Abstract
Bone mass is determined by bone cell differentiation, activity and death, which mainly occur through apoptosis. Apoptosis can be triggered by death receptor Fas (CD95), expressed on osteoblasts and osteoclasts and may be regulated by estrogen. We have previously shown that signaling through Fas inhibits osteoblast differentiation. We here investigate Fas as a possible mediator of bone loss induced by estrogen withdrawal. Four weeks after ovariectomy (OVX), Fas gene expression was greater in osteoblasts and lower in osteoclasts from ovariectomized C57BL/6J (wild-type, wt) mice compared to sham-operated animals. OVX was unable to induce bone loss in mice with a gene knockout for Fas (Fas −/− mice). The number of osteoclasts increased in wt mice after OVX, while they remained unchanged in Fas −/− mice. OVX induced greater stimulation of osteoblastogenesis in Fas −/− than in wt mice, with higher expression of osteoblast specific genes. Direct effects on bone-cell differentiation and apoptosis in vivo were confirmed in vitro, where addition of estradiol decreased Fas expression and partially abrogated the apoptotic and differentiation-inhibitory effect of Fas in osteoblast lineage cells, while having no effect on Fas-induced apoptosis in osteoclast lineage cells. In conclusion, the Fas receptor has an important role in the pathogenesis of postmenopausal osteoporosis by mediating apoptosis and inhibiting differentiation of osteoblast lineage cells. Modulation of Fas effects on bone cells may be used as a therapeutic target in the treatment of osteoresorptive disorders.
Keywords: Apoptosis, CD95, Knockout mice, Osteoblast, Osteoclast, Osteoporosis
Bone mass is determined by the balance between bone formation by osteoblasts and bone resorption by osteoclasts. Among systemic factors, estrogens are major regulators of bone mass, as estrogen deficiency leads to an increase in bone resorption over bone formation and subsequent bone loss; this effect is mediated by increased osteoclastogenesis (1), as well as increased apoptosis of osteoblast lineage cells (2, 3).
Apoptosis, can be triggered by various intra- or extracellular stimuli acting through two basic pathways: internal and external. The internal apoptotic pathway is induced by intracellular events leading to mitochondrial cytochrome c release and activation of caspase 9 (4). The external apoptotic pathway is induced through death receptors of the TNF receptor family, such as Fas (CD95) (5), which transduce the signal to caspase 8 via the Fas-associated death domain (6). The tissue expression of Fas and its main apoptosis-inducing ligand, Fas ligand (Fasl, CD178) may be affected by estrogen (7-9).
In view of the reports that human postmenopausal osteoblasts constitutively express Fas (10), and our previous finding that activation of Fas receptor directly inhibits osteoblast differentiation, with lesser effect on apoptosis (11), we hypothesized that the inhibition of differentiation and apoptosis of osteoblast lineage cells through Fas/Fasl system would mediate the effects of estrogen withdrawal on bone. To test that hypothesis we assessed how estrogen withdrawal induced by OVX affected trabecular bone volume, osteoclast, and osteoblast differentiation in mice with a gene-knockout for Fas (Fas −/−). Also, we tested in vitro whether estrogen was able to directly modulate the effect of Fas on osteoblasts and osteoclasts in vitro. We report here that the inhibition of Fas/Fasl signaling has a protective effect against estrogen withdrawal-induced osteoporosis.
Materials and methods
Mice
Twelve-week old female C57BL/6J mice (wild-type, wt) and mice deficient for the Fas gene on the C57BL/6J background (Fas −/−, 12) were used in the experiments. Fas −/− mice were a kind gift from Prof. Dr. Markus Simon (Max Planck Institute for Immunobiology Freiburg, Germany). All animal protocols were approved by the Ethics Committee of the University of Zagreb School of Medicine (Zagreb, Croatia) and experimentation was conducted in accord with accepted standards of humane animal care. In vivo experiments were performed four times and animals were distributed in sham-operated (SH) and OVX group. First experiment involved 16 wt (8 SH and 8 OVX) and 15 Fas −/− mice (7 SH and 8 OVX). Second experiment involved 13 wt (6 SH and 7 OVX) and 11 Fas −/− mice (5 SH and 6 OVX). Third experiment involved 16 wt (8 SH and 8 OVX) and 13 Fas −/− mice (6 SH and 7 OVX). Fourth experiment involved 25 wt (12 SH and 13 OVX) and 21 Fas −/− mice (10 SH and 11 OVX).
Surgical procedure
Mice were anesthetized with 3-bromoethanol intraperitoneally according to the manufacturer's instructions (Sigma-Aldrich Corp., Milwaukee, MI, USA). Paravertebral lumbar areas were longitudinally incised 5–7 mm, and the ovarian fat pad identified. Ovaries (with oviducts) were removed through a small peritoneal incision, and postoperatively visually confirmed by light microscopy. The procedure was identical for sham-operation, except that ovaries were not removed. Lethality was less than 2%. The animals were sacrificed four weeks after surgery. Estrogen depletion after OVX was confirmed by measuring uterine fresh weight in all animals, with a significant decrease in both mice strains compared with SH mice (102.8±14.9 mg vs. 33.0±7.2 mg in wt mice, p=0.001; and 73.7±14.4 vs. 30.6±5.6 mg in Fas −/− mice, p=0.002).
From each animal, femora were used for histomorphometry and μCT (micro-computerized tomography), lumbar vertebrae were collected for μCT, tibiae were used for riboxynucleic acid (RNA) isolation, and bone marrow was collected for cell cultures.
Histology and histomorphometry
Femora were fixed in 4% paraformaldehyde for 24h at 4 °C, and then demineralized in 14% ethylen-diamine tetraacetic acid in 3% formaldehyde, dehydrated in increasing ethanol concentrations and embedded in paraffin. Six μm sections were cut with a Leica SM 2000 R rotational microtome (Leica, Nussloch, Germany) and stained with Goldner's trichrome or histochemically for tartrate-resistant acid phosphatase (TRAP) activity.
Histomorphometric analysis was performed under Axio Imager microscope (Carl Zeiss Microimaging Inc., Oberkochen, Germany) equipped with a charge-coupled device camera connected to a computer with appropriate software (OsteoMeasure, OsteoMetrics, Decatur, GA, USA).
For static histomorphometry, methaphyseal regions of Goldner's trichrome stained femora, 0.4-1.0 mm distally from the epiphyseal plate were analyzed under 5× magnification. Analyzed variables included trabecular volume (BV/TV), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N/mm) and trabecular separation (Tb.Sp, mm), which were automatically calculated by the OsteoMeasure software.
