 1993). Amphibian larvae develop and metamorphose faster at higher temperatures, so the exposure time to water-borne contaminants may be decreased. Alternatively, changes in temperature may prematurely induce larvae to pass into more sensitive developmental stages during the exposure periods and thereby render larvae more vulnerable.
Atmospheric change affects habitat modification
There are numerous publications supporting the impact of atmospheric change on habitat modification, so we will not review these issues here. In terms of the impact on amphibians, both increased and decreased precipitation affect and can lead to the loss of amphibian habitat (Pounds and Crump, 1994). Changes in temperature will affect habitat as well, in extreme cases contributing to desertification and loss of habitat (Verstraete et al., 2008), which has been shown to affect amphibian distributions (MacNally et al., 2009).
Atmospheric change affects invasive species
Climate change also impacts the establishment of invasive species. In areas where average temperatures increase, species formerly restricted to warmer climates may extend their ranges both away from the equator and into higher elevations (Ficetola et al., 2007). Numerous examples showing changes in species distribution are available, including several studies in amphibians (D'Amore et al., 2010). For example the successful invasion of bullfrogs (R. catesbeiana) in the western United States is in part due to changing climate (Ficetola et al., 2007). Other invasive species are similarly predicted to extend their ranges if warming trends continue (Rodder and Weinsheimer, 2009).
Atmospheric change affects pathogens
Several studies have addressed the potential for atmospheric change to affect the prevalence and impact of pathogens. Some authors have suggested that atmospheric change has contributed to the spread of the disease chytridiomycosis in the neotropics, because increased cloud cover has shifted temperatures towards optimal growth conditions for the fungus that causes the disease (Pounds et al., 2006). Other researchers agree that the correlation is robust, but question whether there is evidence for causation (Rohr et al., 2008a). Regardless, changing climate may contribute to increased disease in two ways: changes in average temperature may impact pathogens directly, or shifts in temperature may affect the ability of exposed amphibians to mount appropriate immune responses (Rohr et al., 2008a). These effects can happen in concert: a given temperature shift may simultaneously increase a pathogen's virulence and decrease the immune response of the amphibian host, or may decrease a pathogen's virulence and increase the immune response in the host. Alternatively, conflicting effects may occur: a pathogen's virulence may be decreased but the disease rate may increase if the immune response of amphibians is decreased over this same temperature shift. Some researchers point out that there may also be seasonal lag times; for example, seasonal temperatures may change in favor of amphibian immune responsiveness but the physiological response may be delayed (Raffel et al., 2006).
Environmental pollutants
The impact of environmental pollutants on amphibian populations is affected by atmospheric change and habitat modification only. Climate change will impact environmental pollutants as described above (see ‘Atmospheric change’ section). Habitat modification, such as draining wetlands, may further increase exposure to chemicals such as pesticides, which may become concentrated as ponds, lakes and rivers desiccate (Hayes et al., 2006a). Various other alterations of ground water supplies and surface water flow will similarly affect the transport of chemicals released into the environment.
Environmental pollutants affect atmospheric change, habitat modification, invasive species and pathogens
Environmental pollutants, in turn, affect all other factors at Level 3: atmospheric change (see above), habitat modification, invasive species and pathogens (see below). Because most of these impacts are discussed elsewhere in this paper, we limit our discussion here. The exception is the impact of environmental pollutants on the survival of pathogens, which we describe now.
At least one study reports that pesticides (atrazine and metolachlor) can affect survivorship of amphibian pathogens (Griggs and Belden, 2008). At the higher doses tested, the mixture of these two herbicides reduced survivorship of free-living cercaria of echinostome trematodes. Environmental pollutants may also affect survivorship of a pathogen's intermediate host (Koprivnikar et al., 2007). For example, the herbicide atrazine suppresses immune function in snails (Russo and Lagadic, 2000). Snails serve as the first host for trematode parasites that induce limb deformities in amphibians. By suppressing immune function in the first host, more cercaria are available to affect amphibians. Further, atrazine suppresses immune function in amphibians, leading to more limb deformity-inducing infections by trematodes (Kiesecker, 2002) and to an increased number of trematode infections in the kidney (Rohr et al., 2008c). In addition, atrazine increases attached algae, which increases snail populations that serve as hosts for the trematodes (Rohr and Crumrine, 2005). Thus, atrazine influences limb deformities by habitat modification (making the habitat more suitable for the intermediate host), by lowering immune function in the intermediate host so that more parasites are available, and by lowering immune function in larval frogs so that a greater number of frogs are infected by a greater number of successful trematodes. Finally, it is also conceivable that environmental pollutants may affect the prevalence of invasive species, as some invasive species may be less susceptible to contaminants than others (Storrs and Semlitsch, 2008).
Habitat modification
Environmental contaminants, climate change and invasive species all affect habitat modification. Environmental pollutants (such as herbicides) are potent agents of habitat modification. In addition to losing plant life due to herbicide application (including aquatic plants that serve as food for anuran larvae), the loss of plants can lead to increased erosion, which will affect water quality. Climate change itself will obviously cause habitat modification and invasive species can modify habitat.
Habitat modification in turn affects several of the factors on Level 3. Habitat modification can determine the success of invasive species (D'Amore et al., 2010). For example, the damming of rivers and streams may be preferable to some species which can then establish themselves and out-compete local species. In addition, habitat modification impacts pathogens (see below), environmental pollutants (see above), atmospheric change (see above) and invasive species (see below).
Invasive species
Pathogens, environmental contaminants, atmospheric change and habitat modification can all potentially influence the success of invasive species. In most cases, invasive species are less susceptible to disturbances, and so these factors increase their success at the expensive of local species (D'Amore et al., 2010; D'Amore et al., 2009; Ficetola et al., 2007; Kats and Ferrer, 2003; Kiesecker and Semlitsch, 2003). In turn, invasive species may potentially affect habitat modification and pathogen success (see below).
Pathogens
Because of the recent focus on pathogens in amphibian declines, this topic is worth significant discussion. Disease is one of the most widely reported causes of the rapid decline of amphibian populations (Berger et al., 1998; Cunningham et al., 2008; Daszak et al., 1999; Lips et al., 2008; Stuart et al., 2004). The greatest volume of evidence that specific pathogens may lead to amphibian deformity, disease or death exists for two species of fungi (Batrachochytrium dendrobatidis and Saprolegnia ferax), an iridovirus (ranavirus), and the trematode parasite (Ribeiroia) (Berger et al., 1998; Bollinger et al., 1999; Green et al., 2002; Jancovich et al., 1997; Johnson et al., 1999; Kiesecker and Blaustein, 1995a; Rohr et al., 2008c; Romansic et al., 2009).
In particular, chytridiomycosis, caused by the fungus B. dendrobatidis (Bd), has been the focus of much attention and deserves special treatment here. Some researchers even suggest that chytridiomycosis is the sole cause of amphibian declines (Collins et al., 2009). Bd was first linked to declines of anurans in Australia and South America in 1993 (Berger et al., 1998). Since then, the fungus has been identified in amphibian communities on all continents where amphibians occur: Europe (Bovero et al., 2008; Garner et al., 2005), North America (Frias-Alvarez et al., 2008; Rachowicz et al., 2006) and Africa (Goldberg et al., 2007). Retrospective analysis of preserved specimens has revealed a longer history between Bd and amphibians than was anticipated, with the earliest evidence from Africa in the 1930s (Weldon et al., 2004). In California, where Bd has been implicated in recent declines of Rana muscosa (Rachowicz et al., 2006), Bd can be traced back to the 1960s in preserved specimens of the native anuran P. regilla (Padgett-Flohr and Hopkins, 2009) with similar introduction times in other localities in North America (Ouellet et al., 2005).
An explanation for the current increase in disease occurrence, especially chytridiomycosis, is debated (Rachowicz et al., 2005). That researchers are documenting recent population declines from Bd in numerous anuran species with very different geographic locations suggests either that some large-scale environmental variable has changed in recent years or that the pathogen itself has evolved to be more virulent. Recently, Bd has been introduced to naïve populations such as those species experiencing declines in relatively undisturbed montane regions of the western United States, Central America and Australia (Lips et al., 2004). Introduction into previously isolated populations, such as these localities, may be enough to decimate exposed populations. At least one study suggests that Bd has recently evolved to be more virulent, however (James et al., 2009).
