Abstract
Background
There is growing use of psychostimulant cognitive enhancers such as methylphenidate (Ritalin). Methylphenidate differs from the psychostimulant cocaine because it does not enhance brain levels of serotonin. We investigated whether exposure to methylphenidate combined with a serotonin-enhancing medication, the prototypical selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac), would produce more “cocaine-like” molecular and behavioral changes.
Methods
We measured the effects of fluoxetine on gene expression induced by the cognitive enhancer methylphenidate in the striatum and nucleus accumbens of rats, by in situ hybridization histochemistry. We also determined whether fluoxetine modified behavioral effects of methylphenidate.
Results
Fluoxetine robustly potentiated methylphenidate-induced expression of the transcription factors c-fos and zif 268 throughout the striatum and to some degree in the nucleus accumbens. Fluoxetine also enhanced methylphenidate-induced stereotypical behavior.
Conclusions
Both potentiated gene regulation in the striatum and the behavioral effects indicate that combining the SSRI fluoxetine with the cognitive enhancer methylphenidate mimics cocaine effects, consistent with an increased risk for substance use disorder.
Keywords: psychostimulant, SSRI antidepressant, cocaine, cognitive enhancer, gene expression
Introduction
Demand for cognitive-enhancing drugs is growing, and these are most often psychostimulants such as methylphenidate (Ritalin) (1,2). Animal studies indicate that psychostimulants can produce long-term adverse effects on brain function and behavior, especially when given during childhood and adolescence (3,4,5). This is, at least in part, because psychostimulants tend to induce changes in gene regulation (6) and other molecular alterations that interfere with normal brain development (4,5). It remains, however, unclear whether proper medical use of drugs such as methylphenidate has detrimental effects on patient health (7,8,9).
Methylphenidate, like cocaine, acts by blocking reuptake of dopamine and norepinephrine and thus enhances their actions at postsynaptic receptors (6). However, unlike cocaine, methylphenidate does not affect serotonin reuptake. This may explain why methylphenidate mimics some but not all molecular effects of cocaine (6). We investigated whether concomitant exposure to methylphenidate and a medication that enhances brain serotonin levels, the SSRI antidepressant fluoxetine (Prozac), produces more “cocaine-like” molecular and behavioral changes. The effects on gene markers for neuroadaptations implicated in drug addiction (6), the transcription factors c-fos and zif 268, were examined in brain regions that subserve reward processing (nucleus accumbens) and habit formation/compulsive behaviors (striatum). We also assessed whether this methylphenidate/SSRI combination would alter drug-induced behavioral effects.
Methods and Materials
Subjects
Drug responses were assessed in five-week-old (adolescent) male Sprague-Dawley rats (Harlan, Madison, WI, USA). Animals were housed under standard laboratory conditions (12:12-hr light/dark cycle; lights on at 7:00 a.m.) and were allowed one week of acclimation, during which they were repeatedly handled. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Drug treatment and behavioral measurements
Rats received a single intraperitoneal injection of vehicle, methylphenidate (methylphenidate HCl, generously provided by the National Institute on Drug Abuse, Bethesda, MD, USA; 5 mg/kg, in 0.02% ascorbic acid, 1 ml/kg), fluoxetine (fluoxetine HCl, Sigma, St. Louis, MO, USA; 5 mg/kg) or methylphenidate (5 mg/kg) plus fluoxetine (5 mg/kg) (n=5-7). After the injection, each rat was placed in the arena of an activity monitor (43 × 43 cm; Truscan, Coulbourn Instruments, Allentown, PA, USA), and the ambulatory distance, rearing rate, and stereotypy counts (local repetitive movements) were measured for 40 min.
In situ hybridization histochemistry
Forty minutes after the injection, the rats were killed with CO2, and the brains were rapidly removed and frozen. After cryostat sectioning (12 μm), the tissue was prepared for in situ hybridization histochemistry (10). Oligonucleotide probes (48-mers; Invitrogen, Rockville, MD, USA) were labeled with [35S]-dATP as described earlier (10). The probes had the following sequence: c-fos, complementary to bases 207-254, GenBank accession number X06769; zif 268, bases 352-399, M18416. Following incubation and washing (10), the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak) for 5-9 days.
