Abstract
Objective/Hypothesis
Mesenchymal stem cells (MSCs) originally isolated from bone marrow, are fibroblast-looking cells that are now assumed to be present in the stromal component of many tissues. MSCs are characterized by a certain set of criteria including their growth culture characteristics, a combination of cell surface markers, and the ability to differentiate along multiple mesenchymal tissue lineages. We hypothesized that human vocal fold fibroblasts (hVFF) isolated from the lamina propria meet the criteria established to define MSCs and are functionally similar to MSCs derived from BM and adipose tissue.
Study Design
In vitro study
Methods
HVFF were previously derived from human vocal fold tissues. MSCs were derived from adipose tissue (AT), and BM of healthy donors, based on their attachment to culture dishes and their morphology, and expanded in culture. Cells were analyzed for standard cell surface markers identified on BM-derived MSCs as well as the ability to differentiate into cells of mesenchymal lineage, i.e. fat, bone and cartilage. We investigated the immunophenotype of these cells before and after interferon-γ (INF- γ) stimulation.
Results
HVFF displayed cell surface markers and multipotent differentiation capacity characteristic of MSCs. Furthermore, these cells exhibited similar patterns of expression of HLA and co-stimulatory molecules, after stimulation with INF- γ compared to MSCs derived from BM and AT.
Conclusions
Based on our findings hVFF derived from lamina propria have the same cell surface markers, immunophenotypic characteristics, and differentiation potential as BM- and AT-derived MSCs. We propose VF fibroblasts are MSCs resident in the vocal fold lamina propria.
Index words: mesenchymal stem cells, human vocal fold stem cells, immune modulation, vocal fold fibroblasts
Introduction
Over the last several years, much attention has been directed at the potential of cell therapy in regenerative medicine, specifically mesenchymal stem cells (MSCs). Mesenchymal stem cell therapy involves the transplantation of autologous or allogeneic cells to patients through local delivery or systemic infusion and offers a novel approach for tissue repair, augmentation, or reconstruction. This new era of cell-based therapies stems from the observation that MSCs, originally isolated from bone marrow (BM), possess the ability to differentiate along multiple tissue lineages, participate in the tissue repair and regeneration process through a variety of paracrine mechanisms, and suppress activation and proliferation of immune and inflammatory cells (1). It is now thought that these stem cells can be isolated from most tissues of the body, including fat; and that MSCs isolated from these diverse tissues possess similar biological characteristics, differentiation potential and immunological properties (2).
The vocal fold lamina propria is composed of a combination of extracellular matrix (ECM) components that account for not only its unique biomechanical characteristics but also challenges that are not adequately matched by current replacement modalities (3,4). Some of the potential treatment modalities proposed/reported for vocal fold scar include injection of biomaterials/hydrogels (5–7), injection of cells – autologous or non-autologous mesenchymal stem cells, autologous fibroblasts, and potentially derivatives of human embryonic stem cells (8–10). Mesenchymal stem cells exhibit fibroblastic morphology and are generally derived using culture methodologies similar to what has been used to derive fibroblasts from different tissues. We hypothesized that human vocal fold fibroblasts (hVFF) are indeed MSCs derived from these tissues. In this study we characterized previously isolated hVFF derived from adult human vocal fold lamina propria, based on the consensus criteria developed to define MSCs (11), and compared them to MSCs derived from AT and BM.
