Abstract
Carbohydrates are known to mediate a large number of biological and pathological events. Small and macromolecules capable of carbohydrate recognition have great potentials as research tools, diagnostics, vectors for targeted delivery of therapeutic and imaging agents, and therapeutic agents. However, this potential is far from being realized. One key issue is the difficulty in the development of “binders” capable of specific recognition of carbohydrates of biological relevance. This review discusses systematically the general approaches that are available in developing carbohydrate sensors and “binders/receptors,” and their applications. The focus is on discoveries during the last five years.
Keywords: boronic acid, carbohydrate sensing, boronolectin, fluorescent sensor, aptamer, lectin
1. INTRODUCTION
Carbohydrates are known to be involved in a wide range of biological and pathological processes,1–8 including cancer metastasis, cell adhesion, cell signaling, embryo development, egg fertilization, protein function regulation, cellular communications, and so on.4,7–14 For example, sialyl Lewis X (sLex) is known to mediate the metastasis of B16 melanoma cancer;15 carbohydrate ligand binding to E-selectin mediates the extravasation of cancer cells and organ selectivity of metastasis;16 sialyl Lewis a (sLea) binding to selectin is involved in the extravasation of human colorectal carcinoma cells;17 endothelial cell adhesion, often mediated by carbohydrate-selectin binding, is correlated with cancer progression;18 and sLea expression on cancer cells is correlated with increased risk of distant hematogenous metastasis,19 presumably a result of carbohydrate-mediated metastasis. In addition, changes in glycosylation patterns often affect the function of a glycoprotein and are biomarkers for pathological or physiological events. For example, the glycosylation patterns of prostate specific antigen (PSA) from cancer cells in culture20 and prostate cancer patients’ tissue and sera21–23 are different from that of normal prostate; human pancreatic RNase 1, a glycoprotein secreted mostly by pancreatic cells, has completely different oligosaccharide chains when produced from pancreatic tumor cells;24–27 and deviation from the normal glycosylation pattern on fibrinogen (Fn), a protein critical to blood coagulation, can lead to coagulation disorders.28 In addition, two recent reviews also discuss stem cell surface glycan biomarkers and show that intricate glycan-dependent modulation of signalling molecules such as FGF-2, Wnt, and Notch plays an important role in stem cell proliferation and differentiation.9,29–32 Table 1 summarizes some additional examples of commonly seen carbohydrate-related biomarkers.
Table I.
Carbohydrate-based Biomarkers
| Carbohydrates | Characteristics |
|---|---|
| Tn antigen67–73 | An oligosaccharide (GalNAcα-O-Ser/Thr) that is a tumor associated antigen and a precursor of the T antigen. It has been used for the development of carbohydrate-based cancer vaccine. |
| sLex 15,63,64,74 | A tetrasaccharide that is usually attached to O-glycans on cell-surface. It is a ligand of the E-selectin family, and is implicated in mediating cancer metastasis. |
| SLX75–78 | Sialylated SSEA-1 (stage-specific embryonic antigen-1) has been identified as a biomarker for pancreatic and lung cancer. |
| SPAN-179 | A sialylated carbohydrate antigen, which is a biomarker for pancreatic cancer. |
| DUPAN280 | Sialyl Lewis C, a sialylated carbohydrate antigen for some cancer. It is commonly used as a marker of pancreatic cancer. |
| α-Gal-α-Gal81 | The causatic antigen of acute tissue rejection for transplantation from animal into human. It has been identified as a cell and matrix surface carbohydrate antigen called the alpha-galactosyl epitope (α-Gal). |
| LPA82–84 | The level of LPA (lysophosphatidic acid) has been suggested as a possible test for ovarian cancer. |
| ST-43985 | A sialylated carbohydrate antigen that is used as a clinical marker for a variety of cancers. |
| CA 12586,87 | Cancer antigen 125, a tumor-associated mucinous glycoprotein, commonly seen in tumors of the ovary. |
| CA 19-9, CA 15-3 CA 27-29, CA 242, CA 50, CA 72-4, CA 195, CA 549, M26, M2928,87–89 |
These carbohydrate cancer antigens are similar to CA 125. For examples, CA 19-9 is used for the diagnosis of pancreatic, colorectal, gastric, or biliary cancer and formonitoring the clinical response to therapy. CA 15-3 and CA 27-29 are used for the diagnosis of breast cancer. |
| TAG-12, TAG-72, TAG-72.390–92 | Tumor associated glycoproteins made by some cancer. TAG-12 is used as a marker for breast cancer; TAG-72 is used for gastric cancer; and TAG-72.3 is used for lung cancer. |
| CEA86,89,90,93–98 | Carcinoembryonic antigen, a glycoprotein produced by gastrointestinal neoplastic epithelium of glandular origin. CEA is a tumor-associated mucin antigen. |
| GP120 (polymannose)99–104 | An envelope glycoprotein essential for HIV-1infection. It is a common target for HIV vaccine research. |
| ABO blood group antigens68,73,97,105,106 | The glycoprotein antigens which determine blood types: O, A, and B. The only difference is the composition of carbohydrates. |
| A2-PAG107,108 | Pregnancy-associated alpha-2 glycoprotein made by some forms of cancer. |
| MCA68,69,72,109 | Mucin-like carcinoma-associated antigen that appears at elevated levels in certain breast cancer. |
| BCM110–112 | Breast cancer mucin and tumor associated glycoproteins that appear at elevated levels in certain forms of breast cancer. |
| CAM17-1, CAM26, CAM29113,114 | Tumor associated antigens. CAM17-1 has been suggested as marker for pancreatic cancer and CAM26 and CAM29 are suggested as breast cancer markers. |
| PMA68 | Prostate carcinoma mucin-like antigen, a high M.W. human tumor-associated mucin antigen and biomarker of prostate cancer. |
| MUC169,109,115–119 | Mucin 1 is a member of the mucin family and a glycosylated phosphoprotein, which is over expressed in carcinomas. It is a tumor-associated antigen and a marker of breast and colon cancer. |
| PEM120,121 | Tumor-associated polymorphic epithelial mucin and a tumor-associated mucin antigen. It is produced by gastrointestinal neoplastic epithelium of glandular origin. |
| M34468 | A high molecular weight, mucin-like antigen over expressed on superficial bladder tumor. It is a tumor-associated mucin antigen and a biomarker of bladder cancer. |
| Galectin-1122–124 | A β-galactoside binding animal lectin and biomarker for colon cancer. |
| Galectin-369,125–127 | An endogenous lectin. It is considered a tool for monitoring cell differentiation in head and neck carcinomas and abiomarker for colon cancer. |
| Homodimeric Galectin-7128–130 | A β-galactoside binding animal lectin on the cell surface, that is capable of inhibiting cancer cell proliferation. |
| Galectin-9131–133 | A β-galactoside binding animal lectin. It is a functional predictive factor for metastasis of breast cancer. |
Conceivably, “binders” of carbohydrates of biological importance such as those listed in Table 1 could be used as medicinal agents in the inhibition of pathological events such as metastasis that are mediated by carbohydrate binding, as diagnostic agents for the detection of disease-related glycoproducts, as vectors for targeted delivery of imaging and therapeutic agents, and as research tools in glycomics work. However, up until recently, there has been essentially no effort in the development of carbohydrate “binders” as potential medicinal and imaging agents. Effort in making fluorescent carbohydrate “binders” (fluorescent sensors) has been largely limited to mono- or disaccharides for potential analytical chemistry work including glucose detection33–61 with a few exceptions.62–65 There had been essentially no activity in developing “binders” for glycoproteins with the ability to differentiate glycoforms until recently when the Wang lab developed a platform approach to select DNA-based aptamers for such applications.66 A major issue in this field that is hindering the development of carbohydrate recognition-based therapeutic and diagnostic agents is the difficulty in the design and synthesis of highly specific and tight carbohydrate “binders.” Chemical approaches to this issue will be addressed in detail later in this review. Another key issue that needs to be addressed is the need for improved “communications” between chemists and glycobiolgists in addressing carbohydrate recognition problems. The detailed descriptions of the carbohydrate recognition sections below also give a glimps of the somewhat “silo” approaches in the literature. For example, when one reads the chemical approaches, lectin approaches, and the biological applications discussed in the following sections, they sometimes seem to come from different worlds. A large percentage of publications on carbohydrate sensing and recognition from chemistry labs are mostly focused on the chemistry issues and not directly addressing important glycobiological problems. Of course, such a situation has its historical reasons. For a long time, the chemistry knowledge and platform tehnologies were not there to apply chemical approaches to address complex glycobiological problems through carbohydrate recognition. However, efforts in recent years have shown great promise for the future development of a large number of carbohydrate binders for various therapeutic, diagnostic, and detection applications. The carbohydrate recognition field is poised to make significant inroads in the biomedical application direction if the significance of such research is widely recognized. In doing so, the chemical and biological fields can and should work together and help each other in addressing some of the most fundmental unanswered questions in carbohydrate recognition and in applying new chemistry tools to solve glycobiology problems. One of the aims of this review is to present carbohydrate recognition studies from different angles, in one place, to facilitate communications among glyco-researchers in different discinplines. The examples will mostly be those published in the last five years. It is hoped that this review will stimulate more research and ideas in taking carbohydrate recognition research to the direction of addressing complex glycobiological and disease problems.
Available carbohydrate “binders” can be classified into the following four categories: (1) antibodies,134,135 (2) lectins,6,136 (3) aptamers based on nucleic acids,137,138 and (4) small molecule lectin mimics.36 Of course, antibodies and derivatives have long been the gold standard in the recognition of a variety of analytes including carbohydrates.68,70,75,79,109,115,116,139 However, in research, antibodies have not been widely used in large scale applications such as in arrays. Presumably, cost and stability are factors. Currently, lectins are the major available tools in research for carbohydrate recognition. However, the available lectins often have cross reactivity issues. Therefore, there is a need to develop alternatives for carbohydrate recognition that meet the following criteria: (1) high affinity, (2) high specificity, and (3) suitable for high throughput analysis. The high affinity and specificity points are easy to understand. The need for high throughput stems from the recent interests in studying carbohydrate/glycan changes at the glycome level.67,140–148 To meet these criteria there have been a great deal of recent interest in developing fluorescent sensing methods for carbohydrates for various applications.34,149–155 In this regard, there have been very active efforts in developing small molecules and “polymeric” “binders” for carbohydrates. Along this line, there have been work in using the boronic acid moiety as the key recognition unit for carbohydrate sensor development.34,149–155 We have termed these boronic acid-based carbohydrate sensors/binders as boronolectins because they mimic the function of lectins and contain the boronic acid unit.36 Therefore, there are small molecule boronolectins (SBL),33,155,156 peptidoboronolectins (PBL),44,157–159 and nucleic acid-based boronolectins (NBL).66,137 There has also been effort in taking advantage of non-covalent interactions such as hydrophobic, hydrogen bond, and ionic interactions,160 and interactions with metals for the design of carbohydrate receptors.34,82,161 Sporadic efforts have also been reported in using aptamers as way to achieve carbohydrate recognition and sensing.162,163 Recently, the Wang lab has developed a platform method for the selection of DNA aptamers for glycoproteins with the ability to differentiate glycosylation variations.66 This review will discuss progress and future prospects of all three areas along with the current state of using lectins for carbohydrate profiling and analysis. Ways to develop antibodies against carbohydrates, though important, will not be covered in this review for two reasons. First, antibody production is a mature method, which does not face the same kind of challenges and issues as the development of small molecule binders and applications of lectins. Second, though there are many commercially available carbohydrate antibodies, they are not described in the kind of mechanistic details with Kd and selectivity information to allow for careful contrast and analysis.
It should be noted that in this review, we do not strive to be comprehensive. Instead, we focus on describing important concepts and approaches, mostly using examples published in the last 5 years. Almost every week there are new publications in areas covered by this review. In order to finish this review in a timely fashion, we had to have an artificial cutoff point of early 2008. Therefore, we ask the understanding and forgiveness of our colleagues and friends in this field who may feel that insufficient weight might have been given to their publications. At last, we should also note that the labs of Wong,164 Bertozzi,165 Kiessling,166 and others have made very important contributions to the field of using chemical approaches to solving glycobiological problems. However such work is beyond the scope of this review, which is focused primarily on carbohydrate “binders.”
2. BORONIC ACID-BASED SENSORS
Boronic acids have a tendency to react with diols and single hydroxyl groups, and therefore, are the most commonly used moiety for the constructure of binders for carbohydrates, which contain many hydroxyl groups. As a prologue to this section, we would like to first note that thus far essentially all boronic acid-based carbohydrate sensing studies were done with arylboronic acids, which sometimes have water solubility and stability problems. α-Amidoboronic acids form an important class of boronic acids with high stability, high water solubility, and known affinity for diols and hydroxyl groups.167–171 They could potentially be very useful in carbohydrate sensor design. However, there has never been a systematic effort to study their binding with diols in a quantitative fashion. Aimed at achieving some fundamental understanding of amidoboronic acid-diol interactions, the Wang lab synthesized and studied the binding of a model α-amidoboronic acid (Figure 1) with various carbohydrates and other diol-containing.172 As expected, this compound showed good water-solubility, comparable affinity for carbohydrates as arylboronic acids, and significant fluorescent property changes upon carbohydrate binding.
Figure 1.
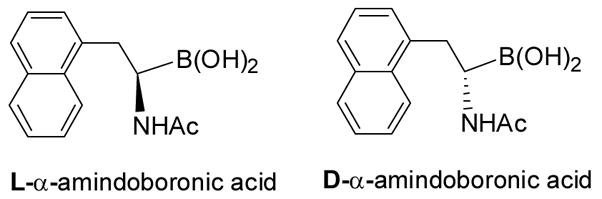
Structures of an enantiomeric pair of α-amidoboronic acids studied for their binding with diols
2-A. Basic chemistry issues
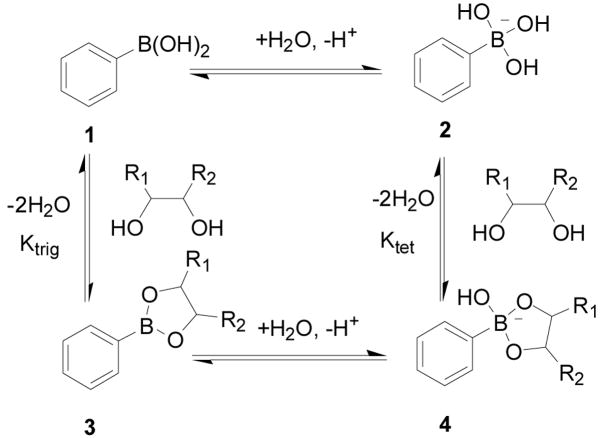
Boronic acids have been known to form tight and reversible complexes with 1,2- and 1,3-substituted Lewis base donors such as hydroxyl, amino and carboxylate groups. The resulting complexes with diols or alcohols are called boronic esters (neutral trigonal boron) or boronate esters (anionic tetrahedral boron) depending on the ionization state of boron atom.3,173–175 Boronic acids are also known to interact with simple Lewis bases such as fluoride176–179 and cyanide ions.180–182 All these properties have made boronic acid an important functional group for sensor development. Indeed, during the last 15 years, there has been a tremendous amount of effort in using the boronic acid group for sensing and recognition of carbohydrates,63,64,183–194 α-hydroxyacids,42,195,196 α-amino alcohols,175,197,198 cyanide,180–182 and fluoride.176–179,199 There have been several excellent reviews on sensor development up to about 2005.33–35,151,156,188 There have also been a review 156 and several in depth studies on the details of the structures of carbohydrate-boronic acid complexes,186,200–203 factors that affect the binding equilibrium,173,174 and issues to consider when designing boronic acid-based carbohydrate sensors.156 There have also been excellent reviews on boronic acid reporters that change fluorescent properties upon binding156,188 and a review on using boronic acid in biological applications with an emphasis on inhibition of hydrolytic enzymes.151 In addition, B-N bond formation, when positioned in a relative 1,5 relationship, occupies a special place in the boronic acid-based sensor field because of the work of Wulff204,205 and Shinkai,184 and has been used as a way to modulate boronic acid fluorescence. The Wang lab has studied in detail how the B-N interactions could change its nature depending on carbohydrate complexation.206,207 Such results are further studied and supported by structural work from the Anslyn lab.208 All such results are detailed in the relevant reviews and extensive research papers and will not be repeated here. Instead, this review will only briefly describe the salient features of the boronic acid-diol complexation reaction, which is directly relevant to carbohydrate recognition, and then focus on new developments in carbohydrate recognitions during the last 2–3 years.
Boronic acid (1) is called an acid because of its boron open shell, which allows for reaction with a protic solvent molecule such as water to form an anionic tetrahedral boronate (2) with the release of one proton (Scheme 1). The deprotonation of the boronic acid hydroxyl group has a higher pKa than the boron open shell reaction with water or alcohol and is actually very hard after anion formation. Therefore, the acidity of a boronic acid is derived from its Lewis acidity per se. In this context, a boronic acid can react with a variety of Lewis bases such as hydroxyl, sulfhydryl, and amino groups as well as fluoride and cyanide. After reacting with a diol, boronic acid is converted to boronic ester (3), which is also an acid much the same way as a boronic acid-it can react with a water molecule, release a proton, and give the boronate ester species (4) (Scheme 1). It is important to note that binding with a diol actually lowers the pKa of the boron atom in most cases.173,174
Scheme 1.
Binding of phenylboronic acid with a diol
Not all boronic acids bind with the diol moiety with the same affinity and not all diols bind to a boronic acid with the same affinity. Understanding the intrinsic preference in binding is very important to the design of boronic acid-based carbohydrate “binders.” Though not always true, boronic acids with low pKa values tend to have high intrinsic affinities.3,156,173,174 For the diol portion, several factors could affect their intrinsic affinities for boronic acids. Low pKa values, small O-C-C-O dihedral angles, and restricted rotations around the C-C bond of the diol moiety all favor binding.174 It is commonly believed that high pH favors boronic acid binding to diols. This is actually not always true. Optimal binding depends on the interplay of the pKa values of the boronic acid and diol, and solution pH.156,174 The optimal pH for binding is often between the pKa values of the boronic acid and diol compounds. With low pKa diols such as catechols, the optimal binding pH could be below physiological pH.156,174 Other factors such as solvent, buffer, and steric hindrance should also be considered when examining the binding between a diol and a boronic acid.156,173,174
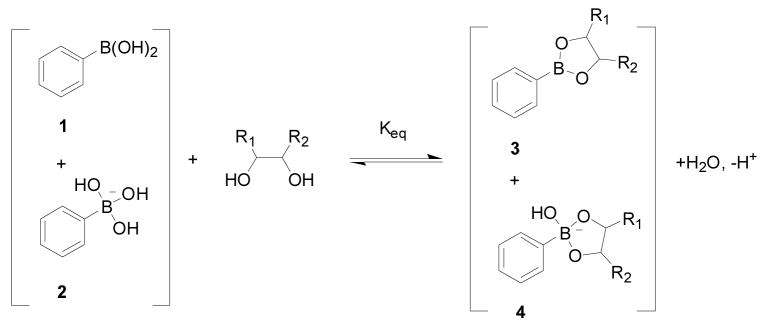
There are two ways of looking at the binding between a boronic acid and a diol. The first way is to look at individual reactions and binding between either the trigonal boronic acid (1, Scheme 1), or the tetrahedral boronate (2, Scheme 1) with a diol. In such a representation, Ktrig represents the binding constant between the trigonal form 1 of boronic acid and the boronic ester 3; Ktet describes the equilibrium between the tetrahedral form 2 of boronic acid and boronate ester 4. The binding equilibrium between phenylboronic acid and a diol can also be presented in Scheme 2, which describes the overall binding equilibrium between boronic acid/boronate 1, 2 and boronic and boronate esters 3, 4.156 Keq is used to describe the overall binding constant (Scheme 2), which of course is an apparent binding constant, but is directly relevant to the equilibrium between a sensor and a carbohydrate. In the literature, binding constants commonly refer to the apparent overall binding constant, Keq, which does not take into consideration of the ionization states of either the complexed or the free form of a boronic acid.173,174
Scheme 2.
Overall binding of phenylboronic acid with a diol
There are several methods for determining binding constants between boronic acids and diols including the pH depression method,3 B-NMR method,209–211 and spectroscopic methods.156,183 The pH depression method is rarely used now because of its requirement for a large amount of sample and the fact it only gives the Ktet values, not the overall binding constants.156,173 The B-NMR method suffers from similar shortcomings as the pH depression method. Furthermore, isotope effects are often not considered in NMR-based methods, which use deuterated solvents. Therefore, spectroscopic methods are normally the preferred choice whenever possible, especially when dealing with boronic acids that change spectroscopic properties upon diol binding.36,155 For those boronic acids that do not change spectroscopic properties upon diol binding and therefore cannot be detected directly by a spectroscopic method, the Wang lab introduced a three-component competition assay by using Alizarin Red S. (ARS) as a fluorescent reporter compound for the sensitive determination of binding constants.156,173,212
From the salient features of the boronic acid-Lewis base (including hydroxyl groups and diols) interactions briefly described above, it is easy to understand why boronic acids are very useful in carbohydrate recognition. However, since monoboronic acids have their intrinsic preference in binding with carbohydrates,3,156,173,174 additional functional interactions are needed if the desired selectivity of the boronic acid-based sensors is different from the intrinsic preference of monoboronic acids, which is essentially all the time. The additional interactions can be boronic acid-based in situations of bis- 63,64,150 or multi-boronic acids213 or the use of other types of interactions such as anionic interactions.214,215 Therefore, in developing boronic acid-based carbohydrates sensors, it is important to have the appropriate scaffold and functional group arrangements. Another important issue is the availability of boronic acid fluorescent reporters, which change fluorescent properties upon binding.36,155 The following section will discuss recent developments in using boronic acids for carbohydrate detection.
2-B. Boronic acid-based fluorescent reporters
Fluorescence-based detection is among the most sensitive methods. Therefore, in carbohydrate recognition and sensing, using fluorescence as a reporting event has been very popular as well.63,64,150,155,178,182–185,206,208,216–240 In designing boronic acid-based fluorescent sensors for carbohydrates, one key requirement is the availability of boronic acids or boronic acid ensembles163,192,195,241–244 that change fluorescent properties upon binding to a diol-containing compound. In the following sections, we describe recent developments in this area in detail.
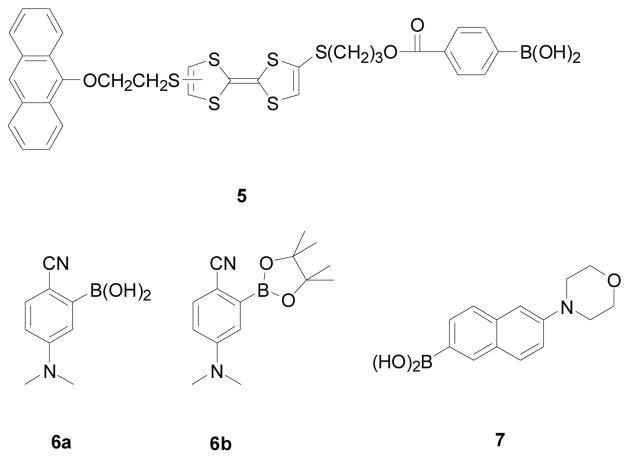
The first class of boronic acid-based fluorescent reporters is the tetrathiafulvaleneanthracenes 5 (Figure 2).195 In this design, the anthracene unit is the fluorophore. It was said that the fluorescence of the anthracene fluorophore can be quenched by the electron-rich tetrathiafulvalene (TTF) through excited state photoelectron transfer (PET). Addition of a sugar such as fructose could increase the fluorescence intensity of the boronic acid reporter by 5-fold at physiological pH in THF/H2O (1:1, v/v). The binding constant of 5 with fructose is 115 M−1. The compound has an excitation wavelength of 370 nm and emission wavelength of 419 nm. The mechanism through which this fluorescent intensity change occurs was proposed to involve the competition of PET between the process involving TTF and boronate and the process involving TTF and the anthracene unit. It was reasoned that binding of the boronic acid moiety results in the conversion of the boronic acid unit to the more acidic boronic ester, which is a stronger acid than the starting boronic acid and therefore should be a better electron “sink” to compete for PET. This increased competition for electron transfer from TTF was thought as the reason for the enhanced fluorescence observed. However, one factor that was not considered was that upon conversion of the boronic to its boronic ester form, it would be converted to the tetrahedral anionic boronate ester because of the increased acidity.36,173,174 Once it is in the anionic tetrahedral form, the boronate ester can no longer accept electrons in a PET process. More studies are needed to elucidate the mechanism through which the fluorescence intensity changes upon sugar binding. In addition, this compound has poor water solubility and requires lengthy synthesis. Such issues could hinder applications in certain situations.
Figure 2.
Structures of boronic acid-based fluorescent reporter compounds 5–7.
Also reported from the same group that developed the tetrathiafulvaleneanthracenes were 4-(N,N-dimethylamine)benzonitrile (DMABN) derivatives with an appended boronic acid (6a) and boronic ester (6b).218 The DMABN boronic acid (6a) showed decreased fluorescent intensity (by over 80%) with the addition of fructose. However, the binding constant is quite high with fructose at 794 M−1.36,173,174 One reason could be because the binding constant was determined in a mixed solvent, THF-H2O (1:1, v/v). Incidentally, the DMABN boronic ester (6b) also exhibits absorption and fluorescence spectral changes upon binding with F−. These two compounds (6a, 6b) have very limited water solubility with most of the binding studies conducted in THF-H2O (1:1, v/v). Their excitation (295 nm) and emission wavelengths (393 nm) were also relatively short, which may limit their applications.
Baker and co-workers developed a water-soluble and high quantum yield (φf 0.453 at pH 7.22) fluorescent carbohydrate reporter (7).245 The fluorescent intensity of this 6-morpholinonaphthalene-2-yl boronic acid 7 decreased by 99% with the addition of 100 mM D-fructose (λex: 300 nm, λem: 420 nm, pH = 7.72). The dissociation constants (KD) for 7 decreased following the order of D-sorbitol (2 × 10−3 M) ≈ D-fructose (3 × 10−3 M) ≫ D-galactose (44 × 10−3 M) > D-glucose (152 × 10−3 M) at pH 7.72.
Lakowicz and co-workers designed, synthesized, and studied spectroscopic properties of boronic acid-based fluorescent sensors (Figure 3), N-(o-, m-, p-boronobenzyl)-6-methoxyquinolinium bromide 8 (o-, m-, p-BMOQBA) for detection of tear glucose concentrations in contact lens polymers.38,246 These compounds showed fluorescent intensity decreases by up to 3 fold upon binding with glucose at 450 nm (λex: 345 nm). These probes have the advantage of having good water solubility and high quantum yields (0.5), which is comparable to that of fluorescein. The mechanism through which fluorescent intensity changes was thought to be due to enhanced electrostatic interactions between the quaternary nitrogen and the boron atom upon sugar binding, which covert the boron atom from its neutral trigonal form to the anionic tetrahedral form.
Figure 3.
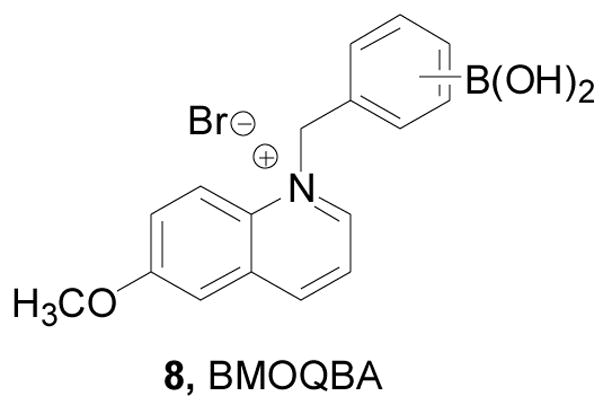
Structure of compound 8
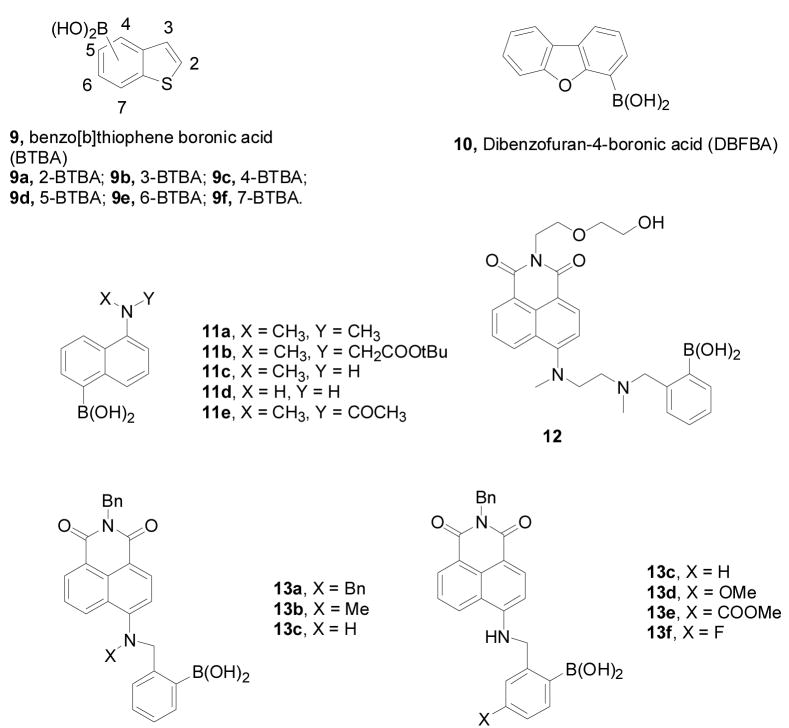
The Wang lab has been working on developing water soluble boronic acid-based fluorescent reporters for a number of years.63,151,218,229,243,247,248 Some of them have been reviewed.36,155 Recent work in the Wang lab includes compounds shown in Figure 4. Among them, six benzo[b]thiophene boronic acid (BTBA) analogs (9a–f) (Figure 4) were reported as fluorescent reporters.249 All six compounds showed significant fluorescent property changes upon sugar binding with good water solubility under physiological condition. Among them, 2-BTBA (9a), 5-BTBA (9d), and 7-BTBA (9f) showed fluorescent property changes at 2 wavelengths (λex: 274 nm, λem: 305 nm and 334 nm). On the other hand, 3-BTBA only showed fluorescent intensity changes at a single wavelength (317 nm). 4-BTBA (9c) and 6-BTBA (9e) were tested as a mixture due to difficulties in separation. This mixture showed a red shift (293 to 303 nm) at one emission wavelength and blue shift (329 to 314 nm) at the other upon sugar binding. Among these reporters, 7-BTBA has the highest binding affinity with sorbitol (Ka = 4561 M−1) and fructose (Ka =1342 M−1). One limitation of this series of reporters is their relatively small fluorescent intensity changes (1 fold) upon sugar binding and the short emission and excitation wavelengths. The fluorescent property changes at two wavelengths on the other hand are an advantage, which allows for ratiometric sensing.
Figure 4.
Structures of boronic acid-based fluorescent reporters 9–13
Another type of water-soluble boronic acid fluorescent reporters is dibenzofuran-4-boronic acid (10, DBFBA, Figure 4) that changes emission intensities at three wavelengths (301, 318, and 327 nm) upon sugar binding under near physiological conditions.250 The apparent binding constant of this reporter with fructose is 514 M−1. One limitation of this fluorescent reporter is its small fluorescent intensity changes upon sugar binding (less than 1 fold) and short excitation wavelength of 286 nm.
The Wang lab also synthesized and evaluated a series of water-soluble fluorescent naphthalene boronic acid-based carbohydrate-reporters (Figure 4).229,248,251,252 In this series, the substitution pattern seems to have a significant influence on the fluorescent properties of the boronic acid. For example, 5-DMANBA (11a) and 5-CMANBA (11b) showed ratiometric fluorescence changes upon saccharide binding. For 5-CMANBA (11b), the excitation wavelength was at 320 nm and the emission wavelength shifted from 490 nm to 440 nm upon sugar binding. 5-MMANBA (11c) and 5-ANBA (11d) on the other hand showed dramatic fluorescence increases (by 66–70 folds) at a single wavelength (438 nm) upon binding with fructose. This was accompanied by a quantum yield increase from 0.056 to 0.72 for 5-MMANBA and from 0.041–0.89 for 5-ANBA upon sugar binding. These compounds also showed color changes upon sugar addition.
