Abstract
The 13C isotope effect for the conversion of prephenate to phenylpyruvate by the enzyme prephenate dehydratase from Methanocaldococcus jannaschii is 1.0334 ± 0.0006. The size of this isotope effect suggests that the reaction is concerted. From the X-ray structure of a related enzyme, it appears that the only residue capable of acting as the general acid needed for removal of the hydroxyl group is threonine-172, which is contained in a conserved TRF motif. The more favorable entropy of activation for the enzyme-catalyzed process (25 eu larger than for the acid-catalyzed reaction) has been explained by a preorganized microenvironment that obviates the need for extensive solvent reorganization. This is consistent with forced planarity of the ring and side chain, which would place the leaving carboxyl and hydroxyl out of plane. Such distortion of the substrate may be a major contributor to catalysis.
Keywords: Prephenate dehydratase, 13C Isotope effect, Transition state structure
1. Introduction
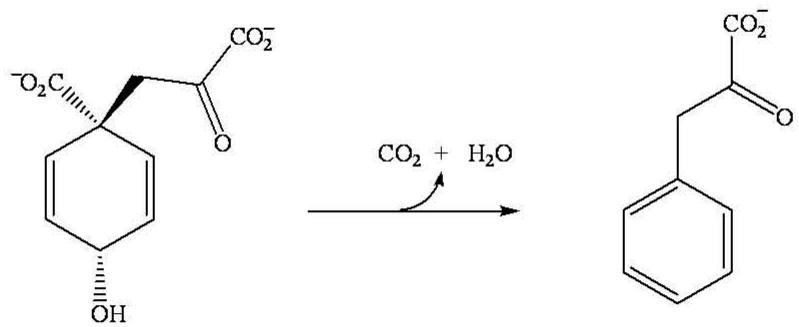
Aromatic amino acid biosynthesis in plants and microorganisms occurs via the shikimate pathway, which produces chorismate [1]. The conversion of chorismate to prephenate is the last common biosynthetic step in the de novo synthesis of both Lphenylalanine and L-tyrosine. The biosynthesis of phenylalanine involves the decarboxylation and dehydration of prephenate to phenylpyruvate catalyzed by prephenate dehydratase (PDT; EC 4.2.1.51), Scheme 1. In a second step phenylpyruvate is subsequently transaminated to form phenylalanine [1,2].
Scheme 1.
Since there is no counterpart of the aromatic amino acid biosynthesis pathway in humans, the enzymes involved are prime sites for drug targets [1]. However, knowledge of the chemical mechanism and the transition state structure is key to creating potent enzyme inhibitors. In the present study, the monofunctional PDT from the extreme thermophile Methanocaldococcus jannaschii (MjPDT) has been used to study the mechanism of the enzymatic conversion of prephenate to phenylpyruvate by measurement of the 13C kinetic isotope effect (KIE) on the decarboxylation.
2. Materials and methods
2.1. Enzyme Production and Purification
MjPDT was recombinantly overproduced in Escherichia coli KA13 that was transformed with the plasmid pAKZ11 and purified as previously described [4]. Its primary structure was confirmed by ESI mass spectrometric analysis, and its concentration was determined by the MicroBC assay (Interchim, France) using bovine serum albumin as a standard. Samples were stored at 4 °C prior to use.
2.2. Materials
Prephenic acid (barium salt) was from Sigma (St. Louis, MO). 13C NMR spectra in D2O showed that the solid contained 87% prephenate, 10% phenylpyruvate and 3% epi-prephenate, an epimer of prephenate at the 4-position on the cyclohexadiene ring. All other chemicals were of the highest purity obtainable.