On TRAP-stained sections, osteoclasts were identified as multinucleated red cells placed adjacent to the bone surface. Osteoclasts were counted in the whole diaphyseal areas, excluding parts ≤1mm from the epiphyseal plate. Total osteoclast number was normalized to the measured bone perimeter, and expressed as number of osteoclasts per milimeter bone perimeter.
For dynamic histomorphometry, mice were injected with calcein and demeclocycline, 20 mg/kg each, at a 3 day interval and sacrificed two days after the demeclocycline injection. Undecalcified femora were fixed in 70% ethanol and embedded in LR White acrylic resin (London Resin Co., London, UK). Nine μm sections were cut and analyzed under a fluorescent microscope (Axio Imager, Zeiss) equipped with a CCD camera connected to a computer with OsteoMeasure software, and mineral apposition rate (MAR, μm/day) was automatically calculated.
Micro-computerized tomography
The distal metaphyses of femora and second lumbar vertebrae were scanned using a μCT system (1172SkyScan, SkyScan, Kontich, Belgium) at 50 kV and 200 μA with a 0.5 aluminum filter using a detection pixel size of 4.3 μm. Images were captured every 0.7° through 180° (vertebrae) and every 0.7° through 360° (femora) rotation of the bone. The scanned images were reconstructed using the SkyScan Recon software and analyzed using SkyScan CT analysis software. Three-dimensional analysis and reconstruction of trabecular bone was performed on the bone region 1 to 5 mm distal to the growth plate. The trabecular bone compartment was delineated from the cortical bone.
The following variables were determined: trabecular bone volume fraction (BV/TV, %), trabecular number (Tb.N/mm), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm).
Cell culture
Osteoblasts and osteoclasts were cultured from bone marrow as previously described (13). Briefly, bone marrow was flushed out from the medullar cavity, and osteoblasts were cultured in 6-well culture plates at a density of 106 cells/mL in 3 mL of minimum essential medium α (α-MEM) supplemented with 10% fetal calf serum (FCS, Invitrogen, Carlsbad, CA, USA). Osteoblast differentiation was induced from culture day 7 by the addition of 50 μg/mL ascorbic acid, 10−8 M dexamethasone, and 8 mM β-glycerophosphate (Sigma-Aldrich Corp.). Osteoblast colonies were identified histochemically by the activity of alkaline phosphatase (AP), using a commercially available kit (Sigma-Aldrich Corp.). Osteoclasts were cultured in 48-well culture plates at a density of 106 cells/mL in 0.5 mL of α-MEM supplemented with 10% FCS (Invitrogen). Osteoclast differentiation was stimulated by the addition of 25 ng/mL recombinant murine (rm) receptor activator of NF-κB ligand (RANKL, gift from Amgen, Thousand Oaks, CA, USA), and 15 ng/mL rm macrophage-monocyte colony stimulating factor (M-CSF, R&D systems, Minneapolis, MN, USA). After 6 days of culture, the plates were stained using a commercially available TRAP assay kit (Sigma-Aldrich Corp.), and osteoclasts were identified histochemically by the activity of TRAP. Cells with three or more nuclei per cell, which were stained positively for TRAP activity, were considered osteoclasts and counted. Osteoclast differentiation was further confirmed by real-time polimerase chain reaction (PCR) for calcitonin receptor (Calcr) gene expression as a marker of mature osteoclasts (14). Osteoclasts did not differentiate in cultures without RANKL and M-CSF (not shown). For the induction of apoptosis, 0.5 μg/mL of hamster anti-mouse Fas antibody (Jo-1, BD Pharmingen), and 5 μg/mL protein G (Sigma-Aldrich Corp.) were added to osteoblastogenic cultures on days 8, 10, and 13, and to osteoclastogenic cultures on days 1 and 4, and incubated for 16 h at 37 °C. Cells treated with 0.5 μg/mL normal hamster IgG (BD Pharmingen), and 5 μg/mL protein G were used as negative controls. Anti-Fas treated osteoblastogenic cultures were also analyzed for number of osteoblast colonies on culture day 14, and the expression of osteoblast differentiation genes on the culture day 9, 11, and 14. The proportion of apoptotic and dead cells after Fas treatment was determined by Annexin V (BD Biosciences, San Jose, CA, USA) and propidium iodide (PI) staining, performed according to the manufacturer's instructions. Data were acquired using the FACSCalibur (BD Biosciences) flow cytometer, and 2×104 events per sample were analyzed using CellQuest software (BD Biosciences).
In some experiments, 10 nM/L estradiol (Sigma-Aldrich Corp.), or a corresponding volume of ethanol (used as a solvent for estradiol) used as a negative control, was added to osteoblastogenic and osteoclastogenic cultures with each medium exchange.
Gene expression analysis
Total RNA was extracted from bone, fresh bone marrow, and cultured cells using TriPure reagent (Roche, Basel, Switzerland). For PCR amplification, 2 μg of total RNA was converted to complementary deoxyribonucleic acid (cDNA) by reverse transcriptase (Applied Biosystems, Foster City, CA, USA). The amount of cDNA corresponding to 20 ng of reversely transcribed RNA was amplified by real-time PCR, using specific amplimer sets designed by Primer Express software (Applied Biosystems) for β-actin (sense 5′CATTGCTGACAGGATGCAGAA3′, antisense 5′GCTGATCCACATCTGCTGGA3′), tumor necrosis factor receptor superfamily member 11a (Tnfrsf11a, RANK, sense 5′GACACTGAGGAGACCACCCAA3′, antisense 5′ACAACGGTCCCCTGAGGACT3′), Tnfsf11(RANKL, sense 5′AAGGAACTGCAACACATTGTGG3′, antisense 5′GCAGCATTGATGGTGAGGTG3′), colony stimulating factor 1 receptor (Csf1r, sense 5′AGTCCACGGCTCATGCTGAT3′, antisense 5′TAGCTGGAGTCTCCCTCGGA3′), and osteocalcin (Bglap2, OC, sense 5′CAAGCAGGAGGGCAATAAGGT3′, antisense 5′AGGCGGTCTTCAAGCCATACT3′), with SYBR Green chemistry (Applied Biosystems). Expression of Fas, runt-related transcription factor 2 (Runx2), alkaline phosphatase (Akp, AP), osteoprotegerin (Tnfrsf11b, OPG), and Calcr was analyzed using commercially available TaqMan Assays (Applied Biosystems). Real-time PCR was conducted using an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Each reaction was performed in duplicate in a 25 μL reaction volume. The expression of specific gene was calculated according to the standard curve of gene expression in the calibrator sample (cDNA from bone, bone marrow, osteoblastogenic or osteoclastogenic culture), and then normalized to the expression level of the β-actin gene (“endogenous” control).