The naphthalimide scaffold has been of interest in the boronic acid field because of its relatively long excitation and emission wavelengths. The Heagy lab reported the first boronic acid compound using this scaffold with very interesting discoveries.253,254 The Mohr lab also reported a very interesting 4-amino-1, 8-naphthalimide boronic acid 12, which showed fluorescent property changes upon sugar binding at long wavelength (λex: 410 nm, λem: 530 nm).255 The Wang lab also worked on several long-wavelength boronic acid fluorescence reporter compounds (13a–c) based on the 4-amino-1, 8-naphthalimide structure (Figure 4).256 In this series, the N-substitution seems to have a significant effect on their fluorescent properties. Among these compounds, 13c has the best water solubility and showed fluorescent intensity increases by up to 2 fold upon sugar binding with an emission wavelength of 570 nm (λex = 493 nm). However, the N-methyl and N-benzyl analogs showed poor water solubility that the binding studies had to be conducted in a buffer solution with 50% methanol. Based on the structure of 13c, the Wang lab also studied substituent effects at the para-position of phenylboronic acid moiety. However, surprisingly the different substituents of 13c–f had little effect on either the binding affinities or fluorescent properties.257
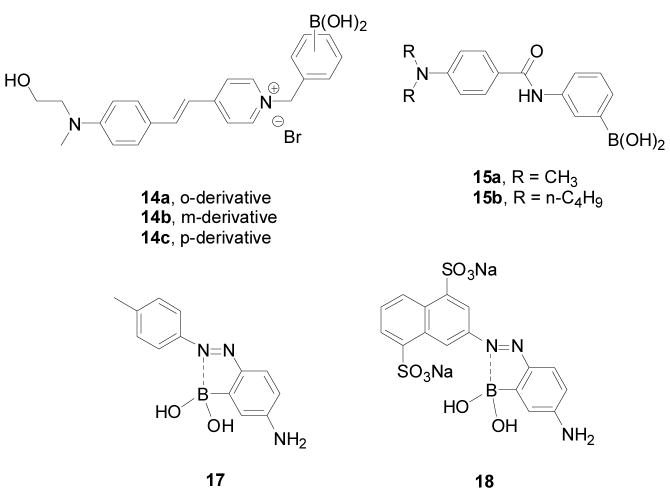
There have also been a number of other fluorescent boronic acid-based carbohydrate reporters, such as 14a–c (Figure 5) using hemicyanine dyes (termed as fluororeactands by the authors) by Mohr and co-workers.258 In such studies, 14a showed the most significant fluorescent intensity increase (by 1 fold) upon binding with fructose at long wavelength (λex = 460 nm, λem = 600 nm). The binding studies were conducted in phosphate buffer solution at pH 7.13. The binding constant between 14a and fructose is 280 M−1, which is larger than that of 14b (40 M−1) and 14c (200 M−1). These boronic acid fluorescent reporters showed good water solubility and significant fluorescent property changes at long wavelength.
Figure 5.
Structures of boronic acid-based reporters 14–18
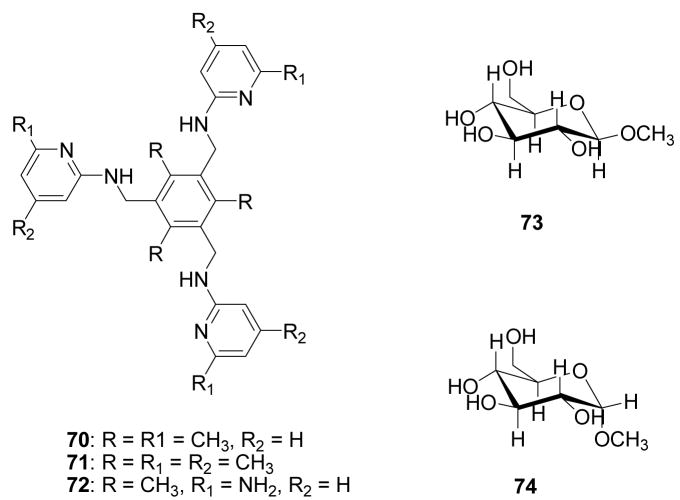
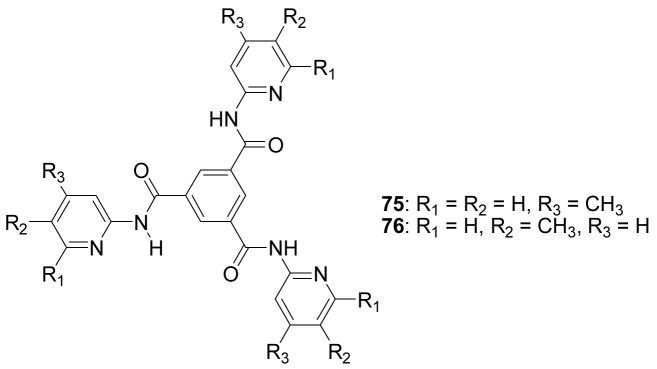
A p-dialkylaminobenzanilide-based boronic acid receptors 15 (Figure 5) was developed by Jiang and co-workers.259 Binding studies were conducted in phosphate buffer-methanol (v/v = 1/1) solution at pH 6.7 (λex = 300 nm). Under such conditions, the fluorescent intensities of both 15a and 15b at around 380–390 nm decreased by 50% upon binding with D-fructose. The binding constants were in the order of D-fructose > D-galactose > D-glucose. The binding constant of reporter 15a with fructose is 1550 M−1 (λem = 392 nm), which much larger than that of 15b (389 M−1, λem = 382 nm).
ortho-Azo substituted phenylboronic acid-based reporters 17–18 (Figure 5) were reported by Egawa and co-workers.260 The UV absorbance of 17 decreased by 70% at 502 nm upon fructose addition in a methanol/water solution (v/v = 1/1) at pH 10.0 and the binding constant was 36 M−1. Reporter 18 had a better water solubility and higher binding affinity for sugars than 17. The UV absorbance of 18 decreased by 80% at 521 nm upon fructose addition in the CHES buffer solution at pH 10.0 and the binding constant was 110 M−1. One concern is that these compounds only exhibited reasonable binding under basic condition. For broad application, it is essential that boronic acids show significant binding under near physiological conditions.
These reporters described should be useful as the basic building blocks for the preparation of fluorescent sensors for sugars. One challenge in this field is the design and synthesis of reporters that have excitation and emission wavelengths beyond 500 nm, low molecular weight, and good stability, and are water soluble and easily functionalizable for the construction of bis- or multi-boronic sensors.
2-C. Small molecule boronic acid sensors for saccharides
Of course, the ultimate goal of developing fluorescent reporter compounds is to use them for the design and synthesis of fluorescent sensors for carbohydrates. In this section, we discuss recent developments in using boronic acids as the key recognition moiety for sensing applications. Again, due to the functional similarity of these boronic acid-based sensors to lectins, we have termed them boronolectins.36
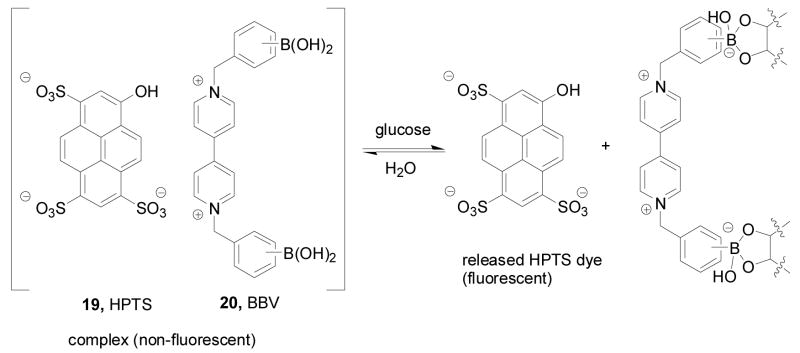
In order to develop a general glucose sensing system in aqueous solution, Singaram and co-workers have used anionic fluorescent dyes in combination with boronic acid-based recognition units.40,42 The viologen bisboronic acid system was selected as a chromophore and a recognition unit. Viologen is a commonly used dye, which is considered electron deficient and redox sensitive. The addition of a substituted viologen boronic acid into a fluorescent anionic dye solution allows the formation of a non-fluorescent complex due to stacking and quenching presumably through charge transfer.42 Upon binding to a sugar, the boronic acid moieties are converted to the corresponding anionic form, which together with the added bulkiness triggers the dissociation of the non-fluorescent complex and causes a significant fluorescent intensity increase upon binding (Scheme 3). The sensitivity can be adjusted by altering the ratio of reporter dye 19 and the bisboronic acid recognition unit 20.
Scheme 3.
Mechanism of sugar sensing by the viologen-boronic acid system
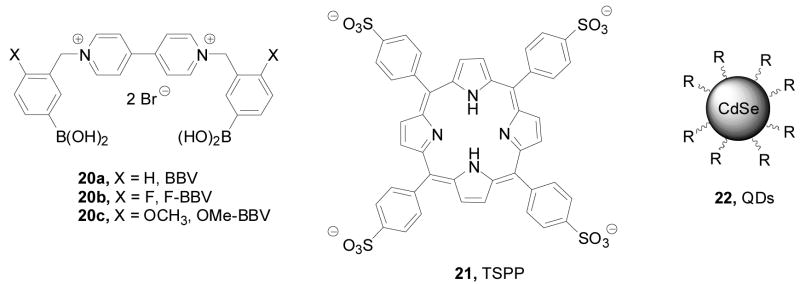
As shown in Figure 6, the Singaram lab prepared several bisboronic acid-substituted viologens 20a–c as optimized fluorescence quenchers. Compare to the other two regioisomers (m- and p-BBV), it was assumed that with ortho-substituted boronic acids (o-BBV) there would be stronger electrostatic interactions between the anionic boronate (after sugar binding) and the positively charged quaternary nitrogen. On the other hand, such interactions would be much weaker with the other regioisomers. It was found that the fluorescent intensity of the complexes between anionic dye 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) trisodium salt (pyranine) and three different boronic acid-substituted benzyl viologens (20a–c) (λex: 460 nm, λem: 510 nm) increased by 9–17% upon addition of glucose.261 The binding constants of these three quenchers with glucose were from 23 M−1 to 37 M−1. When tetrakis(4-sulfophenyl)porphine (TSPP, 21) (λex: 414 nm, λem: 644 nm) was used as the fluorophore, the fluorescent intensity increased by 33% upon sugar addition with the binding constant being 14 M−1 with glucose.40,261 The long wavelength of the TSPP system is also an added advantage, which helps to reduce background interference for applications in biological fluids.
Figure 6.
Structures of compounds 20–22 in the Singaram glucose sensing system
Since fluorescent quantum dots (QDs) have broad absorption, narrow emission, intense brightness, and good photostability, they were used in place of organic dyes for testing the viologen system.61 Two sets of core-shell CdSe QDs (22) coated with ZnS were used (Figure 6). These QDs were decorated with carboxy and amine groups on the surface.61 These QD-viologen ensembles showed fairly narrow fluorescence emission centered at 604 nm (λex = 460 nm) and about 1 fold fluorescent intensity increase upon glucose binding. It was said that concentration dependent fluorescent changes were observed in the range of 2.5 to 20 mM of glucose for QDs (5 × 10−8 M) in their system.
The viologen ensemble systems have the advantage of modular nature with the ability to tune the wavelength by using different fluorophores and being water soluble. It was said that work was on-going in making polymerizable analogs of these dyes for use in glucose sensing. If both components can be immobilized onto hydrogel polymers with similar fluorescent properties as in solution, that would address the issue of multiple components, which could be a limitation for in vivo applications.
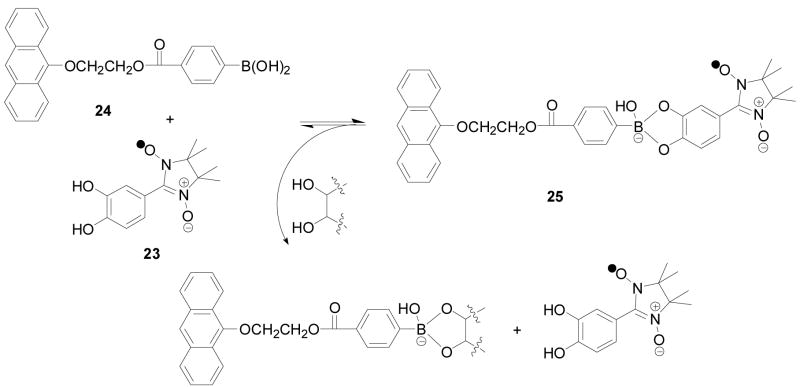
Using a 3-component method similar to that of the alizarin S assay,173,174,212 Zhang and co-workers introduced a nitronyl nitroxide fluorescent quencher 23. In this case, the quencher is the diol component, which can bind to a fluorescent boronic acid 24 and quench its fluorescence through the formation of complex 25 with an apparent association constant of 2410 M−1 (Scheme 4). Addition of a sugar could competitively release the nitronyl nitroxide fluorescent quencher and result in a fluorescent intensity increase.262 In this specific case, an anthracene-based fluorescent boronic acid 24 was used, which has an excitation wavelength of 370 nm and emission wavelength of 419 nm. A maximum of 10 fold fluorescent intensity changes were observed upon sugar addition. In theory, it is possible to use other fluorophores as long as their fluorescence can still be quenched. All studies were conducted in a mixed solvent THF/H2O (1/1, v:v), which indicate possible water solubility problems.
Scheme 4.
Carbohydrate sensing using a 3-component assay with a nitronyl nitroxide quencher
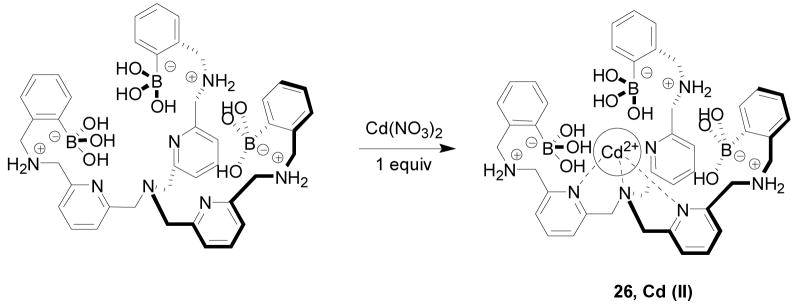
Anslyn and co-workers have synthesized a cadmium-centered tris-boronic acid receptor 26 (Scheme 5) and determined its binding properties toward various carboxyl and phosphorylate sugars using an indicator displacement assay. In this assay, addition of anionic sugars would change the color of the pyrocatechol violet (PV) indicator in the system.263 This receptor showed varying degrees of affinities for different anionic sugars in protic media (methanol/water = 3/1, HEPES buffer 50 mM, pH = 7.4). Among all the sugars tested, gluconic acid showed reasonably good binding with an association constant around 107 M−1.
Scheme 5.
Synthesis of a cadmium-centered tris-boronic acid receptor 26
A bisboronic acid fluorescent sorbitol sensor based on the anthracene fluorophore was synthesized and studied by Yoon and co-workers (Figure 7).264 All fluorescent studies were conducted in 50% MeOH/0.1 M aqueous phosphate buffer at pH 7.4 and by using a 6 μM concentration of compound 27 (λex = 365 nm, λem = 420 nm). The binding constants of chemosensor 27 with sorbitol, xylitol, fructose, galactose and glucose were 1060, 440, 200, 81 and 12 M−1 with fluorescent intensity increases by 2.2, 1.9, 3.9, 2.9 and 2.3 fold, respectively. Such results clearly show that the designed sensor prefers sorbitol, though monoboronic acid also preferentially binds to sorbitol with an over 2-fold selectivity over fructose.173,174,212,264
Figure 7.
Structure of compound 27
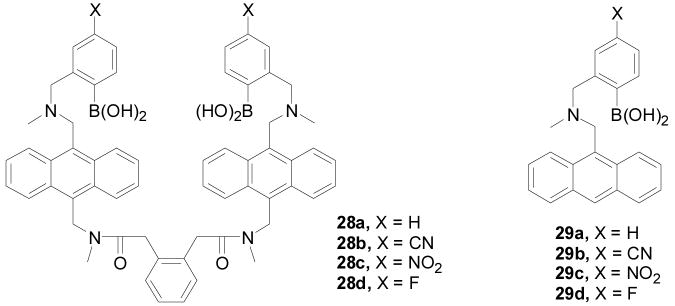
Based on earlier work,63,150 Wang and co-workers synthesized and evaluated additional anthracene-based bisboronic acid sensors for various saccharides.231,265 As shown in Figure 8, a series of new anthracene-based bisboronic fluorescent sensors (28a–d) and three fluorescent monoboronic as controls (29b–c) were synthesized.63,64,156,231 These sensors use the anthracene-based fluorophore (29a) first developed by Shinkai and co-workers.33,266 Compound 28a was published earlier as a selective glucose sensor.150 These new compounds (28b–d) were designed to examine substituent effect on binding and selectivity. Both cyano- (28b) and nitro-substituted (28c) sensors had higher apparent binding constants for glucose (Ka = 2540 and 1808 M−1, respectively) than the un-substituted sensor (28a) (1472 M−1). Although the fluoro-substituted bisfluoroboronic acid (28d) had a lower apparent binding constant (Ka = 630 M−1), it has the best selectivity for glucose under near physiological conditions. Compared to 28a, the selectivity for glucose over fructose did decrease for all the new sensors (28b–c) with the Ka-glucose/Ka-fructose ratio changing from 43 to 3. The binding affinity trend for the bisboronic acids (28a–c) parallels that of the monoboronic acid building blocks (29b–c). Such results suggest that the affinity of the bisboronic acids (29b,c) for glucose was largely correlated with the intrinsic affinity of the boronic acid moiety. Moreover, the introduction of an electron-withdrawing group does not always result in enhanced affinity. Hence, the effect of the electron-withdrawing group on the selectivity and affinity of the bisboronic acid sensors for glucose is hard to predict. In this series, 28b showed the best binding affinity and the highest fluorescent intensity increase (10 fold) upon binding with glucose. Sensor 28a showed the best selectivity of glucose. This series of compounds have an excitation wavelength of 370 nm and emission wavelength of 425 to 430 nm. One issue with these anthracene-based bisboronic acid sensors is their lack of water solubility and the need for organic co-solvent in binding studies. In addition, the fluorescence intensity of the anthracene fluorophore seems to be very sensitive to environmental changes and careful controls have to be done under the same conditions in order for results to be comparable. Temperature, oxygen content, and solvents all seem to significantly affect the fluorescence intensity of these sensors.
Figure 8.
Structures of boronic acid-based sensors 28–29
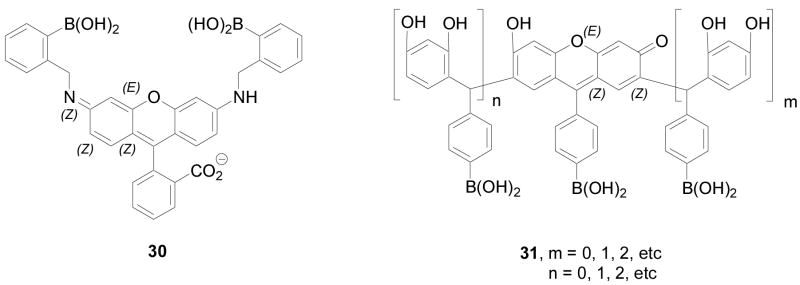
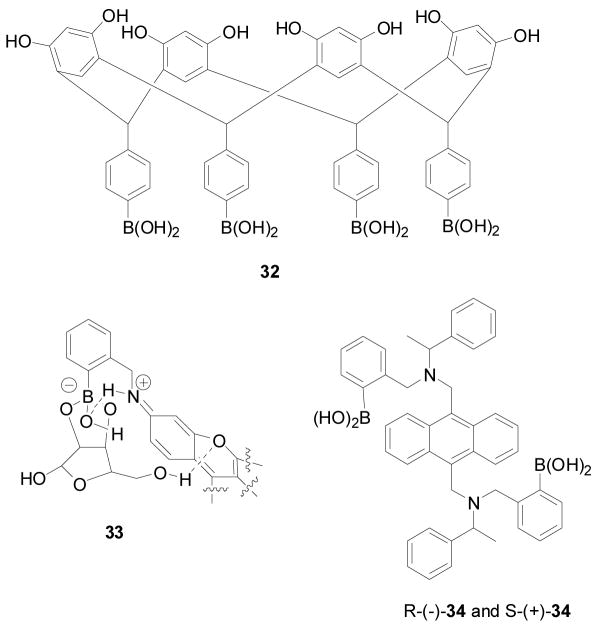
Stereochemical and regiochemical factors play important roles in the selective detection of saccharides with boronic acid-based sensors.44,189,267,268 Several research groups have been working on examining the stereochemical issues in binding. Strongin and co-workers reported several stereoselective and regioselective boronic acid chemosensors (Figure 9 and 10) such as 30, 31 and 32.189 One example is the xanthene dye-based chemosensor 30, which fluoresces at a long wavelength, 597 nm (λex: 565 nm), and showed good selectivity for ribose (Ka = 2400 M−1) comparing to fructose (110 M−1), galactose (310 M−1), and glucose (200 M−1). The fluorescent intensity of 31 increased by 10 fold upon binding with ribose and showed a binding constant of 2400 M−1. Based on stereochemistry studies, the authors proposed that the selectivity of ribose was due to the 2, 3-cis diol structure, and also due to the formation a hydrogen bond between the hydroxyl group of the boronate ester and hydrogen from the amine of 30 (Figure 9, complex 33). Carbohydrates, which can share the same hydroxyl configurations (2, 3-cis diol) as ribofuranose such as allose, psicose, adenosine and nucleotide, may behave similarly as ribose. This is the first example of a boronic acid-based chemosensor for ribose with such significant selectivity. In addition, the solution of 30 (0.07 mM) containing 32 (1.9 mM) was observed to generate micromolar level of chromophore 31 in situ, which gave spectroscopic property changes. Furthermore, sensor 32 (1 × 10−3 M) changed fluorescent intensity by 1 fold upon binding with lactulose and maltulose, comparing to other di- and trisaccharides (1.85 × 10−3 M), such as lactose, maltose, raffinose, and sucrose, which show no fluorescent intensity changes. Their systems possess good sensitivity and selectivity for the target saccharides. Studies were conducted in 9:1 DMSO/phosphate buffer (0.05 M, pH 7.4), which suggests that water solubility may need further improvement.
Figure 9.
Structures of boronic acid-based sensors 30 and 31
Figure 10.
Structures of boronic acid-based sensor 32, complex 33, and sensor 34
The receptor 34 has significant enantioselectivity of sugar alcohols, presumably due to the rigidity of the linker and the crowded binding pockets.268 It also shows very high chemoselectivity for sugar alcohols with six hydroxyl group over those with five or four hydroxyl groups. It can detect monosaccharides at sub-millimolar concentrations. The sensor 34 had enantioselectivity (KR/KS) of 1:20000 in binding with D-mannitol (0.05 M NaCl buffer, 52% CH3OH by weight, pH 8.3). The binding also yielded fluorescence enhancement by 1 fold for the R-sensor (R-34) and 7 fold for the S-sensor (S-34) (λex = 373 nm, λem = 421 nm). Similar to most anthracene-based sensors, water solubility is an issue for biological applications. The same thing is true concerning the chemical and spectroscopic stability of anthracene-based compounds.
During the past decades, the James lab has made very significant contributions towards the boronic acid-based sensing field. They have also been very active in the design and synthesis systems that show stereoselectivity and saccharide selectivity in recognition.45,217,269–271 The most recent one uses a chiral fluorescent BINOL boronic acid.272 Compared with previous ones, this sensor shows improved enantioselectivity as well as chemoselectivity toward sugar alcohols, such as D-sorbitol and D-mannitol. The enantioselectivity of this sensor toward D-sorbitol (K-R/K-S) is 1:35 (pH 9.0), and the chemoselectivity for D-sorbitol/D-mannitol is 20:1.
In conclusion, there has been a great deal of interests in making small molecule boronolectins for various applications. With the rapid development of the glycomics field, we expect to see increased activities in this area.
2-D. Sensors for carbohydrates using oligomeric backbones
One of the most challenging tasks in designing carbohydrate sensors is the construction of the 3-dimensional scaffold with the spacing and orientation of the boronic acid units complementary to that of the diols on a carbohydrate. Taking the de novo design approach is often very hard because of the difficulties in studying saccharide conformations and in designing scaffolds with precise three-dimensional control. Therefore, there have been interests in using peptides or other oligomers as the basic backbone for making boronolectins. These oligomers not only can serve as structural scaffolds, but also provide certain functional groups for complementary interactions. In addition, using oligomers also gives the opportunities for performing combinatorial work. In the following section, we highlight some of the recent developments in this area.
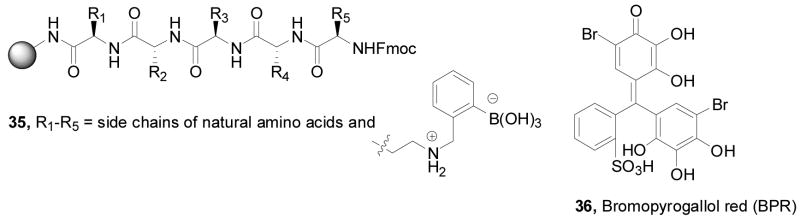
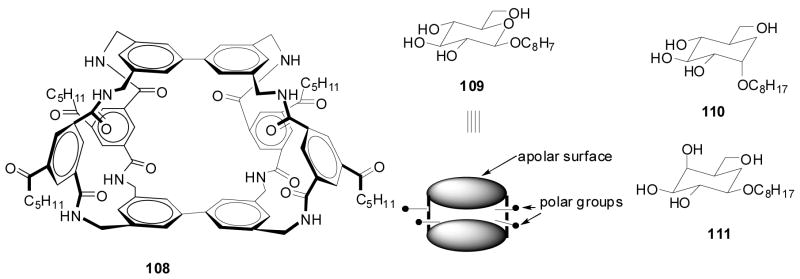
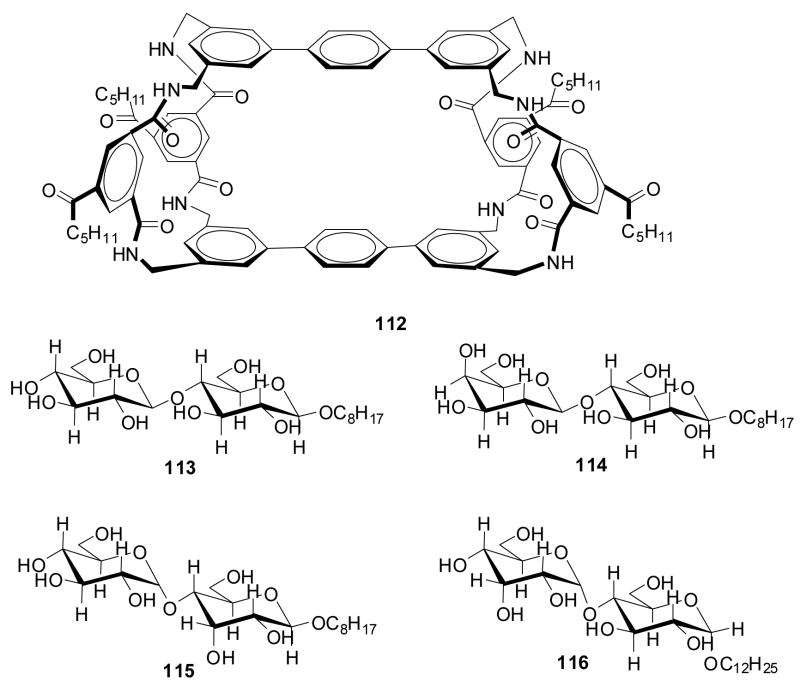
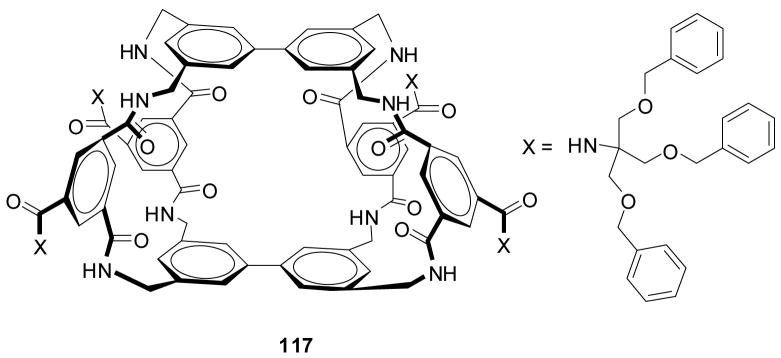
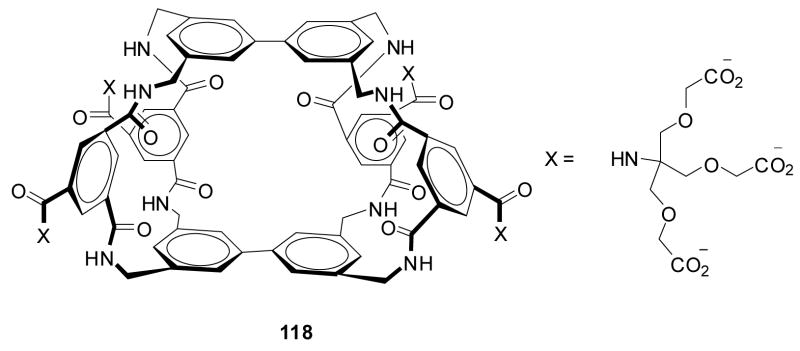
In the first example, Anslyn and co-workers prepared peptide-based boronic acid receptors (35) for pattern-based carbohydrate sensing (Figure 11). In this work, bromopyrogallol red 36 (BPR) was used as an indicator for saccharide binding.159 It should be noted that this type of indicator-based assay ensemble also originated from the Anslyn lab. The binding response signal was recorded by a CCD camera. Linear discriminant analysis (LDA) was used and discrimination between monosaccharides and disaccharide and within various saccharide groups was achieved. This LDA data set could be used for identifying sucralose in a real world beverage sample as well. Their chemosensor assay system has the advantage of good water solubility and high sensitivity. This method was one of the first assays where supramolecular pattern-based sensors were used to identify a specific target in a complex beverage.
Figure 11.
Structures of boronic acid-based sensors 35 and BPR indicator 36
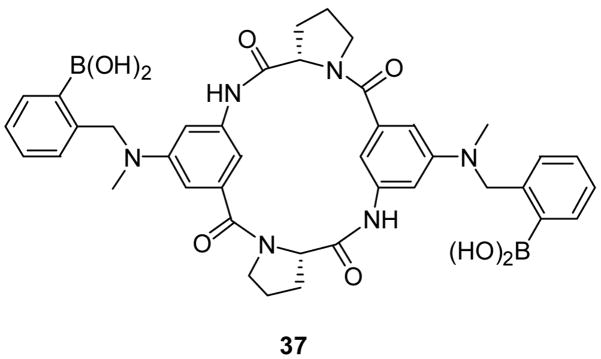
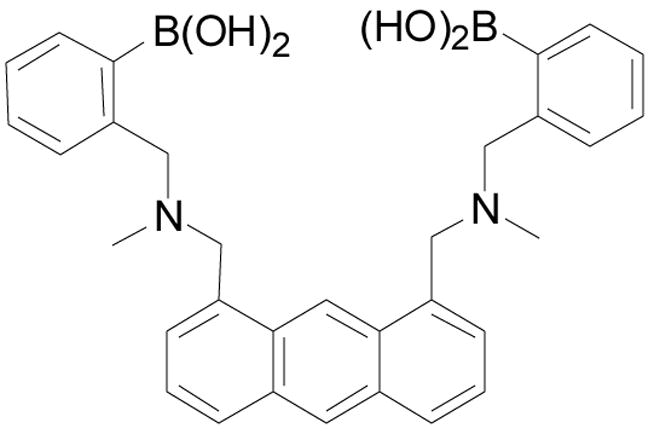
The Kubik lab synthesized and examined the binding affinities of carbohydrate sensor 37 (Figure 12), which contains two boronic acid moieties on the opposite ends of a cyclic tetrapeptide.44 Fluorescent binding studies showed good enantioselectivity of sensor 37 for D-glucose (Ka = 24800 ± 1200 M−1) to L-glucose (Ka = 11900 ± 1600 M−1) in water/methanol (1:1, pH =11.7). From mass spectrometric studies using electrospray ionization (ESI), it seems that this sensor forms significantly more stable 1:1 complexes with D- and L-glucose than with D-galactose, D-mannose, and D-allose. The fluorescent intensity of 37 decreased by half with glucose binding (λex: 285 nm, λem: 480 nm). Though this sensor can sense glucose with high binding affinity, significant fluorescence intensity changes, and good selectivity and enantioselectivity, it does have the disadvantage of being functional only at high pH and requiring an organic solvent for solubilization.
Figure 12.
Structure of boronic acid-based sensor 37
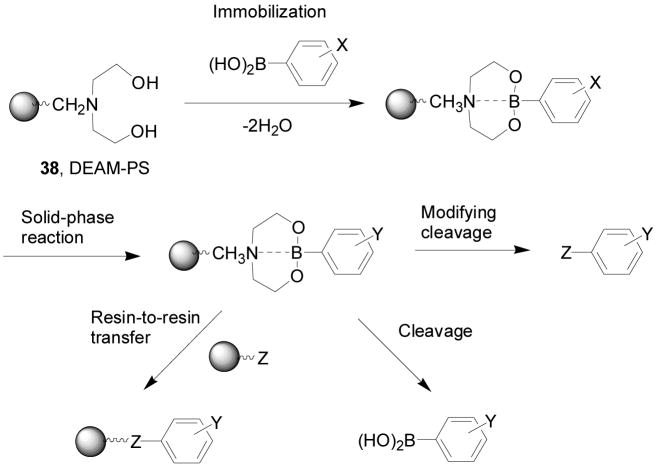
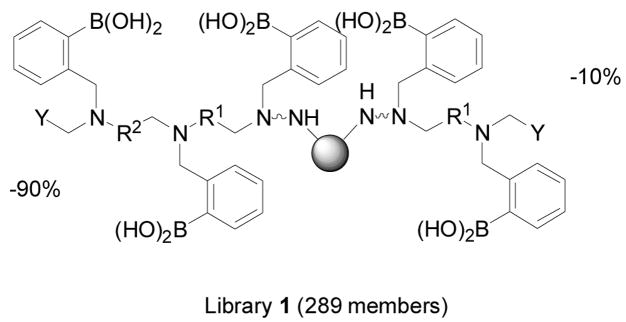
In this section focused on oligomeric boronic acids, it is important to mention the work from the Hall lab on developing a general solid-phase approach to the synthesis and isolation of functionalized boronic acids, which should be very useful in combinatorial library synthesis for boronic acid-based carbohydrate sensors.273 As shown in Scheme 6, N,N-diethanolaminomethyl polystyrene (DEAM-PS, 38) can be used for the immobilization of boronic acids. These DEAM-PS-supported arylboronic acids can be used in resin-to-resin transfer reactions (RRTR) or for further reactions. Along a similar line, the Hall lab has established a prototypic bead-supported split-pool library of triamine-derived triboronic acid receptors (Figure 13).274 Well controlled synthesis, bead decoding by HPLC, and preliminary screening of a combinatorial library of 289 triamine-derived triboronic acid receptors for oligosaccharides were accomplished. The peptide-based boronolectins from single beads were decoded from the library and detected by using electrospray mass spectrometry.