While it seems that some populations, especially relatively isolated ones, such as mountain-top species (Rachowicz et al., 2006; Woodhams et al., 2008), may decline solely because of Bd introduction, in many cases Bd may take its toll on amphibian populations because exposed individuals are stressed by other factors already. For example, in North America, the recently separated temperate species R. muscosa and Rana sierrae (Vredenburg et al., 2007) are currently experiencing dramatic declines from Bd (Rachowicz et al., 2006). Historically, introduced species have been implicated in declines in California (Bradford et al., 1998; Vredenburg et al., 2007) and further fragmentation of R. muscosa populations may have rendered them more susceptible (Bradford et al., 1993). In other words, Bd may simply be spreading advantageously through populations and species already threatened by other factors (Wake and Vredenburg, 2008). There are many amphibian species in a similar predicament, where longstanding threats from habitat modification, environmental pollutants and invasive species have already decreased their population sizes. Thus, even in the absence of the evolution of more virulent pathogen strains, amphibian populations already stressed by other factors may lack the immunological capacity to ward off disease.
The possible significance of impaired amphibian immune function and resulting increased disease susceptibility in global amphibian declines is supported by the survival of some amphibian species despite the introduction of pathogens. For example, not all amphibian species are susceptible to pathogens of global concern. American bullfrogs (R. catesbeiana) and X. laevis carry Bd infections without significant signs of chytridiomycosis (Hanselmann et al., 2004; Schloegel et al., 2009; Weldon et al., 2004). Pseudacris regilla are more resistant to infections with S. ferax than are B. boreas and R. cascadae (Kiesecker and Blaustein, 1995b). These findings suggest that specific immune defenses are required for protection against pathogens and play a key role in population success, rather than success depending on the pathogen's virulence alone. The greatest evidence for the importance of the role of amphibian immune function in global disease will come from pathogen challenge experiments involving other stressors, i.e. pesticides, fertilizers and UV radiation. As described below, amphibians are often more susceptible to disease when challenged with environmentally relevant stressors.
The development of the amphibian immune system and functions used to defend against pathogens have been reviewed elsewhere (Carey et al., 1999; Du Pasquier et al., 1989; Rollins-Smith, 1998; Rollins-Smith, 2009; Rollins-Smith et al., 2002; Rollins-Smith, 2001), so will not be addressed here. Although the impact of other stressors on immune function as they relate to Bd spread has not been fully examined directly, the impacts of stress on immune function and subsequent disease susceptibility, in general, have been demonstrated in numerous cases. Amphibians show particular sensitivity to environmental stressors, both natural and anthropogenic in origin (but see Kerby et al., 2010). In addition to naturally occurring factors that influence disease resistance, such as season, temperature and developmental stage (Maniero and Carey, 1997; Raffel et al., 2006; Rollins-Smith, 1998), host—pathogen ecology may also be affected by anthropogenic activity. For example, altered environmental conditions may increase the success of pathogens by enhancing their growth or virulence. These advantages may be facilitated through increased population size or crowding of hosts, increased populations of secondary host, eutrophication, general increase in pathogen resources, or atmospheric changes toward pathogen optimum conditions. Many of these factors have already been discussed here.
Pathogen success may also be increased due to decreased immune function in the host. This effect could potentially occur through two mechanisms, direct immune suppression or indirect immune suppression. Direct immune suppression may occur through compromised epithelial barriers or chemical interference with immune responses. Immune function may also be compromised indirectly through general disruptions in homeostasis or altered ecology of the host, which culminate in artificially stressful conditions and immune suppression. These indirect stressors may include a lack of resources or prey, increased predators or competitors, or inability to compete for resources with con-specifics. Below, we address the other factors at Level 3 that impact pathogens.
Atmospheric change and pathogens
The effects of UV radiation on immune function in amphibians have not been examined thoroughly. However, exposure of egg masses to environmental UV radiation significantly increased infections with the fungal pathogen S. ferax (Kiesecker et al., 2001; Kiesecker and Blaustein, 1995a; Kiesecker and Blaustein, 1995b). The infections were most pronounced in species experiencing population declines, suggesting species-specific defense mechanisms (Kiesecker and Blaustein, 1995a; Kiesecker and Blaustein, 1995b).
Amphibians must regulate immune function across dynamic temperature regimes without the aid of constant body temperatures. This observation becomes particularly important for amphibians inhabiting temperate climates. Amphibians experimentally held at low temperatures have decreased lymphocyte proliferation, decreased serum complement activity, increased neutrophils and decreased eosinophils (Maniero and Carey, 1997). Season-specific patterns in peripheral blood cell counts were also observed in field-collected amphibians (Raffel et al., 2006; Raffel et al., 2009). These effects may be exacerbated in the face of a changing climate. For example, Kiesecker and colleagues (Kiesecker et al., 2001) studied the impact of precipitation patterns on disease in the Pacific Northwest. The authors found a correlation between decreased rainfall, decreased water levels at oviposition sites of toads, increased exposure to UV radiation, and increased susceptibility of the egg masses to infection. Thus, there is evidence that atmospheric change will affect pathogen virulence and/or disease rates.
Environmental pollutants and pathogens
Numerous studies have documented the effects of environmental pollutants on the amphibian immune system. Nearly all of these studies suggest that amphibians are particularly sensitive to contaminants in laboratory tests, experimental field manipulations and field observations as described below.
Pesticide exposure results in a number of effects that are indicative of decreased immune function, leading to increased disease susceptibility in amphibians. Laboratory exposure of amphibians to agricultural pesticides can result in decreased melanomacrophage aggregates in the liver (Rohr et al., 2008c), altered spleen cellularity (Linzey et al., 2003), decreased lymphocyte proliferation responses (Christin et al., 2003a; Christin et al., 2004), decreased skin peptide defenses (Davidson et al., 2007) and decreased peripheral leukocyte levels (Forson and Storfer, 2006a; Forson and Storfer, 2006b). Langerveld and others (Langerveld et al., 2009) showed that chronic exposure to atrazine resulted in the modulation of genes involved in growth and metabolism, proteolysis, fibrinogen complex formation and immune regulation in X. laevis. Seven genes associated with immune system function, specifically defense molecules present in the skin (e.g. magainin II, levitide A, preprocarulein, skin granule protein), were significantly down-regulated in female tadpoles.
Legacy pesticides also have detrimental effects on immune function in amphibians. DDT, DDE and other organochlorines in soil, amphibian food and liver tissue caused increased melanomacrophage aggregates in liver, increased limb deformities and decreased white pulp in the spleen of exposed marine toad (B. marinus) individuals (Linzey et al., 2003). Albert and others (Albert et al., 2007) showed that dietary exposure to DDT or dieldrin for 10 weeks through eating dosed crickets, suppressed antibody and DTH responses. Other researchers (Barni et al., 2007) showed that several changes in blood cell properties, indicative of impaired immune function, occur in Rana esculenta in polluted rice fields. Gilbertson and others (Gilbertson et al., 2003) found that DDT (923 ng g−1 wet mass), malathion (990 ng g−1 wet mass) and dieldrin (50 ng g−1 wet mass) injections decreased antibody production, decreased oxidative burst of peripheral leukocytes and altered delayed-type hypersensitivity (DTH) response in R. pipiens.
Consistent with their adverse effects on immune function in amphibians, environmental contaminants such as pesticides increase pathogen success and disease rates in exposed amphibians. In particular, the widespread herbicide atrazine impairs immune function and increases disease rates, both alone and when part of a mixture of other pesticides. Christin and others (Christin et al., 2003b) showed that a mixture of pesticides that included atrazine decreased T-cell responses and decreased phagocytosis and spleen cellularity during exposure when frogs were challenged with parasites. This pesticide mixture also increased parasite infections with Rhabdias ranae in R. pipiens (Christin et al., 2003b). Similarly, a pesticide mixture containing atrazine decreased thymus size and cell density, and resulted in the induction of flavo-bacterial meningitis in the same species (Hayes et al., 2006a). A pesticide mixture containing atrazine also decreased spleen cellularity in X. laevis, increased phagocytic cells in X. laevis spleen, and decreased T-cell proliferation in R. pipiens (Christin et al., 2004). Kiesecker (Kiesecker, 2002) showed that wood frogs experienced increased parasitic limb deformities when exposed to atrazine, malathion or esfenvalerate. These pesticides also decreased eosinophils and increased encysted cercariae (Telorchis, Ribeiroia) in exposed amphibians (Kiesecker, 2002). Increased infections with parasites in pesticide-exposed amphibians have also been noted for R. ranae (Christin et al., 2003b; Gendron et al., 2003), Ribeiroia (Kiesecker, 2002) and trematode spp. (Rohr et al., 2008c), and Echinostoma trivolvis (Budischak et al., 2008; Rohr et al., 2008b).