2.3. Preparation of the Potassium Salt of Prephenate
The barium salt of prephenate (300 mg) was dissolved in 7.25 mL of 50 mM KHEPES (pH 7.5) containing 138 mM K2SO4 that had been rigorously sparged with wet, CO2-free N2 gas for 48 hrs. The volume and concentration of salt was calculated to produce a 1.2:1 mol ratio of K2SO4 to barium, resulting in the total precipitation of BaSO4. The precipitated BaSO4 salt was removed by centrifugation at 9000 rpm for 5 min at 4 °C. The resulting potassium salt of prephenate was assayed using both a coupled enzyme assay (using transketolase to interconvert phenylpyruvate between its keto and enolate form which has a higher extinction coefficient) and an end point assay; both assays have been described thoroughly elsewhere [3]. In the end point assay, the amount of phenylpyruvate already in the sample prior to reaction was determined by the addition of 1 N NaOH to an aliquot of the solution in a final volume of 1 mL, followed by spectroscopic analysis of the enolate form of phenylpyruvate at 320 nm and 30 °C (λ320 = 17,500 M−1cm−1). Prephenate was then completely converted to phenylpyruvate by addition of an equal volume of 1 N HCl to an aliquot of the substrate solution, followed by incubation at 25 °C for 15 min. 1 N NaOH was added to an aliquot of the acidified solution, and it was assayed as above. The actual concentration of prephenate in the reaction mixture was determined by subtracting the initial amount of phenylpyruvate from the amount determined after acid hydrolysis. The coupled enzyme and end point assays gave prephenate concentrations that differed by less than 1%.
2.4. 13C Isotope Effects on Prephenate with MjPDT
High conversion reactions were carried out in 4 mL of 100 mM K-HEPES (pH 7.5) in a sealed 50 mL pear-shaped flask with a side arm stopcock. The buffered solution was degassed in vacuo by performing three freeze-pump-thaw cycles using an isopropanol/dry ice bath. Following evacuation, the flask was sparged with wet CO2-free N2 for 18 hrs. An aliquot of the prephenate stock solution (10 μmoles) that had been sparged separately for 4 hrs was added to the sample flask via an airtight needle and syringe. The reaction was initiated by the addition of ~40 ng of MjPDT. Following incubation for 36 hrs at 25 °C, the reaction was quenched by the addition of 300 μL of concentrated H2SO4. Prior to quenching, an aliquot of the reaction mixture was checked for complete conversion of substrate to product using the end-point assay. The CO2 evolved during the reaction was distilled through two isopropanol/dry ice traps and collected using a liquid nitrogen trap. The collected CO2 was analyzed via isotope ratio mass spectrometry to determine the 13C/12C ratio.
Low conversion reactions (~30 μmoles of product) were performed in 100 mM Na-HEPES (pH 7.5) in a 100 mL round bottom flask with an attached side arm stopcock and stir bar. Both the buffer and substrate solutions were sparged to remove endogenous CO2 as described above. The reactions were initiated by addition of ~20 ng of MjPDT to the substrate solution, and the resulting mixture was incubated at 25 °C for a predetermined amount of time. At approximately 50% completion, an aliquot was removed to determine the fraction of reaction. The pH of the reaction was adjusted to 5.9 through the addition of 3M cacodylate buffer of this pH, yielding a final concentration of 300 mM of buffer (adjustment to a lower pH would lead to decarboxylation of residual prephenate). Immediately after the buffer addition, the flask was attached to a gas distillation line and the CO2 quickly collected (for ~15 min) while stirring to facilitate gas evolution. The CO2 collected was analyzed by isotope ratio mass spectrometry to determine the 13C/12C ratio.
The isotope effect was calculated from the mass ratio of 13C in 100% conversion samples (Ro) and low conversion samples (Rp) using the following equation:
where f is the fraction of reaction when Rp is measured.
3. Results and discussion
In acidic medium prephenate decarboxylates to give phenylpyruvate [4-6]. Using 13C NMR to follow decarboxylation of prephenate in H218O, Hermes et al. [6] observed 18O incorporation into prephenate at the 4-OH position, indicating that the acid-catalyzed decarboxylation occurred through the formation of a resonance stabilized carbenium ion which lost CO2 1.75 times faster than it reacted with water to generate prephenate and epi-prephenate. Water added to both faces of this intermediate, resulting in either the reformation of prephenate or the formation of the 4-epimer, epi-prephenate. Rehydration to form prephenate was 1.8 fold faster than formation of the 4-epimer. The observed 13C KIE on the decarboxylation of prephenate in acid was 1.0082, and the intrinsic KIE on the decarboxylation step was calculated to be 1.023, suggesting an early transition state for C – C bond cleavage. By contrast the enzymatic oxidation of prephenate to p-hydroxyphenylpyruvate by prephenate dehydrogenase was a concerted reaction with an intrinsic 13C KIE of 1.0155, showing an even earlier but highly asynchronous transition state with hydride transfer well advanced (intrinsic deuterium KIE of 7.3) [6].