Scheme 6.
Immobilization and derivatization of boronic acids using N, N-diethanolaminomethyl polystyrene (DEAM-PS 38) for combinatorial library synthesis
Figure 13.
Structure frame of a peptide library 1 with 289 members
Other peptide-based boronolectins have also been reported. The Lavigne lab has recently reported their peptide-based boronolectins for glycomics and cancer diagnostics 158. In this study, novel peptide boronolectins (PBLs) were synthesized on beads and used to probe glycoproteins and oligosaccharides with fluorescent labels. A “low” diversity 12-mer PBL library was synthesized on aminomethyl PEG PS resin (100 mm) using a biased split-and-pool combinatorial approach. The theoretical diversity of the PBL library is in the order of 10 million distinct peptide sequences containing a statistical average of 4 boronic acid moieties per peptide. Fluorescent microscope imaging of the beads after mixing with various fluorescently labeled glyco-products allowed for the examination of binding. Though no specific PBL was isolated and examined for binding specificity, binding patterns using microplates allowed the establishment that binding was PBL- and saccharide-dependent (meaning that there was selectivity among the different PBLs in the pool). This approach has the potential for application in pattern recognition and development of diagnostic approaches for multiple glycobiomarkers. Future work was proposed to involve small molecule dye competition assay to make this method amenable for biology tests.
Duggan and co-workers also prepared solid-supported peptide boronic acids derived from 4-borono-L-phenylalanine and studied their affinity for alizarin.157 N-Fmoc-4-pinacolatoborono-l-phenylalanine and standard solid phase peptide synthesis methods were used in the preparation of a library of solid-supported pentapeptide-based bisboronic acids including a ‘lysine series’ and an ‘arginine series’. Twelve diboronic acid sequences that have boronic acid moieties at different positions were obtained at 71–90% yield. Six of them contained two lysine residues for each sequence, while the other six sequences contained two arginine residues for each one. The authors developed a technique for measuring the affinity between a chromophoric diol, alizarin, and the solid-supported peptide–based boronolectins. Binding studies were conducted by measuring absorbance at 507 nm in 50% methanol/50 mM sodium carbonate aqueous solution at pH 10.7. The concentration range of alizarin explored was from 0 to 12 mM. Significant variations in alizarin binding strengths, both within and between arginine and lysine series were observed, with binding constants in the range 200–1100 M−1. The binding affinities of arginine series were 35% stronger than that of the lysine series on average. The binding properties of these peptide-based boronic acids imply their potential application in carbohydrate sensing.
2-E. Other boronic acid-based sugar recognition systems
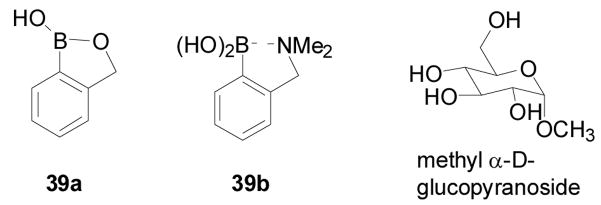
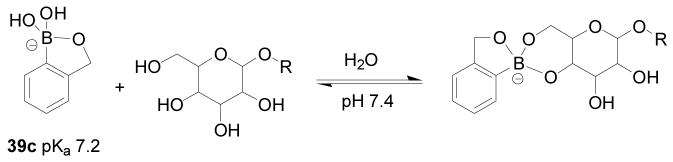
Though boronic acids are known to bind diol-containing compounds, they have different intrinsic preference for diols of different structures.3,173,174 As described earlier, diols with small dihedral angles and low pKa values tend to have a higher intrinsic affinity for boronic acids. Along this line, it is well-recognized that boronic acids bind to diols on aryl and five-membered rings much more tightly than that on six-membered rings. With bisboronic acids, proper design allows for the construction of sensors that can recognize six-membered ring diols with high affinity.208,275 However, with monoboronic acids, the situation is different. Often the binding of a boronic acid with a diol on a six-membered ring is so weak that it is hard to measure a binding constant under near physiological conditions. The only exception is pinanediol,167,276 which is often used as a boronic acid protecting group. Even with bisboronic acid, sometimes the binding would start with the recognition of the six-membered ring form and then slowly rearrange to form the complex with a 5-membered ring diol.150,186,200 The preference by boronic acids for diols on five-membered rings is especially problematic for the recognition of saccharides released from or as part of a glycoprotein/peptide or glycolipid. This is because these glycans tend to have only linear diols and diols on six-membered rings. Because there have been considerable interests in sensors for many types of glycoproteins,62,63,150,277,278 including glycated hemoglobin 160,279,280 and immunoglobulin G,281 there has also been a high level of interest in finding boronic acids that have enhanced ability to recognize diols on a six-membered ring. Along this line, the Hall lab has recently reported an ortho-hydroxymethyl phenylboronic acid (39a, Figure 14),190 which can recognize 1,3-diols on a six-membered ring. The design takes advantage of the known effect of a similarly positioned amino group (39b) reported by Wulff,204 which helps to improve the intrinsic binding affinity of the boronic acid unit. In neutral aqueous media, this boronic acid 39c could complex hexopyranosides primarily using their 4,6-diol, which is presented on most cell-surface glycoconjugates (Scheme 7). The binding constants of 39a with glycopyranosides were obtained by using the ARS UV assay at neutral pH (7.4) in water.173,212 The Ka with methyl α-D-glucopyranoside was 22 M−1, which was slightly lower than the binding constant with glucose. In contrast, the binding constant of phenylboronic acid with glucose is about 5 M−1 at physiological pH.173,212 In addition, the Hall compound also has good water solubility and is structurally simple. One can envision a wide variety of applications of this very special boronic acid.
Figure 14.
Structures of ortho-hydroxymethyl phenylboronic acid 39a and its dialkylamino (Wulff type) analogue 39b as well as methyl α-D-glucopyranoside
Scheme 7.
Binding between ortho-hydroxymethyl phenylboronic acid 39c and glycoconjugates
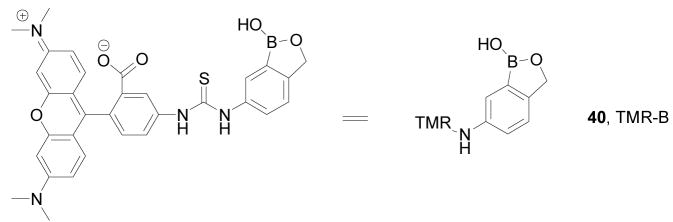
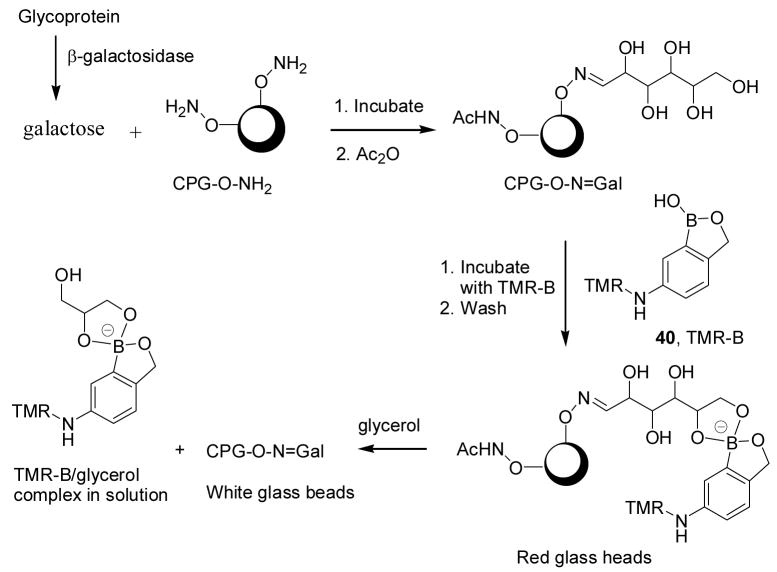
Using the same system (ortho-hydroxymethyl substituted phenylboronic acids) developed by the Hall lab, Hindsgaul and co-workers have developed a method for visual analysis of the terminal glycosylation of glycoproteins.282 The key point of this assay is the application of a boronic acid-based dye reagent, tetramethylrhodamine-boronic acid 40 (TMR-B, λem: 579 nm), as shown in Figure 15 and Scheme 8. In this method, galactose (or other sugars) can be released from a glycoprotein using an enzyme. The released galactose then can be reacted with an immobilized amine. Subsequent reaction with TMR-B through boronic acid-diol complexation then allows for labeling and visualization of the beads based on the presence of the immobilized sugar. The bound TMR-B can also be released by adding a solution of glycerol/MeOH/H2O (1:2:2) to the beads for solution quantitation since the amount of released fluorescent boronate should be proportional to that of immobilized sugar. Using this method, several sugars including galactose (Gal, a hexose), fucose (Fuc, a deoxyhexose), sialic acid (N-acetylneuraminic acid, Neu5Ac), and N-acetylglucosamine (GlcNAc, an aminodeoxyhexose) were analyzed by following the capture/dye-binding/washing sequence for each sugar at 40 μM. The results showed that the relative responses of Gal/Fuc/Neu5Ac/GlcNAc were 1: 0.67: 0.59: 0.36.
Figure 15.
Structure of tetramethylrhodamine-boronic acid (40, TMR-B)
Scheme 8.
A strategy for the visual detection of the terminal glycosylation state of a glycoprotein
There have been many other applications of boronic acid-based sensors for glycoproteins combined with chromatography,283 polymers,284,285 and electrochemistry, which will be discussed in the later sections.279,286,287
2-F. Boronic acid-based polymeric and electrochemical sensors
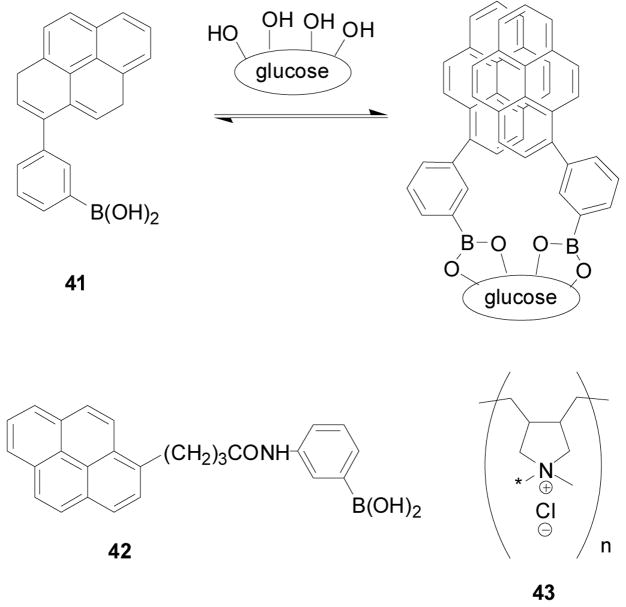
There have been many studies of polymer-based arylboronic acid sensors for saccharides.46 For instance, as shown in Scheme 9, Tao and co-workers invented a new fluorometric glucose detection strategy by utilizing complexation with boronic acid (41,42) on polycation (43). The formation of a 2:1 complex between compound 41 and glucose (Scheme 9) was the general design for this method. In their studies, boronic acid 42 was used for the fluorescent binding evaluation, and polycation 43 was used to enrich 42 along its polymer chain by electrostatic interaction. The fluorescent boronic acid 42 emits at 376 nm as a monomer. After binding with glucose to form a 2:1 complex, two pyrenyl groups from two molecules of 42 along with 43 would stack and generate an excimer. The polymer chain changes its conformation due to a change of the charge state of boron (from the trigonal form to the tetrahedral form) induced by the 2:1 binding with glucose. This conformational change triggers a change of fluorescent intensity, which can be used to monitoring the binding process. Other sugars such as fructose and galactose did not show such a strong tendency to form a 1:2 complex with a boronic acid as did glucose. Therefore this method could be used for sensing glucose selectively. Complexation results in the generation of excimer emission in the range of 430–600 nm (λex: 342 nm).46 The fluorescent studies were conducted in aqueous solution containing 10 mM glucose buffered with 10 mM of NH3 (pH = 10) in the presence of 43 (310 mM). The fluorescent intensity ratio of 482 nm to 376 nm (I482/I376) was increased by 10 fold with glucose binding. On the other hand, the increase of I482/I376 with fructose, galactose and ribose was less than 2 fold. The limit of this system is that it functions only under alkaline conditions, which could be improved by lowering the pKa of the boronic acid through the introduction of electron-withdrawing groups.
Scheme 9.
Arylboronic acid (41, 42)-polymer (43) based sensors for saccharides
Schrader and co-workers developed a fluorescent boronic acid-based polymeric sensor 44 for heparin, a sulfated polysaccharide (Figure 16).216 For this sensor, boronic acid and ammonium ion moieties were used for binding, the dansyl group was used as a fluorophore. The ratio of these three moieties in the sensor was 1.0:2.0:0.3. The fluorescent intensity of polymer 44 decreased by 25% at 510 nm upon heparin addition (30–220 nM) in 25 mM HEPES buffer. Sensor 44 was selective for heparin binding (Ka, 3 × 107 M−1) over other glycans and proteins such as dextran (Ka, 3 × 103 M−1), hyaluronic acid (Ka, 2 × 103 M−1), chondroitin (Ka, 4 × 106 M−1), and ovalbumine (Ka, 1 × 106 M−1). This polymeric sensor had good water-solubility, sensitivity, selectivity, and emitted at long wavelength as well. One limitation for this sensor was the small fluorescent property changes upon sugar binding.
Figure 16.
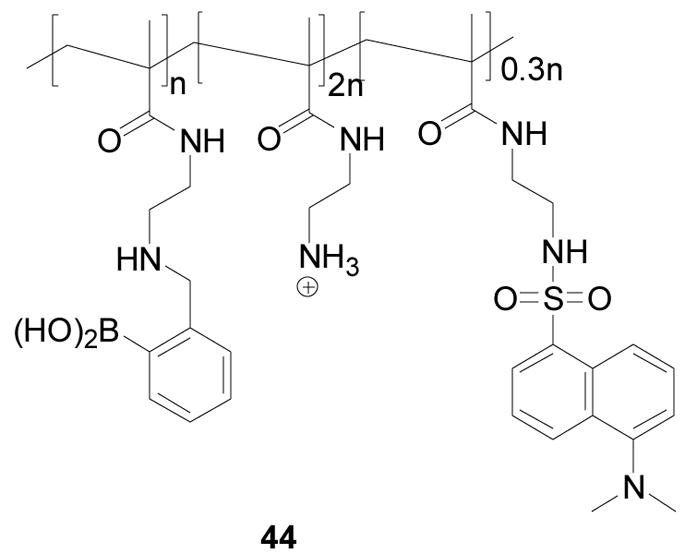
Structure of polymer 44
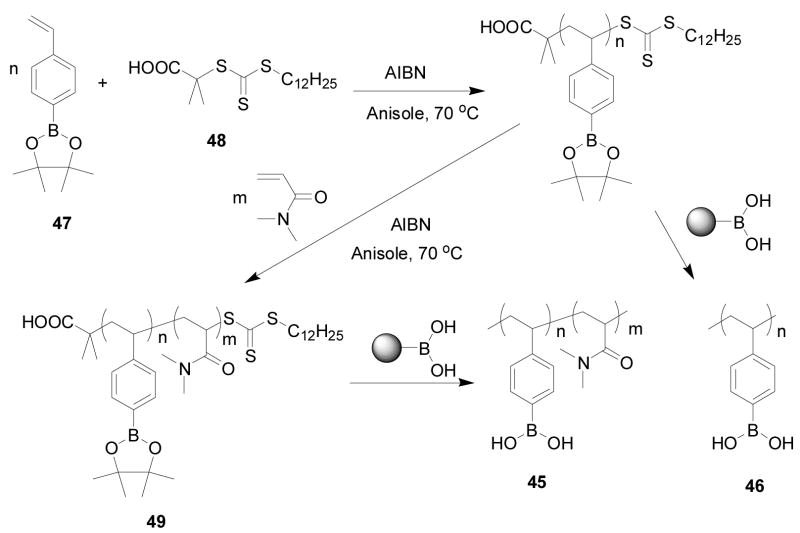
Sumerlin and co-workers prepared a block copolymer (45 or 46) containing boronic acid and acrylamido fragments via atom transfer radical polymerization (ATRP) (Scheme 10). In their studies, 4-pinacolatoborylstyrene (pBSt) (47) was polymerized with 2-dodecylsulfanylthiocarbonylsulfanyl-2-methylpropionic acid (48) as the chain transfer agent (CTA) and 2, 2′-azobisisobutyronitrile (AIBN) as the initiator. Different ratios of monomer/CTA/initiator were studied. The best ratio was determined to be 250/1/0.4, which gave 99% conversion to poly(4-pinacolatoborylstyrene)-β-poly(N,N-dimethylacrylamide) (PpBSt-b-PDMA, 49) with a Mn of 60800 g/mol. This method should be very useful for synthesis of water-soluble boronic acid-containing polymers. Their stated future studies were to focus on the responsive properties of the specific functional boronic acid block copolymers, such as spectroscopic property changes upon binding with sugars.288
Scheme 10.
Formation of a block copolymer (45, 46 and 49) containing boronic acid and acrylamido fragments via atom transfer radical (ATRP) polymerization
There have been a number of studies about supramolecular and polymeric structures, which contain the boronic acid moiety. Such examples include, the synthesis of supramolecular boron complexes with different functional groups and their molecular recognition by Hiratani and co-workers,289 a sugar sensing method based on saccharide-induced conformational changes in copolymers containing boronic acid and a fluorophore by Tao and co-workers,290 a new thermo-sensitive fluorescent strategy by Elmas and coworkers for diol sensing using temperature sensitive copolymer with boronic acid incorporation and alizarin red S as an indicator,291 fluorescent D-glucose sensors based on supramolecular complexes formed between phenylboronic acid modified beta-cyclodextrin and styrylpyridinium dyes (ClSP) by Suzuki and co-workers,292 and using boronate-containing copolymers (BCC) of N-acryloyl-m-aminophenylboronic acid (NAAPBA) with N,N-dimethylacrylamide (DMAA) or N-isopropylacrylamide (NIPAM) for interaction with sugars and yeast cells.293 In addition, a pH dependent equilibrium mechanism of poly(anilineboronic acid)-sugar complex formation in polymer films was examined by Freund and co-workers.294
Since the structure and charge state of the boronic acid moiety could change upon binding with diols, which may induce potential energy changes for the system, there have been boronic acid-based carbohydrate sensors that rely on electrochemical methods for detection. For instance, Yang and co-workers prepared poly(aminophenylboronic acid) (PABA) on gold electrode surface, which showed changes in dielectric characteristics with sugar concentration variations.247 In their studies, electrochemical impedance spectroscopy was used. For the binding studies, 20 mM PBS buffer (pH 7.0) containing 0.1 M NaF was used as the electrolyte solution. Different kinds of saccharides were examined and good linear relationship and high sensitivity (10−9 to 10−2 M) were observed. However, the selectivity was not very high for the system.
Niwa and co-workers used ferrocenylboronic acid and an enzyme-modified electrode for electrochemically recognition of lipopolysaccharides (LPS).295 The gold electrode was modified by a bovine serum albumin membrane with diaphorase. On this electrode, ferrocenylboronic acid derivatives were oxidized and then regenerated by a diaphorase-catalyzed reaction in the presence of NADH. The cycle of consumption and regeneration of ferrocenylboronic acid derivatives induced a current response, which decreased by 225–425 nA/cm2 with the binding of boronic acid and LPS. This current decrease was also amplified by the recycling step. Comparing to LPS, the binding of a monosaccharide such as D-mannose or D-galactose caused no response at the same concentration (1 μg/mL). Furthermore, this electrode showed a rapid response time for LPS. It is faster than the currently used method. The detection limit of LPS from E. coli O127:B38 was as low as 50 ng/mL.
Anzai and co-workers reported a polyphenylboronic acid-modified gold electrode for carbohydrate detection.296 The surface of the gold electrode was coated with a thin film containing poly(allylamine) with the phenylboronic acid (PBA) moiety. The modified gold electrode exhibited a concentration-dependent current decrease with added sugars in the presence of [Fe(CN)6]3− ion at neutral pH. The detection range of the electrode was 1–100 mM for glucose and 0.1–10 mM for fructose.
2-G. Other arylboronic acid-based sensors
There have been quite a few other arylboronic acid-based carbohydrate sensors. For example, Koh and co-workers designed and synthesized phenylboronic acid-based self-assembled monolayers (SAMs) on Au surface for monosaccharide sensing (Figure 17).297 They also characterized the surface properties of the SAMs by atomic force microscopy (AFM). Sugar binding was examined by using surface plasmon resonance (SPR) spectroscopy with good sensitivity (SPR angle shift was 0.133°) at very low concentration of saccharide (1.0 × 10−12 M to 1.0 × 10−4 M). For this study, LED was used as a light source to generate light signal at a maximal wavelength of 650 nm. When the alkyl spacer length of the phenylboronic acid derivatives was at n = 3, the SPR angle shift derived from binding between phenylboronic acid and monosaccharide was amplified by 107 times because of the optimal order of the monolayer structure. However, the selectivity of this system is the same as other monoboronic acids in their binding with various diols. Some other boronic acid-based sensors involving self-assembly have also been reported.287,298–303
Figure 17.
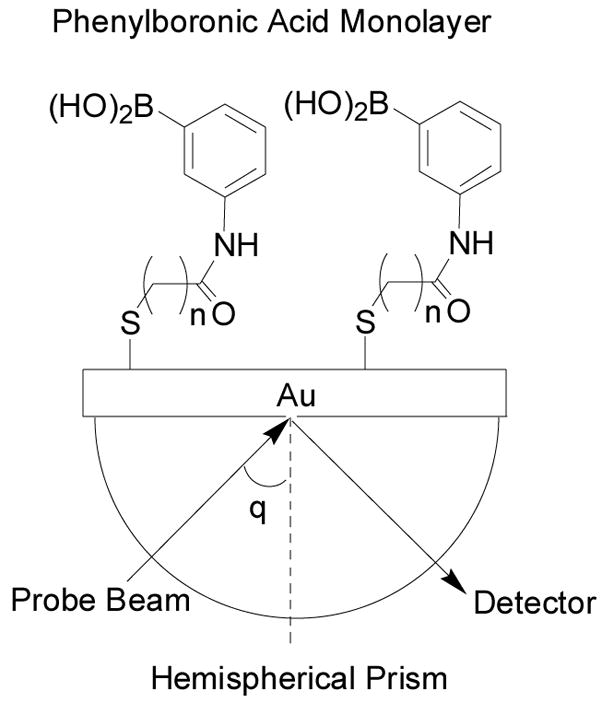
Structures of boronic acid-based sensors on SAMs
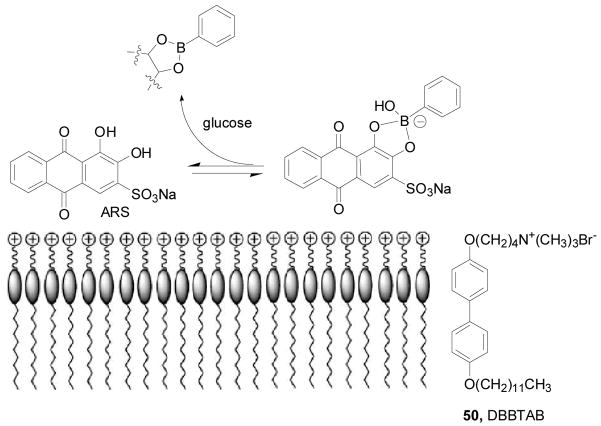
Li and co-workers reported a glucose-responsive vesicular sensor based on boronic acid–glucose recognition in co-vesicles (Scheme 11) 237. The amphiphile, 4-(4-dodecyloxybiphenyl-4-yloxy) butyl trimethylammonium bromide 50 (DBBTAB) is a quaternary ammonium salt, which can form vesicles at low concentrations in aqueous media 237. DBBTAB vesicles have positively charged surface, which attracts negatively charged alizarin red S (ARS) to form co-vesicles. Since ARS has been used as a fluorescent reporter for binding between phenylboronic acid (PBA) and sugar,173,212 the authors prepared a vesicular fluorescent sensor based on phenylboronic acid (PBA)–glucose recognition in the ARS/PBA/DBBTAB co-vesicles. Fluorescence studies of the ARS/PBA/DBBTAB vesicles were conducted in an aqueous phosphate buffer at pH 6.8 ([ARS] = 5 × 10−5 M; λex = 468 nm, λem = 560 nm). Fluorescent intensity increased by 200 fold with the addition of PBA (0–5 × 10−4 M), and then the intensity decreased by 10% with the addition of glucose (0–43 × 10−3 M). Compared with the aqueous PBA/ARS solution, the vesicular sensor system showed increased sensitivity to glucose by 7 to 8 fold. The selectivity of this system was similar to the PBA/ARS system. Although their system worked well in aqueous solution, the small fluorescent intensity change (a 10% decrease) observed with the addition of glucose might become an issue in real life applications.
Scheme 11.
A fluorescent glucose sensing system based on ARS/PBA/DBBTAB co-vesicles
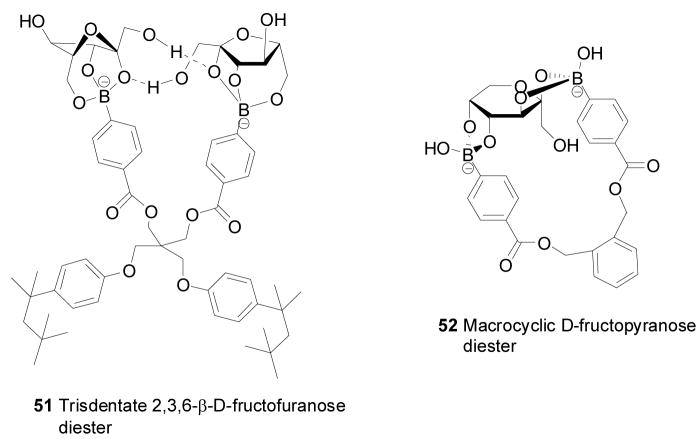
Duggan and co-workers developed fructose-permeable liquid membranes containing boronic acid carriers.304–306 In such a design, the boronic acid contained within the hydrophobic liquid membrane could bind with saccharide at the interface to generate a boronate ester. Then the boronate ester could diffuse through the membrane and hydrolyze back to the boronic acid and release the saccharide to the receiving phase. The transport of fructose and glucose through supported liquid membrane (SLM) promoted by several tetrahedron-shaped lipophilic monoboronic acids and diboronic acids was examined. The selectivity was measured by the ratio of sugar fluxes between fructose and glucose. The diboronic acid gave high fructose/glucose selectivity (7.7:1.0). As shown in Figure 18, their diboronic acid carriers could bind with multiple equivalents of D-fructose to form two types of diesters (51 and 52) in the membrane. The method of transporting D-fructose by 51 showed better selectivity for D-fructose over glucose than did 52. It was proposed that the selectivity was due to the formation of a tridentate 2, 3, 6-β-D-fructofuranose diester 51 and a macrocyclic D-fructopyranose diester 52 within the membrane. This suggestion was supported by computer molecular modeling studies and 13C NMR experiments with labeled 13C-D-fructose.306
Figure 18.
Structures of a tridentate β-D-fructofuranose ester 51 and a macrocyclic β-D-fructopyranose diester 52
Duggan and co-workers also studied the transport of four sialic acid derivatives through lipophilic supported liquid membranes.307 Aliquat 336, a lipophilic quaternary ammonium chloride salt, was added into the membrane to promote the transporting of the sialic acid derivatives. The flux of sialic acid derivatives in the membrane containing Aliquat 336 alone was higher than those in the other two membrane systems (boronic acid alone or boronic acid in combination with Aliquat 336). A mobile-site jumping mechanism was proposed to explain the flux improvement by lipophilic ion pairs. It was proposed that the triol side chain and the amide functional group of sialic acid derivatives form a chloride receptor. The sugar-chloride complex hops from cation to cation in the membrane and then release into the receiving phase. The reason for the impaired transport when a boronic acid is present could be due to the abolishment of the chloride binding ability of the triol side upon boronate formation. It was also interesting to note the very different effect Aliquat 336 has on sialic acid derivatives and D-fructose. For the transport of D-fructose, the flux through ammonium boronate membrane is detectable, while no transport was detected with the membrane containing either boronic acid or ammonium chloride alone. The studies of logP values suggested that D-fructose was more hydrophilic than the four sialic acid derivatives, which explains why only the transport of D-fructose through a lipophilic membrane is favored by the mixture of a lipophilic boronic acid and a lipophilic ammonium salt.
There are other efforts in boronic acid-based saccharide sensing. For examples, Ishihara and co-workers examined the binding mechanism of boronic acid and diols 308. Their studies suggested that boronate ion was not as reactive as boronic acid in an alkaline solution. This was further supported by kinetic evidence such as the estimated upper limit of the rate constants for the reactions of some boronic acids with 2,2′-biphenol and 2,3-dihydroxynaphthalene in a neutral to alkaline (pH 7–14) solution. Tao and co-workers evaluated glucose sensing with boronic acid using enzymatic oxidation.47 In their studies, the enzyme glucose oxidase (GOx) catalyzed the conversion of glucose into gluconic acid, which was able to complex with a fluorescent boronic acid through the hydroxycarboxylate moiety in aqueous media. This method could overcome the weak binding issue between glucose and boronic acid because of the relatively strong interactions between gluconic acid and boronic acid. Kondo and co-workers studied diol adsorption on phenylboronic acid modified silica gel.309 Potentiometric titration was used to determine the pKa of the active site in boronic acid modified gels. Araki and coworkers examined sugar detection by phenylboronic acids in a capillary electrophoresis-chemiluminescence system.310 They examined the enhancing effects of several phenylboronic acid compounds for luminol-hydrogen peroxide-horseradish peroxidase reaction in the system. Compared to other boronic acids, only 4-biphenylboronic acid and p-iodophenylboronic acid showed an enhancing effect over the range of 0.5–10 μM in this system. Suslick and co-workers developed a series of metal loporphyrin appended boronic acid for sugar sensors.311 They synthesized two boronic-acid-appended zinc-loporphyrins as potential colorimetric sensors for carbohydrates. For the synthesis of these two sensors, boronic acid groups were connected either to a β-pyrrolic position or to the meso-position of the porphyrin core. However, no spectroscopic property changes were observed with the addition of sugars.
2-H. Applications of boronic acid in sensing of non-sugar compounds
Since boronic acids can bind with compounds that have two adjacent nucleophilic groups, it can not only be used for recognition of carbohydrates, but also for many other types of compounds such as diamine 312, fluoride 178,313,314, dopamine 315–322, L-DOPA,253 nucleosides,323–325 and amino acids.326 Boronic acids can also be used in drug delivery,327 and as building blocks for synthesis.328 However, such applications are not the focus of this review and will not be discussed in detail.
As a summary to this boronic-acid-based sensor, Table II lists the boronic acid-based sensors described, which are specifically for detection of certain carbohydrates.
Table II.
A summary of boronic acid-based sensors
| Compound No. | Sensor Target | Binding Constants (Ka) | Study conditions |
|---|---|---|---|
| 19, 20a–c | Glucose | 23 M−1 to 37 M−1 | λex: 460 nm, λem: 510 nm, in phosphate buffer, pH = 7.4 |
| 21, 20a–c | Glucose | 14 M−1 | λex: 414 nm, λem: 644 nm, in phosphate buffer, pH = 7.4 |
| 22, 20a–c | Glucose | around 100 M−1 | λex: 460 nm, λem: 604 nm, in phosphate buffer, pH = 7.4 |
| 26 | gluconic acid | 107 M−1 | UV studies in 75% methanol/HEPES buffer, pH = 7.4 |
| 27 | sorbitol | 1060 M−1 (5 fold over fructose) | λex = 365 nm, λem = 420 nm, in50% MeOH/aqueous phosphate buffer, pH = 7.4 |
| 28a | glucose | 1472 M−1 (43 fold over fructose) | λex = 370 nm, λem = 430 nm, in aqueous phosphate buffer, pH = 7.4 |
| 30 | ribose | 2400 M−1 (24 fold over fructose, 8 fold over galactose, and 12 fold over glucose) | λex = 565 nm, λem = 597 nm, in90% DMSO/phosphate buffer, pH = 7.4 |
| 34 | D-mannitol | enantioselectivity (KR/KS) of 1:20000 in binding with D-mannitol | λex = 373 nm, λem = 421 nm, in52% CH3OH/aqueous buffer, pH = 8.3. |
| 37 | D-glucose | D-glucose (24800 ± 1200 M 1) to L-glucose (11900 ± 1600 M 1) | λex = 285 nm, λem = 480 nm, in50% CH3OH/water, pH =11.7 |
| 44 | heparin | Heparin (3 × 107 M−1) over dextran (3 × 103 M−1), hyaluronic acid (2 × 103 M−1), chondroitin (4 × 106 M−1), and ovalbumine (1 × 106 M−1). | λem = 510 nm, in HEPES buffer |
3. OTHER CARBOHYDRATE SENSORS
In addition to boronic acid-based approaches, many other methods have been used for the development of “receptors/binders” for carbohydrates. These include (1) aptamer approaches, (2) non-covalent approaches, and (3) metal chelation approaches. In the following sections, we discuss discoveries in those areas in detail. Since discoveries in these three areas have not been reviewed as extensively as the boronic acid field, the literature covered may go back to earlier times than the coverage of the boronic acid field.