Data analysis and interpretation
All experiments were repeated four times, and the results from representative experiments are presented in figures. Osteoclast and osteoblast colony numbers are expressed as mean±SD of osteoclast number in 6 wells of a 48-well culture plate, or number of osteoblast colonies in 3 wells of 6-well culture plates. Differences in the histomorphometric parameters, numbers of osteoclasts and osteoblast colonies between SH and OVX, B6 and Fas −/− mice were analyzed by two-tailed t-test, and a p value of ≤0.05 was determined as statistically significant. Real-time PCR data shown in figures are expressed as mean±SD of relative messenger (m)RNA quantity in each reaction, for the representative experiment. Since SD values for real-time PCR data present variability between the technical replicates, within the single experiment. The methodological studies of reverse transcription and real-time PCR suggest that the least difference in mRNA that can be reproducibly detected is ~100 % (15), and therefore we assume 100% or higher difference in gene expression, repeating through all experiments, as biologically significant. Biologically significant differences were statistically confirmed by analyzing corresponding variables from repeated experiments as independent samples (n=4), using t-test or ANOVA.
Results
Ovariectomy increases Fas expression by osteoblasts
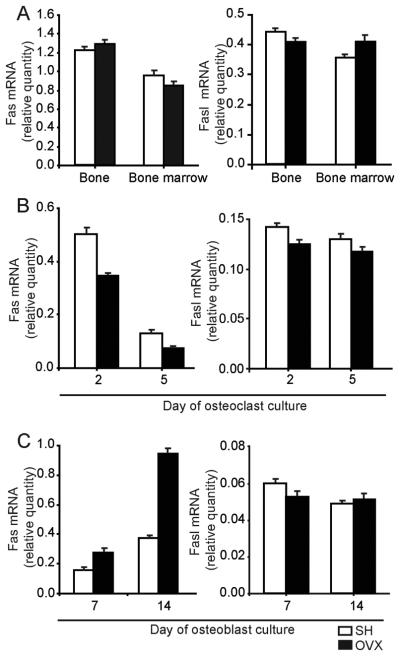
To assess the effect of estrogen withdrawal on the expression of Fas and Fasl in vivo, we analyzed their expression in the bone, bone marrow, and bone marrow-derived osteoblastogenic and osteoclastogenic cultures in wt mice 4 weeks after OVX. One month after surgery, there were no significant differences in Fas or Fasl mRNA levels between OVX and control mice, in either the bone or bone marrow (Fig 1A). The gene expression patterns of Fas and Fasl in osteoclastogenic cultures from bone marrow of OVX mice corresponded to the patterns observed in the cultures from SH animals, but the level of Fas expression in OVX was reduced by half compared with SH mice on culture day 5 (Fig 1B). For this time point, the significant difference in Fas expression was confirmed by analyzing the data obtained in four repeated experiments as independent samples (0.16±0.02 in SH vs. 0.07±0.02 in OVX group, p=0.04, t-test). Fasl gene expression was similar in SH and OVX mice during osteoclast differentiation in vitro (Fig 1B). In four repeated experiments of osteoblastogenic culture, the level of Fas mRNA in OVX mice was almost two-fold higher at day 7 (0.18±0.07 in SH vs. 0.30±0.11 in OVX group, p=0.04, t-test), and even more elevated at later stages of osteoblastogenesis (day 14; 0.42±0.06 in SH vs. 0.88±0.10 in OVX group, p=0.004, t-test) (Fig 1C). At the same time, gene expression of Fasl in the osteoblastogenic culture was not affected by OVX (Fig 1C).
Figure 1. Gene expression of Fas and Fasl in bone, bone marrow, osteoblast, and osteoclast lineage cells in wild-type mice 4 weeks after ovariectomy (OVX).
(A) Expression of Fas/Fasl mRNA in bone and bone marrow. (B) Expression of Fas/Fasl mRNA in osteoclastogenic cultures. (C) Expression of Fas/Fasl mRNA in osteoblastogenic cultures. Expression was calculated according to the standard curve for Fas/Fasl expression in the calibrator sample (cDNA from bone, bone marrow, osteoblastogenic or osteoblastogenic cultures), and normalized to the mRNA quantity for β-actin (“endogenous” control). Results are arithmetic mean±SD of real-time PCR reaction duplicates prepared from the same sample of the representative experiment.
Fas deficient mice do not develop bone loss after estrogen withdrawal
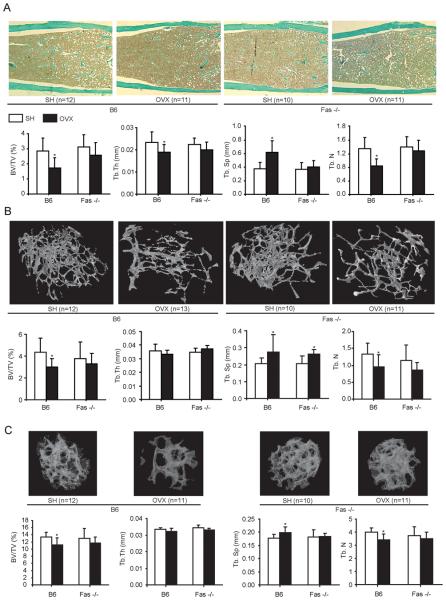
After we demonstrated the changes in the expression of Fas in bone cell cultures from wt mice four weeks after OVX, we tested whether Fas was directly involved in bone loss induced by estrogen withdrawal in vivo. For that purpose we performed OVX in mice with a complete absence of Fas due to a gene knockout (Fas −/− mice) and analyzed the trabecular bone in axial (2nd lumbar vertebra) and appendicular (distal femur) skeleton. As expected, static histomorphometry of distal femora in OVX wt mice revealed a reduction in trabecular bone volume (BV/TV) and trabecular thickness (Tb.Th), as well as increased trabecular separation (Tb.Sp) in comparison with SH wt mice 4 weeks after surgery (Fig 2A). In contrast, there were no statistically significant differences in trabecular bone between OVX and SH Fas −/− mice at the same time point after surgery (Fig 2A). μCT analysis of distal femora and 2nd lumbar vertebrae confirmed trabecular bone loss at all skeletal sites in OVX wt mice, whereas no changes in bone structure was observed after OVX in Fas −/− mice (Fig 2B-C).