Similar to the effects of experimental exposure to pesticides in the laboratory and mesocosms described above, field studies support the view that exposure to agricultural chemicals is associated with impaired immune function and increased disease states in the wild. Agricultural chemical use is correlated with increased parasite infection and limb deformities in the field (Kiesecker, 2002). Amphibian parasite communities have altered ecology in regions of high agricultural pesticide use (King et al., 2007). Rana pipiens showed a decrease in the diversity of parasites with only generalists present in a 2 year study in Canada. In field studies, atrazine and phosphates were correlated with increased trematode loads in R. pipiens (Rohr et al., 2008c). These findings were supported by mesocosm studies that showed that atrazine increased attached algae, resulting in increased snails, increased trematode infections, immuno-suppressed tadpoles and, ultimately, increased infections (Rohr et al., 2008c) (as discussed above). Further, Rohr and others showed that atrazine, glyphosate, carbaryl and malathion increased parasitic infections in Rana clamitans in mesochosm studies (Rohr et al., 2008b). Woodhouse's toads developed clinical disease, hepatomegaly, and died at higher rates when exposed externally to malathion and challenged with the bacterium Aeromonus hydrophila (Taylor et al., 1999). Exposure to atrazine and sodium nitrate decreased peripheral leukocyte levels, and increased infections with Ambystoma tigrinum virus (ATV) in Ambystoma tigrinum nebulosum (Forson and Storfer, 2006a; Forson and Storfer, 2006b). Gendron and others (Gendron et al., 2003) showed that 21 days exposure to atrazine, metribuzin, aldicarb, endosulfan, lindane and dieldrin accelerated the migration of parasites into the lungs and increased the number of parasites in the lungs when pesticide-exposed R. pipiens were subsequently challenged with the parasite R. ranae. After 7 weeks of development in water with no malathion, tadpoles previously exposed as embryos for only 96 h to 60 and 600 μg l−1 malathion suffered from an increased number of E. trivolvis cysts when compared with controls (Budischak et al., 2008).
Habitat modification and pathogens
Habitat modification can increase pathogen virulence. As already discussed above, changes in algae in ponds can create refuges for parasites and/or their hosts, thus increasing parasite prevalence. The impact of habitat modification may be indirect, as degraded habitats may increase stress (references herein), which decreases immune function and thus increases susceptibility to disease.
Invasive species and pathogens
Invasive species may again simply serve as another stressor that contributes to immune function failure, which can increase susceptibility to disease in native amphibians. Invasive species may also increase pathogen prevalence by introducing the pathogens, however. For example the copepod parasite Lernaea cyprinacea is an Asian species that was most likely introduced by fish and then passed to bullfrogs (R. catesbeiana) (Kupferberg et al., 2009), which then introduced the pathogens to the habitat of native frogs, such as Rana boylii in California. The case of Lernaea infections in R. boylii is also an excellent example of the role that multiple interactive factors play in amphibian declines. Here, invasive bullfrogs not only compete for resources and prey on R. boylii but are also responsible for introducing a pathogen that causes disease, which leads to malformations and decreased growth (Kupferberg et al., 2009). In addition, the infections increase with increasing temperature (e.g. atmospheric change). Further, infections were increased in years when there was decreased discharge in the river due to drought (habitat modification). Thus, here we have four interacting factors at Level 3, affecting at least four factors on Level 2, which likely affect both death and recruitment at Level 1. Similarly, chytridiomycosis may have been introduced through an invasive species, X. laevis (which appears resistant) (Weldon et al., 2004), and invasive bullfrogs may serve as reservoirs for the fungus in the western United States (Green and Dodd, 2007; James et al., 2009; Pearl et al., 2007; Schloegel et al., 2009). As discussed earlier, habitat modification and atmospheric changes likely contribute to the success of these invasive species and likely affect the survivorship and virulence of the pathogen itself. So, again, multiple factors likely interact in determining the spread of this disease in this example.
Conclusions
Though much attention has been paid to chytrid recently, and though chytrid and other diseases undoubtedly play important roles in amphibian declines, research on the factors that contribute to pathogen virulence and the subsequent spread of disease is still needed. Further, studies that focus on failed recruitment in addition to examination of factors leading to the death of individuals need to be conducted. A better understanding of the ultimate factors at Level 3 and their interactions with pathogens and a better understanding of the remaining proximate factor (failed recruitment, Level 1) will help us to develop a better approach to mitigate amphibian declines.
Ultimately, the answers may impact more than just amphibian survival. Many scientists argue that amphibians are ‘canaries in the coal mine’ and that the rapid declines in the amphibian population are an environmental warning. Although a recent review argues that amphibians are not more sensitive than other taxa (Kerby et al., 2010), Kerby and colleagues examined sensitivity to chemicals only, e.g. environmental pollutants. Further, they examined only one aspect of the impact of environmental pollutants, direct toxicity (‘relative responses to water-borne toxins’ as described by the authors) and did not consider the greater exposure risk of amphibians as a result of their highly permeable skin. Furthermore, they ignored developmental effects, endocrine-disrupting effects, or interactions between environmental contaminants and other factors. What is more, the authors' conclusion that amphibians are not particularly sensitive is not based on comparisons between amphibians and other vertebrate classes, but rather on comparisons of toxicity between amphibians and invertebrates only. Certainly, it is not surprising that insects, or other invertebrates, are more sensitive to pesticides designed to kill insects. In addition to not considering the developmental and endocrine-disrupting effects of chemicals on amphibians, Kerby and colleagues ignore the fact that the targets of chemicals such as insecticides are prey species for amphibians, thus potentially leading to bioaccumulation of chemicals in some cases and adverse effects that do not manifest as direct toxicity. By analogy, birds are not necessarily more sensitive to DDT, but after consuming exposed insects and other prey, DDT levels accumulated in birds leading to high mortality and failed recruitment (Bernard, 1963).
These issues aside, however, as May points out (May, 2010), when we examine the fraction of species threatened relative to the total number of species that have been examined (as opposed to the total number of known species in a taxon), reptiles and fish exceed amphibians (among vertebrates) and plants and invertebrates exceed amphibians by a factor of two in the proportion of species threatened. Thus, as Kerby and colleagues (Kerby et al., 2010) suggest, perhaps ‘amphibians are not particularly sensitive and might more aptly be described as “miners in a coal mine”’. If this is the case, understanding the factors and interactions that contribute to amphibian declines becomes even more urgent and amphibian studies even more important. At least with amphibians, we have a starting point for defining the global issues that have driven us to the Earth's sixth mass extinction.
Acknowledgements
The Hayes laboratory is thankful for funding from the National Science Foundation, the National Geographic Society, the World Wildlife Fund, the Mitchell Kapor Foundation, the David Foundation, the Homeland Foundation, the Cornell Douglas Foundation, the Wallace Global Fund, the Class of'43 Endowed Chair, the Howard Hughes Medical Institute (Biology Fellows Program), Henry Sam Wheeler, Park Water Co., Ecorisk Inc., Novartis, and Syngenta Crop Protection, all of which have contributed to the research, thoughts and ideas presented in this paper. P.F. was funded by UC Toxic Substance Research and Training Program Fellowship. S.G. was funded by the American Association of University Women, a Soroptimist Founder Region Fellowship, a UCB Mentored Research Award, and a Switzer Environmental Fellowship. M.S. was funded by the National Institutes of Health and a UC Davis fellowship from the Exotics/Invasive Pest Management Program. Deposited in PMC for release after 12 months.