3-A. Aptamer-based carbohydrate sensors
The term “aptamer” is often used to refer to short DNA or RNA sequences that have been selected to bind a chosen target,162,329,330 With the powerful selection methods first developed in the early 1990s, aptamers to small molecules, proteins and nucleic acid structures can be selected with high affinity and specificity. Naturally, the same methods have also been used for the selection of aptamers that bind carbohydrates. Till now, aptamers to carbohydrates were selected for two purposes: (1) as purification tags for RNA or ribonucleoparticles from RNA mixtures74,331,332 and (2) to identify and/or block specific sugars on cell surfaces.333 Herein we mainly focus on the application of aptamers for cell surface carbohydrate recognition and inhibition.
Yu and co-workers first developed RNA aptamers for sialyl Lewis X (sLex, 53, Figure 19) by using in vitro RNA selection.74 sLex is a tetrasaccharide glycan on cell surface and is a mediator of T-cell actions. It sometimes serves as a cancer biomarker.120,139,334,335 During inflammation, the interactions between sLex and selectins on the surface of vascular endothelial cells play a critical role in cell adhesion.120,334 In addition, aberrant expression of sLex has been implicated in cancer metastasis.139,335 Therefore, “receptors” that bind sLex could be effective anti-inflammatory and/or anti-metastasis agents. These were the first isolated RNA aptamers for sLex with high affinity and high specificity. The Kd values were around 10−9 to 10−11 M. These aptamers also showed similar or better binding affinities comparing to that of commercially available antibodies. In addition, the selected RNA aptamers also showed inhibition of adhesion of sLex-expressing HL60 cells to the E- and P-selectins, which suggests the potential for these aptamers to be used as cell adhesion inhibitors for anti-inflammatory therapy. Although it was said that the isolated RNA aptamers could discriminate minor differences in carbohydrates and the binding affinity to sLex is 100 times higher than that of lactose, these aptamers were only able to bind sLex with 5–10 fold higher affinity than with other similar Lewis group sugars.
Figure 19.
sLex structure
In 2006, Paulino and co-workers developed an aptamer-based assay for the detection of neomycin B (54, Figure 20),332 which is an aminoglycoside antibiotic that selectively targets RNA structural motifs. Although several methods including electrophoresis, derivative fluorimetric and pulsed amperometric detection had been reported,336–339 neomycin B detection was still a challenging problem because these reported methods were time consuming and neomycin lacked the needed spectroscopic and electrochemical properties. Therefore, a competitive impedimetric assay for the detection of neomycin B was conducted by using a fully 2′-O-methylated RNA aptamer sensor, which was developed based on previous RNA motifs.340,341 The O-methylation helped to increase the stability of the aptamer against degrading enzymes. In this approach, neomycin B was first immobilized on a self-assembled monolayer of mercaptopropionic acid (MPA) (Scheme 12). Then aptamer was bound to surface linked neomycin B. Neomycin B in solution can competitively displace the bound aptamer and the dissociation was monitored by using faradaic impedance spectroscopy (FIS). With this method, a 1:1 neomycin-aptamer complex was observed under near physiological conditions. High specific affinity was also found by comparing with binding of paromomycin, which differs from neomycin B in the substitution of a single NH2 group by an OH group at C6. Such results confirmed the previous report that the 100-fold lower binding affinity of paromomycin relative to neomycin341 was not affected by the use of 2′-O-Me bases. Neomycin-enriched whole milk was also analyzed to confirm the compatibility of the device with biological fluids. The study showed a linear range between 25 and 2.5 × 103 μM in milk.
Figure 20.
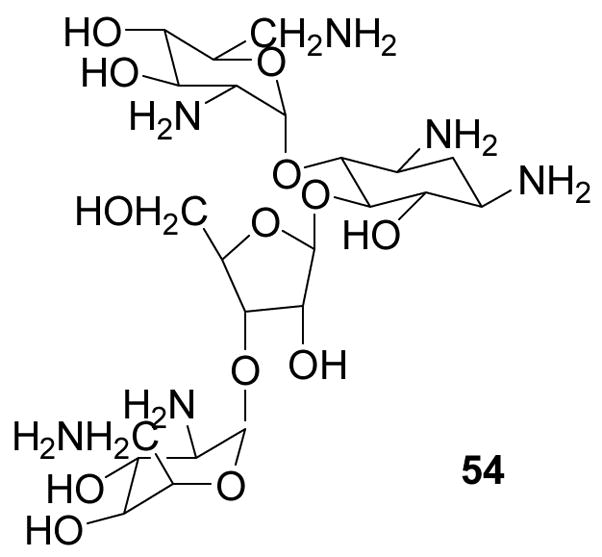
Structure of neomycin B
Scheme 12.
Schematic illustration of the modified electrode and competitive assay
Sialyllactose (α-Neu5Ac-[2–3]- and [2,6]-β-D-Gal-[1–4]-D-Glc) is a ubiquitous saccharide component of mammalian plasma-cell membrane.342,343 It is an important recognition moiety for many animal viruses such as influenza A and C viruses, cardioviruses and Newcastle disease viruses. Thus, blocking the binding of the sialyllactose and animal viruses on cell surface could be a route to the development of anti-viral agent. Sawai and co-workers produced a novel cationic-charged modified DNA aptamer by using modified thymidine residues.344 The idea is to use the cationic protonated amino group at the C5 position to help enhancing the binding affinity with the anionic carboxyl group of sialyllactose. By using the SELEX method, twenty two clones were selected after 13 rounds of selection. The Kd for the aptamer with the highest affinity was 4.9 μM. The author proposed a three-way junction structure for the aptamer with a stem region containing the modified thymidine. The modified aptamer showed a stronger affinity to sialyllactose than the unmodified counterpart, suggesting that the modified thymidine bearing a positively-charged amino group at the C5 position is an important contributing factor to the high binding affinity.
Another example is the modified RNA aptamer, which binds moenomycin A (55, Figure 21). Moenomycin A is an amphiphilic phosphoglycolipid antibiotic and kills bacteria by interfering with the transglycosylation process in peptidoglycan biosynthesis.345,346 It has two building blocks, a branched pentasaccharide moiety and the non-polar lipid part. Hahn and co-workers described 2′-aminopyrimidine RNA aptamers which can specifically bind to the non-polar lipid part of moenomycin A.331,347 The introduction of the 2′-amino group was to increase resistance of the aptamer to enzymatic degradation. All isolated aptamers were shown to be G-rich, indicating a hydrophobic binding site, which might account for the highly specific binding to the non-polar part instead of the saccharide moiety. π-Stacking interactions between the nucleobases and unsaturated side chain of the moenomycin analogs, and “sandwich” formation between hexad (A (GGGG) A) platforms and the lipid moiety were assumed to play a role in stabilizing these complexes. The Kd values of the two aptamers reported in this article were determined to be 350 and 320 nM respectively. Another fact that is remarkable in this study is that the aptamers selectively bind to an unsaturated lipid chain of moenomycin in contrast to the farnesylated Ras aptamer binding with the saturated part chain.348 The authors suggested that the difference might have originated from the different configuration of the isoprenoid moieties.
Figure 21.
Structure of moenomycin A
One challenging area of carbohydrate recognition is the differentiation of glycosylation patterns of a glycoprotein. Conceivably, aptamers can be evolved to recognize glycoproteins. However, conventional aptamer selection approaches do not have the intrinsic ability to do substructure-focused selection, i.e. the selection of aptamers that specifically focus on the recognition of the glycosylation site(s). The Wang lab worked on using boronic acid-modified DNA for the selection of aptamers for the specific recognition of the glycosylation site(s) of a glycoprotein.137,349 As discussed in the previous section, boronic acid is known to high intrinsic high affinity for diols and hydroxyl groups. Therefore, it was reasoned that the incorporation of a boronic acid moiety should allow the aptamer selection process to gravitate toward the recognition of the glycosylation site(s) of a glycoprotein.
The boronic acid moiety was introduced to the 5-position of thymidine.137 Therefore, a modified TTP nucleotide containing a boronic acid functional group (BTTP, 56, Figure 23) was synthesized using copper (I)-catalyzed Huisgen cycloaddition reaction/click chemistry.350–360 This boronic acid labeled nucleoside triphosphate can be incorporated into DNA with a similar efficiency as the natural TTP.137
Figure 23.
Structures of compounds 57, 58a, and 58b
DNA libraries with BTTP (56, Figure 22) were then used for the selection. Fibrinogen was used as a model glycoprotein because of its known association with the risk of major cardiovascular diseases and nonvascular mortality and the critical role of its carbohydrate moiety in fibrinogen function.31,361 The SELEX approach was used for the aptamer selection. Aptamers were selected with Kd in the range of low to mid nM.349 More importantly, removal of the boronic acid, the glycan moiety or both resulted in significantly decreased affinities. Furthermore, sugar and boronic acid can competitively inhibit aptamer binding to fibrinogen. In contrast, aptamers selected without using boronic acid-modified TTP did not bind to the glycosylation sites. All such results suggest that indeed boronic acid modified DNA aptamers can be selected to specifically recognize the glycosylation site(s) of a glycoprotein. The same method can be used for the selection of boronic acid modified DNA based aptamers for other glycoproteins. This represents the very first example that aptamers can be selected to specifically focus on the glycan substructure in a glycoprotein. We refer to these aptamers as nucleic acid-based boronolectins.36
Figure 22.
The chemical structures of BTTP and MTTP
Anslyn and co-workers invented a method of using aptamers to fine-tune the selectivity of synthetic small molecule receptors. Specifically, the Anslyn lab selected an aptamer that could bind to the complex between bis-boronic acid receptor 57a (Figure 23) and tartrate.163 First, organic receptor 57163,362 was immobilized on glyoxal agarose beads through reductive amination. Then the immobilized receptor 58a (Figure 23) in complexation with tartrate was used for the selection and control compound 58b, which was incapable of binding tartrate, was used for counter selection. The progress of the selection was monitored by incorporating a radiolabel into the RNA pool. After 13 round of selection, no further improvement in binding was detected. The selected aptamers were then cloned, sequenced, and used for binding studies. Several interesting observations were made. First, aptamer binding appeared to be dependent on methanol and the optimal methanol concentration for binding was around 20%. This was one of the first examples of aptamer selection that has been carried out in high concentrations of an organic solvent. Second, on its own, 57 was only slightly selective for citrate (1.3, Kd for tartrate = 7.1 × 10−6 M, Kd for citrate = 5.5 × 10−6). However, in the presence of aptamer, the specificity of the receptor was dramatically altered (14 for tartrate, Kd = 2.1 × 10−4 and Kd < 3 × 10−3 for citrate in 20% MeOH). The explanation is that aptamer might form a pocket more precisely accommodate the receptor-tartrate complex, while exclude citrate via steric interactions or charge repulsion. Third, lower affinity of 57 for tartrate in the context of the aptamer was also found. The author explained that at least part of the tartrate-binding energy is used to generate an induced-fit conformation. In summary, by generating an aptamer-receptor complex for the recognition, the author successfully selected an aptamer for a small organic ligand. This work shows a novel route to the development of biosensors for small organic analytes and can serve as a model for future applications to improve the specificity of synthetic receptors.
3-B. Non-covalent sensors
Besides the aptamer approaches, non-covalent sensors is yet another approach for the development of “receptors” for carbohydrates. For the non-covalent sensors, Davis and Wareham published an excellent review in 1999.363 Therefore, earlier work prior to 1999 will not be discussed. Briefly, the review was divided into two main sections, carbohydrate recognition in organic media and carbohydrate recognition in aqueous solution. In the carbohydrate recognition in organic media part, the author discussed “face-to-face” recognition, encapsulation by steroid-based receptors, the cholaphanes alternative frameworks based on cholic acid valleys, bowls, rings and cages, specific motifs for carbohydrate recognition, 2-aminopyridines and related substructures, recognition by anionic centers, and the phosphate-diol motif recognition without pre-organization. In the carbohydrate recognition in aqueous solution part, the discussion was focused on carbohydrate recognition in nature, the driving force for natural carbohydrate recognition, and recognition in model systems.
Biomimetic carbohydrate recognition has drawn much of interest over the past few years. X-ray crystallographic studies of protein-carbohydrate complexes reveal that the polar groups are bound by hydrogen-bond donor and acceptor groups, occasionally to metal ions, while the hydrophobic regions are complemented by nonpolar surfaces. Furthermore, CH-π stacking interactions and van der Waals interactions both contribute significantly to the recognition of carbohydrates by proteins in nature.364 Such information is very important for the design and synthesis of artificial carbohydrate receptors. In addition, it is also very important to understand the driving force for the replacement of water by carbohydrate-OH in a natural binding site. In this part, we will focus on work developed recently by different groups in using non-covalent forces for the design and synthesis of artificial carbohydrate receptors.
3-B-1. Receptors designed by the Mazik lab
In nature, there are many sugar-binding proteins. Such binding often relies on interactions of planar polar side chains with at least two functional groups engaging in cooperative hydrogen bonds and functional groups capable of π-stacking or hydrophobic interactions. Inspired by this, Mazik and co-workers designed and synthesized a series of pyridine-amide/amino and pyrimidine-amide/amino compounds. Such design is to mimic the hydrogen-bond and π-stacking interactions between sugar and proteins in the nature.365–372
As a starting point for the design of monosaccharide receptors, the author first examined the binding affinities of tripyridine 59 and tripyrimidine 60 (Figure 24). For comparative studies, compounds 61 and 62 were also synthesized (Figure 24).365 Octyl-β-D-glucopyranoside (63, Figure 24) and Octyl-α-D-glucopyranoside (64, Figure 24) were selected as model sugars for the evaluation of binding affinity in aprotic solvents such as chloroform. The interactions between hosts 59–62 and glucopyranosides were investigated by 1H NMR and extraction experiments. The NMR studies indicate a 1:1 binding stoichiometry. The binding constants were determined to be 8.7 × 103 M−1 between 63 and receptor 59, and 4.0 × 103 M−1 between 64 and 59 in CDCl3 respectively. Crystallographic studies have also been conducted. The results show that binding involves both hydrogen bond CH-π and van der Waals interactions. Receptor 60 showed similar binding affinity for both anomers with a binding constant of 1.4 × 104 M−1 for 63 and 1.3 × 104 M−1 for 64. In order to test the importance of the presence of pyridine nitrogen in the complex, compound 61 (Figure 24) was further designed and its binding affinity with sugar was studied by extraction experiments. Different from compound 59, compound 61 showed very poor solubility in chloroform even at high concentrations of glucopyranosides. The results suggest that without the hydrogen bond interactions involving the pyridine nitrogen, glucopyranosides could not pull the receptor into the solution, an indication of lack of binding. Due to the solubility problem of compound 61, the dimethylphenyl derivative 65 (Figure 24) with better solubility was also synthesized for binding studies. The Ka value (750 M−1) was more than 11-fold lower than that of the complex between 59 and 63 (Ka = 8.7 × 103 M−1), which further confirmed the importance of hydrogen bonding between pyridine nitrogen and OH groups of glucopyranosides in complex formation. To examine the need for three pyridine rings for tight binding, the binding constants of receptor 62 were also determined. Compared with 59, much lower Ka values (1.1 × 103 M−1 and 460 M−1 for 63 and 64 respectively) were obtained. The smaller binding constants for 62 implied the importance of the three pyridine rings for tight binding.
Figure 24.
Structures of compounds 59–65
Encouraged by the effectiveness of hydrogen-bonding as a way to improve binding affinity toward glucopyranosides, Mazik and co-workers further modified the structures of receptors 59 and 60. Several other receptors 66–69 (Figure 25) were designed to explore which hydrogen bonding motifs are essential for recognition of monosaccharides.371 Again, octyl-β-D-glucopyranoside (63, Figure 24) was selected as a probe for comparison and the interactions of all hosts and 63 were investigated by 1H NMR studies, which also indicate a 1:1 stoichiometry. The binding constants were determined by titration experiments in chloroform at 25 °C. The association constant between compounds 66 and 63 was determined to be 2.7 × 104 M−1. Such results suggest that the three naphthyridine-amide moieties in 66 led to a higher binding constant compared with that of receptor 59 (Ka = 8.7 × 103 M−1). The author contributed the higher binding affinity to secondary hydrogen bonds (Scheme 13).
Figure 25.
Structures of compounds 66–69
Scheme 13.
(a) A cooperative hydrogen-bond pattern in carbohydrate complexation with 59. (b) A cooperative and secondary hydrogen-bond pattern in carbohydrate complexation with 66
Similar to previous experiments, compound 67 was also designed to demonstrate the significance of the “three-point” recognition model. As expected, lower binding affinity (Ka = 4.5 × 103 M−1) was obtained, as compared to compound 66, presumably due to the lack of the third arm for binding. To quantify the influence of the amide NH protons on the formation of binding complex, the binding constant of compound 68 with the replacement of the amide bond by an ester bond was also determined to be 800 M−1, which was more than 10-fold lower than that of the complex between 59 and 63 (Ka = 8.7 × 103 M−1). Such results are consistent with the hypothesis that the amide N-H is very important for binding. Of course, It is also possible that the ester bonds are much more flexible, which would make the receptor molecule less rigid and result in lower binding affinity. Another interesting experiment done by Mazik and co-workers is the design and binding affinity experiments of compound 69 (Figure 25), which has an added coumarin ring for additional van der Waals interactions with 63. The 4-fold higher binding affinity (Ka = 4.3 × 103 M−1) as compared to that of 62 and 63 (Ka = 1.1 × 103 M−1) indicates that appropriately positioned additional hydrophobic interactions, similar as in protein-sugar complexes, are beneficial to binding.
Furthermore, new effective pyridine-based receptors 70–72 (Figure 26) were designed with replacement of the central phenyl ring by a trimethylphenyl ring to involve more effective CH-π interactions with the CH’s of sugar molecules.370 The second methyl group at the 4-position of the pyridine ring in host 71 and an additional amino group in host 72 were incorporated to increase the basicity of the pyridine moiety. The binding constants, again, were determined by 1H NMR experiments. In this study, different ratios were used between the receptor and sugar. This was to examine whether the binding stoichiometry and whether cooperative binding was involved. The results are shown in Table III.
Figure 26.
Structures of compounds 70–74
Table III.
Association constants (Ka) for receptors 70–72 with glucopyranosides 63 and 64
| Host-guest Complex | 70–63 | 70–64 | 71–63 | 71–64 | 72–63 | 72–64 |
|---|---|---|---|---|---|---|
| Ka1 (M−1) | 1.1 × 104 | 690 | 2.1 × 104 | 800 | 9.5 × 103 | 620 |
| Ka2 (M−1) | 250 | NA | 790 | NA | 4.8 × 103 | NA |
The binding constants were determined in chloroform at 25 °C by titration experiments. The stoichiometry of receptor-sugar complexation was obtained by curve-fitting analysis of the titration data and molar ratio plots. Ka1 and Ka2 were defined as binding constant of 1:1 and 1:2 receptor-sugar complexation respectively. The signal in NMR from the amine NH moved downfield and methylene CH2 moved upfield upon binding. Such results suggested the formation of hydrogen bonds between these groups and the sugar hydroxyls. The relative low solubility of the α-anomer glucopyranoside 74 comparing to the β-anomer 73 in the presence of receptor 71 provided additional evidence for the preference of β–anomer. Specifically, during the extraction experiment, 0.7 equiv of β–anomer 73 was extracted into the CDCl3 solution of receptor 71. Besides, after binding with 73, the NH and pyridine CH 1H NMR signals showed significant change in chemical shift, which further indicate complexation. In contrast, α–anomer 74 was not extracted at nearly the same efficiency. It is interesting to note that after addition of a small amount of water (0.07%) to the chloroform solutions, the binding affinity increases by a factor of 2–3. This increase in binding might be due to the formation of water-mediated hydrogen bonds, in line with similar observations in protein-carbohydrate complexes.364
Analogs 75 and 76 were also designed to investigate the influence of steric hindrance at the pyridine nitrogen position on binding affinity (Figure 27).372 The binding constants are shown in Table IV. Again, Ka1 was defined as 1:1 binding constant of receptor-sugar complex, while Ka3 was defined as 2:1 binding constant of receptor-sugar complex. The results showed that when the methyl group was in the α-position (70), lower binding affinity (Ka1 = 8.7 × 103 M−1) was observed as compared to situations when the methyl group is far away from the pyridine nitrogen (Ka3 = 2.4 × 104 for 75–63 and 2.3 × 104 M−1 for 76–63 respectively). For receptor 59, 1:1 binding was observed. Such results indicate that steric hindrance near the pyridine nitrogen plays an important role in affecting the binding. Decreased steric hindrance at the pyridine nitrogen increased the binding affinity of host (Ka3 = 2.4 × 104 for 75–63 and 2.3 × 104 M−1 for 76–63 respectively). One interesting thing is that, unlike 59, compounds 75 and 76 showed inversed selectivity, i.e., binding favors the α-anomer (64, Figure 24) instead of the β-anomer (63, Figure 24). In the case of compound 59, the axial 1-alkoxy group in α-anomer 64 can form intramolecular hydrogen bonds with the 2-OH group more easily than the equatorial 1-alkoxy substituent in β-anomer 63, therefore, the 2-OH group in β-anomer 63 is relatively free from intramolecular bonding, thus can bind with receptors more tightly. However, in the case of binding of compounds 75 or 76 with α-anomer 64, the intermolecular host-guest interactions might play a more important role than the intramolecular H-bonding network, which contributes to the higher binding of 75 or 76 with α-anomer 64.
Figure 27.
Structures of compounds 75 and 76
Table IV.
Association constants Ka for receptors 59, 75–76 and glucopyranosides 63 and 64
| Host-guest Complex | 59–63 | 70–64 | 75–63 | 75–64 | 76–63 | 76–64 |
|---|---|---|---|---|---|---|
| Ka1 (M−1) | 8.7 × 103 | 4.0 × 103 | 660 | 3.6 × 103 | 440 | 2.1 ×103 |
| Ka3 (M−1) | NA | NA | 2.4 × 104 | 8.2 × 104 | 2.3 × 104 | 4.8 × 104 |
Later on, more analogs were further designed (77–85, Figure 28) 369. The binding constants are shown in Table V.
Table V.
Association constants for receptors 77–85 with glucopyranosides 63 and 64
| Host-guest complex | Ka1 (M−1) | Ka2 (M−1) | Ka3 (M−1) |
|---|---|---|---|
| 77–63 | 9.5 × 103 | NA | 4.8 × 103 |
| 77–64 | 620 | NA | NA |
| 78–63 | 4.9 × 104 | 1.3 × 103 | NA |
| 78–64 | 1.3 × 103 | NA | NA |
| 79–63 | 2.0 × 104 | NA | 1.5 × 104 |
| 79–64 | 8.4 × 103 | NA | 8.7 × 103 |
| 80–63 | 650 | NA | NA |
| 81–63 | 1.2 × 103 | NA | NA |
| 82–63 | 1.4 × 103 | NA | 3.9 × 103 |
| 83–63 | NA | NA | NA |
| 84–63 | 950 | NA | NA |
| 85–63 | 190 | NA | NA |
Based on results from crystal structures, the Mazik lab further designed and synthesized additional receptors, 86 and 87 (Figure 29).366 These receptors used 2-aminopyridine groups as heterocyclic analogues of the asparagine/glutamine side chains, while incorporating a phenyl linker to interconnect these recognition units to provide additional nonpolar contacts to a saccharide by CH-π interaction. Carboxylate groups were also incorporated into the receptors to enhance binding through hydrogen bonds and ion-dipole interactions.
Figure 29.
Structures of receptors 86 and 87
Binding studies were again conducted by NMR method. Again, Ka1 is used to designate the binding constant of a 1:1 receptor-sugar complex and Ka2 is used to designate a 1:2 receptor-sugar complex. Incorporation of the carboxylate group improved binding considerably both in organic and aqueous media. Receptor 87 exhibited at least 100-fold higher affinity for β–glucopyranoside (Ka1 = 1.2 × 105 M−1 and Ka2 = 4.7 × 103 M−1) than the previously described tri-armed pyridine-based receptor 70 (Ka1 = 1.1 × 104 M−1 and Ka2 = 250 M−1). Besides, compound 87 shows a preference for the β–anomer in the recognition of glucopyranosides (Ka1 = 1.2 × 105 M−1 for 63 over 2.2 × 104 M−1 for 64). Furthermore, compound 86 shows similar binding affinity as compound 87 despite the fact that it does not have the functional groups for ionic interactions. However, both receptors, 86 and 87, showed dramatically decreased association constants in aqueous solution. For example, compound 87 has Ka1 of 2 M−1 and Ka2 of 72 M−1 with 63 in water. One surprising thing is that compound 87 showed a preference for the disaccharide D-cellobiose with Ka1 of 305 M−1 and Ka2 of 66 M−1 in CDCl3.
By using the same strategy, the Mazik lab also designed and synthesized compounds 88 and 89 (Figure 30).368 In this case, crown ether was incorporated as another binding site instead of a carboxylate group. The crown ether moiety was designed for hydrogen-bonding interactions with sugar. Again binding constants were determined using 1H NMR binding titrations and extraction experiments. The data were fitted into a 1:1 (Ka1) and a 2:1 (Ka3) binding model to give Ka1 of 5.8 × 105 M−1 and Ka3 of 1.4 × 104 M−1 for receptor 88 with 63 and Ka1 of 1.1 × 104 and Ka3 of 4.8 × 104 M−1 for receptor 89. The results showed about 10-fold higher affinity for 63 than its precursor 78 (Ka1 = 1.3 × 103 M−1) without the crown ether group. Besides, the two receptors both showed good selectivity for 63 over 64. For example, receptor 88 has a ratio of 417/1 for binding with 63 over 64. Although the incorporation of crown ether group could enhance the binding affinity, such results were only obtained in CDCl3 solution. It would be interesting to see whether the observed selectivity still exists in aqueous solution.
Figure 30.
Structure of crown ether receptors 88 and 89
Inspired by the binding affinity of designed receptors with disaccharides, Mazik and co-workers further developed a new biphenyl-based receptor 90 for the recognition of disaccharides (91α and 91β, Figure 31).373 The hypothesis for this design was that the four heterocyclic recognition groups based on the 2-aminopyridine unit would provide an excellent structural motif for hydrogen bonding. The biphenyl spacer of 90 should provide additional nonpolar contacts to the saccharides. Binding affinity was determined by 1H NMR with Ka > 106 M−1 for 91β in water-containing chloroform solutions (1:10, 1:20) when the data were fitted into a 2:1 (receptor/maltoside) model. Based on spectroscopic changes, it seems that at the initial stage, a 2:1 complex was formed. This was gradually replaced by a 1:1 complex. Compared with the binding of 91β, 91α has very poor solubility in chloroform in the presence of receptor 90 indicating weak binding affinity and good β/α selectivity. The relative low binding affinity of 90 for 63 (Ka1 = 1.1 × 104 M−1, Ka2 = 300 M−1) indicates preference for disaccharides.
Figure 31.
Structures of compounds 90, 91α and 91β
3-B-2. Receptors designed by the Roelens group
By taking advantage of the well-established hydrogen-bonding property of pyrroles, the Roelens lab described a self-assembled cage receptor containing pyrrole residues for multipoint interactions through hydrogen bonds (95, Scheme 14).374 The pyrrolic cage receptor was designed to provide the conformational and functional requirements for carbohydrate recognition.
Scheme 14.
Synthesis of the macrobicyclic cage 95
The designed compound 95 was synthesized by mixing 92 with 93 in methanol in a 2:3 ratio followed by reduction. The structure of 95 was determined by spectroscopic characterization and single crystal X-ray crystallography. Binding experiments were performed by using 1H NMR in CDCl3 and a 1:1 stoichiometry with an association constant of 4.8 × 104 M−1 with octyl-β-D-glucopyranoside (OctβGlc) was obtained. Interestingly, 95 has very high selectivity for the β anomer showing exclusive binding when observed using NMR. The author ascribed the excellent binding to both a precise size fit of the β-glucoside and an excellent complementarity of the amino/pyrrole group compared to the amide group, which is normally used for hydrogen bonding to a sugar moiety. Another interesting observation is that the precursor compound 94, which has a similar structure as 95, did not exhibit the same binding ability. Addition of OctβGlc did not show evidence of complexation. Solubility experiment was further conducted to probe the binding affinity and selectivity. Methyl-β-D-glucopyranoside (MeβGlc) is insoluble in CDCl3, however, when it was shaken with a 1 mM solution of 95 in CDCl3, the solid was partially dissolved and the NMR spectra showed that over 40% of the cage was present in the complex form. The bound percentage was increased to 50% in CCl4 and 75% in C6D6. Another competitive experiment to study selectivity was performed by feeding 95 with an equal molar mixture of OctβGlc and other selected glycosides. There was less than 10% decrease of binding fraction of 95 with OctβGlc after addition of various glycosides as compared with binding in the absence of competitors. In summary, the Roelens lab designed and successfully synthesized a self-assembled macrobicyclic cage 95, which can specifically recognize the β anomer of D-glucose and its alkyl glucosides with high Ka.
The Roelens lab also designed and synthesized a β-mannoside-selective pyrrolic tripodal receptor 96 (Scheme 15) based on the hydrogen bonding property of the receptor.375 Binding properties were determined by NMR, ITC and ESI-MS techniques and the binding affinities were assessed by the BC50 parameter, which was defined as the total concentration of receptor necessary for binding 50% of the ligand. The BC50 for the designed compound with β-mannose is less than 1 μM in CDCl3 with selectivity of about 800 over α-mannose or α-galactose, and 680 μM in CD3CN solution with selectivity of about 40 over α-galactose.
Scheme 15.
β-Mannoside-selective pyrrolic tripodal receptor
The Roelen’s lab further designed and synthesized a similar tricatecholic receptor for carbohydrate recognition (97, Scheme 16).376 The binding affinity of the receptor was investigated with several monosaccharides and disaccharides and the results showed that the receptor binds with octyl glycosides with BC50 of 0.87–5.2 mM and a 6-fold selectivity factor for α-mannoside over α glucoside.
Scheme 16.
Tricatecholic receptor for carbohydrate recognition
Recently, the Roelen’s group designed and synthesized a series of pyrrolic tripodal receptors 98–105 (Figure 32)377 by incorporating the pyrrolic binding groups into a previous receptor featuring a triethylbenzene scaffold bearing three convergent ureidic H-bonding units.378
Figure 32.
Structures of pyrrolic tripodal receptors 98–105
The pyrrole moiety was conveniently incorporated into the parent triamine by condensation to obtain the Schiff base 100, which was further reduced to amine 101. Compound 102 was designed to assess whether the amino group would be more effective than the amido group in binding monosaccharides. The benzylester moiety at the 5 position of pyrrole was incorporated to overcome the solubility problem of 103 and 104. Compound 105 was designed to ascertain the contribution from the pyrrolic groups to the recognition. Several monosaccharides (Figure 33) were selected as model sugars for binding studies, which were determined by 1H NMR titrations in CDCl3 at room temperature. The results are shown in Table VI.
Figure 33.
Structures of model sugars for binding studies
Table VI.
Binding BC50a (μM) for receptors 98–102, 105 with octylglyosides in CDCl3
| glycoside | BC50 (μM) | |||||
|---|---|---|---|---|---|---|
| 98 | 99 | 100 | 101 | 102 | 105 | |
| αGlc | 1.7 × 103 | NA | 268 | 570 | 8.3 × 103 | NA |
| βGlc | 2.0 × 103 | 3.7 × 103 | 4.8 | 24 | 5.4 × 103 | 6.2 ×104 |
| αGal | 1.5 × 103 | NA | 368 | 790 | NA | NA |
| βGal | 1.2 × 103 | NA | 120 | 70 | NA | NA |
| αMan | 1.6 × 103 | NA | 262 | 43 | NA | NA |
| βMan | 6.0 × 103 | NA | 660 | 37 | NA | NA |
| αGlcNAc | 2.0 × 103 | NA | 1.2 × 103 | 72 | NA | NA |
| βMan | 2.6 × 103 | NA | 30 | 18 | NA | NA |
BC50 was defined as the total concentration of receptor necessary for binding 50% of the ligand
From the results presented in Table VI it can be seen that (1) the incorporation of the pyrrole ring led to improved affinity as demonstrated by the higher binding affinity of 100 and 101 in contrast to that of 99; (2) compound 100 showed a remarkable binding affinity with βGlc, which is over 400-fold higher than that of 99, and the highest selectivity for βGlc than all the other compounds studied; (3) The amino receptor 101 generally has a higher affinity than the imino receptor 100 except for αGlc and αGal, where compound 100 shows higher affinities; and (4) somewhat surprisingly, receptor 102 showed lower binding affinity than 99 and no selectivity. In an effort to understand the structural features of these receptors, the X-ray crystal structures of receptors 100 and 104 were also determined. Alternate arrangement of substituents was observed with the three pyrrole arms on the same side of the aromatic ring forming a cleft. In the center of it is a captured ethanol molecule. The crystal structure conformations were as expected and reaffirmed the original design principles.