Figure 2. Histomorphometry and μCT analysis of femora and lumbar vertebrae from wild-type (B6) and Fas deficient (Fas −/−) mice 4 weeks after ovariectomy (OVX).
The following histomorphometric variables were analyzed: trabecular bone volume (BV/TV), trabecular number (Tb.N/mm), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm). (A) Upper panels, representative femoral diaphyseal sections from sham-operated (SH) and OVX B6 and Fas −/− mice, stained with Goldner-trichrome; lower panels, histomorphometric variables in SH and OVX B6 and Fas −/− mice assessed by histomorphometric analysis of distal femoral sections. * p≤0.001 (t-test) vs. SH mice. (B) Upper panels, representative images of distal femora from SH and OVX B6 and Fas −/− mice; lower panels, histomorphometric variables in distal femora from SH and OVX B6 and Fas −/− mice assessed by μCT analysis. *p≤0.001 (t-test) vs. SH mice. (C) Upper panels, representative images of second lumbar vertebrae from SH and OVX B6 and Fas −/− mice; lower panels, histomorphometric variables in distal femora from SH and OVX B6 and Fas −/− mice assessed by μCT analysis. *p≤0.001 (t-test) vs. SH mice. Results are arithmetic mean±SD of variables measured in single bone of each group of mice.
Estrogen deficiency does not stimulate osteoclastogenesis in Fas deficient mice
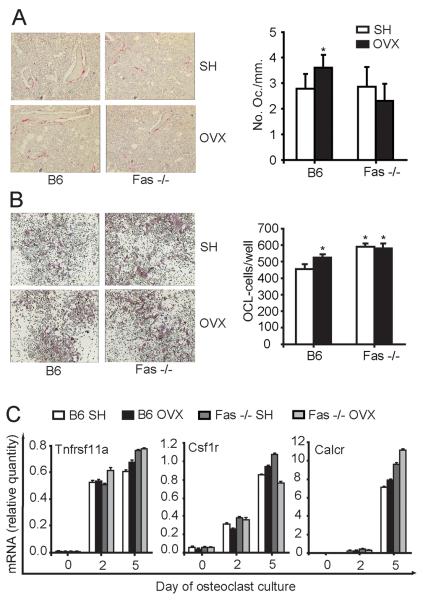
Since bone loss after OVX is a consequence of an increase in the number of osteoclasts (1), we investigated whether an increase in osteoclast number could be detected in bones of OVX Fas −/− mice. Bone resorption activity on femoral sections was assessed through the number of bone-lining osteoclasts histochemically positive for TRAP activity. The number of osteoclasts per milimeter bone surface was significantly higher in OVX wt mice (compared to SH controls), whereas the number of osteoclasts per milimeter bone surface in OVX Fas −/− mice was unchanged, compared to SH Fas −/− mice (Fig 3A).
Figure 3. Osteoclastogenesis in wild-type (B6) and Fas deficient (Fas −/−) mice 4 weeks after ovariectomy (OVX).
Osteoclastogenesis in vivo was assessed by histomorphometric analysis of distal femoral sections from sham-operated (SH) and OVX B6 and Fas −/− mice stained histochemically for the activity of tartrate-resistant acid phosphatase (TRAP). Osteoclastogenesis in vitro was assessed in cultures prepared from bone marrow of SH and OVX B6 and Fas −/− mice. (A) Number of TRAP positive osteoclasts per millimeter bone perimeter in distal femoral metaphyseal sections (mean±SD, t-test, *p≤0.03 vs. SH mice). (B) Number of TRAP positive osteoclasts (mean±SD, t-test, *p≤0.02 vs. SH B6 mice) on day 6 of cell culture. (C) Gene expression pattern in osteoclastogenic cultures from SH and OVX B6 and Fas −/− mice. For each time point in each group, cells were cultured in quadruplicates and pooled for RNA isolation. Day 0 represents gene expression in freshly isolated bone marrow cells. Values were calculated according to the standard curve of gene expression in the calibrator sample (cDNA from osteoclastogenic cultures) and normalized to the expression of the gene for β-actin (“endogenous” control). Results are arithmetic mean±SD of real-time PCR reaction duplicates prepared from the same sample of the representative experiment. Csf1r, colony stimulating factor 1 receptor; Calcr, calcitonin receptor; Tnfrsf11a, tumor necrosis factor receptor superfamily member 11a, RANK.
Although SH Fas −/− mice had a more robust osteoclastogenesis from the bone marrow progenitors in vitro compared to wt mice (590.0±20.0 per well, vs. 457.5±26.3 per well in wt mice, t-test, p≤0.001), the number of osteoclasts differentiated from Fas −/− bone marrow remained unchanged after OVX (Fig 3B). To assess the osteoclast differentiation sequence from bone marrow cells, we followed the dynamics of expression of three key osteoclast-related genes: Tnfrsf11a (RANK), Csf1r, and Calcr. As shown in Fig. 3C, the expression pattern of these genes was comparable in all four groups of animals, indicating that the differentiation sequence was not affected by the OVX in both wt and Fas −/− mice.
Estrogen deficiency stimulates osteoblast activity and differentiation in Fas deficient mice
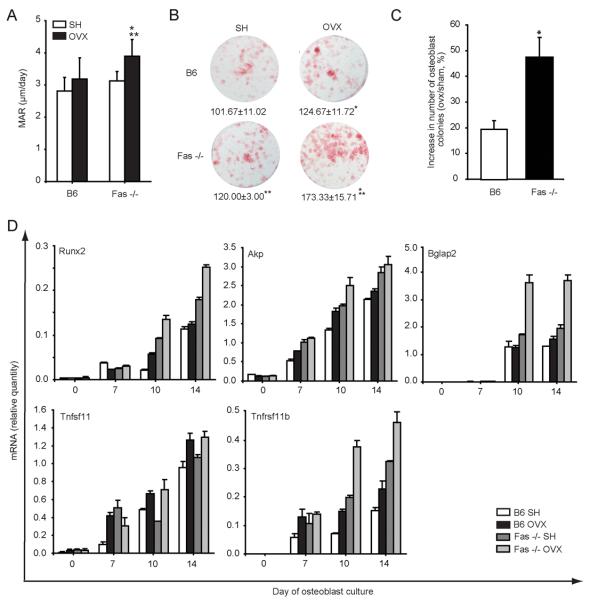
MAR was used to estimate osteoblast activity in vivo. Although MAR increased after OVX in wt and Fas −/− mice, this increase was statistically significant only in Fas −/− mice (Fig 4A).