Materials and methods
Human mesenchymal stem cell isolation and culture
Human MSCs were derived from BM and AT of healthy donors based on protocols approved by the University of Wisconsin Health Sciences Institutional Review Board (IRB), after obtaining informed consent from the donors. We used discarded BM filters after BM harvest from normal HLA-matched sibling donors. Briefly, BM-MSCs were isolated from cell pellet fractions collected from filters and washed with phosphate buffered saline (PBS) by centrifugation. Mononuclear cells were isolated by Ficoll Hypaque 1.073 (GE Healthcare Biosciences) and Leucosep tube (Greiner Bio-one, Monroe, NC, USA) according to manufacturer’s protocol. Red blood cells (RBC) were lysed with 3 minute incubation in RBC lysis buffer and mononuclear cells were suspended in alpha minimum essential media (α-MEM) supplemented with 10% fetal bovine serum (FBS- Hyclone, Logan, UT, USA), 1X non-essential amino acids (NEAA, Sigma Inc., St. Louis, MO), and 4 mM L-glutamine (Sigma Inc.), 1X penicillin/streptomycin (Sigma Inc., St. Louis, MO, USA). Human AT-MSCs were isolated from tissues excised or from aspirates from abdominoplasty flaps. The excised tissue was rinsed with PBS, minced and digested with type I collagenase/PBS (Sigma Inc, St Louis, MO) at 37 °C for 45 minutes. The sample was neutralized with an equal volume of α-MEM and centrifuged at 1200 RPM for 5 minutes. Red blood cells were lysed with 3 minute incubations in RBC lysis buffer and mononuclear cells were suspended in supplemented α-MEM as above and plated on culture flasks. After adherence of stromal cells to plastic plates, the culture media was changed to remove non-adherent cells. The cells were cultured in a 37 °C incubator with 5% CO2 - 95% humidified atmosphere. Media was changed every 3 days and the cells were expanded until passage 4, at which time they were used for flow cytometry, differentiation experiments and immunophenotype assays.
Human vocal fold fibroblast isolation and cell line derivation
Two primary, normal hVFF lines – p59 and p21 – were obtained from specimens excised from non-cancerous donors in accordance with our IRB approved protocol; their derivation has been previously described (4). Additionally two immortalized hVFF lines – T59 and T21 – whose transduction is reported elsewhere (12) were used in this investigation. For current experiments, primary and immortalized hVFFs were grown in Dulbecco’s modified essential medium (DMEM) with 10% FBS and 1X NEAA. All experiments performed on primary and immortalized cells ranged between passages four through six.
Fluorescent activated cell sorting analysis
For fluorescent activated cell sorting (FACS) analysis, single cell suspensions of cells were stained and analyzed, within 24 hour of staining. Data was acquired using FACScalibur (Becton Dickinson, Franklin Lakes, NJ, USA) or Accuri C6 flow cytometer (Accuri, Ann Arbor, MI, USA) and acquired data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA) or CFlow software (Accuri, Ann Arbor, MI). Antibodies used were human specific fluorescently labeled mouse monoclonal antibodies as follows: anti-CD3 allophyocyanin (APC), anti-CD14 APC, anti-CD31 APC, anti-CD45 phycoerithrin (PE) labeled, anti-CD90 PerCP-Cy5.5, anti-CD105 APC, anti-CD163 PE, anti-HLA-ABC Fluorescein Isothiocyanate (FITC) (all eBioscience, San Diego, CA, USA), anti-CD14 FITC, anti-CD29 PE, anti-CD34 FITC, anti-CD44 PE, anti-CD73 PE, anti-CD90 APC, anti-HLA-DR FITC (all BD Pharmingen, San Diego, CA, USA). For surface staining, Fc receptors were blocked with Fc Receptor Blocking agent (Miltenyi Biotech, Auburn, CA, USA) for 15 min at 4°C. Surface antibodies were added and incubated for 30 minutes at 4°C in the dark and then cells were washed with autoMACS separation buffer (PBS, 2 mM EDTA, 0.5% BSA, pH 7.2, from Miltenyi Biotech, Auburn, CA) and fixed with 1% paraformaldehyde (PFA) in PBS until ready for analysis within 24 hours. Control staining with directly labeled-isotype-matched monoclonal antibodies was included in all FACS experiments.