3-B-3. Receptors designed by the Inoue group
Inoue and co-workers developed water soluble extended analogs 3poly (107, Figure 34)379 of polypyridine structures based on their previous studies including 1poly (106, Figure 34).380,381 The design was based on the reason that the polymers used are expected to spontaneously form a helical structure by solvophobic interactions in aqueous solution between one pyridine ring and another at an interval of one pitch (Scheme 17). In the helix form, pyridine rings line up in a cisoid conformation and all the pyridine nitrogen pointed to the inside of pore. Since the pore provides a ”low-entropy” location compared to the protic solvent molecules outside, which can form hydrogen bonds with each other, the included solvent molecules in the pore thus can be substituted by other hydrogen-bonding substrate such as carbohydrate. The structure of amphiphilic polymer 107 in solution was investigated by UV/vis, 1H NMR and fluorescence spectra. The results indicated that the major contribution for the highly ordered structure form comes from the intramolecular π-stacking interactions of 107 in polar solvents.
Figure 34.
Structures of 106 and 107
Scheme 17.
Hydrogen-bond-driven saccharide recognition
Binding of the water soluble polymer 107 with some common monosaccharides was investigated by using circular dichroism (CD) in MeOH/water (10/1). It seemed that the chirality of the saccharide was transferred to the helical sense of the polymer as evidenced by the appearance of induced CDs (ICDs) in the absorptive region of the polymer. The binding constants were determined to be 14, 5.5 and 4.4 M−1 with D-mannose, D-fructose and D-allose, respectively. However, no significant binding was found with saccharides in 100% of water. The problem of weak binding in aqueous solution can be attributed to two conflicting factors: (1) solvophobic interactions, which cause the helix formation, are favored in water and disfavored in MeOH, and (2) hydrogen bonding, which is the driving force for saccharide recognition, is advantageous in MeOH and disadvantageous in water. More work is needed to improve binding affinity in aqueous environment.
3-B-4. Receptors designed by the A. P. Davis group
In 1998, Davis and co-workers reported a carbohydrate receptor 108 (Figure 35).382 The design of 108 drew lessons from sugar-protein interactions, which again rely on both hydrogen bond and hydrophobic interactions. The target of 108, the β–D-glucopyranoside unit 109, possesses axial CH groups and equatorial directed hydroxyl groups (Figure 35). Therefore, for complementary interactions, it is desirable that the binding cavity have parallel aryl rings, appropriately spaced and linked by moieties capable of hydrogen-binding. The tricyclic structure of 108 has two biphenyl rings and eight amide groups that appear to fit these criteria quite well. Molecular modeling on the analogous tetramethyl ester indicated that the cavity defined by the biphenyl and benzene 1, 3-dicarboxamide was able to accept a β–D-glucopyranoside molecule by forming hydrogen bonds and CH-π interactions.
Figure 35.
Structures of compounds 108 and monosaccharides 109–111
Binding was examined using monosaccharides 109–111 as models (Figure 35) and 1H NMR in CDCl3/CD3OH (92:8). Data were consistent with 1:1 binding with Ka of 980, 20 and 220 M−1 for 109, 110, and 111 respectively. Binding affinities in organic solvent CDCl3 were also determined by using fluorescence spectroscopy. Using a 1:1 binding model, the binding constants were 3.0 × 105, 1.3 × 104 and 1.1 × 105 for saccharides 109, 110, and 111, respectively. Good selectivity of the β-anomer was also observed. For example, the binding constant for β-glucoside 109 was 980 M−1, which is about 45-fold higher than that of α-glucoside 110 (Ka = 20 M−1). Besides, the binding affinity in CDCl3/CD3OH system gave a good reason to hope that tricyclic polyamide receptors upon further optimization could be developed as receptors for saccharides in aqueous solution.
Inspired by the remarkable selectivity for the all-equatorial β–glucoside as against α-anomers, Davis and co-workers studied another receptor 112, an “extended analogue” of 108, for the recognition of disaccharides through non-covalent interactions (Figure 36).383 Similar to receptor 108, receptor 112 possesses extended, parallel non-polar surface linked through spacers containing hydrogen-bond donor and acceptor groups. This geometry is compatible with equatorially substituted carbohydrate derivatives. Based on this, the all equatorially n-octyl-β-D-cellobioside 113 (Figure 36) was first used for initial binding studies. Titration 1H NMR experiments showed a 1:1 binding with a Ka of 7.0 × 103 M−1 in CDCl3/CD3OH (92:8), which is higher than that of 108 with β-monosaccharide (Ka = 980 M−1). No detectable binding was observed when using octyl β–D-lactoside 114, octyl β-D-maltoside 115, and dodecyl α–D-maltoside 116 as substrates (Figure 37). Furthermore, binding with 113 was also studied using a fluorescent method. The results also supported a 1:1 binding model with Ka of 2.5 × 103 M−1 with 113 (Figure 37). In contrast, no detectable binding was observed for 114, 115 and 116. The fluorescence titration of 112 versus 113 was also repeated in a less competitive solvent system, CHCl3/MeOH (98:2). As expected, the binding constant was much higher under such conditions (6.4 × 104 M−1). Moreover, weak but unambiguous induced CD was observed when 113 was added to receptor solution in CHCl3/MeOH (98:2), although signals were too noisy for quantitative analysis. Once again, there was no binding observed with 114, 115 and 116. All results support high selectivity for 113.
Figure 36.
Structures of compounds 112–116
Figure 37.
Structure of 117
Since the solubility properties of 108 was low in organic solvent and did not permit carbohydrate extraction studies from aqueous solution, a new variant 117 with a highly lipophilic exterior array of 12 benzyloxy substituents was designed (Figure 37).384 In homogeneous solution, 117 shows similar binding affinity as that of 110 with Ka of 720 M−1 in CDCl3/MeOH (92:8) and 980 M−1 in CDCl3/MeOH (98:2). In extraction experiments, three hexoses (glucose, galactose and mannose), two pentoses (ribose and xylose) and two methyl glucosides were all extracted into chloroform from 1 M aqueous solutions. Receptor 117 even showed notable affinity and selectivity for glucose by extracting detectable amount even from 0.1 M aqueous solutions. In contrast, there was no extraction observed for other sugars under the same condition (0.1 M).
In 2005, Davis and co-workers reported another variant of 108, receptor 118 (Figure 38), with dodecacarboxylate side chains to provide the opportunity to study carbohydrate recognition in homogenous aqueous solution.385 It is important to note that this is the first tricyclic polyamide receptor that shows binding affinity in aqueous solution. Using an NMR titration method with freshly dissolved glucose with an anomeric ratio (α/β) of 72:28, a Ka of 4.6 M−1 was obtained in D2O. For glucose equilibrated overnight, which gave an α/β ratio of 40:60, the binding constant was 9.2 M−1. Such results suggest possible selectivity for the β anomer. The binding of 118 with other substrates was also studied (Table VII). The studies with glycosides also show a notable preference for β–glucose units. For example, the binding affinity for methyl-β-D-glucoside is 27.3 M−1. In contrast, the binding affinity for methyl-α-D-glucoside is 6.9 M−1.
Figure 38.
Structure of compound 118
Table VII.
Association constants (Ka) between receptor 121 and carbohydrates as measured by 1H NMR titration in D2O
| Carbohydrates | Ka [M−1] | Carbohydrates | Ka [M−1] |
|---|---|---|---|
| D-glucose(α/β = 72: 28) | 4.6 | 2-deoxy-D-glucose | 7.2 |
| D-glucose(α/β = 40: 60) | 9.2 | D-xylose | 4.6 |
| D-galactose | 2.1 | D-lyxose | v.sa |
| D-mannose | v.sa | L-rhamnose | v.sa |
| D-arabinose | 2.2 | L-fucose | 2.1 |
| D-ribose | 3.1 | D-cellobiose | 16.6 |
| methyl-β-D-glucoside | 27.3 | D-lactose | v.sa |
| methyl-α-D-glucoside | 6.9 | D-maltose | v.sa |
Very small. Minor signal movements, almost linear with substrate concentration.
The binding affinity of 118 was also determined by means of fluorescence spectroscopy. Similar results were obtained (Table IIX). Although the affinities of receptor 118 are relatively low, the selectivity observed is significant and suggests that further optimization could lead to improve non-boronic acid-based receptors for saccharides that are functional in aqueous solution.
Table IIX.
Association constants (Ka) between receptor 121 and carbohydrates as measured by fluorescence titration in D2O
| Carbohydrates | Ka [M−1] | Carbohydrates | Ka [M−1] |
|---|---|---|---|
| D-glucose (α/β = 72: 28) | 9.5 | methyl-α-D-glucoside | 6.9 |
| methyl-β-D-glucoside | 27.3 | D-xylose | 4.6 |
Later on, Davis and co-workers successfully applied their strategy to all-equatorial disaccharides such as cellobiose, designed and synthesized a tetracyclic disaccharide receptor 119 (Figure 39) with good affinity and selectivity.386 Receptor 119 has two building blocks: a-meta-tertphenyl structure providing the roof and floor and defining the length of the cavity, and isophthalamide units serving as pillars. Each pillar involves two amide linkages, with potential to form hydrogen bond with the OH groups in cellobiose. Besides, the pillars were also furnished with externally directed tricarboxylate units to promote solubility. Furthermore, five rigid isophthalamides were also incorporated to prevent the cavity from collapsing.
Figure 39.
Structure of compound 119
Binding was studied by using an NMR titration method, induced circular dichroism, fluorescence spectroscopy and calorimetry. Ten disaccharides and three monosaccharides were selected as models for selectivity test. Binding constants between receptor 119 and selecting carbohydrates were in the range of 5–910 M−1 (Table IX). The most intriguing result is the excellent selectivity of receptor 119. Minor changes to disaccharide structures could cause almost complete suppression of complex formation. For example, for the result of gentibiose, which contains an all-equatorial structure that is slightly longer than D-cellobiose, the change from D-cellobiose was enough to reduce the binding constant to 12 M−1 from 580 M−1. The NOESY contacts observed for the complex between β–D-cellobiose and receptor 119 and the computational model of the complex gave a good sandwich-like, 2:1 binding model (Figure 39). It features eight intermolecular hydrogen bonds and about ten CH-π interactions, giving a persuasive explanation for the high binding affinity and selectivity.
Table IX.
Carbohydrate substrates used with receptor 119
| Carbohydrates | Ka (M−1) | ||
|---|---|---|---|
| 1H NMR | ICD | Fluorescence | |
| D-cellobiose (1β-OH: 1α-OH= 3:2) | 600a | 580 | 560 |
| Methyl β-D-cellobioside | B | 910 | 850 |
| D-Xylobiose | B | 250 | 270 |
| D-N, N′-diacetylchitobiose | 120 | NA | 120 |
| D-lactose | B | 11 | 14 |
| D-Mannobiose | B | 13 | 9 |
| D-Maltose | B | 15 | 11 |
| D-Gentiobiose | NA | 12 | 5 |
| D-Trehalose | C | c | NA |
| D–Sucrose | NA | c | c |
| D-Glucose | 11a,d | 12 | c |
| D-Ribose | NA | c | c |
| D-N-acetylglucosamine | 24d | NA | 19 |
T = 298 K,
Intermediate exchanges led to peak broadening and prevented the determination of Ka,
No change in spectra upon addition of carbohydrate,
Fast exchanges on the NMR time scale
3-B-5. Other receptors belonging to different structural classes
The Yu lab reported two relatively simple receptors based on a naphthyridine core: 2,7-di(3′-pyridyl)-1,8-naphthyridine (DPN, 120, Figure 41) and 2,7-di(3′-quinolyl)- 1,8-naphthyridine (DQN, 121, Figure 41). Both 120 and 121 possess a naphthyridine core moiety, in which two pyridine nitrogen atoms serve as the hydrogen bond acceptors. The binding studies were conducted using fluorescence and UV-vis. Binding constants of about 500–9.0 × 103 M−1 were obtained for β-D-fructoside, α-D-riboside, α(β)-D-glucoside, α(β)-D-galactoside and α(β)-D-mannoside in CHCl3. Although these compounds showed good binding affinity, none showed good selectivity. For example, the binding constants for β-D-glucoside and α-D-glucoside are 6.3 × 103 M−1 and 4.5 × 103 M−1 respectively. Indeed, the binding constants for all the specific sugars used as model saccharides are similar.
Figure 41.
Structures of compounds 120 and 121
Based on the hypothesis that rigid, folding structures might be used as artificial receptors for molecular recognition or sensing, the Li lab designed two new oligoamides 122 and 123 (Figure 42).387 The oligomers were incorporated with phenyl subunits via amide linkages. The existence of three-centered hydrogen bonds in the oligomers and consequently the folding formation in solution were characterized by 1H NMR. The binding affinities were investigated using 1H NMR, fluorescence, and CD spectroscopy. The association constants obtained by fluorescence titration experiments for four selected saccharides were about 1.0 × 103 – 7.0 × 103 M−1 with 1:1 binding mode in chloroform. The results support the hypothesis that the new folding structures can efficiently complex saccharide derivatives in chloroform through intermolecular multiple hydrogen bonds.
Figure 42.
Structures of hydrogen bonding-driven foldamers 122 and 123
Lipopolysaccharides (LPS) are the major constituents of the outer cellular membrane of Gram-negative bacteria, which are also known as bacterial endotoxin. They play an important role in the septic shock syndrome.388 The LPS of Gram-negative bacteria normally have a conserved portion, the oligosaccharide core and lipid A part. Lipid A part has an anionic amphiphilic nature, which is able to bind with cationic hydrophobic ligands to neutralize the toxicity of the bacteria. Based on the property of Gram-negative bacteria, Cunsolo and co-workers design and synthesized three calix[4]arene-based ligands as endotoxin receptors, which could be used as potential antimicrobial agents 124–126 (Figure 43).389 A tetraproxy-tetraamino-calix[4]arene was chosen as the amphiphilic building block. Tyrosine and tryptophan amino acid residues were used to enhance the hydrophobic and basic properties. Moreover, a 6-amino-hexanoyl arm was designed as a spacer between the calix[4]arene scaffold and the acyl active residue to endow the receptors with high conformational mobility for induced fit recognition of substrates.
Figure 43.
Calix[4]arene-based ligands as endotoxin receptors 124–126
Binding of these receptors towards LPS of Gram-negative bacteria was studied by NMR and UV titrations. However, no binding constant was obtained by these two methods. The author suggested that it might be due to non-specific binding caused by the high stoichiometric ratios. For compound 125, the molar ratio was 3:1 and 10:1 respectively between the receptor and LPS in the NMR and UV titration experiments.
Another application is the development of highly selective recognition of diols by a self-regulating fine-tunable methylazacalix[4]pyridine (127, MACP-4, Figure 44) cavity.390 Gong and co-workers reported the highly selective recognition of diols and benzenediols by MACP-4. The central hypothesis is that the multi-pyridine-containing feature might serve as a strong hydrogen-bond acceptor and form a complex with diols and benzenediols through intermolecular hydrogen bond. Pyridine rings could also serve as other binding units through π-π stacking and CH-π interactions. Besides, the fine-tunable cavity of MACP-4 might be able to recognize marginally different diol and benzene diol derivatives based on the property that it can self-regulate its conformation during binding. For example, the inversion of MACP-4 by resorcinol (1, 3-benzenediol) could be observed by using dynamic 1H NMR spectroscopic study (Figure 45). Such properties might be attributed to the decreased activation energy of the transition state B in the presence of excess amount of resorcinol.
Figure 44.
Structure of MACP-4
Figure 45.
Transition states of MACP-4-resorcinol complex
Molecular recognition of MACP-4 towards various diols was investigated by using 1H NMR and X-ray diffraction analysis. MACP-4 exhibited excellent selectivity in the recognition of resorcinol (1, 3-benzenediol) with a binding constant of 6.0 × 103 M−1 in CDCl3. A very stable 1:1 sandwich complex was formed and the predominant driving force for the formation is the intermolecular hydrogen bonding between the pyridine nitrogen atoms of the host and the hydroxyl groups of the guest.
3-C. Metal chelator-based sensors
Another important approach for carbohydrate recognition is the coordination of a carbohydrate ligand to a metal center. For example, in nature, C-type lectins recognize saccharides in a calcium-dependent manner.391 In this part, several carbohydrate sensors based on the idea of bearing both boronic acid for binding diols and metal-chelation for binding of other parts are discussed. Besides, simple water-soluble lanthanum and europium complexes, which are effective at detecting neutral sugars as well as glycolipids and phospholipids, and sugar discriminating binuclear copper complex, will also be mentioned.
Conversion of glucose to glucose-6-phosphate (G-6-P) and the inter-conversion between G-6-P and α-D-glucose-1-phosphate (G-1-P) are essential steps to produce glycogen.392 Thus selective recognition and detection of G-6-P and G-1-P could be used in the study of relevant biological processes. Shinkai and co-workers developed an artificial receptor with high selectivity using boronic-acid-appended zinc (II) porphyrin (128, Figure 46).393 When zinc (II) porphyrin was bound to G-6-P, the δp value in 31P NMR markedly shifts to a higher magnetic field. A strong excitation-coupling band also appeared in CD spectroscopy. However, such big changes are not observable for zinc (II) porphyrin with D-glucose, G-1-P and for boronic-acid-appended porphyrin in the presence of these glucose derivatives. Such result was thought to be due to two reasons: (1) the phosphate in G-6-P can interact with the central metal zinc, (2) the 1,2-diols can bind with the boronic acid.
Figure 46.
Complex Structure proposed for boronic-acid-appended Zinc (II) porphyrin plus G-6-P
An artificial receptor for the detection of uronic acids and sialic acids was also developed by the Shinkai lab (129, Figure 47).215 Uronic acids play an important role in the oxidation process of monosaccharides or in the biosynthetic process of L-ascorbic acid, while sialic acids are the recognition unit by influenza viruses. The central idea for the design of artificial receptor is based on both boronic acid recognition of diols and Zn recognition of the carboxylate unit of the respective analytes.
Figure 47.
Complexation mode proposed for the two-points binding of uronic acids to the Zn(II) complex
Specifically, the Zn (II) complex of new receptors 130 and 131 were also developed by the Shinkai lab (Figure 48).215 They contain a fluorescent group bearing both an o-aminomethylphenyl boronic acid group for diol binding and the 1, 10-phenanthroline-Zn (II) chelate moiety for carboxylate binding. The association constants (determined in water:MeOH = 1:2 at pH = 8) of 130 were better than that of compound 131. For example, the log Ka of 130 for D-galacturonic acid is 3.1 in comparison with that of 131 (1.9). The results of 130 also showed some selectivity for uronic acids in aqueous solution, about 2.5:1 in logKa over D-galactose. The studies were further extended to sialic acids, which also showed similar affinity (log Ka = 2.3 for 130 and selectivity 1.7:1 in log Ka over D-galactose). In conclusion, the described receptor showed a good example for the design concept utilizing cooperative actions of boronic acid and a metal chelate, which can be used for further application.
Figure 48.
Structure of 130 and 131
Inspired by calcium-saccharide interactions found in C-type lectins, the Strongin group successfully developed lanthanum and europium receptors 132 and 133 (Figure 49)82 based on the similar properties of lanthanides and calcium.394 The utility of water-soluble salophene-lanthanide complexes addressed three current challenges. (1) the detection of neutral carbohydrate at physiologically relevant pH; (2) the selective detection of gangliosides; (3) the selective detection of lysophosphatidic acid (LPA), which is a biomarker for several pathological conditions including ovarian cancer and is selectively detected by the europium complex even in human plasma samples. The author proposed that the 2-sn-OH moiety of LPA might play a key role in promoting binding to the metal center. Besides, the europium complex showed good selectivity between sialic acid-containing gangliosides and asialoganglioside at physiologically relevant pH value without the interference of other molecules found in common brain ganglioside and phospholipids extracts. The author attributed the selectivity to the cooperative complexation of the oligosaccharide and sialic acid residues to the metal center.
Figure 49.
Structures of salophene-lanthanide (europium) complexes
Another example for the metal chelator-based sensor is a sugar discriminating binuclear copper (II) complex 134 developed by Striegler and co-workers (Figure 50).34,395 In this work, complex formation between carbohydrates and the Cu complex was investigated by a combined approach of UV/vis and CD spectroscopy. The copper complex showed good discrimination of different carbohydrate such as mannose and glucose due to different binding complexes (135, 136, Figure 50). However, it was only functional at high pH value (>12), which will put a sever limit on potential applications.
Figure 50.
Complexes modes with mannosed glucose
As a summary for this section, Table X lists the non-boronic acid-based sensorsdescribed together with their salient binding properties.
Table X.
A summary of non-boronic acid-based sensors
| Sensor type | Sensor target | Binding affinities |
|---|---|---|
| RNA aptamers | sialyl Lewis X (sLex, 53) | Kd values were around 10−9 to 10−11 M, 100 fold selectivity over lactose, 5–10 fold over other similar Lewis group sugars. |
| aptamer-based assay development | neomycin B (54) | Showed a linear range between 25 and 2.5 ×103 μM in milk, 100-fold binding selectivity over paromomycin, |
| Cation-charged modified DNA aptamers | Sialyllactose | The best Kd was 4.9 μM. |
| 2′-aminopyrimidine RNA aptamers | moenomycin A | The Kd values of the aptamers were 350 and 320 nM. |
| boronic acid-modified DNA aptamers | Fibrinogen was used as a model glycoprotein | Kd in the range of low to mid nM |
| compound 66 | octyl-β-D-glucopyranosi de (63) | The association constant was 2.7 × 104 M−1 in chloroform at 25 °C as determined by 1H NMR. |
| compound 87 | glucopyranosi des (63 and 64) | Ka = 1.2 × 105 M−1 for 63, 2.2 × 104 M−1 for 64. |
| compound 88 and 89 | glucopyranosi des (63 and 64) | Ka1 (1:1 binding) of 5.8 × 105 M−1 and Ka3 (2:1 binding) of 1.4 × 104 M−1 for receptor 88 with 63; Ka1 of 1.1 × 104 and Ka3 of 4.8 × 104 M−1 for receptor 89. |
| biphenyl-based receptor 90 | disaccharides (91β) | Binding affinity was determined by 1H NMR with Ka > 106 M−1 for 91β in water-containing chloroform solutions (1:10, 1:20) |
| a self-assembled cage receptor containing pyrrole residues (95) | OctβGlc | An association constant of 4.8 × 104 M−1 with OctβGlc was determined by using 1H NMR in CDCl3 assuming a 1:1 stoichiometry |
| β-mannoside- selective pyrrolic tripodal receptor96 | β-mannose | The BC50 with β-mannose is less than 1 μM in CDCl3 with selectivity of about 800 fold over α-mannose or α-galactose, and 680 μM in CD3CN solution with selectivity of about 40 fold over α-galactose. |
| tricatecholic receptor for carbohydrate recognition (97, Scheme 16) | octyl glycosides (α-mannoside) | BC50 of 0.87 5.2 mM and 6-fold selectivity over α glucoside. |
| a carbohydrate receptor 108 | β-glucoside109 | binding constant was determined to be 980 M−1 by using 1H NMR in CDCl3/CD3OH (92:8) |
| receptor 112 | n-octyl-β-D- cellobioside 113 | In CHCl3/MeOH (98:2), the binding constant was6.4 × 104 M−1. |
| Metal chelator- based sensors: Zn(II) complex of new receptors 130 and 131 | D- galacturonic acid | The log Ka for binding with D-galacturonic acid is 3.1 for 130 and 1.9 for 131 as determined in water:MeOH = 1:2 at pH = 8. |
4. LECTINS AND CARBOHYDRATE RECOGNITION
In addition to the man-made molecules described in previous sections that can recognize carbohydrates, nature also has its own set of readily available carbohydrate-binding proteins, which can be used for carbohydrate recognition in lab research and potential clinical applications. Currently, several hundred lectins have been identified, and about 60 lectins are commercially readily available from companies such as Sigma-Aldrich and Fisher Scientific. They all have certain specificity based on the overall topology and sugar compositions. For example, there are lectins that recognize poly mannose saccharides and others that can recognize galactose connected to different structures (Table XI). However, almost all lectins have cross reactivity issues. There are many good reviews and books on lectins in general.396,397 Therefore, readers are referred to those comprehensive sources for general matters related to lectins. This following section will not try to be comprehensive. Instead, we will focus on ways that lectins can be used for carbohydrate recognition in research and for potential clinical applications. We will also discuss the major issues to address and potential pitfalls.
Table XI.
| Abbreviation | Common name/Species | Specificity |
|---|---|---|
| AAA | Fresh water eel/Anguilla anguilla | α-Fucose |
| AHA | Actinohivin/Longispora albida | Man |
| AIA | Jack fruit, jaca/Artocarpus integrifolia | Galactose |
| APA | Leek/Allium porrum | Man |
| AUA | Ramsons lectin/Allium ursinum | Man |
| BPA | Camels foot tree/Bauhinia purpurea | GalNAc/Gal |
| CHA | None/Cymbidium hybrid | Man |
| Con A | Jack bean/Canavalia ensiformis | Man, Glc, GlcNAc |
| CVL | None/Chaetopterus variopedatus | β-Gal |
| CV-N | Cyanovirin-N/Nostoc ellipososporium | α(1,2)Man |
| DBA | Horse gram/Dolichos biflorus | GalNAc |
| DSA | Jimson weed/Datura stramonium | GalNac |
| ECA | Cocks comb coral tree/Erythrina cristagalli | Gal β-1, 4GlcNAc olig |
| EHA | Broad-leaved helleborine/Epipactis helleborine | Man |
| GNA | Snowdrop/Galanthus nivalis | α(1,3)Man |
| GRFT | Griffithsin/Griffithsia spp. | Man, Glc, GlcNAc |
| GSI | None/Griffonia simplicifolia –I | α-galactose |
| GSII | None/Griffonia simplicifolia –II | GlcNAc |
| GSL | None/Gerardia savaglia | D-Mann |
| HBA | Rubber tree/Hevea brasiliensis | GlcNAc |
| HHA | Amaryllis/Hippeastrum hybrid | α(1,3)-α(1,6)Man |
| HPA | Roman snail/Helix pomatia | α-GalNAc |
| LCA | Lentil/Lens culinaris | Man, Glc, GlcNAc |
| LOA | Twayblade/Listera ovata | α-(1,3)Man |
| Lotus A | Lotus, asparagus pea/Lotus tetragonolobus | α-Fucose |
| MAA | Maackia/Maackia amurensis | α-2.3 sialic acid |
| MHA | Myrianthin/Myrianthus holstii | GlcNAc |
| MVL | None/Microcystis viridis | Manβ(1,4) GlcNAc |
| None | Mermaid/Laxus oneistus | Man |
| None | Sweet pea/Lathyrus odaratus | Man, Glc, GlcNAc |
| NPA | Daffodil/Narcissus pseudonarcissus | α(1,6)Man |
| PAA | Avocado/Persea Americana | unknown |
| PNA | Peanut/Arachis hypogaea | Gal-B-OR |
| PSA | Garden pea/Pisum sativum | Man, Glc/GlNAc |
| SBA | Soya bean/Glycine max | GalNAc |
| SNA | Elderberry/Sambucus nigra | α-2,6 sialic acid |
| STA | Potato/Solanum tuberosum | GlcNAc oligomers |
| SVN | Scytovirin/Scytonema varium | α(1,2)-α(1,6)Man |
| UDA | Stinging nettle/Urtica dioica | GlcNAc oligomers |
| UEA-I | Gorse, Furze/Ulex europaeus-I | α-Fucose |
| VFA | Broad bean, faba bean/Vicia faba | Man, Glc, GlcNAc |
Before the discussion of using lectins in biological and analytical analysis, it is important to give a brief overview of the structural basis for lectin binding. The legume lectin family is the most widely researched lectin group and much of what is known about lectin binding mechanism comes from the study of these lectins. The legume family shows a wide range of specificity for carbohydrates as compared to other lectin families. Here only the salient features of lectin-carbohydrate interactions are presented. The structural basis of the binding of lectins of the legume family has been reviewed.398 A few other good reviews have also been written about the mechanism of lectin binding.399,400 Therefore readers are referred to these reviews for more in depth discussions.
It is interesting to note that in boronolectins, the strong and reversible interactions between the boronic acid group and hydroxyl groups play a key role in bindng. In contrast, lectin binding relies mostly on hydrophobic and hydrogen bond interactions. The carbohydrate binding sites of lectins are typically a shallow depression on the lectin surface. There are four amino acids that are generally conserved in all lectins regardless of their binding specificity; these are asparagines, aspartic acid, glycine (arginine in Con A) and an aromatic residue. Site-directed mutagenesis in which aspartic acid and asparagines residue is replaced results in loss of the lectin-carbohydrate binding ability.36 The actual carbohydrate binding site consists of four loops that are typically named A, B, C, and D.399 Aspartic acid is in loop A, glycine in loop B, while asparagines and the aromatic or hydrophobic residue reside in loop C. The amino acids in loop D are highly variable in conformation, length and sequence and have a role to play in the specificity of a lectin. Lectins typically contain a Ca2+ and a transition metal ion (usually Mn2+). The two cations are 4 Å apart and are near to the carbohydrate-binding site. These cations are thought to be important in positioning the amino acids residues that interact with the carbohydrate. The correct orientation for the asparagine residue is mediated by a cisoid-peptide bond between the asparagine and the preceding amino acid.343
The binding of the lectin to the carbohydrate involve a number of hydrogen bonds as well as hydrophobic interactions. For example, the X-ray crystal structure of Con A with mannose shows in detail the typical interactions of legume lectins with carbohydrates.401 The main features of the carbohydrate-lectin interaction of ConA involved key hydrogen bonds with conserved residues. These are an aspartate (Asp 208), that is preceded by the cisoid peptide bond and held in place by a water bridge with Ca2+, an asparagine (Asn 14) residue that interacts with the calcium ion and the backbone NH of Arg 228 (in other lectins this is glycine). Hydrophobic interactions mainly involve a number of Van der Waals forces that contribute to the overall binding of the carbohydrate. This is demonstrated in which there is a Van der Waals interaction with aromatic residue Tyr 12 and the sugar ring as well as an interaction between the loop segment (Thr97-Glu102).
It is believed that lectin-carbohydrate interactions typically have moderate affinity. However, there have not been a vast number of methods in the literature used for studying the binding constants of lectins to their corresponding carbohydrates. Some recent examples of lectin binding constant determination using surface plasmon resonance (SPR), flouorescence polarization (FP), fluorescence correlation spectroscopy (FCS), and quartz crystal microbalance (QCM) techniques give a glimps of the binding strength. In one study, high mannose glycosylasparagines were prepared and labeled with a fluorescence group such as the dansyl (Dns) group, fluorescein (Fl), or tetramethylrhodamine (TMR).402 It was found that the association constants of Con A with Dns-Asn(M6)-OH and TMR-Asn(M6)-OH were 2.94 × 103 and 1.79 × 103 M−1, respectively. Similarly, the Ka values of a mannose/glucose binding lectin, Castanea crenata agglutinin (CCA), for mannose, glucose and a-D-Man-(1→3)-D-Man were estimated to be 4.18 × 102 M−1, 1.39 × 102 M−1, and 2.07 × 103 M−1, respectively.403 Using QCM, the binding constants between ConA and maltose and Jacalin and fetuin were determined as 4.5 × 102 M−1 and 6.4 ×104 M−1, respectively.404 All these are modest binding affinities.
Lectin-monosaccharide binding is relatively weak. This is primarily due to the shallow binding site. However, lectins still show higher affinity for oligosaccharides. Therefore, multivalent interactions between lectin and carbohydrates are usually involved in a recognition event leading to the higher affinity. For example, there are 13 mannose residues in the carboxypeptidase Y (CaY). The association constant between Con A and CaY in saturation binding experiments was 2.7 × 106 M−1, which was much higher than binding with a monomer.405 There had been previous studies indicating the association equilibrium constants of Con A with oligosaccharides containing mannose in the range of 2 × 106 to 30 × 106 M−1, depending on the type of ligands studies studied.402,406–408 Overall, lectin-carbohydrate binding constants are commonly seen in the range of 102–106 M−1 range with monosaccahrde binding in the lower range and polysaccharide/multivalent binding in the higher range.
Since glycosylation is an important and abundant post-translational and co-translational protein modification409 and carbohydrates have been shown to be important in tumor formation and metastasis, cell-cell adhesion, infection, autoimmunity and inflammation, there is a need for methods that allow for the examination of glycans individually as well as in an array format to allow for global glycan profiling. Developing methods to evaluate glycosylation can be very challenging due to the diversity and complexity of glycans.165 There is currently limited technology to identify carbohydrates (glycans). Current methods for analyzing glycans such as western blotting, LCMS and MS requires advanced expertise, is time consuming and requires the glycans to be removed from proteins. Such methods only allow for the analysis of glycans individually. To examine a large number of glycans simultaneously, one will need to have arrays of carbohydrate “binders” with certain specificity and affinity. One such approach is the use of carbohydrate-binding lectins.
In recent years, there have been a number of examples of the use of lectin microarrays for recognition of glycosylation patterns. General information about the makeup of the glycans can be determined such as whether it is N-glycosylated, O-glycosylated, high-mannose, core-fucosylated and fully or partially sialylated. These microarrays can also monitor changes in glycosylation patterns associated with changes in cell-adhesion and tumor cell states.410,411 The advantage of microarrays is that it allows for a profile or fingerprint to be taken of the carbohydrate landscape.