Figure 4. Osteoblast differentiation and activity in wild-type (B6) and Fas deficient (Fas −/−) mice 4 weeks after ovariectomy (OVX).
Osteoblast activity in vivo was assessed by dynamic histomorphometry. Osteoblastogenic cultures were prepared from bone marrow of sham-operated (SH) and OVX B6 and Fas −/− mice. (A) Mineral apposition rate (MAR) in SH and OVX B6 and Fas −/− mice was assessed by histomorphometric analysis of distal femoral sections:* p≤ 0.001 (t-test) vs. SH mice, ** p≤ 0.001 (t-test) vs. B6 mice. (B) Osteoblastic colonies from SH and OVX B6 and Fas −/− mice on day 14 of cell culture, stained red for alkaline phosphatase (AP). Number of osteoblast colonies is presented as mean±SD of culture duplicates: t-test, * p≤ 0.05 vs. SH mice, ** p≤ 0.05 vs. B6 mice (C) Percent increase in the number of osteoblast colonies in OVX mice compared to SH mice (mean±SD increase in four repeated experiments; t-test, * p=0.002 vs. B6 mice). (D) Gene expression pattern during in vitro osteoblast differentiation. For each time point in each group, cells were cultured in triplicates pooled for RNA isolation. Day 0 represents gene expression in freshly isolated bone marrow cells. Values were calculated according to the standard curve of gene expression in the calibrator sample (cDNA from osteoblastogenic culture), and normalized to the expression of the gene for β-actin (“endogenous” control). Results are arithmetic mean±SD of real-time PCR reaction duplicates prepared from the same sample of the representative experiment. Akp, alkaline phosphatase gene; AP, alkaline phosphatase; Bglap2, osteocalcin; Runx2, runt-related transcription factor 2; Tnfsf11, tumor necrosis factor receptor superfamily member 11, RANKL; Tnfrsf11b, tumor necrosis factor receptor superfamily member 11b, OPG.
Osteoblastogenesis in vitro was assessed by the number of formed osteoblast colonies histochemically positive for AP activity and by determining the expression of osteoblast differentiation genes: Runx2, Akp, Tnfsf11(RANKL), Tnfrsf11b (OPG), and Bglap2 (OC). The number of osteoblast colonies was higher in Fas −/− mice, either SH or OVX, in comparison with the wt animals (Fig 4B). Bone marrow from both wt and Fas −/− mice had more AP-positive colonies 4 weeks after OVX (Fig 4B), but this increase in Fas −/− mice was twice greater than that in wt mice (19.8±3.7% increase in wt mice vs. 47.5±7.6% in Fas −/− mice, p=0.002, t-test, Fig 4C).
OVX led to an increase in the expression of osteoblast differentiation genes in both wt and Fas −/− mice; this increase being significant in Fas −/− mice especially for OPG and OC at days 10 and 14 of osteoblastogenic cultures (p<0.05, ANOVA and Student-Newman-Keuls post-hoc test, Fig 4D). The expression pattern of osteoblast-differentiation genes confirmed enhanced osteoblastogenesis in Fas −/− mice, and this enhancement was significant for Runx2 at late stages (day 10 and 14) of osteoblast differentiation (p<0.05, ANOVA and Student-Newman-Keuls post-hoc test, Fig 4D). In addition, the expression of RANKL was similar in all groups of mice, giving the significant 3.5 fold decrease in RANKL/OPG ratio in Fas −/− mice, compared to wt mice on culture day 14 (p=0.01, ANOVA and Student-Newman-Keuls post-hoc test).
Estrogen decreases expression of Fas and abrogates in vitro effect of Fas activation in osteoblast lineage cells
Since we found that the absence of Fas in vivo had a protective effect on bone loss after OVX and resulted in a stimulation of the osteoblastogenic response to estrogen withdrawal, we then assessed whether estrogen could directly modulate the effects of Fas activation on osteoblast lineage cells.
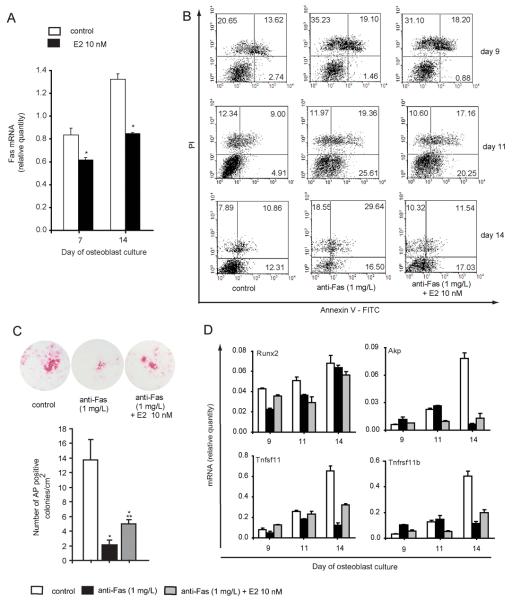
We first analyzed the effect of exogenous estradiol on Fas gene expression in osteoblast lineage cells, and demonstrated that it negatively regulates the Fas gene expression in both immature (day 7, 70% of the level in the control cultures) and mature (day 14, 60% of the level in the control cultures) osteoblastogenic cultures (Fig 5A). We then assessed whether estradiol could rescue differentiating osteoblasts from Fas-induced apoptosis. As shown in Fig. 5B, treatment with estradiol 24 h prior to Fas activation by Jo-1 antibody reduced the proportion of apoptotic and dead cells by 7.5% in immature osteoblasts (culture day 11), as measured by Annexin V/PI labeling. That effect was most pronounced in mature osteoblasts (culture day 14), where anti-Fas treatment doubled the proportion of dead and apoptotic cells, and estradiol pretreatment reduced that number to the level of the control cells (Fig 5B).
Figure 5. Estradiol modifies the effect of Fas on osteoblast differentiation and apoptosis.