Differentiation of cells toward adipogenic, osteogenic, and chondrogenic lineages
Cell differentiation into adipogenic, osteogenic and chondrogenic lineages and subsequent detection was performed using established reported methodologies (2). Briefly, human cells – hVFF (primary and immortalized lines) and AT- and BM- derived MSCs were treated with osteogenic medium or adipogenic medium (Miltenyi Biotech, Auburn, CA) for 21 days, with media changes every 3–4 days. Cultures were assayed for mineral content by Alizarin red S staining (Acros Organics, NJ, USA) and for lipid accumulation by Oil red O staining (Sigma Inc. St Louis, MO). For chondrogenic differentiation, we used cell pellet 3D culture methodology with chondrogenic media (Miltenyi Biotech, Auburn, CA) changes every 3–4 days for 28 days. Paraffin-embedded sections were stained with Safranin-O (Sigma Inc, St Louis, MO) to detect glycosaminoglycans.
Cell surface marker change after IFN-γ challenge
Bone marrow derived MSCs, primary hVFF p59 and p21, and immortalized hVFF T59 and T21 were treated with 200 IU of IFN-γ for two and four days respectively. Cells were then harvested by trypsinization and stained with HLA-ABC FITC, HLA-DR PE, CD80 FITC and CD86 PE (all eBioscience, San Diego, CA) by using the protocol described above. Cells untreated with IFN-γ were used as control. Staining for each group was done in triplicate.
Results
Cell surface phenotype characterization of hVFFs
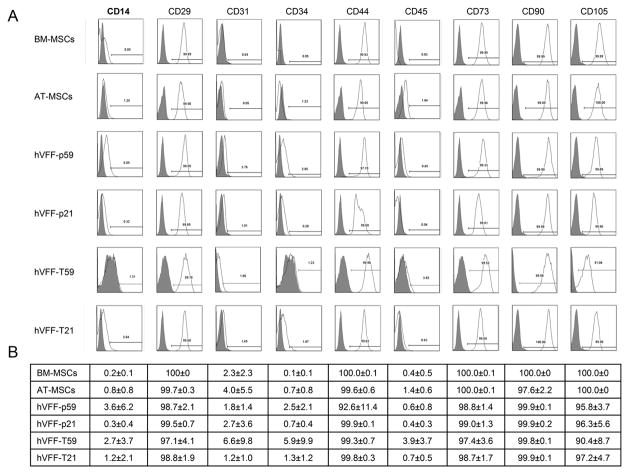
HVFF primary lines – p59 and p21 – previously isolated from normal healthy tissue donors, and their telemorase transduced counterparts – T59 and T21 respectively – were expanded to passage four and compared to BM- and AT- MSCs expanded under similar culture conditions (Figure 1). All cell types were used for characterization by flow cytometry, differentiation studies and immunophenotype assays. All hVFF were positive for MSC markers such as CD29, CD44, CD73, CD90, and CD105 and negative for hematopoietic markers such as CD14, CD31, CD34, CD45 similar to MSCs derived from BM or AT (Figure 2, Panel A). Panel B of Figure 2 shows the mean values ± standard errors of the percentages of MSCs and hVFF that were tested for each cell surface antigen expression by flow cytometry.
Figure 1. Morphology of hVFF.
Representative microscopic views (5X) of human bone marrow-derived mesenchymal stem cells (MSCs) (upper left), human adipose tissue-derived MSCs (lower left); human vocal fold fibroblasts (hVFF) p59 (upper middle); hVFF p21 (lower middle); hVFF T59 (upper right); hVFF T21 (lower right).
Figure 2. Phenotype of hVFF.
Panel A shows representative fluorescent activated cell sorting analysis of hVFF cell lines p59, p21, T59 and T21 compared to BM- and AT- derived MSCs for different cell surface markers. Panel B shows a table of the mean value (± standard error) of the expression of corresponding markers. These experiments were performed in triplicate.