In one such example from the Mahal lab, nine common lectins were arrayed on aldehyde or epoxide derivatized glass slides144 and the glycoproteins to be examined were labeled by conjugation to lysines carrying a fluorescent dye, Cy3. The array was then used to analyze the protein ovalbumine that is known to have high mannose and/or hybrid N-linked glycans.412 The glycopattern observed showed positive signals for the lectins WGA, ConA and GS-II. This was consistent with the known glycosylation pattern for ovalbumine since these three lectins are known to bind to N-linked glycans and ConA to mannose. This pattern is visible to the detection limit of 10 μg/mL. In order to test the ability of the microarray to differentiate between different glycans, the glycopatterns for ovalbumine was compared to that of two other glycoproteins, bovine submaillary mucin (BSM) and porcine gastric mucin (PGM). The observed pattern for ovalbumine is different from that of BSM or PGM. The observed glycosylation patterns using this nine-lectin array demonstrate its ability to distinguish minimal differences in glycosylation of proteins.
This group utilized this same microarray method to monitor cell-surface glycosylation of Escherichia coli.413 The glycosylation of bacterial cell surfaces has been shown to be important in symbiosis, immunity and cell-cell interaction.144,414 However, the limitations of currently available techniques hinder a more detailed examination of the role of glycans in these processes. The method presented by the Mahal group uses a lectin microarray approach to analyze bacterial cell-surface carbohydrates in an efficient manner. The lectin microarray consisted of 21 lectins. Each of the glass slides were printed with 14 isolated subarrays containing five spots per lectin using a SpotBot Arrayer (95 μM spot size). They examined the binding of fluorescently labeled bacteria (stained by nucleic-acid dye SYTO 85) to the microarray. Significant differences were not observed for the uptake of the dye by different bacterial cell lines; however these strains showed unique binding patterns to the lectin arrays. This observation was especially significant for closely related E. coli strains JM101 and HB101 that could not be distinguished using traditional hemagglutination assays. The two showed reproducible differences in their lectin fingerprints. Both strains showed strong binding to lectins GSII, HPA and WGA, however only JM101 showed moderate binding to MAA and weak binding to BPA, DBA and ECA. They also examined two pathogenic bacteria E. coli RS218 (neonatal meningitis pathogen) and Salmonella tryphimurium LT2. Both of these bacteria showed binding to α-2, 3-IMAA and α-2-6-(SNA)-sialic acid residues as well as for α-1,2-fucose binding as indicated by lectin UEA I. The presence of sialic acids and fucose has been implicated in immune evasion of pathogens.415 RS218 also showed binding to BPA, DBA, ECA and GSI. To further probe the specificity of these interactions, binding of certain lectins was inhibited by a known inhibitor; lactose.
The same microarray technology was also used to examine real-time glycosylation changes in bacterial surfaces. It is known that alterations in bacterial carbohydrates occur in response to environmental stimuli and play a role in pathogenesis of bacteria such as E.coli and Salmonella.416 Therefore, glycosylation of RS218 was monitored as a function of growth, where OD600 of less than 0.4 represented the lag phase, OD600 between 0.4 and 2.0 were considered exponential growth phase and OD600 above 2.0 was the stationary phase. A decrease in the binding of all positive lectins was observed associated with bacterial growth. These results suggest a possible role of glycosylation in growth dependent invasion of RS218.
Although a very useful microarray technology, several limitations were indicated. Firstly, only accessible carbohydrate motifs rather than the entire glycome were observed using the microarrays, though the most relevant glycans are typically on the accessible surface. Secondly, there is a lack of availability of lectins that recognize unique bacterial sugars. We hope that as more lectins that bacteria recognize are discovered, so will the detection capabilities of this array technology. Despite the limitations, the microarray technology presented can provide a powerful tool to quickly determine changes in surface glycans of bacteria in response to environmental stimuli.
Conventional microarray methods already discussed can analyze microgram quantities while the use of evanescent-field fluorescence detection microarray technology417,418 offers a more sensitive method of glycoprofiling using lectin microarray technology with the ability of analyzing picogram quantities of glycoprotein samples.140 Essentially, an electromagnetic wave called an evanescent-field is propagated within a wavelength distance from the sensor surface (~100–200 nm) in the lower refractive index sample medium. The evanescent-field technology is able to analyze relatively weak interactions (such as those between lectins and glycoproteins with Kd values in the 10−4 – 10−7 M range) because it can detect ongoing interactions under equilibrium conditions in situ without the need to wash after a probing reaction. The evanescent field lectin microarray has been applied to profile glycans of Lec mutants.419 In this application, cell glycan analysis was performed for Chinese hamster ovary (CHO) cells and their glycan profile was compared to those of their glycosylation-defective Lec mutants. Lec1 (defect in GlcNAc-T1), Lec2 (CMP-sialic acid transporter) and Lec8 (UDP-Gal transporter) were chosen since their biosynthetic features are well characterized. The Lec mutant and CHO cells were grown harvested and washed with PBS to remove any glycoprotein containing ingredient. The cell-surfaces were then labeled with Cy3-succimidyl ester, and a 20 μL aliquot of each sample was subjected to lectin microarray. The result of the lectin microarray of the CHO cells and the Lec mutants each showed different profiles. Lec1 showed a decrease in signals for a number of lectins such as MAL, ECA, RCA 120, PHS (L), PHA (E), WGA, DSA and LEL and an increase in signals for GNA and HHL. The similarity among the profiles were evaluated using correlation coefficients, that is, a high similarity is indicated by a correlation coefficient higher than 0.9 and a low similarity if the coefficient is lower than 0.5. Keeping these coefficient limits in mind, the resemblance of CHO and Lec1 cells is relatively low with a correlation coefficient of 0.57 whereas Lec2 is relatively similar with a coefficient of 0.81. Lec8 showed the biggest difference among the Lec mutants with a coefficient of 0.46. Lec 8 showed significant decreases in signal for MAL, ECA, RCA120, ACL and PHA (E) which recognize non-reducing terminal sialic acid or galactose. In addition, the Lec 8 mutant showed increased binding with WFA, ABA, EEL, NPA and SBA lectins. These results were compared to results from flow cytometric analysis and glycosidase digestion with neuraminidase. For the latter technique the cells were washed extensively after treatment with neuraminidase and before Cy3 labeling, resulting in a substantial change in glycan profile for CHO cells. On the other hand, no difference were observed for any of the three Lec mutants which can be explained by the fact that Lec mutants do not have sialic acid and are not affected by neuraminidase treatment.
For flow cytometry, 11 lectins were used as well as anti-Tn (αGAlNAc) antibody. Both lectin and flow cytometry gave similar results; however this lectin microarray has an advantage over flow cytometry because a number of lectin-biding experiments can be carried out at the same time with increased sensitivity. Their results determined that evanescent field microarray technology can be applied to glycan profiling of crude samples.
To address the need for better interpretation of the resulting glycan fingerprints created by the lectin microarray, one group developed a convenient and rapid lectin-microarray method, Qproteome™ GlycoArray kit. In this method, glycoanalysis is performed on intact glycoproteins that require about 4– 6 hours in most cases. The major advantage of this kit over other methods is that it uses a set of proprietary algorithms that can provide analysis of the resulting histogram with the click of a mouse.420 The technology consists of arrays of 24 plant lectins with overlapping specificities. Binding of a glycoprotein to the array results in a characteristic fingerprint of glycan composition of the protein. The use of such a large number of lectins ensures a high sensitivity to changes in glycosylation pattern. The lectins were spotted in duplicate arrays on nitrocellulose membrane-coated glass slides with 6 replicate sports each and the array was then incubated with intact glycoprotein. The arrays are scanned and the resulting images are analyzed using propriety image analysis software that converts the images into a fingerprint, that is, a histogram of the signal obtained for each lectin. The robust average of the signals is obtained from the 6 replicates and the fingerprint is interpreted by proprietary knowledge-based algorithms. The algorithm creates a list of epitopes with their relative abundance. To develop the algorithm, the specificity of lectins was determined by studying their binding to a large collection of carefully characterized glycoproteins. The glycoproteins in which literature information was not available were fully characterized by mass spectrometric and chromatographic methods. From this information, thousands of fingerprints were created of several hundred glycoform mixtures and resulted in detailed specificity definitions for many lectins. The performance of this microarray analysis kit was demonstrated by the analysis of a series of glycoproteins and their glycovariants. For example, the glycoprofile of a well-studied protein bovine pancreatic ribonuclease B (RNase B) was examined that contains a single N-linked glycosylation site at Asn34 and also contains high-mannose-type-N-linked glycans. Glycoanalysis of RNase B showed the presence of high levels of high-mannose type N-linked glycans only, which is in agreement with literature data. Strong signals were observed for Glc/Man-and Man-recognizing lectins and essentially no signals for complex-type and antenna-termini-recognizing lectins. The glyco-array kits method also stayed consistent to literature data in the analysis of prostate-specific antigen, porcine thyroglobulin, Tamm Horsfall glycoprotein and recombinant human erythropoietin. This method has been provided as a useful tool for glycobiologists for more detailed analyses and characterization of their proteins of interest.
The use of lectin microarray technology has far reaching implication in the understanding the role of glycans in cell processes and diseases. This technology can be especially useful in understanding the changes in glycosylation that accompany some diseases. Advances in interpreting glycan profile fingerprints with algorithms have improved the gathering of structural information that will enhance this field.
Because of the ability for lectins to specifically recognize certain glycan biomarkers, the potential of using them for targeting has been recognized.421–423 Glycotargeting using lectins have applications in antiviral-therapy424 and anti-tumor therapy.425–427 Lectins have been explored for application in selective drug targeting systems. Since carbohydrates are the most abundant modification in cells and lectins have the ability to bind to and recognize terminal sugar residues, they provide a means to introduce small drug molecules into cells. A comprehensive review of lectins in drug delivery has been presented by Woodley and co-workers.428 Glycotargeting with lectins is exemplified by the potential use of lectins as anti-HIV therapies.
The increased risk and fatalities associated with viral diseases such as hepatitis C and HIV-1 demands the development of novel anti-viral treatments, especially since current therapies have only limited success. Carbohydrates on viral envelopes play an important role in viral entry into the cells. Therefore, if an agent can bind to these glycans it may interrupt the function of the virus. Many such avenues have been explored in which lectins are used to develop anti-HIV therapy. The infection of cells by HIV-1 requires the fusion of the viral membrane with cellular membranes. This fusion is mediated by viral envelope proteins such as gp120 and gp41 along with cell surface receptors (CD4 and chemokine receptor) on the target cells 429. Therefore, the viral envelope proteins are attractive targets for anti-HIV-1 therapy. Agents that interact with the viral envelope may interfere with the entry into target cells. The binding of lectins to the glycans on the viral envelope may also force the virus to delete a portion of its glycans shield, making the virus more susceptible to attack.102
Several lectins had been isolated, identified and studied for anti-HIV activity. Cyanovirin (CV-N) is a 11kDa protein with 101 amino acids. It is isolated from the cyano bacterium Nostoc ellisposporum430 and has affinity for high-mannose glycans especiallyα-(1,2)-linked mannose oligomers.431,432 CV-N inactivates T-lymphocyte-tropic, laboratory strains of HIV type 1 and HIV type 2, as well as T-tropic, M-tropic and dual tropic primary clinical isolates of HIV-1. The anti-HIV activity of CV-N is related, in some part, to its binding to envelope protein gp120. As a result, it prevents in vitro fusion and transmission of HIV-1 between infected and uninfected cells. Continuous treatment of uninfected CEM-SS cells in the presence of high concentrations (9000 nM) of CV-N did attenuate the lethal effect of the virus. CV-N had an anti-HIV activity in CEM-SS cells with an EC50 of 0.1 nM. SVN (scytovirm) is a 9.7kDa peptide with 95 amino acids and has been shown to have affinity for α(1,2)-α(1,6)-mannose trisaccharide units. SVN inhibit HIV infection in T-tropic laboratory strain HIV-1 in CEM-SS cells with an EC50 of 0.3 nM.433 According to ELISA studies performed, the anti HIV activity is related to its binding to the glycosylated viral core proteins gp120, gp160 and to a lesser extent gp41. However, SVN did not bind to the CD4 receptor. Additional experiments also showed that SVN had to be present in the first eight hours of virus infection for it to be effective. Also pretreatment and removal of SVN led to normal HIV infection in uninfected CEM-SS cells. Altogether these studies suggest that SVN interferes with binding/fusion mechanism of the virus.
The lectin actinohivin has been shown to recognize mannose-type glycans103 and can inhibit T-cell and macrophage infection by HIV-1 in cell culture. Actinohivin inhibits T-tropic and M-tropic syncytium formation in HeLa/T-env/Tat and HeLa/CD4/Lac-Z cells with an IC50 of 60 nM and in HeLa/M-env/Tat and HOS/CD4/CCr-5/Lac-Z cells with an IC50 of 700 nM.
There are many other similar examples. For example Gerardia Savaglia (GSA) is a D-mannose – calcium dependent specific lectin dimer that has shown complete suppression of HIV-1 infection in the H9 cell line at a concentration of 0.2 μM103 and plant lectins from Galanthus nivalis (GNA) and Urtica dioica (UDA) have activities against HIV-1 in CEM cell cultures with an EC50 of 0.01 and 0.1 μM respectively.101,424,434,435 A lectin derived from an invertebrate Chaetopterus variapedatus (CVL) is specific to β-galactose and has an anti HIV activity in the range of 0.004–0.06 μM.436
All the examples above demonstrate that indeed binding of glycans important in pathological processes is a feasible strategy for developing therapeutics. If small molecule glycan “binders’ can be developed as described in the previous sections, they will also have the potential as therapeutic agents.
Overall, lectins are also very useful tool and potential therapeutics due to their ability to recognize carbohydrates with certain specificity. One potential problem is the size and protein nature of lectins, which may present delivery problems and may limit their application in vivo and under harsh conditions where stability might be an issue.
5. CONCLUSIONS
Carbohydrates are known to play important roles in a large number of biological and pathological processes. Therefore, high affinity and specificity “binders” for biologically important carbohydrates are potential medicinal and diagnostic agents. They can also be important research tools. The use of lectins in various applications has shown some initial promise. Progress in recent years in boronic acid-based carbohydrate binders (boronolectins), non-covalent binders, and metal-based binders have laid an excellent foundation for the future development of medicinal and diagnostic agents based on carbohydrate recognition. Especially important and new are ways of making peptide-based boronolectins and nucleic acid-based boronolectins, which give tremendous modularity and structural diversity for a wide range of application. The use of boronic acid-modified DNA aptamers for glycoproteins with the ability to differentiate glycoforms represents a very significant progress in glycoprotein detections. However, it is also true that peptide- and nucleic acid-based boronolectins may only be useful for in vitro applications and for targeting of cell surface biomarkers because of their potential membrane permeability problems. In this regard, small molecule lectin mimics including boronolectins continue to hold an advantage for intracellular applications. Because of the need for carbohydrate “binders” for a wide range of applications including concentration determination, cell-surface labeling, as diagnostic agents, delivery of imaging agents to cell surface, binding to cell surface carbohydrates involved in pathological events (such as metastasis) for therapeutic applications, and intracellular recognition and labeling, all the different methods of developing carbohydrate “binders” discussed will be very useful. We hope with this review, we can pursued more people in the carbohydrate sensing field to work on problems directly related to therapeutic and diagnostic applications.
Figure 28.
Structures of compounds 77–85
Figure 40.
NOESY contacts observed for the complex between β-cellobiose and receptor 119
Acknowledgments
Financial support for the work conducted in the authors’ lab from the Georgia Cancer Coalition, Georgia Research Alliance, the National Institutes of Health (CA123329, CA113917, DK55062, CA88343, NO1-CO-27184, GM084933, GM086925, and CA122536), and the Molecular Basis of Disease Program at GSU is gratefully acknowledged.
Biographies
Binghe Wang was born in 1962 in Beijing, China. He obtained his B.S. degree in medicinal chemistry from Beijing Medical College (Now Beijing University Health Sciences Center) in 1982, and his Ph.D. degree in medicinal chemistry from the University of Kansas, School of Pharmacy, in 1991. Subsequently, he did postdoctoral work with Professor Victor Hruby of the University of Arizona and Professor Ronald T. Borchardt of the University of Kansas. He started his independent career in 1994 as an Assistant Professor of Medicinal Chemistry at the University of Oklahoma, College of Pharmacy. In 1996, he moved to the Department of Chemistry, North Carolina State University, and was promoted to Associate Professor with tenure in 2000. In 2003, he moved to his current institution, Georgia State University, as Professor of Chemistry, Georgia Research Alliance Eminent Scholar in Drug Discovery, and Georgia Cancer Coalition Distinguished Scientist. He is Editor-in-Chief of Medicinal Research Reviews, published by John Wiley and Sons, and the chief editor of a book series entitled “Wiley Series in Drug Discovery and Development.” His research expertise includes drug delivery, drug design and synthesis, bioorganic chemistry and molecular recognition, fluorescent sensor design and synthesis, and new diagnostics. He has co-edited two books together with his colleagues: “Pharmaceutical Profiling in Drug Discovery for Lead Selection” by AAPS and “Drug Delivery: Principles and Applications” by John Wiley and Sons.
Shan Jin was born in Beijing, China. He received a bachelor’s degree in Chemistry from Peking University in Beijing, China in 2004. In the same year, he joined Professor Binghe Wang’s group at Georgia State University as a graduate student. He earned his M.S. degree from Georgia State University in 2007 and continued to pursue a PhD degree in the same group. His research interests focus on boronic acid-based carbohydrate sensors.
Yunfeng Cheng received his B.S. degree in chemistry from Zhejiang University of Technology in 2001 and M.S. degree in medicinal chemistry from Zhejiang University in 2004. He is presently a Ph.D. graduate student at Georgia State University. His current research directions are to study aptamer-based carbohydrate sensors and the antagonists of AI-2-mediated bacterial quorum sensing.
Suazette Reid received her B.S. degree in chemistry from Morgan State University in Baltimore, Maryland and her M.S. degree in Organic Chemistry from Georgia Institute of Technology. She is presently a Ph.D. candidate at Georgia State University. Her research involves the design and synthesis of novel small molecule inhibitors of the Hypoxia Inducible Factor (HIF) pathway.
Dr. Minyong Li is an adjunct assistant professor in the Department of Chemistry, Georgia State University. He received his BS and PhD degrees from China Pharmaceutical University in 1999 and in 2005, respectively. He began his research career in 2005 with Dr. Binghe Wang at Department of Chemistry, Georgia State University as postdoctoral research associate. In 2007, he was appointed adjunct assistant professor. He is a member of Sigma Xi-the Scientific Research Society (2006), America Chemical Society (1999) and QSAR and Modeling Society (2006). His research interests are in the general areas of medicinal chemistry and chemical biology. He has published about 30 peer-reviewed papers.
References
- 1.Alavi A, Axford JS, editors. Glycoimmunology. Vol. 376. New York: Plenum Press; 1995. [Google Scholar]
- 2.Timmer MS, Stocker BL, Seeberger PH. Probing Glycomics. Curr Opin Chem Biol. 2007;11(1):59–65. doi: 10.1016/j.cbpa.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Manilla G, Warren NL, Abney T, Atwood J, 3rd, Azadi P, York WS, Pierce M, Orlando R. Tools for Glycomics: Relative Quantitation of Glycans by Isotopic Permethylation using 13CH3I. Glycobiology. 2007;17(7):677–687. doi: 10.1093/glycob/cwm033. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M. Cell Surface Carbohydrates: Cell-type Specific Expression. In: Fukuda M, Hindsgaul O, editors. Molecular Glycobiology . New York: Oxford University Press; 1994. pp. 1–52. [Google Scholar]
- 5.Dennis JW. Changes in Glycosylation Associated with Malignant Transformation and Tumor Progression. In: Fukuda M, editor. Cell Surface Carbohydrates and Cell Development . Boca Raton: CRC Press; 1992. pp. 161–194. [Google Scholar]
- 6.Gabius H-J, Gabius S, editors. Lectins and Glycobiology . New York: Springer-Verlag; 1993. [Google Scholar]
- 7.Taylor ME, Drickamer K. Introduction to Glycobiology . New York: Oxford University Press, USA; 2006. [Google Scholar]
- 8.Fukuda M, Hindsgaul O. Molecular Glycobiology . New York: Oxford University Press; 1994. pp. 1–52. [Google Scholar]
- 9.Varki A. Glycan-based Interactions involving Vertebrate Sialic-acid-recognizing Proteins. Nature. 2007;446(7139):1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 10.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-Mediated Cell Adhesion in Cancer Metastasis and Angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey RC, Oegema TR, Skubitz KM, Pambuccian SE, Grindle SM, Skubitz AP. Cell Membrane Glycosylation Mediates the Adhesion, Migration, and Invasion of Ovarian Carcinoma Cells. Clin Exp Metastasis. 2003;20:143–152. doi: 10.1023/a:1022670501667. [DOI] [PubMed] [Google Scholar]
- 12.Catterall JB, Jones LMH, Turner GA. Membrane Protein Glycosylation and CD44 Content in the Adhesion of Human Ovarian Cancer Cells to Hyaluronan. Clin Exp Metastasis. 1999;17:583–591. doi: 10.1023/a:1006756518500. [DOI] [PubMed] [Google Scholar]
- 13.Takano R, Muchmore E, Dennis JW. Sialylation and Malignant Potential in Tumor-Cell Glycosylation Mutants. Glycobiology. 1994;4:665–674. doi: 10.1093/glycob/4.5.665. [DOI] [PubMed] [Google Scholar]
- 14.Pasmatzi E, Badavanis G, Monastirli A, Georgiou S, Sagriotis A, Sakkis T, Mantagos S, Varakis J, Stamatiou G, Tsambaos D. Qualitative and Quantitative Alterations of Cell Surface Carbohydrate Residues During Epidermal Morphogenesis. Anat Embryol. 2005;209:207–215. doi: 10.1007/s00429-004-0440-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Nakayama J, Ohyama C, Suzuki M, Suzuki A, Fukuda M, Fukuda MN. Cancer Res. Vol. 62. Sialyl: Lewis; 2002. X-dependent Lung Colonization of B16 Melanoma Cells through a Selectin-like Endothelial Receptor Distinct from E- or P-selectin; pp. 4194–4198. [PubMed] [Google Scholar]
- 16.Gout S, Tremblay PL, Huot J. Selectins and Selectin Ligands in Extravasation of Cancer Cells and Organ Selectivity of Metastasis. Clin Exp Metastasis. 2008;25(4):335–344. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 17.Ben-David T, Sagi-Assif O, Meshel T, Lifshitz V, Yron I, Witz IP. The Involvement of the sLe-a Selectin Ligand in the Extravasation of Human Colorectal Carcinoma Cells. Immunol Lett. 2008;116(2):218–224. doi: 10.1016/j.imlet.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H, Boelte KC, Lin PC. Endothelial Cell Adhesion Molecules and Cancer Progression. Curr Med Chem. 2007;14(4):377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 19.Kannagi R. Chang Gung Med J. 3. Vol. 30. Carbohydrate Antigen Sialyl: Lewis; 2007. A--its Pathophysiological Significance and Induction Mechanism in Cancer Progression; pp. 189–209. [PubMed] [Google Scholar]
- 20.Peracaula R, Tabares G, Royle L, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. Altered Glycosylation Pattern Allows the Distinction between Prostate-Specific Antigen (PSA) from Normal and Tumor Origins. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 21.Tabares G, Radcliffe CM, Barrabes S, Ramirez M, Aleixandre RN, Hoesel W, Dwek RA, Rudd PM, Peracaula R, de Llorens R. Different Glycan Structures in Prostate-specific Antigen from Prostate Cancer Sera in Relation to Seminal Plasma PSA. Glycobiology. 2006;16(2):132–145. doi: 10.1093/glycob/cwj042. [DOI] [PubMed] [Google Scholar]
- 22.Kyselova Z, Mechref Y, Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. Alterations in the Serum Glycome Due to Metastatic Prostate Cancer. J Proteome Res. 2007 doi: 10.1021/pr060664t S1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabares G, Jung K, Reiche J, Stephan C, Lein M, Peracaula R, de Llorens R, Hoesel W. Free PSA forms in Prostatic Tissue and Sera of Prostate Cancer Patients: Analysis by 2-DE and Western Blotting of Immunopurified Samples. Clin Biochem. 2007;40:5–6. 343–350. doi: 10.1016/j.clinbiochem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Barrabes S, Pages-Pons L, Radcliffe CM, Tabares G, Fort E, Royle L, Harvey DJ, Moenner M, Dwek RA, Rudd PM, De Llorens R, Peracaula R. Glycosylation of Serum Ribonuclease 1 Indicates a Major Endothelial Origin and Reveals an Increase in Core Fucosylation in Pancreatic Cancer. Glycobiology. 2007;17(4):388–400. doi: 10.1093/glycob/cwm002. [DOI] [PubMed] [Google Scholar]
- 25.Peracaula R, Tabares G, Lopez-Ferrer A, Brossmer R, de Bolos C, de Llorens R. Role of Sialyltransferases Involved in the Biosynthesis of Lewis Antigens in Human Pancreatic Tumour Cells. Glycoconjugate J. 2005;22(3):135–144. doi: 10.1007/s10719-005-0734-2. [DOI] [PubMed] [Google Scholar]
- 26.Peracaula R, Royle L, Tabares G, Mallorqui-Fernandez G, Barrabes S, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. Glycosylation of Human Pancreatic Ribonuclease: Differences between Normal and Tumor States. Glycobiology. 2003;13:227–244. doi: 10.1093/glycob/cwg019. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Salas E, Peracaula R, Frazier ML, De Llorens R. Ribonucleases Expressed by Human Pancreatic Adenocarcinoma Cell Lines. Eur J Biochem. 2000;267:1484–1494. doi: 10.1046/j.1432-1327.2000.01148.x. [DOI] [PubMed] [Google Scholar]
- 28.Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, Muro-Cacho CA, Yeatman TJ. Fibrinogen γ Overexpression in Pancreatic Cancer Identified by Large-scale Proteomic Analysis of Serum Samples. Cancer Res. 2006;66(5):2592–2599. doi: 10.1158/0008-5472.CAN-05-3659. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi R, Inoue S, Inagaki K, Sakai M, Miyamoto T, Komori T, Inagaki M, Isobe R. Biologically Active Glycosides from Asteroidea, 42. Isolation and Structure of a New Biologically Active Ganglioside Molecular Species from the Starfish Asterina pectinifera. Chem Pharm Bull (Tokyo) . 2006;54:287–291. doi: 10.1248/cpb.54.287. [DOI] [PubMed] [Google Scholar]
- 30.Sahni A, Khorana AA, Baggs RB, Peng H, Francis CW. FGF-2 Binding to Fibrin(ogen) is Required for Augmented Angiogenesis. Blood. 2006;107(1):126–131. doi: 10.1182/blood-2005-06-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosesson MW. Fibrinogen and Fibrin Structure and Functions. J Thromb Haemost. 2005;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 32.Trosset JY, Dalvit C, Knapp S, Fasolini M, Veronesi M, Mantegani S, Gianellini LM, Catana C, Sundstrom M, Stouten PF, Moll JK. Inhibition of Protein-Protein Interactions: The Discovery of Druglike beta-Catenin Inhibitors by Combining Virtual and Biophysical Screening. Proteins. 2006;64(1):60–67. doi: 10.1002/prot.20955. [DOI] [PubMed] [Google Scholar]
- 33.James TD, Shinkai S. Artificial Receptors as Chemosensors for Carbohydrates. Top Curr Chem. 2002;218:159–200. [Google Scholar]
- 34.Striegler S. Selective Carbohydrate Recognition by Synthetic Receptors in Aqueous Solution. Curr Org Chem. 2003;7:81–102. [Google Scholar]