Osteoblastogenic cultures were prepared from the bone marrow of wt mice. Osteoblastogenic cultures were treated with 0.5 μg/mL of anti-Fas antibody (Jo-1) and 5 μg/mL protein G at days 8, 10, and 13. Cells treated with 0.5 μg/mL normal hamster IgG and 5 μg/mL protein G were used as negative controls. 10 nM estradiol was added to osteoblastogenic cultures with each medium exchange and a corresponding volume of ethanol (which was used as a solvent for estradiol) was used as a negative control. (A) Expression of Fas mRNA in osteoblastogenic cultures after treatment with estradiol. Values were calculated according to the standard curve of gene expression in the calibrator sample (cDNA from osteoblastogenic cultures) and normalized to the expression of the gene for β-actin (“endogenous” control). Cell treatment was performed in triplicate wells and results are arithmetic mean±SD of relative Fas mRNA expression in each well (* p≤ 0.05 vs. control group, t-test). (B) Representative data of the Annexin V-FITC/PI labeling of apoptotic cells. Numbers represent percentages of cells in each quadrant. Apoptotic cells are in the lower right quadrant, and dead cells are in the upper right quadrant. (C) Effect of estradiol on the number of osteoblast colonies after treatment with anti-Fas antibody. Histograms represent the number of osteoblast colonies per square centimeter plate surface (mean±SD, n=3; * p<0.05 vs. control group, ** p<0.05 vs. anti-Fas and control group, t-test). Figures show representative osteoblast colonies on day 14 of cell culture, stained red for AP. (D) Gene expression pattern during in vitro osteoblastic differentiation. Values were calculated according to the standard curve of gene expression in the calibrator sample (cDNA from osteoblastogenic culture) and normalized to the expression of the gene for β-actin (“endogenous” control). Results are arithmetic mean±SD of real-time PCR reaction duplicates prepared from the same sample of the representative experiment. Akp, alkaline phosphatase gene; AP, alkaline phosphatase; Runx2, runt-related transcription factor 2; Tnfsf11, tumor necrosis factor receptor superfamily member 11, RANKL; Tnfrsf11b, tumor necrosis factor receptor superfamily member 11, OPG.
Treatment with an activating anti-Fas antibody resulted in a decrease of AP-positive osteoblast colonies on culture day 14, which is in accordance with our previous study (11). This was not only a result of increased apoptosis, but also of a direct effect on osteoblast differentiation. Estradiol treatment prior to the addition of anti-Fas antibody was able to partially abrogate inhibitory effect of Fas activation and increased the number of AP positive colonies compared with the cultures treated with anti-Fas only (Fig 5C). Expression of Runx2 decreased in immature (day 7 and 10) osteoblastogenic cultures treated with anti-Fas antibody, and that effect of Fas activation could be blocked by estradiol pretreatment, but only when added early in the culture course (Fig 5D). In contrast, down-regulation of Akp, Tnfrsf11b, and Tnfsf11genes by anti-Fas treatment could not be abrogated with estradiol pretreatment (Fig 5D).
Estrogen has no effect on Fas induced-apoptosis of immature osteoclast lineage cells
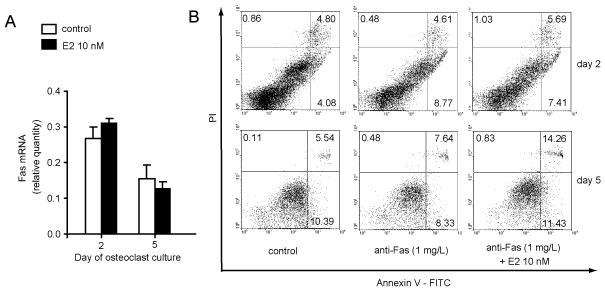
Since our earlier study demonstrated that Fas activation doubled the number of apoptotic cells in early stages of osteoclastogenic cultures, and did not affect differentiation of osteoclasts (11), we analyzed the effect of estradiol on the expression of Fas and Fas-mediated apoptosis in osteoclastogenic cultures. We found no significant changes in the expression of Fas in osteoclastogenic cultures treated with estradiol compared with control cultures (Fig 6A). Anti-Fas antibody doubled the number of apoptotic cells on day 2 of osteoclastogenic cultures, while it had no effect on the number of apoptotic cells in mature osteoclastogenic cultures (day 5, Fig 6B). Estradiol pretreatment had no effect on the number of apoptotic or dead cells on day 2 of osteoclastogenic cultures, but it doubled the number of dead cells in mature osteoclastogenic cultures (day 5, Fig 6B).
Figure 6. Estradiol does not influence Fas mediated osteoclast apoptosis.
Osteoclastogenic cultures were prepared from bone marrow of wt mice and treated with 0.5 μg/mL of anti-Fas antibody (Jo-1) and 5 μg/mL protein G at day 1 and 4. Cells treated with 0.5 μg/mL normal hamster IgG, and 5 μg/mL protein G were used as negative controls. 10 nM estradiol was added to osteoclastogenic cultures with each medium exchange and a corresponding volume of ethanol (used as a solvent for estradiol) was used as a negative control. (A) Expression of Fas in osteoclastogenic cultures after treatment with estradiol. Values were calculated according to the standard curve of gene expression in the calibrator sample (cDNA from osteoclastogenic cultures) and normalized to the expression of the gene for β-actin (“endogenous” control). Cell treatment was performed in six wells and three wells were pooled for RNA isolation. Results are arithmetic mean±SD of relative Fas mRNA expression in two pooled samples. (B) Representative data of the Annexin V-FITC/PI labeling of apoptotic cells. Numbers represent percentages of cells in each quadrant. Apoptotic cells are in the lower right quadrant, and dead cells are in the upper right quadrant.
Discussion
The results of our study clearly demonstrated that effects of estrogen withdrawal were mediated by Fas. Estrogen withdrawal stimulated Fas expression in cultured osteoblasts. Fas has already been shown to be expressed on mature osteoblasts (11, 16-18), where it induces apoptosis and suppresses differentiation (11). It has been further demonstrated that Fas is constitutively expressed in osteoblasts cultured from bones of postmenopausal women, and its activation is able to induce their apoptosis (10). Increased Fas expression on osteoblast lineage cells observed in our study after OVX may thus represent an effective mechanism for limiting osteoblast activity and contributing to the development of the osteoporotic phenotype.
On the other hand, we found a moderate decrease in Fas expression in osteoclastogenic cultures, which may have explain, at least in part, the expansion of the progenitor pool and increased in vitro osteoclastogenesis in response to estrogen withdrawal. Fas expression on osteoclast lineage cells has been described, but the data are conflicting and depending on culture conditions. They vary from absent (19) to strongly expressed and efficient in inducing apoptosis of mature osteoclasts (20). In our model, the expression of Fas mRNA on osteoclasts lineage cells was basally low (between 220- and 219-fold lower than that of β-actin) and Fasl had a limited effect of inducing apoptosis in a small proportion of cells in immature osteoclastogenic cultures (11). Therefore, the absence of Fas-mediated osteoclast apoptosis may not be an important mechanism for OVX induced increase in osteoclastogenesis.