Analysis of autoradiograms
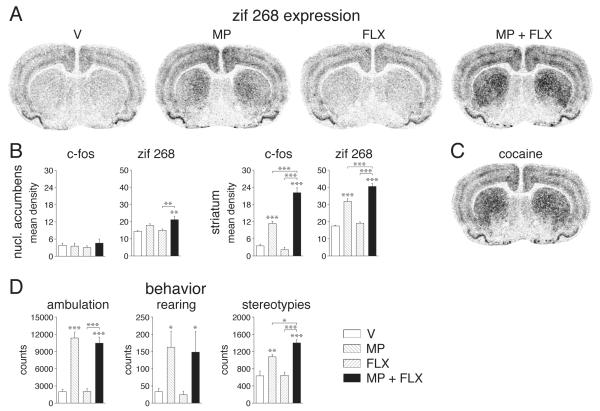
Gene expression was assessed in sections from 3 rostrocaudal levels: rostral, approximately at +1.6 mm relative to bregma (11); middle, +0.4; caudal, −0.8. Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA) across the total area of the nucleus accumbens (rostral level) and striatum (rostral, middle and caudal levels), using previously described procedures (10). Treatment effects were determined by two-factor ANOVA, followed by Newman-Keuls post hoc tests to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). The illustrations of film autoradiograms displayed in Figure 1 are computer-generated images. Maximal hybridization signal is black.
Fig. 1.
Fluoxetine potentiates methylphenidate-induced gene regulation and behavior. (a) Illustrations of film autoradiograms depict expression of zif 268 in the striatum on the middle striatal level for rats that received a single injection of vehicle (V), methylphenidate (MP, 5 mg/kg, i.p.) or fluoxetine (FLX, 5 mg/kg), or a combination of methylphenidate+fluoxetine. (b) Mean density values (mean±SEM) for c-fos and zif 268 expression in the nucleus accumbens (left) and middle striatum (right) are given for rats that were treated with vehicle, methylphenidate, fluoxetine, or methylphenidate+fluoxetine (n=5-7). (c) For comparison, zif 268 expression in the striatum after acute cocaine administration (25 mg/kg; 19) is shown. (d) Ambulation (left), rearing (middle) and stereotypy counts (right) are given for animals that received the above methylphenidate and/or fluoxetine treatments and were then tested in a novel open field (40 min). Fluoxetine selectively potentiated methylphenidate-induced stereotypies. * P<0.05, ** P<0.01, *** P<0.001, vs. vehicle controls or as indicated.
Results
Gene regulation in the striatum and nucleus accumbens
Pronounced effects on gene regulation were present in the striatum on all three rostrocaudal levels (Fig. 1; Tab. 1) and were most robust in sensorimotor domains of the striatum (Fig. 1). Administration of methylphenidate alone significantly increased the expression of c-fos and zif 268 on all three levels (P<0.001), consistent with previous findings (12,13). Fluoxetine alone did not produce changes in the expression of these genes (Fig. 1a, b, Tab. 1). However, adding fluoxetine to methylphenidate treatment potentiated methylphenidate-induced c-fos and zif 268 expression in the striatum on all levels; the increase in hybridization signals in the methylphenidate plus fluoxetine group was approximately twice as high as that produced by methylphenidate alone (P<0.01; Fig. 1a, b, Tab. 1). In the nucleus accumbens, effects were more modest. Neither methylphenidate alone nor fluoxetine alone produced statistically significant changes in zif 268 or c-fos mRNA levels. However, concomitant administration of both significantly increased zif 268 expression (P<0.01; Fig. 1b). A similar tendency for c-fos did not reach statistical significance (Fig. 1b).
Table 1.
Fluoxetine potentiates methylphenidate-induced gene regulation in the striatum. Mean density values (mean±SEM) for c-fos (top) and zif 268 expression (bottom) in the striatum on rostral, middle and caudal levels are given for rats that were treated with vehicle, methylphenidate, fluoxetine, or methylphenidate+fluoxetine (n=5-7).