Differentiation of cells toward osteogenic, adipogenic, and chondrogenic lineages
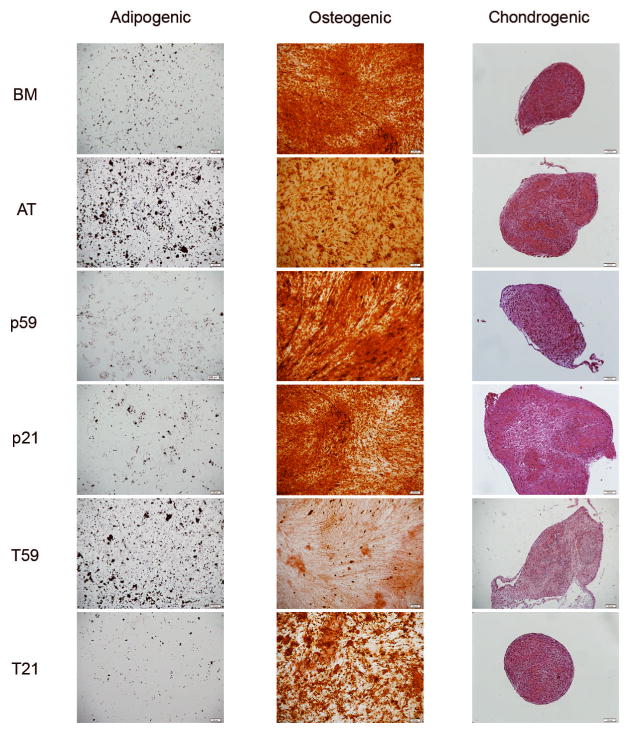
Undifferentiated hVFF, both primary and immortalized lines, as well as BM- and AT-derived MSCs were subjected to adipogenic, osteogenic, and chondrogenic culture conditions. HVFF, both parent cells and immortalized lines, differentiated into adipocytes, osteocytes and chondrocytes. Oil red O staining shows lipid vacuoles stained red (Figure 3, left column); Alizarin red S staining shows deposits of calcium crystals stained orange to brown (Figure 3, middle column), and Safranin O staining shows cartilage-specific glycosaminoglycans stained pink (Figure 3, right column).
Figure 3. Differentiation Potential.
Representative microscopic images views of the differentiation of hVFF cell lines p59, p21, T59 and T21 compared to BM- and AT derived MSCs into adipogenic (left column), osteogenic (middle column), and chondrogenic lineages (right column). Oil red O staining shows lipid vacuoles stained red; Alizarin red S staining shows deposits of calcium crystals stained orange to brown, and Safranin O staining shows cartilage-specific glycosaminoglycans.
Effect of IFN-γ on HLA antigen and co-stimulatory cell surface markers
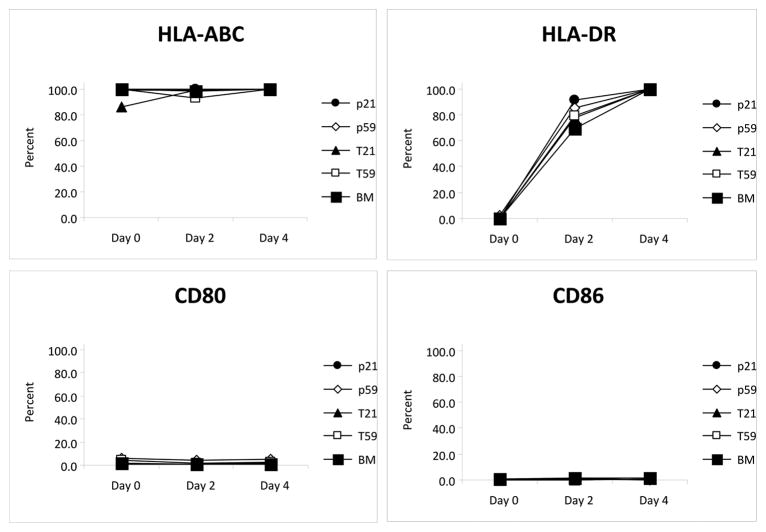
Unstimulated BM-MSCs and hVFF were positive for HLA-ABC (BM-MSCs 99.4%, hVFF-p59 98.8%, hVFF-p21 99.6%, hVFF-T59 99.5% and hVFF-T21 86.1%) while negative for HLA-DR, and co-stimulatory molecules CD80 and CD86. However, after two days of stimulation with 200 IU of IFN-γ, cultured cells began to express HLA-DR (BM-MSCs 69.1%, hVFF-p59 84.9%, hVFF-p21 91.5%, hVFF-T59 79.4% and hVFF-T21 77.8%), but remained negative for CD80 or CD86. Stimulation for four days resulted in almost all the cells staining positive for HLA-DR (BM-MSCs 99.4%, hVFF-p59 99.5%, hVFF-p21 100%, hVFF-T59 99.9% and hVFF-T21 99.5%), however, CD80 and CD86 remained negative overall. Figure 4 summarizes the data from three different sets of experiments.