The enzyme-catalyzed decarboxylation and dehydration of prephenate by PDT has been proposed to occur in a concerted manner. While several authors have suggested that protonation of the substrate hydroxyl must occur prior to decarboxylation [7,8], a typical pK value for the protonation of an alcohol is about -2, well outside the pK range of any of the enzyme's active site residues. Thus a stepwise reaction similar to the acid-catalyzed reaction is unlikely.
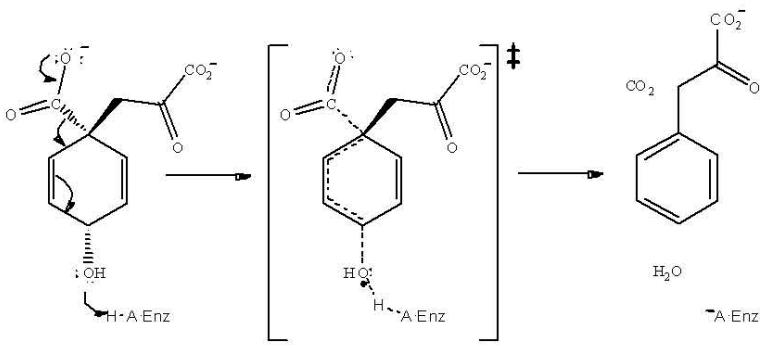
In the present study, the 13C KIE on the decarboxylation of prephenate by MjPDT was determined to be 1.0334, Table 1. It is not known whether there is a forward commitment in this reaction, and if there is, the intrinsic isotope effect would be larger than 1.0334. If 1.0334 is the intrinsic isotope effect, the transition state is somewhat earlier than that of typical decarboxylations, which show values about 1.05, while if there is a forward commitment, the transition state is later. With the acid-catalyzed reaction showing a 13C isotope effect of 1.0082, the significantly larger value for the enzymatic reaction is consistent with our conclusion that the reaction is not stepwise. We think that the observed value thus supports a concerted reaction, with general acid assistance for removal of the OH group (Scheme 2).
Table 1.
13C Kinetic Isotope Effects for MjPDT-Catalyzed Decarboxylation of Prephenate
|
13/12δ Partial |
fa |
13(V/K) (klight/kheavy)b |
|---|---|---|
| −24.308 | 0.513 | 1.0337 |
| −21.412 | 0.594 | 1.0324 |
| −26.757 | 0.405 | 1.0336 |
| −21.163 | 0.622 | 1.0333 |
| −25.488 | 0.471 | 1.0339 |
| 1.0334 ± 0.0006 |
Reactions were carried out in 100 mM HEPES, pH 7.5, 25 °C.
Determined via end-point assay in 1 N NaOH at 30 °C.
Corrected for fraction of reaction.
Scheme 2.
Multiple sequence alignments have been performed by several authors in an effort to determine possible residues responsible for the catalytic and regulatory activity of PDT [3,8,9]. In one report, 15 residues were found to be conserved among 12 different PDT-containing proteins [8], including a strictly conserved TRF motif. Mutation of these residues revealed the importance of Thr172 and Phe174 (MjPDT numbering) for catalysis, whereas Arg 173 was surprisingly tolerant to substitution [8,10]. Another mutational study using the PDT domain of the E. coli P-Protein provided independent evidence for the importance of Thr172 in catalysis [7]. Remarkably, glutamate and aspartate residues, which could act as a general acid in the active site, were not essential for activity. Based on the kinetic studies of mutant and wild-type enzyme it was suggested that Thr172 may form a hydrogen bond to the substrate, while Phe174 is part of a hydrophobic pocket that would help to anchor the substrate in the active site.
In a recent publication, Tan et al. reported the crystal structure of the active state of PDT from Staphylococcus aureus [11]. The structure revealed that the biologically active form of PDT was a tetramer formed from a dimer of dimers with the active site located in a cleft between the two dimer subunits. Located at the bottom of the cleft is the conserved TRF motif with the side chains of Thr172 and Phe174 pointing into the cleft. Although many of the other conserved residues were located within or lining the active site cleft, Arg173 was oriented away from the pocket, precluding a role in substrate recognition or catalysis [10]. In this structure, Thr172 had hydrogen-bonding interactions with a solvent molecule as well as the backbone oxygen of Asn57. The structure suggests that Thr172 is the key general acid, providing a proton to the leaving hydroxide group.