| striatum | vehicle | methyl- phenidate |
fluoxetine | methylphenidate +fluoxetine |
|
|---|---|---|---|---|---|
| c-fos | rostral | 2.4 ± 0.5 | 8.8 ± 0.3 *** | 3.6 ± 0.3 | 14.5 ± 1.6 *** ††† ••• |
| middle | 3.5 ± 0.6 | 11.3 ± 0.8 *** | 2.2 ± 0.8 | 22.1 ± 1.8 *** ††† ••• | |
| caudal | 2.9 ± 0.4 | 12.0 ± 1.7 *** | 2.7 ± 0.5 | 20.4 ± 1.8 *** ††† ••• | |
| zif 268 | rostral | 18.8 ± 0.6 | 30.2 ± 0.7 *** | 21.4 ± 1.0 | 37.6 ± 2.2 *** ††† ••• |
| middle | 17.5 ± 0.5 | 31.7 ± 1.6 *** | 19.0 ± 0.9 | 40.5 ± 1.8 *** ††† ••• | |
| caudal | 13.8 ± 1.0 | 28.4 ± 1.9 *** | 15.5 ± 0.9 | 35.3 ± 2.5 *** ††† •• | |
P<0.001, vs. vehicle
P<0.001, vs. fluoxetine
P<0.01
P<0.001, vs. methylphenidate
Behavioral effects in the open field
We also investigated effects of these drug treatments on open-field behavior. As seen before (6), methylphenidate alone increased measures of locomotion (ambulation; P<0.001) and rearing (P<0.05), but fluoxetine had no effect on these behavioral variables, neither alone nor in combination with methylphenidate (Fig. 1d). In contrast, behavioral stereotypies, a measure of striatal dysfunction related to compulsive behavior (14), were potentiated by the methylphenidate plus fluoxetine treatment (Fig. 1d). Thus, methylphenidate alone increased stereotypy counts (P<0.01), while fluoxetine alone had no effect. When given together with methylphenidate, however, fluoxetine further elevated stereotypy levels over those induced by methylphenidate alone (P<0.05).
Discussion
Our present findings are the first to demonstrate that concomitant administration of the serotonin reuptake blocker fluoxetine robustly potentiates gene regulation by methylphenidate, consistent with the notion that serotonin facilitates dopamine-mediated gene regulation (6). Here, we show enhanced induction of transcription factors (c-fos, zif 268) in the striatum and the nucleus accumbens. Such acute gene induction often serves as a marker for long-term neuroadaptations after repeated psychostimulant treatment, which are thought to underlie addiction (14). This potentiated gene induction was most robust in sensorimotor domains of the striatum, which mediate motor learning/habit formation and are implicated in compulsive aspects of drug addiction (15,16). These fluoxetine effects on gene regulation were associated with selective potentiation of motor stereotypies, which are thought to reflect dysfunction in sensorimotor striatal circuits and may be related to compulsive behavior (17). In the nucleus accumbens, gene induction was mostly enhanced in the lateral part of the shell (Van Waes et al., in preparation), which receives input (among other areas) from the insular cortex, which is implicated in craving and relapse in drug addiction (18). Together, these molecular and behavioral changes are consistent with an increased liability for drug addiction or other compulsive disorders (4,6,17).
In summary, our findings show that concomitant exposure to psychostimulant-cognitive enhancers such as methylphenidate together with SSRIs such as fluoxetine produces cocaine-like effects, suggestive of an increased risk for substance use disorder. This is of concern as surveys indicate that millions of patients in the US alone are being treated with SSRIs and psychostimulants (2,8), and concomitant exposure to these drugs will likely increase considerably with spreading popularity (1) of psychostimulants as cognitive enhancers.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DA011261 (H.S.) and DA020654 (M.M.). We would like to thank Joel Beverley for excellent technical assistance.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interests.
REFERENCES
- 1.Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- 2.Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 4.Carlezon WAJ, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- 9.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 10.Steiner H, Kitai ST. Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- 12.Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Yano M, Steiner H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- 14.Steiner H. Psychostimulant-induced gene regulation in corticostriatal circuits. In: Steiner H, Tseng KY, editors. Handbook of Basal Ganglia Structure and Function. Elsevier; New York: 2010. in press. [Google Scholar]
- 15.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 16.Belin D, Everitt BJ. Drug addiction: The neural and psychological basis of a compulsive incentive habit. In: Steiner H, Tseng KY, editors. Handbook of Basal Ganglia Structure and Function. Elsevier; New York: 2010. in press. [Google Scholar]
- 17.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 18.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unal CT, Beverley JA, Willuhn I, Steiner H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: Effects on zif 268 and homer 1a. Eur J Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]