- 35.Wang W, Gao X, Wang B. Boronic Acid-based Sensors for Carbohydrates. Curr Org Chem. 2002;6:1285–1317. [Google Scholar]
- 36.Adhikari P, Bachhawat-Sikder K, Thomas CJ, Ravishankar R, Jeyaprakash AA, Sharma V, Vijayan M, Surolia A. Mutational Analysis at Asn-41 in Peanut Agglutinin. A Residue Critical for the Binding of the Tumor-Associated Thomsen-Friedenreich Antigen. J Biol Chem. 2001;276(44):40734–40739. doi: 10.1074/jbc.M103040200. [DOI] [PubMed] [Google Scholar]
- 37.Hall DG. Boronic Acids. Wiley-VCH; 2004. [Google Scholar]
- 38.Badugu R, Lakowicz JR, Geddes CD. Boronic Acid Fluorescent Sensors for Monosaccharide Signaling based on the 6-methoxyquinolinium Heterocyclic Nucleus: Progress toward Noninvasive and Continuous Glucose Monitoring. Bioorg Med Chem. 2005;13(1):113–119. doi: 10.1016/j.bmc.2004.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boduroglu S, El Khoury JM, Reddy VD, Rinaldi PL, Hu J. A Colorimetric Titration Method for Quantification of Millimolar Glucose in a pH 7.4 Aqueous Phosphate Buffer. Bioorg Med Chem Lett . 2005;15:3974–3977. doi: 10.1016/j.bmcl.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 40.Cordes DB, Miller A, Gamsey S, Sharrett Z, Thoniyot P, Wessling R, Singaram B. Optical Glucose Detection across the Visible Spectrum using Anionic Fluorescent Dyes and a Viologen Quencher in a Two-Component Saccharide Sensing System. Org & Biomolecular Chem . 2005;3:1708–1713. doi: 10.1039/b418953a. [DOI] [PubMed] [Google Scholar]
- 41.Gamsey S, Baxter NA, Sharrett Z, Cordes DB, Olmstead MM, Wessling RA, Singaram B. The Effect of Boronic Aacid-positioning in an Optical Glucose-sensing Ensemble. Tetrahedron. 2006;62(26):6321–6331. [Google Scholar]
- 42.Gamsey S, Miller A, Olmstead MM, Beavers CM, Hirayama LC, Pradhan S, Wessling RA, Singaram B. Boronic Acid-Based Bipyridinium Salts as Tunable Receptors for Monosaccharides and alpha-Hydroxycarboxylates. J Am Chem Soc. 2007;129(5):1278–1286. doi: 10.1021/ja066567i. [DOI] [PubMed] [Google Scholar]
- 43.Gamsey S, Suri JT, Wessling RA, Singaram B. Continuous Glucose Detection using Boronic Acid-substituted Viologens in Fluorescent Hydrogels: Linker Effects and Extension to Fiber Optics. Langmuir. 2006;22(21):9067–9074. doi: 10.1021/la0617053. [DOI] [PubMed] [Google Scholar]
- 44.Heinrichs G, Schellentrager M, Kubik S. An Enantioselective Fluorescence Sensor for Glucose based on a Cyclic Tetrapeptide containing Two Boronic Acid Binding Sites. Eur J Org Chem. 2006(18):4177–4186. [Google Scholar]
- 45.Kabilan S, Marshall AJ, Sartain FK, Lee MC, Hussain A, Yang X, Blyth J, Karangu N, James K, Zeng J, Smith D, Domschke A, Lowea CR. Holographic Glucose Sensors. Biosen Bioelectron. 2005;20:1602–1610. doi: 10.1016/j.bios.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Kanekiyo Y, Tao H. Selective Glucose Sensing Utilizing Complexation with Fluorescent Boronic Acid on Polycation. Chem Lett . 2005;34:196. [Google Scholar]
- 47.Kanekiyo Y, Tao H. Glucose-specific Sensing with Boronic Acid Utilizing Enzymatic Oxidation. Chem Lett. 2006;35(8):852–853. [Google Scholar]
- 48.Kaur G, Fang H, Gao X, Li H, Wang B. Diboronic Glucose Sensors Tetrahedron. 2006;62:2583–2589. [Google Scholar]
- 49.Kaur G, Lin N, Fang H, Wang B. Boronic Acid-based Glucose Sensors. In: Geddes CD, Lakowicz JR, editors. Glucose Sensing Volume 11, Topics in Fluorescence Spectroscopy . Springer Press; 2006. pp. 377–397. [Google Scholar]
- 50.Murota K, Sakamoto S, Kudo K. Glucose Responsive Two-step Release of Hydrogel-immobilized Protein. Chem Lett. 2008;37(6):582–583. [Google Scholar]
- 51.Nativi C, Cacciarini M, Francesconi O, Vacca A, Moneti G, Ienco A, Roelens S. Pyrrolic Tripodal Receptors Effectively Recognizing Monosaccharides. Affinity Assessment through a Generalized Binding Descriptor. J Am Chem Soc. 2007;129(14):4377–4385. doi: 10.1021/ja068754m. [DOI] [PubMed] [Google Scholar]
- 52.Peng B, Qin Y. Lipophilic Polymer Membrane Optical Sensor with a Synthetic Receptor for Saccharide Detection. Anal Chem. 2008;80(15):6137–6141. doi: 10.1021/ac800946p. [DOI] [PubMed] [Google Scholar]
- 53.Roy D, Cambre JN, Sumerlin BS. Sugar-responsive Block Copolymers by Direct RAFT Polymerization of Unprotected Boronic Acid Monomers. Chem Commun. 2008(21):2477–2479. doi: 10.1039/b802293c. [DOI] [PubMed] [Google Scholar]
- 54.Rzayev ZMO, Beskardes O. Boron-containing Functional Copolymers for Bioengineering Applications. Coll Czech Chem Commun. 2007;72(12):1591–1630. [Google Scholar]
- 55.Samoie G, Wang W, Xu X, Escobedo JO, Schneider H-J, Strongin RM. A Chemomechanical Polymer that Functions in Blood Plasma with High Glucose Selectivity. Angew Chem Int Ed. 2006;45:5319–5322. doi: 10.1002/anie.200601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharrett Z, Gamsey S, Levine P, Cunningham-Bryant D, Vilozny B, Schiller A, Wessling RA, Singaram B. Boronic Acid-appended Bis-viologens as a New Family of Viologen Quenchers for Glucose Sensing. Tetrahedron Lett. 2008;49(2):300–304. [Google Scholar]
- 57.Takahashi S, Anzai J. Voltammetric Response of Au Electrodes Modified with Layer-by-layer Film Composed of Phenylboronic Acid Polymer and Carboxymethylcellose to D-Glucose and D-Fructose. Bunseki Kagaku. 2007;56(11):951–955. [Google Scholar]
- 58.Terraneo G, Potenza D, Canales A, Jimenez-Barbero J, Baldridge KK, Bernardi A. A Simple Model System for the Study of Carbohydrate--Aromatic Interactions. J Am Chem Soc. 2007;129(10):2890–2900. doi: 10.1021/ja066633g. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Li Y. Luminescent Nanocrystals for Nonenzymatic Glucose Concentration Determination. Chemistry. 2007;13(15):4203–4207. doi: 10.1002/chem.200700005. [DOI] [PubMed] [Google Scholar]
- 60.Wang QS, Li GQ, Mao WY, Qi HX, Li GW. Glucose-responsive Vesicular Sensor Based on Boronic Acid-glucose Recognition in the ARS/PBA/DBBTAB Covesicles. Sensor Actuat B-Chem . 2006;119(2):695–700. [Google Scholar]
- 61.Cordes DB, Gamsey S, Singaram B. Fluorescent Quantum Dots with Boronic Acid Substituted Viologens To Sense Glucose in Aqueous Solution. Angew Chem Int Ed. 2006;45:1–4. doi: 10.1002/anie.200504390. [DOI] [PubMed] [Google Scholar]
- 62.Burnett TJ, Peebles HC, Hageman JH. Synthesis of a Fluorescent Boronic Acid which Reversibly Binds to Cell Walls and a Diboronic Acid which Agglutinates Erythrocytes. Biochem Biophy Res Comm. 1980;96:157–162. doi: 10.1016/0006-291x(80)91194-8. [DOI] [PubMed] [Google Scholar]
- 63.Yang W, Fan H, Gao S, Gao X, Ni W, Karnati V, Hooks WB, Carson J, Weston B, Wang B. The First Fluorescent Diboronic Acid Sensor Specific for Hepatocellular Carcinoma Cells Expressing Sialyl Lewis X. Chem Biol. 2004;11:439–448. doi: 10.1016/j.chembiol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Yang W, Gao S, Gao X, Karnati VR, Ni W, Wang B, Hooks WB, Carson J, Weston B. Diboronic Acids as Fluorescent Probes for Cells Expressing Sialyl Lewis X. Bioorg Med Chem Lett. 2002;12:2175–2177. doi: 10.1016/s0960-894x(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZY, Smith BD. Anionic Saccharides Activate Liposomes Containing Phospholipids Bearing a Boronic Acid for Ca2+-Dependent Fusion. J Am Chem Soc. 1998;120:7141–7142. [Google Scholar]
- 66.Li M, Lin N, Huang Z, Du L, Fang H, Altier C, Wang B. Selecting Aptamers for a Glycoprotein through the Incorporation of the Boronic Acid Moiety. J Am Chem Soc. 2008;130(38):12636–12638. doi: 10.1021/ja801510d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manimala JC, Li Z, Jain A, VedBrat S, Gildersleeve JC. Carbohydrate Array Analysis of Anti-Tn Antibodies and Lectins Reveals Unexpected Specificities: Implications for Diagnostic and Vaccine Development. ChemBioChem. 2005;6:2229–2241. doi: 10.1002/cbic.200500165. [DOI] [PubMed] [Google Scholar]
- 68.Beckett ML, Wright GL., Jr Characterization of A Prostate Carcinoma Mucin-like Antigen (PMA) Int J Cancer. 1995;62:703–710. doi: 10.1002/ijc.2910620610. [DOI] [PubMed] [Google Scholar]
- 69.Byrd JC, Bresalier RS. Mucins and Mucin Binding Proteins in Colorectal Cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 70.Davidson B, Berner A, Nesland JM, Risberg B, Kristensen GB, Trope CG, Bryne M. Carbohydrate Antigen Expression in Primary Tumors, Metastatic Lesions, and Serous Effusions from Patients Diagnosed with Epithelial Ovarian Carcinoma: Evidence of Up-Regulated Tn and Sialyl Tn Antigen Expression in Effusions. Human Pathol. 2000;31:1081–1087. doi: 10.1053/hupa.2000.9776. [DOI] [PubMed] [Google Scholar]
- 71.Davidson B, Gotlieb WH, Ben-Baruch G, Kopolovic J, Goldberg I, Nesland JM, Berner A, Bjamer A, Bryne M. Expression of Carbohydrate Antigens in Advanced-Stage Ovarian Carcinomas and their Metastases - A Clinicopathologic Study. Gynecol Oncol. 2000;77:35–43. doi: 10.1006/gyno.1999.5708. [DOI] [PubMed] [Google Scholar]
- 72.Kim YJ, Varki A. Perspectives on the Significance of Altered Glycosylation of Glycoproteins in Cancer. Glycoconjugate J. 1997;14:569–576. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 73.Zanetti M, Lenert G, Springer GF. Idiotypes of Pre-existing Human Anti-carcinoma Anti-T and Anti-Tn antibodies. Int Immunol. 1993;5:113–119. doi: 10.1093/intimm/5.2.113. [DOI] [PubMed] [Google Scholar]
- 74.Jeong S, Eom TY, Kim SJ, Lee SW, Yu J. In vitro Selection of the RNA Aptamer Against the Sialyl Lewis X and Its Inhibition of the Cell Adhesion. Biochem Biophys Res Commun. 2001;281(1):237–243. doi: 10.1006/bbrc.2001.4327. [DOI] [PubMed] [Google Scholar]
- 75.Farmer RW, Richtsmeier WJ, Scher RL. Identification of Sialyl Lewis-X in Squamous Cell Carcinoma of the Head and Neck. Head Neck. 1998;20:726–731. doi: 10.1002/(sici)1097-0347(199812)20:8<726::aid-hed11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 76.Fujiwara M, Satoh H, Fujimoto M, Yazawa T, Ishikawa H, Horiguchi H, Ogata T, Matsui M, Kamma H. Lung Cancer Cell Line Producing Cytokeratin 19 Fragment and Sialyl Lewis X-I Antigen. Anticancer Res. 1998;18:1043–1046. [PubMed] [Google Scholar]
- 77.Tanaka K, Togo S, Nanko M, Ishikawa T, Ichikawa Y, Yamaoka H, Shimada H. Hepatogastroenterol. Vol. 46. Sialyl: Lewis; 1999. X Expression in Vascular Permeating Lesions as a Factor for Predicting Colorectal Cancer Metastasis; pp. 875–882. [PubMed] [Google Scholar]
- 78.Yamada N, Chung YS, Maeda K, Sawada T, Ikehara T, Nishino H, Okuno M, Sowa M. Increased Expression of Sialyl Lewis A and Sialyl Lewis X in Liver Metastases of Human Colorectal Carcinoma. Invasion Metastasis. 1995;15:95–102. [PubMed] [Google Scholar]
- 79.Yamada N, Chung YS, Sawada T, Okuno M, Sowa M. Role of SPan-1 Antigen in Adhesion of Human Colon Cancer Cells to Vascular Endothelium. Dig Dis Sci. 1995;40:1005–1012. doi: 10.1007/BF02064189. [DOI] [PubMed] [Google Scholar]
- 80.Yasuda M, Murakami M, Muramatsu T, Itoh J, Saito K, Kamoshida S, Kajiwara H, Osamura RY. Immunohistochemical Expression of Type-1 Carbohydrate Antigens: Availability of DU-PAN-2 on Pathological and Clinical Aspects. Acta Histochem Cytochem. 2003;36(3):185–192. [Google Scholar]
- 81.Osman N, McKenzie IFC, Ostenried K, Ioannou YA, Desnick RJ, Sandrin MS. Combined Transgenic Expression of α-Galactosidase and α1,2-Fucosyltransferase Leads to Optimal Reduction in the Major Xenoepitope Galα(1,3)Gal. Proc Nat Acad Sci USA. 1997;94(26):14677–14682. doi: 10.1073/pnas.94.26.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alpturk O, Rusin O, Fakayode SO, Wang WH, Escobedo JO, Warner IM, Crowe WE, Kral V, Pruet JM, Strongin RM. Lanthanide Complexes as Fluorescent Indicators for Neutral Sugars and Cancer Biomarkers. Proc Nat Acad Sci USA. 2006;103(26):9756–9760. doi: 10.1073/pnas.0603758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen ZZ, Wu MZ, Elson P, Kennedy AW, Belinson J, Casey G, Xu Y. Fatty Acid Composition of Lysophosphatidic Acid and Lysophosphatidylinositol in Plasma from Patients with Ovarian Cancer and other Gynecological Diseases. Gynecol Oncol. 2001;83:25–30. doi: 10.1006/gyno.2001.6357. [DOI] [PubMed] [Google Scholar]
- 84.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-Derived Lysophosphatidic Acid Supports the Progression of Osteolytic Bone Metastases in Breast Cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yonemori K, Katsumata N, Noda A, Uno H, Yunokawa M, Nakano E, Kouno T, Shimizu C, Ando M, Tamura K, Takeuchi M, Fujiwara Y. Development and Verification of a Prediction Model Using Serum Tumor Markers to Predict the Response to Chemotherapy of Patients with Metastatic or Recurrent Breast Cancer. J Cancer Res Clin Oncol. 2008;134(11):1199–1206. doi: 10.1007/s00432-008-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antila R, Jalkanen J, Heikinheimo O. Comparison of Secondary and Primary Ovarian Malignancies Reveals Differences in their Pre- and Perioperative Characteristics. Gynecol Oncol . 2006;101:97–101. doi: 10.1016/j.ygyno.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 87.Tserkezoglou A, Kontou S, Hadjieleftheriou G, Apostolikas N, Vassilomanolakis M, Sikiotis K, Salamalekis E, Tseke P, Magiakos G. Primary and Metastatic Ovarian Cancer in Patients with Prior Breast Carcinoma. Pre-Operative Markers and Treatment Results. Anticancer Res. 2006;26:2339–2344. [PubMed] [Google Scholar]
- 88.Kuusela P, Jalanko H, Roberts P, Sipponen P, Mecklin JP, Pitkanen R, Makela O. Comparison of CA 19-9 and Carcinoembryonic Antigen (CEA) Levels in the Serum of Patients with Colorectal Diseases. Br J Cancer. 1984;49:135–139. doi: 10.1038/bjc.1984.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ugrinska A, Bombardieri E, Stokkel MPM, Crippa F, Pauwels EKJ. Circulating Tumor Markers and Nuclear Medicine Imaging Modalities: Breast, Prostate and Ovarian Cancer. Quart J Nuclear Med. 2002;46:88–104. [PubMed] [Google Scholar]
- 90.Chhieng DC, Rodriguez-Burford C, Talley LI, Sviglin H, Stockard CR, Kleinberg MJ, Barnes MN, Partridge EE, Khazaeli MB, Grizzle WE. Expression of CEA, Tag-72, and Lewis-Y Antigen in Primary and Metastatic Lesions of Ovarian Carcinoma. Human Pathology. 2003;34:1016–1021. doi: 10.1053/s0046-8177(03)00355-1. [DOI] [PubMed] [Google Scholar]
- 91.Myers RB, Grizzle WE. Biomarker Expression in Prostatic Intraepithelial Neoplasia. Eur Urol. 1996;30:153–166. doi: 10.1159/000474165. [DOI] [PubMed] [Google Scholar]
- 92.Thor A, Gorstein F, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Tumor-associated Glycoprotein (TAG-72) in Ovarian Carcinomas defined by Monoclonal Antibody B72.3. J Natl Cancer Inst. 1986;76:995–1006. [PubMed] [Google Scholar]
- 93.Hammarstrom S. The Carcinoembryonic Antigen (CEA) Family: Structures, Suggested Functions and Expression in Normal and Malignant Tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 94.Kammerer U, Thanner F, Kapp M, Dietl J, Sutterlin M. Expression of Tumor Markers on Breast and Ovarian Cancer Cell Lines. Anticancer Res. 2003;23:1051–1055. [PubMed] [Google Scholar]
- 95.Ono M, Sakamoto M, Ino Y, Moriya Y, Sugihara K, Muto T, Hirohashi S. Cancer Cell Morphology at the Invasive Front and Expression of Cell Adhesion-related Carbohydrate in the Primary Lesion of Patients with Colorectal Carcinoma with Liver Metastasis. Cancer. 1996;78:1179–1186. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1179::AID-CNCR3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 96.Radhi JM, Lukie BE. Pancreatic Cancer and Fibrinogen Storage Disease. J Clin Pathol. 1998;51(11):865–867. doi: 10.1136/jcp.51.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanders DS, Kerr MA. Mol Pathol. Vol. 52. Lewis; 1999. Blood Group and CEA Related Antigens; Coexpressed Cell-cell Adhesion Molecules with Roles in the Biological Progression and Dissemination of Tumours; pp. 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scorilas A, Chiang PM, Katsaros D, Yousef GM, Diamandis EP. Molecular Characterization of a New Gene, CEAL1, Encoding for a Carcinoembryonic Antigen-like Protein with a Highly Conserved Domain of Eukaryotic Translation Initiation Factors. Gene. 2003;3:79–89. doi: 10.1016/s0378-1119(03)00499-2. [DOI] [PubMed] [Google Scholar]
- 99.McFadden K, Cocklin S, Gopi H, Baxter S, Ajith S, Mahmood N, Shattock R, Chaiken I. A Recombinant Allosteric Lectin Antagonist of HIV-1 envelope gp120 Interactions. Proteins: Struct, Funct Bioinf . 2007;67(3):617–629. doi: 10.1002/prot.21295. [DOI] [PubMed] [Google Scholar]
- 100.Wong NK, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the Oligosaccharides Associated with the Human Ovarian Tumor Marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 101.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Peumans W, Van Damme E, Schols D. Carbohydrate-binding Agents cause Deletions of Highly Conserved Glycosylation Sites in HIV GP120 - A New Therapeutic Concept to Hit the Achilles Heel of HIV. J Biol Chem. 2005;280(49):41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 102.Balzarini J. Targeting the Glycans of gp120: a Novel Approach Aimed at the Achilles Heel of HIV. Lancet Infect Dis. 2005;5(11):726–731. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- 103.Muller WE, Renneisen K, Kreuter MH, Schroder HC, Winkler I. The D-mannose-specific Lectin from Gerardia Savaglia Blocks Binding of Human Immunodeficiency Virus Type I to H9 Cells and Human Lymphocytes In Vitro. J Acquir Immune Defic Syndr. 1988;1(5):453–458. [PubMed] [Google Scholar]
- 104.Ji X, Gewurz H, Spear GT. Mannose Binding Lectin (MBL) and HIV. Mol Immunol. 2005;42(2):145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 105.Kochibe N, Furukawa K. Purification and Properties of a Novel Fucose-specific Hemagglutinin of Aleuria Aurantia. Biochemistry. 1980;19(13):2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- 106.Yamazaki N, Kojima S, Bovin NV, Andre S, Gabius S, Gabius HJ. Endogenous Lectins as Targets for Drug Delivery. Adv Drug Deliv Rev. 2000;43:2–3. 225–244. doi: 10.1016/s0169-409x(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 107.Anderson JM, Stimson WH, Gettinby G, Jhunjhunwala SK, Burt RW. Detection of Mammary Micrometastases by Pregnancy-Associated alpha 2-Glycoprotein (PAG, alpha 2-PAG or PAM) and Carcinoembryonic Antigen (CEA) Eur J Cancer. 1979;15(5):709–714. doi: 10.1016/0014-2964(79)90145-2. [DOI] [PubMed] [Google Scholar]
- 108.Wardman AG, Bowen M, Struthers LPL, Cooke NJ. The Diagnosis of Pleural Effusions - Are Cancer Markers Clinically Helpful. Med Pediatr Oncol. 2006;12(1):68–72. doi: 10.1002/mpo.2950120116. [DOI] [PubMed] [Google Scholar]
- 109.Hanisch FG, Stadie TRE, Deutzmann F, PeterKatalinic J. MUC1 Glycoforms in Breast Cancer - Cell Line T47D as a Model for Carcinoma-Associated Alterations of O-Glycosylation. Eur J Biochem. 1996;236:318–327. doi: 10.1111/j.1432-1033.1996.00318.x. [DOI] [PubMed] [Google Scholar]
- 110.Daly L, Ferguson J, Cram GP, Jr, Hars V, George SL, McCarty KS, Jr, Bast RC., Jr Comparison of a Novel Assay for Breast Cancer Mucin to CA15-3 and Carcinoembryonic Antigen. J Clin Oncol. 1992;10:1057–1065. doi: 10.1200/JCO.1992.10.7.1057. [DOI] [PubMed] [Google Scholar]
- 111.Seregni E, Coli A, Mazzucca N. Italian Group RIA-IRMA Test IAoNMA. Circulating Tumour Markers in Breast Cancer. Eur J Nucl Med Mol Imag. 2004;31:S15–22. doi: 10.1007/s00259-004-1523-z. [DOI] [PubMed] [Google Scholar]
- 112.Garcia MB, Blankenstein MA, van der Wall E, Nortier JWR, Schornagel JH, Thijssen JHH. Comparison of Breast Cancer Mucin (BCM) and CA 15-3 in Human Breast Cancer. Breast Cancer Res Treat. 1990;17(2):69–76. doi: 10.1007/BF01806286. [DOI] [PubMed] [Google Scholar]
- 113.Ecclestona DW, Miltonc JD, Hoffmana J, Barab J, Rhodes JM. Pancreatic Tumour Marker Anti-Mucin Antibody CAM 17.1 Reacts with a Sialyl Blood Group Antigen, Probably I, Which Is Expressed throughout the Human Gastrointestinal Tract. Digestion. 1998;59(6):665–670. doi: 10.1159/000007573. [DOI] [PubMed] [Google Scholar]
- 114.Hadden JW. Review Article The Immunology and Immunotherapy of Breast Cancer: an Update. Int J Immunopharmacol. 1999;21(2):79–101. doi: 10.1016/s0192-0561(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 115.Ferreira CS, Matthews CS, Missailidis S. DNA Aptamers that Bind to MUC1 Tumour Marker: Design and Characterization of MUC1-binding Single-stranded DNA Aptamers. Tumour Biol. 2006;27(6):289–301. doi: 10.1159/000096085. [DOI] [PubMed] [Google Scholar]
- 116.Iida S, Tsuiji H, Nemoto Y, Sano Y, Reddish MA, Irimura T. Expression of Mucin Genes and Carbohydrate Epitopes in 19 Human Colon Carcinoma Cell Lines. Oncol Res. 1998;10:407–414. [PubMed] [Google Scholar]
- 117.Pieve CD, Iley JN, Perkins A, Missailidis S. Development of Anti-MUC1 DNA Aptamers for the Imaging and Radiotherapy of Breast Cancer. Breast Cancer Res . 2006;8:32. [Google Scholar]
- 118.Zhang K, Baeckstrom D, Brevinge H, Hansson GC. Secreted MUC1 Mucins Lacking Their Cytoplasmic Part and Carrying Sialyl-Lewis a and x Epitopes from a Tumor Cell Line and Sera of Colon Carcinoma Patients Can Inhibit HL-60 Leukocyte Adhesion to E-selectin-expressing Endothelial Cells. J Cell Biochem. 1996;60:538–549. doi: 10.1002/(SICI)1097-4644(19960315)60:4%3C538::AID-JCB10%3E3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 119.Zhang K, Baeckstrom DHB, Hansson GC. Comparison of Sialyl-Lewis-a-carrying CD43 and MUC1 Mucins Secreted from a Colon Carcinoma Cell Line for E-selectin Binding and Inhibition of Leukocyte Adhesion. Tumor Biol. 1997;18:175–187. doi: 10.1159/000218028. [DOI] [PubMed] [Google Scholar]
- 120.Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and Common Glycoprotein Kigands for E-selectin and P-selectin on Myeloid Cells. J Cell Biol. 1994;125(2):471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Charrier JP, Tournel C, Michel S, Dalbon P, Jolivet M. Two-dimensional Electrophoresis of Prostate-specific Antigen in Sera of Men with Prostate Cancer or Benign Prostate Hyperplasia. Electrophoresis. 1999;20:4–5. 1075–1081. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<1075::AID-ELPS1075>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 122.Hittelet A, Legendre H, Nagy N, Bronckart Y, Pector JC, Salmon I, Yeaton P, Gabius HJ, Kiss R, Camby I. Upregulation of Galectins-1 and-3 in Human Colon Cancer and their Role in Regulating Cell Migration. Inter J Cancer. 2003;103:370–379. doi: 10.1002/ijc.10843. [DOI] [PubMed] [Google Scholar]
- 123.Nagy N, Legendre H, Engels O, Andre S, Kaltner H, Wasano K, Zick Y, Pector JC, Decaestecker C, Gabius HJ, Salmon I, Kiss R. Refined Prognostic Evaluation in Colon Carcinoma using Immunohistochemical Galectin Fingerprinting. 2003;97:1849–1858. doi: 10.1002/cncr.11268. [DOI] [PubMed] [Google Scholar]
- 124.Yang RY, Hsu DK, Yu L, Ni J, Liu FT. Cell Cycle Regulation by Galectin-12, a New Member of the Galectin Superfamily. J Biol Chem. 2001;276:20252–20260. doi: 10.1074/jbc.M010914200. [DOI] [PubMed] [Google Scholar]
- 125.Betka J, Plzak J, Smetana K, Gabius HJ. Galectin-3, an Endogenous Lectin, as a Tool for Monitoring Cell Differentiation in Head and Neck Carcinomas with Implications for Lectin-Glycan Functionality. Acta Oto-Laryngol . 2003;123:261–263. [PubMed] [Google Scholar]
- 126.Kayser K, Hoeft D, Hufnagl P, Caselitz J, Zick Y, Andre S, Kaltner H, Gabius HJ. Combined Analysis of Tumor Growth Pattern and Expression of Endogenous Lectins as a Prognostic Tool in Primary Testicular Cancer and its Lung Metastases. Histol Histopathol. 2003;18:771–779. doi: 10.14670/HH-18.771. [DOI] [PubMed] [Google Scholar]
- 127.Legendre H, Decaestecker C, Nagy N, Hendlisz A, Schuring MP, Salmon I, Gabius HJ, Pector JC, Kiss R. Prognostic Values of Galectin-3 and the Macrophage Migration Inhibitory Factor (MIF) in Human Colorectal Cancers. Modern Pathol. 2003;16:491–504. doi: 10.1097/01.MP.0000068235.45178.C1. [DOI] [PubMed] [Google Scholar]
- 128.Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D’Egidio M, Natoli CMP, Iacobelli S. 90K (Mac-2 BP) and Galectins in Tumor Progression and Metastasis. Glycoconjugate J. 2002;19:551–556. doi: 10.1023/B:GLYC.0000014085.00706.d4. [DOI] [PubMed] [Google Scholar]
- 129.Kopitz J, Andre S, von Reitzenstein C, Versluis K, Kaltner H, Pieters RJ, Wasano K, Kuwabara I, Liu FT, Cantz M, Heck AJ, Gabius HJ. Homodimeric Galectin-7 (p53-Induced Gene 1) is a Negative Growth Regulator for Human Neuroblastoma Cells. Oncogene. 2003;22:6277–6288. doi: 10.1038/sj.onc.1206631. [DOI] [PubMed] [Google Scholar]
- 130.Brewer CF. Thermodynamic Binding Studies of Galectin-1,-3 and-7. Glycoconjugate J. 2002;19:459–465. doi: 10.1023/B:GLYC.0000014075.62724.d0. [DOI] [PubMed] [Google Scholar]
- 131.Pielage JF, Cichon C, Greune L, Hirashima M, Kucharzik T, Schrnidt MA. Reversible Differentiation of Caco-2 Cells Reveals Galectin-9 as a Surface Marker Molecule for Human Follicle-associated Epithelia and M Cell-like Cells. Int J Biochem Cell Biol. 2007;39(10):1886–1901. doi: 10.1016/j.biocel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi T, Kageshita T, Saita N, Nakamura T. Galectin-9 in Physiological and Pathological Conditions. Glycoconjugate J. 2004;19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f. [DOI] [PubMed] [Google Scholar]
- 133.Yamauchi A, Irie A, Kihara M, Yokomise H, Hirashima M. Galectin-9 as a Predictive Factor for Metastasis of Breast Cancer. Breast Cancer Res Treat. 2002;76(Suppl 1) [Google Scholar]
- 134.Dexlin L, Ingvarsson J, Frendeus B, Borrebaeck CAK, Wingren C. Membrane Protein Profiling of Intact Cells Using Recombinant Antibody Microarrays. J Proteome Res. 2008;7:319–327. doi: 10.1021/pr070257x. [DOI] [PubMed] [Google Scholar]
- 135.Wong NK, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the Oligosaccharides Associated with the Human Ovarian Tumor Marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 136.Brooks SA, Leathem AJC. In: Lectin Histochemistry : A Concise Practical Handbook . Schumacher U, editor. Oxford: BIOS Scientific in association with the Royal Microscopical Society ; 1997. [Google Scholar]
- 137.Lin N, Yan J, Huang Z, Altier C, Li M, Carrasco N, Suyemoto M, Johnston L, Wang S, Wang Q, Fang H, Caton-Williams J, Wang B. Design and Synthesis of Boronic-acid-labeled Thymidine Triphosphate for Incorporation into DNA. Nucleic Acids Res. 2007;35(4):1222–1229. doi: 10.1093/nar/gkl1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klussmann S, editor. The Aptamer Handbook-Functional Oligonucleotides and Their Applications . Weinheim: Wiley-VCH Werlag GMbH & Co. KGaA; 2006. [Google Scholar]
- 139.Hanski C, Hanski ML, Zimmer T, Ogorek D, Devine P, Riecken EO. Characterization of the Major Sialyl-le(x)-Positive Mucins Present in Colon, Colon-Carcinoma, and Sera of Patients with Colorectal-Cancer. Cancer Res. 1995;55(4):928–933. [PubMed] [Google Scholar]
- 140.Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, Hirabayashi J. Evanescent-field Fluorescence-assisted Lectin Microarray: A New Strategy for Glycan Profiling. Nat Methods. 2005;2(11):851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- 141.Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Efficiency and Fidelity in a Click-chemistry Route to Triazole Dendrimers by The Copper(I)-catalyzed Ligation of Azides and Alkynes. Angew Chem Int Ed. 2004;43(30):3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 142.Miyamoto S. Clinical Applications of Glycomic Approaches for the Detection of Cancer and Other Diseases. Curr Opin Mol Ther. 2006;8(6):507–513. [PubMed] [Google Scholar]
- 143.Mahal LK, Pilobello K, Krishnamoorthy L. Development of a Lectin Microarray for the Glycomic Profiling of Cells. Glycobiology . 2004;14:1203. [Google Scholar]
- 144.Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. Development of a Lectin Microarray for the Rapid Analysis of Protein Glycopatterns. ChemBioChem. 2005;6(6):985–989. doi: 10.1002/cbic.200400403. [DOI] [PubMed] [Google Scholar]
- 145.Hsu K-L, Pilobello KT, Mahal LK. Analyzing the Dynamic Bacterial Glycome with a Lectin Microarray Approach. Nature Chem Biol. 2006;2:153–157. doi: 10.1038/nchembio767. [DOI] [PubMed] [Google Scholar]
- 146.Zheng T, Peelen D, Smith LM. Lectin Arrays for Profiling Cell Surface Carbohydrate Expression. J Am Chem Soc. 2005;127:9982–9983. doi: 10.1021/ja0505550. [DOI] [PubMed] [Google Scholar]
- 147.Goodarzi MT, Turner GA. A Lectin Method for Investigating the Glycosylation of Nanogram Amounts of Purified Glycoprotein. Glycoconjugate J. 1997;14:493–496. doi: 10.1023/a:1018507703589. [DOI] [PubMed] [Google Scholar]
- 148.Rosenfeld R, Bangio H, Gerwig GJ, Rosenberg R, Aloni R, Cohen Y, Amor Y, Plaschkes I, Kamerling JP, Maya RB. A Lectin Array-Based Methodology for the Analysis of Protein Glycosylation. J Biochem Biophys Methods. 2006;70:415–426. doi: 10.1016/j.jbbm.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 149.Hartley JH, James TD, Ward CJ. Synthetic Receptors. J Chem Soc, Perkin Trans I. 2000:3155–3184. [Google Scholar]
- 150.Karnati V, Gao X, Gao S, Yang W, Sabapathy S, Ni W, Wang B. A Selective Fluorescent Sensor for Glucose. Bioorg Med Chem Lett. 2002;12:3373–3377. doi: 10.1016/s0960-894x(02)00767-9. [DOI] [PubMed] [Google Scholar]
- 151.Yang W, Gao X, Wang B. Boronic Acid Compounds as Potential Pharmaceutical Agents. Med Res Rev. 2003;23:346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 152.Arimori S, Bell ML, Oh CS, Frimat KA, James TD. Modular Fluorescence Sensors for Saccharides. J Chem Soc, Perkin Trans. 2002;1 :803–808. [PubMed] [Google Scholar]
- 153.Badugu R, Lakowicz JR, Geddes CD. Enhanced Fluorescence Cyanide Detection at Physiologically Lethal Levels: Reduced ICT-Based Signal Transduction. J Am Chem Soc. 2005;127:3635–3641. doi: 10.1021/ja044421i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao H, McGill T, Heagy MD. Substituent Effects on Monoboronic Acid Sensors for Saccharides Based on N-Phenyl-1,8-Naphthalenedicarboximides. J Org Chem. 2004;69:2959–2966. doi: 10.1021/jo035760h. [DOI] [PubMed] [Google Scholar]
- 155.Cao HS, Heagy MD. Fluorescent Chemosensors for Carbohydrates: A Decade’s Worth of Bright Spies for Saccharides in Review. J Fluorescence. 2004;14:569–584. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 156.Yan J, Fang H, Wang B. Boronolectins and Fluorescent Boronolectins-An Examination of the Detailed Chemistry Issues Important for their Design. Med Res Rev. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 157.Duggan PJ, Offermann DA. The Preparation of Solid-supported Peptide Boronic Acids Derived from 4-borono-L-phenylalanine and their Affinity for Alizarin. Aust J Chem. 2007;60(11):829–834. [Google Scholar]