Figure 4. Immunophenotype of hVFF.
Unstimulated BM-MSCs and hVFF cells are positive for HLA-ABC but do not express HLA-DR, CD80 or CD86. Treatment of cells with IFN-γ induces the cell surface expression of HLA-DR while CD80 and CD86 remain unchanged. Panel A shows the trend in cell surface marker expression over four days of treatment with IFN-γ for BM-MSCs, hVFF-p59, hVFF-p21, hVFF-T59 and hVFF-T21. Panel B shows a table of the mean value (± standard error) of the expression of surface markers. These experiments were performed in triplicate.
Discussion
We investigated whether hVFF will be similar to AT- and BM-derived MSCs in their cell surface markers, differentiation potential, and importantly, their immunological phenotypes. We found that hVFF meet the definition of MSCs based on a strict set of criteria widely accepted for defining MSCs (11). From a clinical standpoint, the similarity in BM- and AT-derived MSCs and hVFF, now characterized as VF-MSCs in this study, indicates that either BM- or AT- derived MSCs could be suitable for novel therapies in vocal fold repair and regeneration. Some of the functional characteristics of MSCs include their ability to migrate to the site of injury or inflammation, stimulate proliferation and differentiation of resident progenitor cells, and promote recovery of injured cells through growth factor secretion and matrix remodeling. Furthermore, there is an emerging body of evidence that MSCs possess the ability to suppress activation and proliferation of T-lymphocytes, B-lymphocytes, natural killer (NK) cells and dendritic cells. Such properties have been the basis of using allogeneic MSCs without HLA matching in a variety of clinical applications such as for treatment of graft versus host disease and other inflammatory or immune system disorders (1). Importantly, we propose the apparent lack of immunogenicity and their potential applicability as a universal stem cell donor without the need for tissue matching make MSCs a desirable cell-based therapeutic modality in a variety of pathophysiologic processes including voice disorders such as vocal fold scarring or paralysis.
Voice disorders affect 3–9% Americans yearly and have a significant impact on quality of life. Vocal fold scarring may cause a deformity of the vocal fold edge, a disruption of the viscoelastic layered structure of the lamina propria and increase in stiffness of the vibratory structure and glottic incompetence. Vocal fold scarring has been suggested by Hirano (13) as one of the major problems awaiting improvement in the future. Treatment for dysphonias caused by connective tissue or ECM injury or loss has been limited. The foremost reason for the inability to adequately treat these dysphonias is that present surgical options do not adequately address the ECM biomechanical tissue properties and do not mimic the complex composition of the ECM. The composition of the ECM is a central issue because of the crucial contributions of this component to the biomechanical properties of the tissue and the resultant voice quality. Collagen injections, fat injections, steroid injections, microflaps with dissection and fat implants have all been tried for ECM disorders with varying success (13). Of unique interest is autologous fat injection as it potentially manipulates the undifferentiated MSC population. First reported in 1991, intracordal fat injection (14) has been clinically useful though the amount of adipose resorption is unpredictable. While there are inherent advantages to using autologous fat for head and neck reconstruction, the technique is also restricted by mechanical damage during harvest, cyst formation, and localized necrosis. Mesenchymal stem cells, derived from AT or BM, offer the same regenerative potential as autologous fat with the additional immunomodulatory effects described above and also of a higher number of cells generated through ex vivo culture expansion. Nevertheless, it is quite possible that MSCs from these different sources will have different gene expression signatures; these investigations are currently underway in our lab.