References
- Adams M. (1999). Correlated factors in amphibian decline: Exotic species and habitat change in western Washington. J. Wildlife Manage. 63, 1162-1171 [Google Scholar]
- Albert A., Drouillard K., Haffner G. D., Dixon B. (2007). Dietary exposure to low pesticide doses causes long-term immunosuppression in the leopard frog (Rana pipiens). Environ. Toxicol. Chem. 26, 1179-1185 [DOI] [PubMed] [Google Scholar]
- Barni S., Boncompagni E., Grosso A., Bertone V., Freitas I., Fasola M., Fenoglio C. (2007). Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aqua. Toxicol. 81, 45-54 [DOI] [PubMed] [Google Scholar]
- Becker C. G., Fonseca C. R., Haddad C. F. B., Batista R. F., Prado P. I. (2007). Habitat split and the global decline of amphibians. Science 318, 1775-1777 [DOI] [PubMed] [Google Scholar]
- Belden L. K. (2006). Impact of eutrophication on wood frog, Rana sylvatica, tadpoles infected with Echinostoma trivolvis cercariae. Can. J. Zool Rev. Can. Zool. 84, 1315-1321 [Google Scholar]
- Berger L., Speare R., Daszak P., Green D., Cunningham A. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 95, 9031-9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R. F. (1963). Studies on the effects of DDT on birds. Publ. Mus. Mich. State Univ. Biol. Ser. 2, 159-191 [Google Scholar]
- Blaustein A. R., Kiesecker J. (2002). Complexity in conservation: Lessons from the global decline of amphibian populations. Ecol. Lett. 5, 597-608 [Google Scholar]
- Blaustein A. R., Wake D. B. (1990). Amphibian declines: Judging stability, persistence and susceptibility of populations to local and global extinction. Trends Ecol. Evol. 5, 203-204 [Google Scholar]
- Bollinger T., Mao J., Schock D., Brigham R., Chinchar V. (1999). Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J. Wildlife Dis. 35, 413-429 [DOI] [PubMed] [Google Scholar]
- Boone M. D., James S. (2003). Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecol. App. 13, 829-841 [Google Scholar]
- Boone M. D., Semlitsch R. D. (2002). Interactions of an insecticide with competition and pond drying in amphibian communities. Ecol. App. 12, 307-316 [Google Scholar]
- Boone M. D., Semlitsch R. D., Little E. E., Doyle M. C. (2007). Multiple stressors in amphibian communities: Effects of chemical contamination, bullfrogs, and fish. Ecol. App. 17, 291-301 [DOI] [PubMed] [Google Scholar]
- Bovero S., Sotgiu G., Angelini C., Doglio S., Gazzaniga E., Cunningham A. A., Garner T. W. J. (2008). Detection of chytridiomycosis caused by Batrachochytrium dendrobatidis in the endangered Sardinian newt (Euproctus platycephalus) in Southern Sardinia, Italy. J. Wild. Dis. 44, 712-715 [DOI] [PubMed] [Google Scholar]
- Bradford D. F., Tabatabai F., Graber D. M. (1993). Isolation of remaining populations of the native frog, Rana muscosa, by introduced fishes in Sequoia and King's Canyon National Parks, California. Conserv. Biol. 7, 882-888 [Google Scholar]
- Bradford D. F., Cooper S. D., Jenkins T. M., Kratz K., Sarnelle O., Brown A. D. (1998). Influences of natural acidity and introduced fish on faunal assemblages in California alpine lakes. Can. J. Fish. Aqua. Sci. 55, 2478-2491 [Google Scholar]
- Brodeur J., Sherwood G., Rasmussen J., Hontela A. (1997). Impaired cortisol secretion in yellow perch (Perca flavescens) from lakes contaminated by heavy metals: In vivo and in vitro assessment. Can. J. Fish. Aqua. Sci. 54, 2752-2758 [Google Scholar]
- Budischak S. A., Belden L. K., Hopkins W. A. (2008). Effects of malathion on embryonic development and latent susceptibility to trematode parasites in ranid tadpoles. Environ. Toxicol. Chem. 27, 2496-2500 [DOI] [PubMed] [Google Scholar]
- Burmeister S., Somes C., Wilczynski W. (2001). Behavioral and hormonal effects of exogenous vasotocin and corticosterone in the green treefrog. Gen. Comp. Endocrinol. 122, 189-197 [DOI] [PubMed] [Google Scholar]
- Carey C., Cohen N., Rollins-Smith L. (1999). Amphibian declines: An immunological perspective. Dev. Comp. Immunol. 23, 459-472 [DOI] [PubMed] [Google Scholar]
- Carr J., Gentles A., Smith E., Goleman W., Urquidi L., Thuett K., Kendall R., Giesy J., Gross T., Solomon K., et al. (2003). Response of larval Xenopus laevis to atrazine: Assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ. Toxicol. Chem. 22, 396-405 [PubMed] [Google Scholar]
- Chen C., Yang S., Guo Y., Sun C., Gu C., Xu B. (2009). Photolytic destruction of endocrine disruptor atrazine in aqueous solution under UV irradiation: Products and pathways. J. Hazardous Mat. 172, 675-684 [DOI] [PubMed] [Google Scholar]
- Chipman R. K. (1966). Cotton rat age classes during a population decline. J. Mammal. 47, 138-141 [Google Scholar]
- Christin M. S., Gendron A., Brousseau P., Ménard L., Marcogliese D., Cyr D., Ruby S., Fournier M. (2003a). Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. 22, 1127-1133 [PubMed] [Google Scholar]
- Christin M. S., Gendron A. D., Brousseau P., Menard L., Marcogliese D. J., Cyr D., Ruby S., Fournier M. (2003b). Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. 22, 1127-1133 [PubMed] [Google Scholar]
- Christin M. S., Menard L., Gendron A. D., Ruby S., Cyr D., Marcogliese D. J., Rollins-Smith L., Fournier M. (2004). Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aqua. Toxicol. 67, 33-43 [DOI] [PubMed] [Google Scholar]
- Collins J. P., Crump M. L., Lovejoy T. E. (2009). Extinction In Our Times: Global Amphibian Declines Oxford: Oxford University Press; [Google Scholar]
- Crump M. L., Hensley F. R., Clark K. L. (1992). Apparent decline of the golden toad: underground or extinct? Copeia 1992, 413-420 [Google Scholar]
- Cunningham A. A., Daszak P., Stuart S. N., Hoffmann M., Chanson J., Cox N., Berridge R., Ramani P., Young B. (2008). Chytridiomycosis: driver of amphibian declines and extinctions. In Threatened Amphibians of the World (eds Stuart S. N., Hoffman M., Chanson J. S., Cox N. A., Berridge R. J., Ramani P., Young B. E.), pp. 49-50 Barcelona, Spain: Lynx Edicions; [Google Scholar]
- D'Amen M., Bombi P. (2009). Global warming and biodiversity: Evidence of climate-linked amphibian declines in Italy. Biol. Conserv. 142, 3060-3067 [Google Scholar]
- D'Amore A., Kirby E., McNicholas M. (2009). Invasive species shifts ontogenetic resource partitioning and microhabitat use of a threatened native amphibian. Aqua. Conserv. Mar. Fresh. Eco. 19, 534-541 [Google Scholar]
- D'Amore A., Hemingway V., Wasson K. (2010). Do a threatened native amphibian and its invasive congener differ in response to human alteration of the landscape? Biol. Inv. 12, 145-154 [Google Scholar]
- Daszak P., Berger L., Cunningham A. A., Hyatt A. D., Green D. E., Speare R. (1999). Emerging infectious diseases and amphibian population declines. Emerging Infect. Dis. 5, 735-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C., Shaffer H., Jennings M. (2001). Declines of the California red-legged frog: Climate, UV-B, habitat, and pesticides hypotheses. Ecol. Appl. 11, 464-479 [Google Scholar]
- Davidson C., Mahony N., Struger J., Ng P., Pettit K. (2002). Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate change hypothesis for California amphibian declines. Conserv. Biol. 16, 1588-1601 [Google Scholar]
- Davidson C., Benard M., Shaffer H., Parker J., O'Leary C., Conlon J., Rollins-Smith L. (2007). Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ. Sci. Technol. 41, 1771-1776 [DOI] [PubMed] [Google Scholar]
- Delis P., Mushinsky H., McCoy E. (1996). Decline of some west-central Florida anuran populations in response to habitat degradation. Biodivers. Conserv. 5, 1579-1595 [Google Scholar]
- Di Candia M. R., Routman E. J. (2007). Cytonuclear discordance across a leopard frog contact zone. Mol. Phylogen. Evol. 45, 564-575 [DOI] [PubMed] [Google Scholar]