The question then is, what leads to the catalysis? The hydroxyl group of Thr172 is not a strong enough acid to protonate the hydroxyl group of prephenate so that it could be released as water to give a carbenium ion, as occurs in the non-enzymatic reaction. However, if geometric distortion promotes decarboxylation, the driving force for aromatization will tend to loosen the bond to the hydroxyl group, thus raising its pK towards that of hydroxide. As the hydroxyl group becomes hydroxide, one would have a proton from Thr172 poised between the oxygen of Thr172 and the hydroxyl of prephenate. As the pK's become close, this could become a strong low-barrier hydrogen bond. Formation of such a strong hydrogen bond in a concerted elimination would lower the activation energy of the reaction. This pairing would then pick up a proton from the buffer either before, or certainly after dissociation of phenylpyruvate.
The major difference between the enzymatic and acid-catalyzed reactions lies in the ΔS‡ values (−3 vs. −28 cal mol−1 K−1, respectively; the ΔH‡ values are 15 and 17 kcal/mol [4]). This suggests that catalysis by the enzyme involves accurate orientation, as well as general acid catalysis. Forcing planarity of the ring and the attached pyruvate side chain so that the ring carboxyl and hydroxyl groups are out of the ring plane should go a long way toward promoting aromatization.
4. Acknowledgments
This work was supported in part by the ETH Zurich (DH) and the Swiss National ScienceFoundation (DH) and by an NIH grant to WWC (GM18938).
Abbreviations
- PDT
prephenate dehydratase
- MjPDT
PDT from Methanocaldococcus jannaschii
- KIE
kinetic isotope effect
- IRMS
isotope ratio mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haslam E. Shikimic acid metabolism and metabolites. John Wiley & Sons; New York: 1993. [Google Scholar]
- 2.Euverink GLW, Wolters DJ, Dijkuizen L. Prephenate dehydratase of the actinomycete Amycolatopsis methanolica: purification and characterization of wild-type and deregulated mutant proteins. Biochem. J. 1995;308:313–320. doi: 10.1042/bj3080313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleeb AC, Kast P, Hilvert D. A monofunctional and thermostable prephenate dehydratase from the archaeon Methanocaldococcus jannaschii. Biochemistry. 2006;45:14101–14110. doi: 10.1021/bi061274n. [DOI] [PubMed] [Google Scholar]
- 4.Katagiri M, Sato R. Accumulation of phenylalanine by a phenylalanineless mutant of Escherichia coli. Science. 1953;118:250–251. doi: 10.1126/science.118.3061.250. [DOI] [PubMed] [Google Scholar]
- 5.Zamir LO, Tiberio R, Jensen RA. Differential acid-catalyzed aromatization of prephenate, arogenate, and spiro-arogenate. Tetrahedron Lett. 1983;24:2815–2818. [Google Scholar]
- 6.Hermes JD, Tipton PA, Fisher MA, O'Leary MH, Morrison JF, Cleland WW. Biochemistry. 1984;23:6263–6275. doi: 10.1021/bi00320a057. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Wilson DB, Ganem B. Probing the catalytic mechanism of prephenate dehydratase by site-directed mutagenesis of the Escherichia coli P-protein dehydratase domain. Biochemistry. 2000;39:4722–4728. doi: 10.1021/bi9926680. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S-K, Lin L-L, Lo H-H, Hsu W-H. Mutational analysis of feedback inhibition and catalytic sites of prephenate dehydratase from Corynebacterium glutamicum. Arch. Microbiol. 2004;181:237–244. doi: 10.1007/s00203-004-0649-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Pohnert G, Kongsaeree P, Wilson DB, Clardy J, Ganem B. Chorismate mutase-prephenate dehydratase from Escherichia coli. Study of catalytic and regulatory domains using genetically engineered proteins. J. Biol. Chem. 1998;273:6248–6253. doi: 10.1074/jbc.273.11.6248. [DOI] [PubMed] [Google Scholar]
- 10.Kleeb AC, Hansson-Edalat M, Gamper M, Haugstetter J, Giger L, Neuenschwander M, Kast P, Hilvert D. Metabolic engineering of a genetic selection system with tunable stringency. Proc. Natl. Acad. Sci. USA. 2007;104:13907–13912. doi: 10.1073/pnas.0705379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan K, Li H, Zhang R, Gu M, Clancy ST, Joachimiak A. Structures of open (R) and close (T) states of prephenate dehydratase (PDT) - Implication of allosteric regulation by L-phenylalanine. J. Struct. Biol. 2008;162:94–107. doi: 10.1016/j.jsb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]