Changes in Fas expression seem to be the main regulatory effect of estrogen withdrawal in bone cells, since expression of Fasl in bone cells was unchanged by OVX. However, Fasl is expressed in other cells present within the bone marrow microenvironment, such as activated T lymphocytes (21), NK cells (22), monocytes (23), erythroid lineage cells (24), which may provide sufficient levels of endogenous Fasl to induce apoptosis and inhibit differentiation of osteoblasts.
To confirm the physiological importance of changes in Fas expression after OVX, we performed OVX in Fas −/− mice and demonstrated that the absence of Fas would protect them from the estrogen withdrawal-induced bone loss. OVX significantly reduced the trabecular bone volume in wt mice, whereas it was unable to induce significant bone loss in Fas −/− mice. These findings strongly suggest that Fas is required for bone loss after OVX in vivo, and are in line with our previous finding that mice with a point mutation for Fasl (gld) also do not lose bone after OVX (13).
Fas deficiency influenced both osteoclastic and osteoblastic responses to estrogen withdrawal. OVX increased the number of osteoclasts in vivo as well as osteoclastogenesis in vitro in wt mice, while such an increase was not observed in Fas −/− mice. Several studies already demonstrated the importance of Fas/Fasl system in the apoptosis of mature osteoclasts or osteoclast progenitors in the pathogenesis of osteoresorptive disorders (13, 20, 25, 26). Increased osteoclastogenesis is a well documented response to estrogen deficiency (1), and there are many mechanisms proposed to be involved in this response, such as IL-6 (1), absence of a direct inhibitory effect of estrogen on mature osteoclast apoptosis (27), and stimulation of osteoclastogenesis by increased TNF production from lymphocytes (28), among others. Since Fas was found to be expressed on osteoclasts, apoptosis induced by Fasl may be an effective mechanism limiting the osteoclast progenitor pool. Also, a decrease in Fas expression on osteoclasts after OVX may contribute to an increased osteoclastogenesis. However, in vivo absence of Fas resulted in failure of a normal osteoclastogenic response to estrogen withdrawal. One of the potential mechanisms for this osteoprotective effect is an altered pattern of osteoblast differentiation in Fas deficiency. We have previously described increased osteoblast differentiation as a result of Fas or Fasl deficiency (11, 13). Such increased differentiation is characterized by increase in the expression of OPG (11, 13), which is not followed by an increase in the expression of RANKL, suggesting that the altered differentiation pattern of osteoblasts in Fas deficient mice results in a lower RANKL/OPG ratio compared to wt mice, which may negatively regulate osteoclastogenesis. Furthermore, Park et al. reported enhanced osteoclastogenesis in response to Fasl in vitro, but as a consequence of increased levels of TNF and IL-1 in osteoclast cultures due to Fas activation (29). Although the significance of this mechanism in vivo is unknown, it may at least partially contribute to the absence of an osteoclastogenic response to ovarietomy in Fas −/− mice.
Increased osteoblastogenesis, with the highest increase between week 2 and 8 after OVX, is also a well documented response to OVX and has been ascribed to stimuli additional to factors released from bone matrix during osteoclastic resorption, as occurs during normal remodeling (30). In our model, the increase in osteoblastogenesis was more prominent in Fas deficient than in wt mice, suggesting involvement of Fas in limiting osteoblastic response after OVX, and thus contributing to bone loss. Fas has also been shown to be expressed by human osteoblasts and necessary for their apoptosis (31). TNF and IL-1 may increase the sensitivity of human osteoblasts to Fas induced apoptosis (32), but estradiol or raloxifene could not abrogate the effect of TNF in stimulating the expression of Fas and induction of postmenopausal human osteoblast apoptosis (10). This process could be in vitro successfully inhibited by the antioxidant molecules fraxetin and myrecitin (33, 34). Since increased TNF production is a well described consequence of estrogen deficiency (28), such small molecules may be potentially used as antiresorptive agents.
The osteoprotective effect of Fas absence may be explained by a direct influence of estrogen on Fas expression and activity on bone cells, or by indirect mechanisms involving other cells and molecules which are able to influence bone cell differentiation, activity and death.
To distinguish which of our findings resulted from direct interaction of estrogen and Fas in bone cells, we analyzed whether estrogen is able to modify the expression of Fas in osteoblastogenic and osteoclastogenic cultures. Estradiol was able to reduce the expression of Fas in immature and mature osteoblastogenic cultures and pretreatment with estradiol before Fas stimulation resulted in fewer dead cells in mature osteoblastogenic cultures, while having no effect on Fas induced apoptosis of immature osteoclasts. A partial abrogation of Fas-induced inhibition of osteoblast differentiation assessed by the number of AP-positive osteoblast colonies and expression of osteoblast differentiation genes was also observed when osteoblastogenic cultures were pretreated with estradiol prior to Fas ligation. This confirms that estrogen withdrawal may have a direct effect on the activity of the Fas/Fasl system in osteoblast lineage cells. A similar effect of estradiol on the expression of Fas was also described in T lymphocytes as a mechanism of stimulation of thymocyte apoptosis and increased sensitivity to autoimmunity (35). On the other hand, estradiol had no effect on the expression of Fas in osteoclasts, which points to the conclusion that a decrease in the osteoclastogenic response in Fas deficient mice is not driven by a direct influence of Fas/Fasl system on the osteoclast life span. Estradiol pretreatment even increased the number of dead cells in mature osteoclastogenic cultures, which is not surprising since apoptosis of mature osteoclasts has already been described as a direct effect of estradiol (36).
Taken together, our results demonstrate the important role of Fas/Fasl in in vivo regulation of bone mass by estrogens. Our results demonstrated that estrogen supports Fas-mediated osteoclast apoptosis and counteract negative effect of Fas on osteoblast differentiation and survival in vivo. We also showed that estrogen withdrawal requires an active Fas receptor for the development of osteoporotic phenotype, and that this effect involves mainly osteoblast lineage cells. Further investigation of the molecules involved in the regulatory effect of Fas on osteoblast-lineage cell survival and differentiation is needed to better define specific targets for the treatment of osteoresorptive disorders.
Acknowledgements
RANKL was a kind gift from Amgen Inc. (Thousand Oaks, USA). We thank Prof. Dr. Markus M. Simon for providing us the Fas −/− mice. We thank Mrs. Katerina Zrinski-Petrovic for her technical assistance.
This work was supported by the grant from the Welcome Trust (CRIGS 073828/Z/03/Z) and the grants from the Croatian Ministry of Science, Education and Sports (108-1080229-0140, 108-1080229-0142, and 108-1080229-0341).