Not only are MSCs being investigated in novel cellular therapies, this cell type offers many favorable characteristics for tissue engineering strategies as well. Recently, Macchiarini and colleagues published their results with the transplantation of a tissue-engineered airway (15). The construct reported was a decellularized allograft seeded with autologous chondrocytes derived from BM-MSCs. Such reports provide novel solutions for serious clinical disorders; however, there is much potential for cell-based therapies in other disorders of the head and neck. Given the unique composition of the vocal folds and laryngotracheal tree, there is much to be learned about cellular differentiation and immunobiology of MSCs residing in the tissues of interest when considering such cellular therapies. Fibroblasts are derived from the mesodermal germ layer and therefore may have an immunophenotype similar to mesenchymal cells. We have shown that fibroblasts cultured from human vocal folds are indeed, by definition, MSCs that possess properties similar to BM- and AT- derived MSCs. The results of this study provide insight to the biology of stromal components of vocal folds, the true identity of fibroblasts isolated from these tissues, and the immunomodulatory phenotype of these cells. Research of this type begins to set the foundation to address challenging clinical problems in the head and neck population, both reconstructive and cosmetic, through identification of an optimal MSC tissue source for head and neck bioengineering strategies from direct cell injection to complex cell-scaffold constructions.
Conclusion
Mesenchymal stem cells have unique functional and immunomodulatory characteristics that make them attractive for regenerative medicine. This work, for the first time, shows that hVFF indeed are MSCs as defined by cell surface markers and differentiation potential; furthermore, these VF-MSCs express an immunophenotype similar to BM-MSCs. In-depth genetic characterization of MSCs from hVFF and their comparison with BM and AT-MSCs is needed before we can optimize a cell population for development of tissue bioengineered constructs or other cellular therapy modalities for vocal fold lamina propria reconstruction.
Acknowledgments
Source of Funding: NIDCD - NIH grant R01 DC4336; NHLBI – NIH grant K08 HL081076; NCI – NIH grant T32 CA009614
This work was supported in part by NIH/NIDCD Grant R01 DC4336 (S.L. Thibeault), NIH/NHLBI HL081076 K08 (P. Hematti), and NIH T32 Physician-Scientist Training Grant CA009614 (S.E. Hanson).
Footnotes
Conflict of Interest: None
References
- 1.Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando) 2008;22(4):262–273. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36(3):350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thibeault SL, Klemuk SA, Smith ME, Leugers C, Prestwich G. In Vivo Comparison of Biomimetic Approaches for Tissue Regeneration of the Scarred Vocal Fold. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibeault SL, Li W, Bartley S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol Head Neck Surg. 2008;139(6):816–822. doi: 10.1016/j.otohns.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114(9):662–670. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 6.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12(8):2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 7.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12(11):3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 8.Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112(11):915–920. doi: 10.1177/000348940311201101. [DOI] [PubMed] [Google Scholar]
- 9.Chhetri DK, Head C, Revazova E, Hart S, Bhuta S, Berke GS. Lamina propria replacement therapy with cultured autologous fibroblasts for vocal fold scars. Otolaryngol Head Neck Surg. 2004;131(6):864–870. doi: 10.1016/j.otohns.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Cedervall J, Ahrlund-Richter L, Svensson B, et al. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: short-term results. Laryngoscope. 2007;117(11):2075–2081. doi: 10.1097/MLG.0b013e3181379c7c. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Thibeault SL. Novel Isolation and Biochemical Characterization of Immortalized Fibroblasts for Tissue Engineering Vocal Fold Lamina Propria. Tissue Eng Part C Methods. 2008 doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano S, Bless DM, Heisey D, Ford CN. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2003;112(7):617–624. doi: 10.1177/000348940311200708. [DOI] [PubMed] [Google Scholar]
- 14.Mikaelian DO, Lowry LD, Sataloff RT. Lipoinjection for unilateral vocal cord paralysis. Laryngoscope. 1991;101(5):465–468. doi: 10.1288/00005537-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]