- Distel C. A., Boone M. D. (2009). Effects of aquatic exposure to the insecticide carbaryl and density on aquatic and terrestrial growth and survival in American toads. Environ. Toxicol. Chem. 28, 1963-1969 [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Schwager J., Flajnik M. (1989). The immune system of Xenopus. Annu. Rev. Immunol. 7, 251-275 [DOI] [PubMed] [Google Scholar]
- Du Preez L. H., Solomon K., Carr J., Giesy J., Gross C., Kendall R. J., Smith E., Van Der Kraak G., Weldon C. (2005). Population structure characterization of the clawed frog (Xenopus laevis) in corn-growing versus non-corn-growing areas in South Africa. Afr. J. Herp. 54, 61-68 [Google Scholar]
- Eigenbrod F., Hecnar S. J., Fahrig L. (2008). Accessible habitat: an improved measure of the effects of habitat loss and roads on wildlife populations. Landscape Ecol. 23, 159-168 [Google Scholar]
- Fenelon J., Moore R. (1998). Transport of agrichemicals to ground and surface waters in a small central Indiana watershed. J. Environ. Qual. 27, 884-894 [Google Scholar]
- Ficetola G. F., Thuiller W., Miaud C. (2007). Prediction and validation of the potential global distribution of a problematic alien invasive species-the American bullfrog. Divers. Distrib. 13, 476-485 [Google Scholar]
- Forson D., Storfer A. (2006a). Atrazine increases Ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecol. Appl. 16, 2325-2332 [DOI] [PubMed] [Google Scholar]
- Forson D., Storfer A. (2006b). Effects of atrazine and iridovirus infection on survival and lifehistory traits of the long-toed salamander (Ambystoma macrodatylum). Environ. Toxicol. Chem. 25, 168-173 [DOI] [PubMed] [Google Scholar]
- Frias-Alvarez P., Vredenburg V. T., Familiar-Lopez M., Longcore J. E., Gonzalez-Bernal E., Santos-Barrera G., Zambrano L., Parra-Olea G. (2008). Chytridiomycosis survey in wild and captive Mexican amphibians. Ecohealth 5, 18-26 [DOI] [PubMed] [Google Scholar]
- Garcia T., Romansic J., Blaustein A. (2003). Interacting effects of ultraviolet radiation and disease on the survivorship of three amphibian species. Ecol. Soc. Am. Ann. Meet. Abstr. 88, 27-28 [Google Scholar]
- Garner T. W. J., Walker S., Bosch J., Hyatt A. D., Cunningham A. A., Fisher M. C. (2005). Chytrid fungus in Europe. Emerging Infect. Dis. 11, 1639-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron A. D., Marcogliese D. J., Barbeau S., Christin M. S., Brousseau P., Ruby S., Cyr D., Fournier M. (2003). Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia 135, 469-476 [DOI] [PubMed] [Google Scholar]
- Gerlach J. (2008). Fragmentation and demography as causes of population decline in Seychelles freshwater turtles (Genus Pelusios). Chelonian Conserv. Biol. 7, 78-87 [Google Scholar]
- Gilbertson M. K., Haffner G. D., Drouillard K. G., Albert A., Dixon B. (2003). Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environ. Toxicol. Chem. 22, 101-110 [PubMed] [Google Scholar]
- Goldberg T. L., Readel A. M., Lee M. H. (2007). Chytrid fungus in frogs from an equatorial African montane forest in western Uganda. J. Wildlife Dis. 43, 521-524 [DOI] [PubMed] [Google Scholar]
- Goleman W. L., Carr J. A., Anderson T. A. (2002a). Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ. Toxicol. Chem. 21, 590-597 [PubMed] [Google Scholar]
- Goleman W. L., Urquidi L. J., Anderson T. A., Smith E. E., Kendall R. J., Carr J. A. (2002b). Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ. Toxicol. Chem. 21, 424-430 [PubMed] [Google Scholar]
- Green D. E., Dodd C., Jr (2007). Presence of amphibian chytrid fungus Batrachochytrium dendrobatidis and other amphibian pathogens at warmwater fish hatcheries in southeastern North America. Herpetol. Conserv. Biol. 2, 43-47 [Google Scholar]
- Green D. E., Converse K. A., Schrader A. K. (2002). Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996-2001. In The Domestic Animal/Wildlife Interface: Issues for Disease Control, Conservation, Sustainable Food Production, and Emerging Diseases (eds Gibbs E. P. J., Bokma B. H.), pp. 323-339 New York: New York Academy of Sciences; [DOI] [PubMed] [Google Scholar]
- Griggs J. L., Belden L. K. (2008). Effects of atrazine and metolachlor on the survivorship and infectivity of Echinostoma trivolvis trematode cercariae. Arch. Environ. Contam. Toxicol. 54, 195-202 [DOI] [PubMed] [Google Scholar]
- Gutierrez J. B., Teem J. L. (2006). A model describing the effect of sex-reversed YY fish in an established wild population: the use of a Trojan Y chromosome to cause extinction of an introduced exotic species. J. Theor. Biol. 241, 333-341 [DOI] [PubMed] [Google Scholar]
- Hanselmann R., Rodriguez A., Lampo M., Fajardo-Ramos L., Aguirre A., Kilpatrick A., Rodriguez J., Daszak P. (2004). Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol. Conserv. 120, 115-119 [Google Scholar]
- Harper E. B., Rittenhouse T. A. G., Semlitsch R. D. (2008). Demographic consequences of terrestrial habitat loss for pool-breeding amphibians: predicting extinction risks associated with inadequate size of buffer zones. Conserv. Biol. 22, 1205-1215 [DOI] [PubMed] [Google Scholar]
- Hatfield J. L., Wesley C. K., Prueger J. H., Pfeiffer R. L. (1996). Herbicide and nitrate distribution in central Iowa rainfall. J. Environ. Qual. 25, 259-264 [Google Scholar]
- Hayes T. (1997). Hormonal mechanisms as potential constraints on evolution: Examples from the Anura. Am. Zool. 37, 482-490 [Google Scholar]
- Hayes T., Licht P. (1992). Gonadal involvement in sexual size dimorphism in the African bullfrog (Pyxicephalus adspersus). J. Exp. Zool. 264, 130-135 [Google Scholar]
- Hayes T., Chan R., Licht P. (1993). Interactions of temperature and steroids in growth, development, and metamorphosis in a toad (Bufo boreas). J. Exp. Zool. 266, 206-215 [DOI] [PubMed] [Google Scholar]
- Hayes T., Collins A., Lee M., Mendoza M., Noriega N., Stuart A. A., Vonk A. (2002a). Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 99, 5476-5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T., Haston K., Tsui M., Hoang A., Haeffele C., Vonk A. (2002b). Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): Laboratory and field evidence. Environ. Health Perspect. 111, 568-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T., Haston K., Tsui M., Hoang A., Haeffele C., Vonk A. (2002c). Feminization of male frogs in the wild. Nature 419, 895-896 [DOI] [PubMed] [Google Scholar]
- Hayes T., Case P., Chui S., Chung D., Haefele C., Haston K., Lee M., Mai V.-P., Marjuoa Y., Parker J., et al. (2006a). Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ. Health Perspect. 114, 40-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T., Stuart A., Mendoza G., Collins A., Noriega N., Vonk A., Johnston G., Liu R., Kpodzo D. (2006b). Characterization of atrazine-induced gonadal malformations and effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (estradiol 17β): Support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 114, 134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. A., Mendonca M. T., Congdon J. D. (1997). Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gen. Comp. Endocrinol. 108, 237-246 [DOI] [PubMed] [Google Scholar]
- Howe C., Berrill M., Pauli B., Helbing C., Werry K., Veldhoen N. (2004). Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 23, 1928-1938 [DOI] [PubMed] [Google Scholar]
- James T. Y., Litvintseva A. P., Vilgalys R., Morgan J. A. T., Taylor J. W., Fisher M. C., Berger L., Weldon C., du Preez L., Longcore J. E. (2009). Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. Plos Pathogens 5, e1000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancovich J., Davidson E., Morado J., Jacobs B., Collins J. (1997). Isolation of a lethal virus from the endangered tiger salamander Ambystoma tigrinum stebbinsi. Dis. Aqua. Organis. 31, 161-167 [Google Scholar]