Abbreviations
- Akp
alkaline phosphatase gene
- AP
alkaline phosphatase
- α-MEM
minimum essential medium α
- Bglap2
osteocalcin gene
- BV/TV
trabecular bone volume
- cDNA
complementary deoxyribonucleic acid
- Csf1r
colony stimulating factor 1 receptor
- Calcr
calcitonin receptor
- Fasl
Fas ligand
- FCS
fetal calf serum
- MAR
mineral apposition rate
- M-CSF
macrophage-monocyte colony stimulating factor
- μCT
micro-computerized tomography
- OC
osteocalcin
- OPG
osteoprotegerin
- OVX
ovariectomy-induced estrogen withdrawal
- PCR
polimerase chain reaction
- PI
propidium iodide
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- Runx2
runt-related transcription factor 2
- RNA
riboxynucleic acid
- SH
sham-operation
- Tb.N.
trabecular number
- Tb.Th.
trabecular thickness
- Tb.Sp.
trabecular separation
- Tnfsf11
tumor necrosis factor superfamily member 11, RANKL gene
- Tnfrsf11a
RANK gene
- Tnfrsf11b
OPG gene
- TRAP
tartrate-resistant acid phosphatase
- wt
wild-type mice
References
- 1.Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 2.Kousteni S, Chen JR, Bellido T, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 3.Tomkinson A, Reeve J, Shaw RW, et al. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 4.Denault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev. 2002;102:4489–4500. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 5.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RM, Chan FK, Chun HJ, et al. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 7.Ishimaru N, Saegusa K, Yanagi K, et al. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren's syndrome through fas-mediated apoptosis. Am J Pathol. 1999;155:173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaita G, Candolfi M, Zaldivar V, et al. Estrogens up-regulate the Fas/FasL apoptotic pathway in lactotropes. Endocrinology. 2005;146:4737–4744. doi: 10.1210/en.2005-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki A, Enari M, Eguchi Y, et al. Involvement of Fas in regression of vaginal epithelia after ovariectomy and during an estrous cycle. EMBO J. 1996;15:211–215. [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Moreno C, Catalan MP, Ortiz A, et al. Modulation of survival in osteoblasts from postmenopausal women. Bone. 2004;35:170–177. doi: 10.1016/j.bone.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic N, Lukic IK, Grcevic D, et al. The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol. 2007;178:3379–3389. doi: 10.4049/jimmunol.178.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 13.Katavic V, Lukic IK, Kovacic N, et al. Increased bone mass is a part of the generalized lymphoproliferative disorder phenotype in the mouse. J Immunol. 2003;170:1540–1547. doi: 10.4049/jimmunol.170.3.1540. [DOI] [PubMed] [Google Scholar]
- 14.Grcevic D, Lee SK, Marusic A, et al. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165:4231–4238. doi: 10.4049/jimmunol.165.8.4231. [DOI] [PubMed] [Google Scholar]
- 15.Stahlberg A, Hakansson J, Xian X, et al. Advanced quantitative real-time PCR in clinical diagnostics and cDNA microarray validation; 1st European Conference in Functional Genomics and Diseases; Prague. Czech Republic, 2003. [Google Scholar]
- 16.University of Connecticut Health Cennter: Genome anatomy project for the osteoprogenitor lineage. Available from: http://skeletalbiology.uchc.edu/30_ResearchProgram/304_gap/index.htm.
- 17.Bu R, Borysenko CW, Li Y, et al. Expression and function of TNF-family proteins and receptors in human osteoblasts. Bone. 2003;33:760–770. doi: 10.1016/j.bone.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Hatakeyama S, Tomichi N, Ohara-Nemoto Y, et al. The immunohistochemical localization of Fas and Fas ligand in jaw bone and tooth germ of human fetuses. Calcif Tissue Int. 2000;66:330–337. doi: 10.1007/s002230010069. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa Y, Ohtsuki M, Uzuki M, et al. Suppression of osteoclastogenesis in rheumatoid arthritis by induction of apoptosis in activated CD4+ T cells. Arthritis Rheum. 2003;48:3350–3358. doi: 10.1002/art.11322. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, McKenna MA, Feng X, et al. Osteoclast apoptosis: the role of Fas in vivo and in vitro. Endocrinology. 2003;144:5545–5555. doi: 10.1210/en.2003-0296. [DOI] [PubMed] [Google Scholar]
- 21.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 22.Eischen CM, Schilling JD, Lynch DH, et al. Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J Immunol. 1996;156:2693–2699. [PubMed] [Google Scholar]
- 23.Kiener PA, Davis PM, Rankin BM, et al. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- 24.De Maria R, Testa U, Luchetti L, et al. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93:796–803. [PubMed] [Google Scholar]
- 25.Krum SA, Miranda-Carboni GA, Hauschka PV, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Kameda T, Mano H, Yuasa T, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cenci S, Weitzmann MN, Roggia C, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H, Jung YK, Park OJ, et al. Interaction of Fas ligand and Fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol. 2005;175:7193–7201. doi: 10.4049/jimmunol.175.11.7193. [DOI] [PubMed] [Google Scholar]
- 30.Jilka RL, Takahashi K, Munshi M, et al. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101:1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawakami A, Eguchi K, Matsuoka N, et al. Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Miner Res. 1997;12:1637–1646. doi: 10.1359/jbmr.1997.12.10.1637. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi M, Kawakami A, Nakashima T, et al. Tumor necrosis factor-alpha and interleukin-1beta increase the Fas-mediated apoptosis of human osteoblasts. J Lab Clin Med. 1999;134:222–231. doi: 10.1016/s0022-2143(99)90201-9. [DOI] [PubMed] [Google Scholar]
- 33.Kuo PL. Myricetin inhibits the induction of anti-Fas IgM-, tumor necrosis factor-alpha- and interleukin-1beta-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Life Sci. 2005;77:2964–2976. doi: 10.1016/j.lfs.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Kuo PL, Huang YT, Chang CH, et al. Fraxetin inhibits the induction of anti-Fas IgM, tumor necrosis factor-alpha and interleukin-1beta-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Int Immunopharmacol. 2006;6:1167–1175. doi: 10.1016/j.intimp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Do Y, Ryu S, Nagarkatti M, et al. Role of death receptor pathway in estradiol-induced T-cell apoptosis in vivo. Toxicol Sci. 2002;70:63–72. doi: 10.1093/toxsci/70.1.63. [DOI] [PubMed] [Google Scholar]
- 36.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]