- Jancovich J., Davidson E., Parameswaran N., Mao J., Chinchar V., Collins J., Jacobs B., Storfer A. (2005). Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Molec. Ecol. 14, 213-224 [DOI] [PubMed] [Google Scholar]
- Johnson P., Lunde K. R., Ritchie E. G., Launer A. (1999). The effect of trematode infection on amphibian limb development and survivorship. Science 284, 802-804 [DOI] [PubMed] [Google Scholar]
- Johnson P. T. J., Chase J. M., Dosch K. L., Hartson R. B., Gross J. A., Larson D. J., Sutherland D. R., Carpenter S. R. (2007). Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Nat. Acad. Sci. USA 104, 15781-15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurani M., Murgas K., Mikulaj L., Babusiko F. (1973). Effect of stress and environmental-temperature on adrenal function in Rana esculenta. J. Endocrinol. 57, 385-391 [DOI] [PubMed] [Google Scholar]
- Kats L. B., Ferrer R. P. (2003). Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Div. Distrib. 9, 99-110 [Google Scholar]
- Kerby J. L., Richards-Hrdlicka K. L., Storfer A., Skelly D. K. (2010). An examination of amphibian sensitivity to environmental contaminants: are amphibians poor canaries? Ecol. Lett. 13, 60-67 [DOI] [PubMed] [Google Scholar]
- Kiesecker J. L. (2002). Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proc. Natl. Acad. Sci. USA 99, 9900-9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker J. M., Blaustein A. R. (1995a). The prevalence of the pathogenic fungus, Saprolegnia on amphibian embryos in the Pacific Northwest. Bull. Ecol. Soc. Am. 76, 350 [Google Scholar]
- Kiesecker J. M., Blaustein A. R. (1995b). Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc. Natl. Acad. Sci. USA 92, 11049-11052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker J. M., Blaustein A. R. (1999). Pathogen reverses competition between larval amphibians. Ecology 80, 2442-2448 [Google Scholar]
- Kiesecker J. M., Semlitsch R. D. (2003). Invasive species as a global problem. Toward understanding the worldwide decline of amphibians. In Amphibian Conservation (ed. Semlitsch R. D.), pp. 113-126 London: Harper Collins Publisher; [Google Scholar]
- Kiesecker J. M., Blaustein A. R., Belden L. (2001). Complex causes of amphibian population declines. Nature 410, 681-684 [DOI] [PubMed] [Google Scholar]
- King K. C., McLaughlin J. D., Gendron A. D., Pauli B. D., Giroux I., Rondeau B., Boily M., Juneau P., Marcogliese D. J. (2007). Impacts of agriculture on the parasite communities of northern leopard frogs (Rana pipiens) in southern Quebec, Canada. Parasitology 134, 2063-2080 [DOI] [PubMed] [Google Scholar]
- Koprivnikar J., Baker R. L., Forbes M. R. (2007). Environmental factors influencing community composition of gastropods and their trematode parasites in southern Ontario. J. Parasitol. 93, 992-998 [DOI] [PubMed] [Google Scholar]
- Kupferberg S. J., Catenazzi A., Lunde K., Lind A. J., Palen W. J. (2009). Parasitic copepod (Lernaea cyprinacea) outbreaks in Foothill Yellow-legged Frogs (Rana boylii) linked to unusually warm summers and amphibian malformations in Northern California. Copeia 529-537 [Google Scholar]
- Lampo M., Bayliss P. (1996). The impact of ticks on Bufo marinus from native habitats. Parasitol. 113, 199-206 [Google Scholar]
- Langerveld A. J., Celestine R., Zaya R., Mihalko D., Ide C. F. (2009). Chronic exposure to high levels of atrazine alters expression of genes that regulate immune and growth-related functions in developing Xenopus laevis tadpoles. Environ. Res. 109, 379-389 [DOI] [PubMed] [Google Scholar]
- Langlois V., Carew A., Pauli B., Wade M., Cooke G., Trudeau V. (2009). Low levels of the herbicide atrazine alters sex ratios and reduces metamorphic success in Rana pipiens tadpoles raised in outdoor mesocosms. Environ. Health Perspect. doi:10.1289/ehp.0901418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNoir J., McConnell L., Fellers G., Cahill T., Seiber J. (1999). Summertime transport of current-use pesticides from California's central valley to the Sierra Nevada mountain range, USA. Environ. Toxicol. Chem. 18, 2715-2722 [Google Scholar]
- Licht P., McCreery B. R., Barnes R., Pang R. (1983). Seasonal and stress related changes in plasma gonadotropins, sex steriods, and corticosterone in the bullfrog, Rana catebeiana. Gen. Comp. Endocrinol. 50, 124-145 [DOI] [PubMed] [Google Scholar]
- Ling R. W., Vanamberg J. P., Werner J. K. (1986). Pond acidity and its relationship to larval development of Amystoma maculatumi and Rana sylvatica in upper Michigan. J. Herpetol. 20, 230-236 [Google Scholar]
- Linzey D. W., Burroughs J., Hudson L., Marini M., Robertson J., Bacon J. P., Nagarkatti M., Nagarkatti P. S. (2003). Role of environmental pollutants on immune functions, parasitic infections and limb malformations in marine toads and whistling frogs from Bermuda. Int. J. Environ. Health Res. 13, 125-148 [DOI] [PubMed] [Google Scholar]
- Lips K., Mendelson J. I., Munoz-Alonso A., Canseco-Marquez L., Mulcahy D. (2004). Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biol. Conserv. 119, 555-564 [Google Scholar]
- Lips K. R., Mendelson J. M., III, Stuart S. N., Hoffmann M., Chanson J., Cox N., Berridge R., Ramani P., Young B. (2008). Spread of disease in Latin American amphibian populations. In Threatened Amphibians of the World (eds Stuart S. N., Hoffman M., Chanson J. S., Cox N. A., Berridge R. J., Ramani P., Young B. E.), pp. 105 Barcelona, Spain: Lynx Edicions; [Google Scholar]
- Lode O., Eklo O., Holen B., Svensen A., Johnsen A. (1995). Pesticides in precipitation in Norway. Sci. Total Env. 160/161, 421-431 [Google Scholar]
- Mackey M. J., Boone M. D. (2009). Single and interactive effects of malathion, overwintered green frog tadpoles, and cyanobacteria on gray treefrog tadpoles. Environ. Toxicol. Chem. 28, 637-643 [DOI] [PubMed] [Google Scholar]
- MacNally R., Horrocks G., Lada H., Lake P. S., Thomson J. R., Taylor A. C. (2009). Distribution of anuran amphibians in massively altered landscapes in south-eastern Australia: effects of climate change in an aridifying region. Glob. Ecol. Biogeog. 18, 575-585 [Google Scholar]
- Maniero G. D., Carey C. (1997). Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J. Comp. Physiol. B 167, 256-263 [DOI] [PubMed] [Google Scholar]
- Marcogliese D. J., King K. C., Salo H. M., Fournier M., Brousseau P., Spear P., Champoux L., McLaughlin J. D., Boily M. (2009). Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat. Toxicol. (Amsterdam) 91, 126-134 [DOI] [PubMed] [Google Scholar]
- Mast M. A., Foreman W. T., Skaates S. V. (2007). Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch. Environ. Contamin. Toxicol. 52, 294-305 [DOI] [PubMed] [Google Scholar]
- May R. M. (2010). Ecological science and tomorrow's world. Philos. Trans. R Soc. B Biol. Sci. 365, 41-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays H. L., Doucet S. M., Yao C. T., Yuan H. W. (2006). Sexual dimorphism and dichromatism in Steere's Liocichla (Liocichla steerii). J. Field Ornithol. 77, 437-443 [Google Scholar]
- McCoy K. A., Bortnick L. J., Campbell C. M., Hamlin H. J., Guillette L. J., St Mary C. M. (2008). Agriculture alters gonadal form and function in the toad Bufo marinus. Environ. Health Perspect. 116, 1526-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel T. V., Martin P. A., Struger J., Sherry J., Marvin C. H., McMaster M. E., Clarence S., Tetreault G. (2008). Potential endocrine disruption of sexual development in free ranging male northern leopard frogs (Rana pipiens) and green frogs (Rana clamitans) from areas of intensive row crop agriculture Aquat. Toxicol. 90, 82 [DOI] [PubMed] [Google Scholar]
- Mckoy K. A., Sepulveda M. S., Gross T. S. (2002). Atrazine exposure and reproductive system abnormalities in field collected Bufo marinus. Soc. Environ. Toxicol. Chem., 23rd Annual Meeting in North America Salt Lake City, UT: [Google Scholar]
- Mendez S. I. S., Tillitt D. E., Rittenhouse T. A. G., Semlitsch R. D. (2009). Behavioral response and kinetics of terrestrial atrazine exposure in American toads (Bufo americanus). Arch. Environ. Contam. Toxicol. 57, 590-597 [DOI] [PubMed] [Google Scholar]
- Moore F. L. (1983). Behavioral endocrinology of amphibian reproduction. Bioscience 33, 557-561 [Google Scholar]
- Moore F. L., Miller L. J. (1984). Stress-induced inhibition of sexual behavior — corticosterone inhibits courtship behaviors of a male amphibian (Taricha granulosa). Hormone. Behav. 18, 400-410 [DOI] [PubMed] [Google Scholar]
- Moore F. L., Zoeller R. T. (1985). Stress-induced inhibition of reproduction — evidence of suppressed secretion of LHRH in an amphibian. Gen. Comp. Endocrinol. 60, 252-258 [DOI] [PubMed] [Google Scholar]
- Moore I. T., Jessop T. S. (2003). Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Hormone. Behav. 43, 39-47 [DOI] [PubMed] [Google Scholar]
- Müller S., Berg M., Ulrich M., Schwarzenbach R. P. (1997). Atrazine and its primary metabolites in Swiss lakes: input characteristics and long-term behavior in the water column. Environ. Sci. Tech. 31, 2104-2113 [Google Scholar]
- Murphy M. B., Hecker M., Coady K. K., Tompsett A. R., Jones P. D., Du Preez L. H., Everson G. J., Solomon K. R., Carr J. A., Smith E. E., et al. (2006). Atrazine concentrations, gonadal gross morphology and histology in ranid frogs collected in Michigan agricultural areas. Aquat. Toxicol. 76, 230-245 [DOI] [PubMed] [Google Scholar]
- Ortiz-Santaliestra M. E., Sparling D. W. (2007). Alteration of larval development and metamorphosis by nitrate and perchlorate in southern leopard frogs (Rana sphenocephala). Arch. Environ. Contam. Toxicol. 53, 639-646 [DOI] [PubMed] [Google Scholar]
- Orton F., Carr J., Handy R. (2006). Effects of nitrate and atrazine on larval development and sexual differentiation in the northern leopard frog Rana pipiens. Environ. Toxicol. Chem. 25, 65-71 [DOI] [PubMed] [Google Scholar]
- Ouellet M., Mikaelian I., Pauli B., Rodrigue J., Green D. (2005). Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv. Biol. 19, 1431-1440 [Google Scholar]
- Padgett-Flohr G. E., Hopkins R. L. (2009). Batrachochytrium dendrobatidis, a novel pathogen approaching endemism in central California. Dis. Aquat. Organis. 83, 1-9 [DOI] [PubMed] [Google Scholar]
- Pearl C. A., Bull E. L., Green D. E., Bowerman J., Adams M. J., Hyatt A., Wente W. H. (2007). Occurrence of the amphibian pathogen Batrachochytrium dendrobatidis in the Pacific Northwest. J. Herpetol. 41, 145-149 [Google Scholar]
- Pounds J. A., Crump M. L. (1994). Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv. Biol. 8, 72-85 [Google Scholar]
- Pounds J. A., Bustamante M. R., Coloma L. A., Consuegra J. A., Fogden M. P. L., Foster P. N., La Marca E., Masters K. L., Merino-Viteri A., Puschendorf R., et al. (2006). Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161-167 [DOI] [PubMed] [Google Scholar]
- Puschendorf R., Alford R. A., Rowley J. J. L., Stuart S. N., Hoffmann M., Chanson J., Cox N., Berridge R., Ramani P., Young B. (2008). Climate change and amphibian declines. In Threatened Amphibians of the World (eds Stuart S. N., Hoffman M., Chanson J. S., Cox N. A., Berridge R. J., Ramani P., Young B. E.), pp. 50-51 Barcelona, Spain: Lynx Edicions; [Google Scholar]
- Quaranta A., Bellantuono V., Cassano G., Lippe C. (2009). Why amphibians are more sensitive than mammals to xenobiotics. PLOS ONE 4, e7699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachowicz L. J., Hero J. M., Alford R. A., Taylor J. W., Morgan J. A. T., Vredenburg V. T., Collins J. P., Briggs C. J. (2005). The novel and endemic pathogen hypotheses: competing explanations for the origin of emerging infectious diseases of wildlife. Conserv. Biol. 19, 1441-1448 [Google Scholar]
- Rachowicz L. J., Knapp R. A., Morgan J. A. T., Stice M. J., Vredenburg V. T., Parker J. M., Briggs C. J. (2006). Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87, 1671-1683 [DOI] [PubMed] [Google Scholar]
- Raffel T., Rohr J., Kiesecker J., Hudson P. (2006). Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 20, 819-828 [Google Scholar]
- Raffel T. R., LeGros R. P., Love B. C., Rohr J. R., Hudson P. J. (2009). Parasite age-intensity relationships in red-spotted newts: does immune memory influence salamander disease dynamics? Int. J. Parasitol. 39, 231-241 [DOI] [PubMed] [Google Scholar]
- Rajakaruna R. S., Piyatissa P. M. J. R., Jayawardena U. A., Navaratne A. N., Amerasinghe P. H. (2008). Trematode infection induced malformations in the common hourglass treefrogs. J. Zool. 275, 89-95 [Google Scholar]
- Relyea R. A. (2003). Predator cues and pesticides: A double dose of danger for amphibians. Ecol. App. 13, 1515-1521 [Google Scholar]
- Relyea R. A. (2004a). Growth and survival of five amphibian species exposed to combinations of pesticides. Environ. Toxicol. Chem. 23, 1737-1742 [DOI] [PubMed] [Google Scholar]
- Relyea R. A. (2004b). Synergistic impacts of malathion and predatory stress on six species of North American tadpoles. Environ. Toxicol. Chem. 23, 1080-1084 [DOI] [PubMed] [Google Scholar]
- Relyea R. A. (2005). The lethal impact of Roundup on aquatic and terrestrial amphibians. Ecol. App. 15, 1118-1124 [Google Scholar]
- Relyea R. A. (2009). A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 159, 363-376 [DOI] [PubMed] [Google Scholar]
- Relyea R. A., Hoverman J. T. (2008). Interactive effects of predators and a pesticide on aquatic communities. Oikos 117, 1647-1658 [Google Scholar]
- Relyea R. A., Jones D. K. (2009). The toxicity of Roundup Original Max to 13 species of larval amphibians. Environ. Toxicol. Chem. 28, 2004-2008 [DOI] [PubMed] [Google Scholar]
- Rodder D., Weinsheimer F. (2009). Will future anthropogenic climate change increase the potential distribution of the alien invasive Cuban treefrog (Anura: Hylidae)? J. Nat. Hist. 43, 1207-1217 [Google Scholar]
- Roelants K., Gower D. J., Wilkinson M., Loader S. P., Biju S. D., Guillaume K., Moriau L., Bossuyt F. (2007). Global patterns of diversification in the history of modern amphibians. Proc. Nat. Acad. Sci. USA 104, 887-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr J. R., Crumrine P. (2005). Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. App. 15, 1135-1147 [Google Scholar]
- Rohr J. R., Palmer B. (2005). Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ. Toxicol. Chem. 24, 1253-1258 [DOI] [PubMed] [Google Scholar]
- Rohr J. R., Elskus A., Shepherd B., Crowley P., McCarthy T., Niedzwiecki J., Sager T., Sih A., Palmer B. (2003). Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma. barbouri). Environ. Toxicol. Chem. 22, 2385-2392 [DOI] [PubMed] [Google Scholar]
- Rohr J. R., Elskus A., Shepherd B., Crowley P., McCarthy T., Niedzwiecki J., Sager T., Sih A., Palmer B. (2004). Multiple stressors and salamanders: Effects of an herbicide, food limitation, and hydroperiod. Ecol. Appl. 14, 1028-1040 [Google Scholar]
- Rohr J. R., Sager T., Sesterhenn T. M., Palmer B. D. (2006). Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environ. Health. Perspect. 114, 46-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr J. R., Raffel T. R., Romansic J. M., McCallum H., Hudson P. J. (2008a). Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl. Acad. Sci. USA 105, 17436-17441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr J. R., Raffel T. R., Sessions S. K., Hudson P. J. (2008b). Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 18, 1743-1753 [DOI] [PubMed] [Google Scholar]
- Rohr J. R., Schotthoefer A. M., Raffel T. R., Carrick H. J., Halstead N., Hoverman J. T., Johnson C. M., Johnson L. B., Lieske C., Piwoni M. D., et al. (2008c). Agrochemicals increase trematode infections in a declining amphibian species. Nature 455, 1235-1239 [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. A. (1998). Metamorphosis and the amphibian immune system. Immunol. Rev. 166, 221-230 [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. A. (2001). Neuroendocrine-immune system interactions in amphibians-Implications for understanding global amphibian declines. Immunol. Res. 23, 273-280 [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. A. (2009). The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim. Biophys. Acta 1788, 1593-1599 [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. A., Doersam J., Longcore J., Taylor S., Shamblin J., Carey C., Zasloff M. (2002). Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev. Comp. Immunol. 26, 63-72 [DOI] [PubMed] [Google Scholar]
- Romansic J. M., Diez K. A., Higashi E. M., Johnson J. E., Blaustein A. R. (2009). Effects of the pathogenic water mold Saprolegnia ferax on survival of amphibian larvae. Dis. Aqua. Org. 83, 187-193 [DOI] [PubMed] [Google Scholar]
- Russo J., Lagadic L. (2000). Effects of parasitism and pesticide exposure on characteristics and functions of hemocyte populations in the freshwater snail Lymnaea. palustris (Gastropoda, Pulmonata). Cell. Biol. Toxicol. 16, 15-30 [DOI] [PubMed] [Google Scholar]
- Schloegel L. M., Picco A. M., Kilpatrick A. M., Davies A. J., Hyatt A. D., Daszak P. (2009). Magnitude of the US trade in amphibians and presence of Batrachochytrium. dendrobatidis and Ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 142, 1420-1426 [Google Scholar]
- Semlitsch R. D., Conner C. A., Hocking D. J., Rittenhouse T. A. G., Harper E. B. (2008). Effects of timber harvesting on pond-breeding amphibian persistence: testing the evacuation hypothesis. Ecol. Appl. 18, 283-289 [DOI] [PubMed] [Google Scholar]
- Sheriff M. J., Krebs C. J., Boonstra R. (2009). The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 78, 1249-1258 [DOI] [PubMed] [Google Scholar]
- Shine R. (1979). Sexual selection and sexual dimorphism in the amphibia. Copeia 1979, 297-306 [Google Scholar]
- Shine R. (1989). Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quart. Rev. Biol. 64, 419-461 [DOI] [PubMed] [Google Scholar]
- Smith K. G., Lips K. R., Chase J. M. (2009). Selecting for extinction: nonrandom disease-associated extinction homogenizes amphibian biotas. Ecol. Lett. 12, 1069-1078 [DOI] [PubMed] [Google Scholar]
- Sodhi N. S., Bickford D., Diesmos A. C., Lee T. M., Koh L. P., Brook B. W., Sekercioglu C. H., Bradshaw C. J. A. (2008). Measuring the meltdown: drivers of global amphibian extinction and decline. PLOS one 3, e1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon K., Baker D., Richards R., Dixon K., Klaine S., LaPoint T., Kendall R., Weisskopf C., Giddings J., Giesy J., et al. (1996). Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 15, 31-76 [DOI] [PubMed] [Google Scholar]
- Sparling D. W., Fellers G. M. (2009). Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environ. Toxicol. Chem. 28, 1696-1703 [DOI] [PubMed] [Google Scholar]
- Sparling D. W., Fellers G., McConnell L. (2001). Pesticides and amphibian population declines in California, USA. Environ. Toxicol. Chem. 20, 1591-1595 [DOI] [PubMed] [Google Scholar]
- Storrs S. I., Semlitsch R. D. (2008). Variation in somatic and ovarian development: predicting susceptibility of amphibians to estrogenic contaminants. Gen. Comp. Endocrinol. 156, 524-530 [DOI] [PubMed] [Google Scholar]
- Stuart S., Chanson J., Cox N., Young B., Rodrigues A., Fischman D., Waller R. (2004). Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783-1786 [DOI] [PubMed] [Google Scholar]
- Sullivan K., Spence K. (2003). Effects of sublethal concentrations of atrazine and nitrate on metamorphosis of the African clawed frog. Environ. Toxicol. Chem. 22, 627-635 [PubMed] [Google Scholar]
- Tavera-Mendoza L., Ruby S., Brousseau P., Fournier M., Cyr D., Marcogliese D. (2002a). Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ. Toxicol. Chem. 21, 527-531 [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L., Ruby S., Brousseau P., Fournier M., Cyr D., Marcogliese D. (2002b). Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ. Toxicol. Chem. 21, 1264-1267 [PubMed] [Google Scholar]
- Taylor S. K., Williams E. S., Mills K. W. (1999). Effects of malathion on disease susceptibility in Woodhouse's toads. J. Wildlife. Dis. 35, 536-541 [DOI] [PubMed] [Google Scholar]
- Theodorakis C. W., Rinchard J., Carr J. A., Park J. W., McDaniel L., Liu F. J., Wages M. (2006). Thyroid endocrine disruption in stonerollers and cricket frogs from perchlorate-contaminated streams in east-central Texas. Ecotoxicology 15, 31-50 [DOI] [PubMed] [Google Scholar]
- Thurman E., Cromwell A. (2000). Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ. Sci. Tech. 34, 3079-3085 [Google Scholar]
- Todd B. D., Luhring T. M., Rothermel B. B., Gibbons J. W. (2009). Effects of forest removal on amphibian migrations: implications for habitat and landscape connectivity. J. App. Ecol. 46, 554-561 [Google Scholar]
- Turner W. R., Oppenheimer M., Wilcove D. S. (2009). A force to fight global warming. Nature 462, 278-279 [DOI] [PubMed] [Google Scholar]
- Verstraete M. M., Brink A. B., Scholes R. J., Beniston M., Smith M. S. (2008). Climate change and desertification: where do we stand, where should we go? Glob. Plan. Chang. 64, 105-110 [Google Scholar]
- Vogel J. R., Majewski M. S., Capel P. D. (2008). Pesticides in rain in four agricultural watersheds in the United States. J. Env. Qual. 37, 1101-1115 [DOI] [PubMed] [Google Scholar]
- Voyles J., Young S., Berger L., Campbell C., Voyles W. F., Dinudom A., Cook D., Webb R., Alford R. A., Skerratt L. F., et al. (2009). Pathogenesis of Chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582-585 [DOI] [PubMed] [Google Scholar]
- Vredenburg V. T., Bingham R., Knapp R., Morgan J. A. T., Moritz C., Wake D. (2007). Concordant molecular and phenotypic data delineate new taxonomy and conservation priorities for the endangered mountain yellow-legged frog. J. Zool. 271, 361-374 [Google Scholar]
- Vredenburg V. T., Koos M. S., Wake D. B., Stuart S. N., Hoffmann M., Chanson J., Cox N., Berridge R., Ramani P., Young B. (2008). Amphibian declines in California. In Threatened Amphibians of the World (eds Stuart S. N., Hoffman M., Chanson J. S., Cox N. A., Berridge R. J., Ramani P., Young B. E.), pp. 91 Barcelona, Spain: Lynx Edicions; [Google Scholar]
- Wake D. (1991). Declining amphibian populations: A global phenomenon. Science 253, 860 [DOI] [PubMed] [Google Scholar]
- Wake D. B., Vredenburg V. T. (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 105, 11466-11473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon C., du Preez L., Hyatt A., Muller R., Speare R. (2004). Origin of the amphibian chytrid fungus. Emerg. Infect. Dis. 10, 2100-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C., Sapolsky R. M. (2003). Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 15, 711-724 [DOI] [PubMed] [Google Scholar]
- Woodhams D. C., Kilburn V. L., Reinert L. K., Voyles J., Medina D., Ibanez R., Hyatt A. D., Boyle D. G., Pask J. D., Green D. M., et al. (2008). Chytridiomycosis and amphibian population declines continue to spread eastward in Panama. Ecohealth 5, 268-274 [DOI] [PubMed] [Google Scholar]