- 158.Liu X, Li X, Chan K, Zou W, Pribil P, Li XF, Sawyer MB, Li J. One-Pot Methylation in Glycomics Application: Esterification of Sialic Acids and Permanent Charge Construction. Anal Chem. 2007 doi: 10.1021/ac070091j S0003. [DOI] [PubMed] [Google Scholar]
- 159.Edwards NY, Sager TW, McDevitt JT, Anslyn EV. Boronic Acid Based Peptidic Receptors for Pattern-based Saccharide Sensing in Neutral Aqueous Media, An Application in Real-life Samples. J Am Chem Soc. 2007;129(44):13575–13583. doi: 10.1021/ja073939u. [DOI] [PubMed] [Google Scholar]
- 160.Halamek J, Wollenberger U, Stocklein W, Scheller FW. Development of a Biosensor for Glycated Hemoglobin. Electrochimica Acta. 2007;53(3):1127–1133. [Google Scholar]
- 161.Striegler S, Dittel M. A Sugar’s Choice: Coordination to a Mononuclear or a Dinuclear Copper(II) Complex. Inorg Chem. 2005;44(8):2728–2733. doi: 10.1021/ic048724p. [DOI] [PubMed] [Google Scholar]
- 162.Carothers JM, Oestreich SC, Szostak JW. Aptamers Selected for Higher-Affinity Binding Are Not More Specific for the Target Ligand. J Am Chem Soc. 2006:A–I. doi: 10.1021/ja060952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manimala JC, Wiskur SL, Ellington AD, Anslyn EV. Tuning the Specificity of a Synthetic Receptor using a Selected Nucleic Acid Receptor. J AM Chem SOc. 2004;126(50):16515–16519. doi: 10.1021/ja0478476. [DOI] [PubMed] [Google Scholar]
- 164.Liang PH, Wang SK, Wong CH. Quantitative Analysis of Carbohydrate-protein Interactions Using Glycan Microarrays: Determination of Surface and Solution Dissociation Constants. J Am Chem Soc. 2007;129(36):11177–11184. doi: 10.1021/ja072931h. [DOI] [PubMed] [Google Scholar]
- 165.Bertozzi CR, Kiessling LL. Chemical Glycobiology Science. 2001;291(5512):2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 166.Smith EA, Thomas WD, Kiessling LL, Corn RM. Surface Plasmon Resonance Imaging Studies of Protein-Carbohydrate Interactions. J Am Chem Soc. 2003;125:6140–6148. doi: 10.1021/ja034165u. [DOI] [PubMed] [Google Scholar]
- 167.Matteson DS. Alpha-amido Boronic Acids: A Synthetic Challenge and their Properties as Serine Protease Inhibitors. Med Res Rev. 2008;28(2):233–246. doi: 10.1002/med.20105. [DOI] [PubMed] [Google Scholar]
- 168.Zou Y, Broughton DL, Bicker KL, Thompson PR, Lavigne JJ. Peptide Borono Lectins (PBLs): A New Tool for Glycomics and Cancer Diagnostics. Chembiochem. 2007;8(17):2048–2051. doi: 10.1002/cbic.200700221. [DOI] [PubMed] [Google Scholar]
- 169.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee SL, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang YC, Pazdur R. Approval Summary for Bortezomib for Injection in the Treatment of Multiple Myeloma. Clin Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 170.Richardson PG. A Review of the Proteasome Inhibitor Bortezomib in Multiple Myeloma. Expert Opin Pharmacoth. 2004;5:1321–1331. doi: 10.1517/14656566.5.6.1321. [DOI] [PubMed] [Google Scholar]
- 171.Groll M, Berkers CR, Ploegh HL, Ovaa H. Crystal Structure of the Boronic Acid-based Proteasome Inhibitor Bortezomib in Complex with the Yeast 20S Proteasome. Structure. 2006;14(3):451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 172.Jin S, Zhu C, Li M, Wang B. Identification of the First Fluorescent α-Amidoboronic Acids that Change Fluorescent Properties upon Sugar Binding. Bioorg Med Chem Lett. 2008 doi: 10.1016/j.bmcl.2009.02.011. manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Springsteen G, Wang B. A Detailed Examination of Boronic Acid-Diol Complexation. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 174.Yan J, Springsteen G, Deeter S, Wang B. The Relationship among pKa, pH, and Binding Constants in the Interactions between Boronic Acids and Diols-It is not as Simple as It Appears. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 175.Jabbour A, Steinberg D, Dembitsky VM, Moussaieff A, Zaks B, Srebnik M. Synthesis and Evaluation of Oxazaborolidines for Antibacterial Activity against Streptococcus Mutans. J Med Chem. 2004;47:2409–2410. doi: 10.1021/jm049899b. [DOI] [PubMed] [Google Scholar]
- 176.DiCesare N, Lakowicz JR. New Sensitive and Selective Fluorescent Probes for Fluoride Using Boronic Acids. Anal Biochem. 2002;301:111–116. doi: 10.1006/abio.2001.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Badugu R, Lakowicz JR, Geddes CD. A Wavelength-Ratiometric Fluoride-Sensitive Probe based on the Quinolinium Nucleus and Boronic Acid Moiety. Sensors Actuators B-Chem . 2005;104:103–110. doi: 10.1016/j.snb.2004.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Swamy KMK, Lee YJ, Lee HN, Chun J, Kim Y, Kim SJ, Yoon J. A New Fluorescein Derivative Bearing a Boronic Acid Group as a Fluorescent Chemosensor for Fluoride Ion. J Org Chem. 2006;71(22):8626–8628. doi: 10.1021/jo061429x. [DOI] [PubMed] [Google Scholar]
- 179.Oehlke A, Auer AA, Jahre I, Walfort B, Ruffer T, Zoufala P, Lang H, Spange S. Nitro-substituted Stilbeneboronate Pinacol Esters and their Fluoro-adducts. Fluoride Ion Induced Polarity Enhancement of Arylboronate Esters. J Org Chem. 2007;72(12):4328–4339. doi: 10.1021/jo070084v. [DOI] [PubMed] [Google Scholar]
- 180.Badugu R, Lakowicz JR, Geddes CD. Excitation and Emission Wavelength Ratiometric Cyanide-Sensitive Probes for Physiological Sensing. Anal Chem. 2004;327:82–90. doi: 10.1016/j.ab.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Badugu R, Lakowicz JR, Geddes CD. Fluorescence Intensity and Lifetime-Based Cyanide Sensitive Probes for Physiological Safeguard. Analytica Chimica Acta. 2004;522:9–17. doi: 10.1016/j.aca.2004.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Badugu R, Lakowicz JR, Geddes CD. Cyanide-Sensitive Fluorescent Probes. Dyes Pigments. 2005;64:49–55. doi: 10.1016/j.dyepig.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoon J, Czarnik AW. Fluorescent Chemosensors of Carbohydrates. A Means of Chemically Communicating the Binding of Polyols in Water Based on Chelation-Enhanced Quenching. J Am Chem Soc. 1992;114:5874–5875. [Google Scholar]
- 184.James TD, Sandanayake KRAS, Iguchi R, Shinkai S. Novel Saccharide-Photoinduced Electron Transfer Sensors Based on the Interaction of Boronic Acid and Amine. J Am Chem Soc. 1995;117:8982–8987. [Google Scholar]
- 185.Eggert H, Frederiksen J, Morin C, Norrild JC. A New Glucose-selective Fluorescent Bisboronic Acid. First Report of Strong α-Furanose Complexation in Aqueous Solution at Physiological pH. J Org Chem. 1999;64:3846–3852. [Google Scholar]
- 186.Bielecki M, Eggert H, Norrild JC. A Fluorescent Glucose Sensor Binding Covalently to All Five Hydroxy Groups of α-D-Glucose. A Reinvestigation. J Chem Soc, Perkin Trans. 1999;2 :449–455. [Google Scholar]
- 187.Asher SA, Alexeev VL, Goponenko AV, Sharma AC, Lednev IK, Wilcox CS, Finegold DN. Photonic Crystal Carbohydrate Sensors: Low Ionic Strength Sugar Sensing. J Am Chem Soc. 2003;125:3322–3329. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 188.Cao HS, Heagy MD. Fluorescent Chemosensors for Carbohydrates: A Decade’s worth of Bright Spies for Saccharides in Review. J Fluor. 2004;14:569–584. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 189.Jiang S, Rusin O, Escobedo JO, Kim KK, Yang Y, Fakode S, Warner IM, Strongin RM. Stereochemical and Regiochemical Trends in the Selective Spectrophotometric Detection of Saccharides. J Am Chem Soc. 2006;128:12221–12228. doi: 10.1021/ja063651p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dowlut M, Hall DG. An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water. J Am Chem Soc. 2006;128:4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- 191.Draffin SP, Duggan PJ, Duggan SAM. Highly Fructose Selective Transport Promoted by Boronic Acids Based on a Pentaerythritol Core. Org Lett. 2001;3:917–920. doi: 10.1021/ol015560x. [DOI] [PubMed] [Google Scholar]
- 192.Zhong Z, Anslyn EV. A Colorimetric Sensing Ensemble for Heparin. J Am Chem Soc. 2002;124:9014–9015. doi: 10.1021/ja020505k. [DOI] [PubMed] [Google Scholar]
- 193.Mulla HR, Agard NJ, Basu A. 3-Methoxycarbonyl-5-nitrophenyl Boronic Acid: High Affinity Diol Recognition at Neutral pH. Bioorg Med Chem Lett. 2004;14(1):25–27. doi: 10.1016/j.bmcl.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 194.Badugu R, Lakowicz JR, Geddes CD. A Wavelength-Ratiometric pH Sensitive Probe Based on the Boronic Acid Moiety and Suppressed Sugar Response. Dyes and Pigments. 2004;61:227–234. doi: 10.1016/j.dyepig.2003.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang Z, Zhang DQ, Zhu DB. A New Saccharide Sensor Based on a Tetrathiafulvalene-Anthracene Dyad with a Boronic Acid Group. J Org Chem. 2005;70:5729–5732. doi: 10.1021/jo050682e. [DOI] [PubMed] [Google Scholar]
- 196.Gray CW, Jr, Houston TA. Boronic Acid Receptors for x-Hydroxycarboxlates: High Affinity of Shinkai’s Glucose Receptor for Tartrate. J Org Chem. 2002;67:5426–5428. doi: 10.1021/jo025876y. [DOI] [PubMed] [Google Scholar]
- 197.Cho BT. Oxazaborolidines as Asymmetric Inducers for the Reduction of Ketones and Ketimines. In: Hall DG, editor. Boronic Acids . Weinheim: Wiley-VCH; 2005. pp. 411–439. [Google Scholar]
- 198.Aharoni R, Bronstheyn M, Jabbour A, Zaks B, Srebnik M, Steinberg D. Oxazaborolidine Derivatives Inducing Autoinducer-2 Signal Transduction in Vibrio Harveyi. Bioorg Med Chem. 2008;16(4):1596–1604. doi: 10.1016/j.bmc.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 199.Cooper CR, Spencer N, James TD. Selective Fluorescence Detection of Fluoride Using Boronic Acids. Chem Commun. 1998:1365–1366. [Google Scholar]
- 200.Norrild JC, Eggert H. Evidence for Mono- and Bisdentate Boronate Complexes of Glucose in the Furanose Form. Application of 1JC-C Coupling Constants as a Structural Probe. J Am Chem Soc. 1995;117:1479–1484. [Google Scholar]
- 201.Norrild JC, Eggert H. Boronic Acids as Fructose Sensors. Structure Determination of the Complexes Involved Using 1J(CC) Coupling Constants. J Chem Soc Perkin Trans. 1996;2 :2583–2588. [Google Scholar]
- 202.Norrild JC. An Illusive Chiral Aminoalkylferroceneboronic Acid. Structural Assignment of a Atrong 1:1 Sorbitol Complex and New Insight into Boronate-polyol Interactions. J Chem Soc Perkin Trans. 2001;2 :719–726. [Google Scholar]
- 203.Norrild JC, Sotofte I. Crystal Structures of 2-(N, N-Dimethylaminoalkyl)ferroceneboronic Acids and Their Diol Derivatives The Quest for a B-N Intramolecular Bond in the Solid State. J Chem Soc Perkin Trans. 2001;2 :727–732. [Google Scholar]
- 204.Burgemeister T, Grobe-Einsler R, Grotstollen R, Mannschreck A, Wulff G. Fast Thermal Breaking and Formation of a B-N Bond in 2-(aminomethyl)benzene Boronates. Chem Ber. 1981;114:3403–3411. [Google Scholar]
- 205.Wulff G, Lauer M, Bohnke H. On the Chemistry of Binding-Sites. 5. Rapid Proton-Transfer as Cause of an Unusually Large Neighboring Group Effect. Angew Chem Int Ed. 1984;23:741–742. [Google Scholar]
- 206.Franzen S, Ni W, Wang B. A Study of the Mechanism of Electron Transfer Quenching by Boron-Nitrogen Adducts in Fluorescent Sensors. J Phys Chem B. 2003;107:12942–12948. [Google Scholar]
- 207.Ni W, Kaur G, Springsteen G, Wang B, Franzen S. Regulating the Fluorescence Intensity of an Anthracene Boronic Acid System: A B-N Bond or a Hydrolysis Mechanism. Bioorgan Chem. 2004;32:571–581. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 208.Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV. A Structural Investigation of the N-B Interaction in an o-(N, N-Dialkylaminomethyl)arylboronate System. J Am Chem Soc. 2006;128:1222–1223. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 209.Vandenberg R, Peters JA, Vanbekkum H. The Structure and (Local) Stability-Constants of Borate Esters of Monosacchharides and Disaccharides as Studied by B-11 and C- 13 NMR-Spectroscopy. Carbohydr Res. 1994;253:1–12. doi: 10.1016/0008-6215(94)80050-2. [DOI] [PubMed] [Google Scholar]
- 210.Vanduin M, Peters JA, Kieboom APG, Vanbekkum H. Studies on Borate Esters. 2. Structure and Stability of Borate Esters of Polyhydroxycarboxylates and Related Polyols in Aqueous Alkaline Media as Studied by B-11 NMR. Tetrahedron. 1985;41:3411–3421. [Google Scholar]
- 211.Vanduin M, Peters JA, Kieboom APG, Vanbekkum H. The Ph-Dependence of the Stability of Esters of Boric-Acid and Borate in Aqueous-Medium as Studied by B-11 NMR. Tetrahedron. 1984;40(15):2901–2911. [Google Scholar]
- 212.Springsteen G, Wang B. Alizarin Red as a General Fluorescent Reporter for Studying the Binding of Boronic Acids and Carbohydrates. Chem Commun. 2001:1608–1609. doi: 10.1039/b104895n. [DOI] [PubMed] [Google Scholar]
- 213.Stones D, Manku S, Lu X, Hall DG. Modular Solid-phase Synthetic Approach to Opitmizae Structural and electronic Properties of Oligoboronic Acids Receptors for the Aqueous Recognition of Oligosaccharides. Chem Eur J. 2004;10:92–100. doi: 10.1002/chem.200305400. [DOI] [PubMed] [Google Scholar]
- 214.Yang W, Yan J, Fang H, Wang B. The First Fluorescent Sensor for D-Glucarate Based on the Cooperative Action of Boronic Acid and Guanidinium Groups. Chem Commun. 2003:792–793. doi: 10.1039/b300098b. [DOI] [PubMed] [Google Scholar]
- 215.Yamamoto M, Takeuchi M, Shinkai S. Molecular Design of a PET-Based Chemosensor for Uronic Acids and Sialic Acids Utilizing a Cooperative Action of Boronic Acid and Metal Chelate. Tetrahedron. 1998;54:3125–3140. [Google Scholar]
- 216.Sun W, Bandmann H, Schrader T. A Fluorescent Polymeric Heparin Sensor. Chemistry. 2007;13(27):7701–7707. doi: 10.1002/chem.200700677. [DOI] [PubMed] [Google Scholar]
- 217.James TD. Saccharide-selective Boronic Acid based Photoinduced Electron Transfer (PET) Fluorescent Sensors. Creative Chemical Sensor Systems. 2007;277:107–152. Topics in Current Chemistry. [Google Scholar]
- 218.Tan W, Zhang DQ, Wang Z, Liu CM, Zhu DB. 4-(N, N-dimethylamine)benzonitrile (DMABN) Derivatives with Boronic Acid and Boronate Groups: New Fluorescent Sensors for Saccharides and Fluoride Ion. J Mater Chem. 2007;17(19):1964–1968. [Google Scholar]
- 219.Badugu R, Lakowicz JR, Geddes CD. Boronic Acid Fluorescent Sensors for Monosaccharide Signaling based on the 6-methoxyquinolinium Heterocyclic Nucleus: Progress toward Noninvasive and Continuous Glucose Monitoring. Bioorg Med Chem Lett. 2005;13(1):113–119. doi: 10.1016/j.bmc.2004.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Arimori S, Bosch LI, Ward CJ, James TD. Fluorescent Internal Charge Transfer (ICT) Saccharide Sensor. Tetrahedron Lett. 2001;42:4553–4555. [Google Scholar]
- 221.Arimori S, Bosch LI, Ward CJ, James TD. A D-glucose Selective Fluorescent Internal Charge Transfer (ICT) Sensor. Tetrahedron Lett. 2002;43(5):911–913. [Google Scholar]
- 222.Arimori S, Phillips MD, James TD. Probing Disaccharide Selectivity with Modular Fluorescent Sensors. Tetrahedron Lett. 2004;45:1539–1542. [Google Scholar]
- 223.Bissell RA, Bryan AJ, de Silva AP, McCoy CP, Fluorescent PET. (Photoinduced Electron Transfer) Sensors with Targeting/Anchoring Modules as Molecular Versions of Submarine Periscopes for Mapping Membrane-bounded Protons. J Chem Soc, Chem Commun . 1994:405–407. [Google Scholar]
- 224.Cooper CR, James TD. Synthesis and Eluation of D-Glucosamine-Selective Fluorescent Sensors. J Chem Soc-Perkin Trans. 2000;1 :963–969. [Google Scholar]
- 225.De Silva AP, Gunaratne HQN, Fluorescent PET. (Photoinduced Electron Transfer) Sensors Selective for Submicromolar Calcium with Quantitatively Predictable Spectral and Ion-Binding Properties. J Chem Soc, Chem Commun . 1990:186–188. [Google Scholar]
- 226.DiCesare N, Lakowicz JR. Spectral Properties of Fluorophores Combining the Boronic Acid Group with Electron Donor or Withdrawing Groups. Implication in the Development of Fluorescence Probes for Saccharides. J Phys Chem A. 2001;105:6834–6840. doi: 10.1021/jp010076x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.DiCesare N, Lakowicz JR. Fluorescent Probe for Monosaccharides Based on a Functionalized Boron-dipyrromethene with a Boronic Acid Group. Tetrahedron Lett. 2001;42:9105–9108. doi: 10.1016/S0040-4039(01)02022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.DiCesare N, Lakowicz JR. Chalcone-analogue Fluorescent Probes for Saccharides Signaling Using the Boronic Acid Group. Tetrahedron Lett. 2002;43:2615–2618. doi: 10.1016/s0040-4039(02)00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gao X, Zhang Y, Wang B. New Boronic Acid Fluorescent Reporter Compounds II. A Naphthalene-based Sensor Functional at Physiological pH. Org Lett. 2003;5:4615–4618. doi: 10.1021/ol035783i. [DOI] [PubMed] [Google Scholar]
- 230.James TD, Linnane P, Shinkai S. Fluorescent Saccharide Receptors: A Sweet Solution to the Design, Assembly and Evaluation of Boronic Acid Derived PET Sensors. Chem Commun. 1996:281–288. [Google Scholar]
- 231.Kaur G, Fang H, Gao X, Li H, Wang B. Substituent Effect on Anthracene-based Bisboronic Acid Glucose Sensors. Tetrahedron. 2006;62:2583–2589. [Google Scholar]
- 232.Kral V, Rusin O, Schmidtchen FP. Novel Porphyrin-Cryptand Cyclic Systems: Receptors for Saccharide Recognition in Water. Org Lett. 2001;3:873–876. doi: 10.1021/ol007069w. [DOI] [PubMed] [Google Scholar]
- 233.Luis GP, Granda M, Badia R, Diaz-Garcia ME. Selective Fluorescent Chemosensor for Fructose Form. Analyst. 1959;123(1):155–158. [Google Scholar]
- 234.Mizutani T, Kurahashi K, Murakami T, Matsumi N, Ogoshi H. Molecular Recognition of Carbohydrates by Zinc Porphyrins: Lewis Acid Lewis Base Combinations as a Dominant Factor for their Selectivity. J Am Chem Soc. 1997;(119):8991–9001. [Google Scholar]
- 235.Tong AJ, Yamauchi A, Hayashita T, Zhang ZY, Smith BD, Teramae N. Boronic Acid Fuorophore/beta-Cyclodextrin Complex Sensors for Selective Sugar Recognition in Water. Anal Chem. 2001;73(7):1530–1536. doi: 10.1021/ac001363k. [DOI] [PubMed] [Google Scholar]
- 236.Viguier RFH, Hulme AN. Sensitized Europium Complex Generated by Micromolar Concentrations of Copper(I): Toward the Detection of Copper(I) in Biology. J Am Chem Soc. 2006;128:11370–11371. doi: 10.1021/ja064232v. [DOI] [PubMed] [Google Scholar]
- 237.Wang Q, Li G, Xiao W, Qi H, Li G. Glucose-responsive Vesicular Sensor Based on Boronic Acid glucose Recognition in the ARS/PBA/DBBTAB Covesicles. Sens Actuators, B . 2006;119:695–700. [Google Scholar]
- 238.Yang W, Springsteen G, Yan J, Deeter S, Wang B. A Novel Type of Fluorescent Boronic Acid that Shows Large Fluorescence Intensity Changes upon Binding with a Diol in Aqueous Solution at Physiological pH. Bioorg Med Chem Lett. 2003;13:1019–1022. doi: 10.1016/s0960-894x(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 239.Schiller A, Wessling RA, Singaram B. A Fluorescent Sensor Array for Saccharides Based on Boronic Acid Appended Bipyridinium Salts. Angew Chem Int Ed. 2007;46(34):6457–6459. doi: 10.1002/anie.200701888. [DOI] [PubMed] [Google Scholar]
- 240.Sandanayake KRAS, Shinkai S. Novel Molecular Sensors for Saccharides Based on the Interaction of Boronic Acid and Amines: Saccharide Sensing in Neutral Water. J Chem Soc, Chem Commun . 1994:1083–1084. [Google Scholar]
- 241.Lavigne JJ, Anslyn EV. Teaching Old Indicators New Tricks: A Colorimetric Chemosensing Ensemble for Tartrate/Malate in beverages. Angew Chem Int Ed. 1999;38:3666–3669. [PubMed] [Google Scholar]
- 242.Cabell LA, Monahan M-K, Anslyn EV. A Competition Assay for Determining Glucose-6-phosphate Concentration with a Tris-Boronic Acid Receptor. Tetrahedron Lett. 1999;40:7753–7756. [Google Scholar]
- 243.Lu WB, Zhang LH, Ye XS, Su JC, Yu ZX. Molecular Receptors for Monosaccharides: Di(pyridyl)naphthyridine and Di(quinolyl)naphthyridine. Tetrahedron. 2006;62(8):1806–1816. [Google Scholar]
- 244.Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MRK, Sanders S, Plattner JJ. Discovery of a New Boron-Containing Antifungal Agent, 5-Fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), for the Potential Treatment of Onychomycosis. J Med Chem. 2006:A–D. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]
- 245.Schertzer BM, Baker SN, Diver ST, Baker GA. A General, Modular Approach to a New Family of Amine-Substituted Arylboronic Acid Saccharide Chemosensors. Aust J Chem. 2006;59(9):633–639. [Google Scholar]
- 246.Badugu R, Lakowicz JR, Geddes CD. Wavelength-ratiometric near Physiological pH Sensors based on 6-aminoquinolinium Boronic Acid Probes. Talanta. 2005;66(3):569–574. doi: 10.1016/j.talanta.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ma Y, Yang XR. One Saccharide Sensor Based on the Complex of the Boronic Acid and the Monosaccharide using Electrochemical Impedance Spectroscopy. J Electroanalytical Chem. 2005;580(2):348–352. [Google Scholar]
- 248.Gao X, Zhang Y, Wang B. A Highly Fluorescent Water-soluble Naphthalene-based Boronic Acid Reporter for Saccharide Sensing that also Shows Ratiometric UV Changes. Tetrahedron. 2005;61:9111–9117. [Google Scholar]
- 249.Akay S, Yang W, Wang J, Lin L, Wang B. Synthesis and Evaluation of Dual Wavelength Fluorescent Benzo[b]thiophene Boronic Acid Derivatives for Sugar Sensing. Chem Biol Drug Design. 2007;70(4):279–289. doi: 10.1111/j.1747-0285.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 250.Wang J, Jin S, Wang B. A New Boronic Acid Fluorescent Reporter that Changes Emission Intensity at Three Wavelengths upon Sugar Binding. Tetrahedron Lett. 2005;46:7003–7006. [Google Scholar]
- 251.Gao X, Zhang Y, Wang B. Naphthalene-Based Water-Soluble Fluorescent Boronic Acid Isomers Suitable for Ratiometric and Off-On Sensing of Saccharides at Physiological pH. New J Chem. 2005;29:579–586. [Google Scholar]
- 252.Zhang Y, Gao X, Hardcastle K, Wang B. Water-soluble Fluorescent Boronic Acid Compounds for Saccharide Sensing: Structural Effect on Their Fluorescence Properties. Chem Eur J. 2006;12:1377–1384. doi: 10.1002/chem.200500982. [DOI] [PubMed] [Google Scholar]
- 253.Coskun A, Akkaya EU. Three-Point Recognition and Selective Fluorescence Sensing of L-DOPA. Org Lett. 2004;6(18):3107–3109. doi: 10.1021/ol0488744. [DOI] [PubMed] [Google Scholar]
- 254.Hutt K, Hernandez R, Heagy MD. Toward Intrinsically Fluorescent Proteomimetics: Fluorescent Probe Response to Alpha Helix Structure of Poly-gamma-benzyl-L-glutamate. Bioorg Med Chem Lett. 2006;16(20):5436–5438. doi: 10.1016/j.bmcl.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 255.Trupp S, Schweitzer A, Mohr JG. A Fluorescent Water-soluble Naphthalimide-based Receptor for Saccharides with Highest Sensitivity in the Physiological pH Range. Org Biomol Chem. 2006;4:2965–2968. doi: 10.1039/b604716e. [DOI] [PubMed] [Google Scholar]
- 256.Wang J, Jin S, Akay S, Wang B. Design and Synthesis of a Long-wavelength Fluorescent Boronic Acid Reporter Compound. Eur J Org Chem. 2007:2091–2099. [Google Scholar]
- 257.Jin S, Wang J, Li M, Wang B. Synthesis, Evaluation, and Computational Studies of Naphthalimide-Based Long-Wavelength Fluorescent Boronic Acid Reporters. Chem-Eur J . 2008;14:2795–2804. doi: 10.1002/chem.200701785. [DOI] [PubMed] [Google Scholar]
- 258.Trupp S, Schweitzer A, Mohr GJ. A Fluorescent Water-Soluble Naphthalimide-Based Receptor For Saccharides With Highest Sensitivity In The Physiological pH Range. Org Biomol Chem. 2006;4:2965–2968. doi: 10.1039/b604716e. [DOI] [PubMed] [Google Scholar]
- 259.Liu LH, Zhang H, Zhang X, Jiang YB. Ratiometric Fluorescent Receptors for Monosaccharides Based on the Intramolecular Charge Transfer in p-dialkylaminobenzanilides. Chinese J Chem. 2005;23(4):421–426. [Google Scholar]
- 260.Egawa Y, Gotoh R, Niina S, Anzal J. Ortho-azo Substituted Phenylboronic Acids for Colorimetric Sugar Sensors. Bioorg Med Chem Lett. 2007;17(13):3789–3792. doi: 10.1016/j.bmcl.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 261.Cordes DB, Miller A, Gamsey S, Singaram B. Simultaneous Use of Multiple Fluorescent Reporter Dyes for Glucose Sensing in Aqueous Solution. Anal Bioanal Chem. 2007;(387):2767–2773. doi: 10.1007/s00216-007-1128-z. [DOI] [PubMed] [Google Scholar]
- 262.Yu Y, Zhang D, Tan W, Wang Z, Zhu D. Formation of the Intermediate Nitronyl Nitroxide Anthracene Dyad Sensing Saccharides. Bioorg Med Chem Lett. 2007;17:94–96. doi: 10.1016/j.bmcl.2006.09.081. [DOI] [PubMed] [Google Scholar]
- 263.Zhang T, Anslyn EV. A Colorimetric Boronic Acid Based Sensing Ensemble for Carboxy and Phospho Sugars. Org Lett. 2006;8:1649–1652. doi: 10.1021/ol060279+. [DOI] [PubMed] [Google Scholar]
- 264.Swamy KMK, Jang YJ, Park MS, Koh HS, Lee SK, Yoon YJ, Yoon J. A Sorbitol-selective Fluorescence Sensor. Tetrahedron Lett. 2005;46(20):3453–3456. [Google Scholar]
- 265.Fang H, Kaur G, Wang B. Progress in Boronic Acid-Based Fluorescent Glucose Sensors. J Fluorescence. 2004;14(5):481–489. doi: 10.1023/b:jofl.0000039336.51399.3b. [DOI] [PubMed] [Google Scholar]
- 266.James TD, Sandanayake KRAS, Shinkai S. Novel Photoinduced Electron-transfer Sensor for Saccharides Based on the Interaction of Boronic Acid and Amine. J Chem Soc, Chem Commun . 1994:477–478. [Google Scholar]
- 267.Houston TA, Levonis SM, Kiefel MJ. Tapping into Boron/alpha-hydroxycarboxylic Acid Interactions in Sensing and Catalysis. Aust J Chem. 2007;60(11):811–815. [Google Scholar]
- 268.Zhao J, James TD. Chemoselective and Enantioselective Fluorescent Recognition of Sugar Alcohols by a Bisboronic Acid Receptor. J Mater Chem. 2005;15:2896–2901. [Google Scholar]
- 269.Koskelaa SJM, Fylesb TM, James TD. A Ditopic Fluorescent Sensor for Potassium Fluoride. Chem Commun. 2005:945–947. doi: 10.1039/b415522j. [DOI] [PubMed] [Google Scholar]
- 270.Zhao J, James TD. Enhanced Fluorescence and Chiral Discrimination for Tartaric Acid in a Dual Fluorophore Boronic Acid Receptor. Chem Commun. 2005;14:1889–1891. doi: 10.1039/b418279k. [DOI] [PubMed] [Google Scholar]
- 271.Zhao J, Davidson MG, Mahon MF, Kociok-Kohn G, James TD. An Enantioselective Fluorescent Sensor for Sugar Acids. J Am Chem Soc. 2004;126(49):16179–16186. doi: 10.1021/ja046289s. [DOI] [PubMed] [Google Scholar]
- 272.Liang XF, James TD, Zhao JZ. 6,6′-Bis-substituted BINOL Boronic Acids as Enantio Selective and Chemoselective Fluorescent Chemosensors for D-sorbitol. Tetrahedron. 2008;64(7):1309–1315. [Google Scholar]
- 273.Gravel M, Kim A, Mark Zak T, Berube C, Hall DG. Universal Solid-Phase Approach for the Immobilization, Derivatization, and Resin-to-Resin Transfer Reactions of Boronic Acids. J Org Chem. 2002;67:3–15. doi: 10.1021/jo0106501. [DOI] [PubMed] [Google Scholar]
- 274.Manku S, Hall DG. Synthesis, Decoding, and Preliminary Screening of a Bead-supported Split-pool Library of Triboronic Acid. Receptors for Complex Oligosaccharides. Aust J Chem. 2007;60(11):824–828. [Google Scholar]
- 275.Yang W, He H, Drueckhammer DG. Computer-Guided Design in Molecular Recognition: Design and Synthesis of a Glucopyranose Receptor. Angew Chem, Int Ed . 2001;40:1714–1718. [PubMed] [Google Scholar]
- 276.Snow RJ, Bachovchin WW, Barton RW, Campbell SJ, Coutts SJ, Freeman DM, Gutheil WG, Kelly TA, Kennedy CA, Krolikowski DA, Leonard SF, Pargellis CA, Tong I, Adams J. Studies on Proline Boronic Acid Dipeptide Inhibitors of Dipeptidyl Peptidase-iv - Identification of a Cyclic Species Containing a B-N Bond. J Am Chem Soc. 1994;116:10860–10869. [Google Scholar]
- 277.Geele G, Garrett E, Hageman HJ. Effect of Benzene Boronic Acids on Sporulation and on Production of Enzymes in Bacillus subtilis Cells. Spores VI Am Soc Micro. 1975:391–396. [Google Scholar]
- 278.Davis-Mancini K, Lopez IP, Hageman JH. Benzeneboronic Acid Selectivity Inhibits Sporulation. J Bacteriol. 1978;136:625–630. doi: 10.1128/jb.136.2.625-630.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu SQ, Wollenberger U, Katterle M, Scheller FW. Ferroceneboronic Acid-based Amperometric Biosensor for Glycated Hemoglobin. Sens Actuator, B . 2006;113(2):623–629. [Google Scholar]
- 280.Adamczyk M, Chen YY, Johnson DD, Mattingly PG, Moore JA, Pan Y, Reddy RE. Chemiluminescent Acridinium-9-carboxamide Boronic Acid Probes: Application to a Homogenous Glycated Hemoglobin Assay. Bioorg Med Chem Lett. 2006;16(5):1324–1328. doi: 10.1016/j.bmcl.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 281.Halamek J, Wollenberger U, Stocklein WFM, Warsinke A, Scheller FW. Signal Amplification in Immunoassays using Labeling via Boronic Acid Binding to the Sugar Moiety of Immunoglobulin G: Proof of Concept for Glycated Hemoglobin. Anal Lett. 2007;40(7):1434–1444. [Google Scholar]
- 282.Sorensen MD, Martins R, Hindsgaul O. Assessing the Terminal Glycosylation of a Glycoprotein by the Naked Eye. Angew Chem Int Ed Engl. 2007;46(14):2403–2407. doi: 10.1002/anie.200604936. [DOI] [PubMed] [Google Scholar]
- 283.Monzo A, Bonn GK, Guttman A. Boronic Acid-lectin Affinity Chromatography. 1. Simultaneous Glycoprotein Binding with Selective or Combined Elution. Anal Bioanal Chem. 2007;389:7–8. 2097–2102. doi: 10.1007/s00216-007-1627-y. [DOI] [PubMed] [Google Scholar]
- 284.Liu SQ, Bakovic L, Chen AC. Specific Binding of Glycoproteins with Poly(aniline boronic acid) Thin Film. J Electroanalytical Chem. 2006;591(2):210–216. [Google Scholar]
- 285.Ivanov AE, Shiomori K, Kawano Y, Galaev IY, Mattiasson B. Effects of Polyols, Saccharides, and Glycoproteins on Thermoprecipitation of Phenylboronate-containing Copolymers. Biomacromolecule. 2006;7(4):1017–1024. doi: 10.1021/bm050208i. [DOI] [PubMed] [Google Scholar]
- 286.Rick J, Chou TC. Amperometric Protein Sensor - Fabricated as a Polypyrrole, Poly- aminophenylboronic Acid Bilayer. Biosen Bioelectron. 2006;22(3):329–335. doi: 10.1016/j.bios.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 287.Liu SQ, Miller B, Chen AC. Phenylboronic Acid Self-assembled Layer on Glassy Carbon Electrode for Recognition of Glycoprotein Peroxidase. Electrochem Commun. 2005;7(12):1232–1236. [Google Scholar]
- 288.Cambre JN, Roy D, Gondi SR, Sumerlin BS. Facile Strategy to Well-defined Water-soluble Boronic Acid (Co)Polymers. J Am Chem Soc. 2007;129(34):10348–10349. doi: 10.1021/ja074239s. [DOI] [PubMed] [Google Scholar]
- 289.Kameta N, Hiratani K. Synthesis of Supramolecular Boron Complexes and their Molecular Recognition. Journal of Synthetic Organic Chemistry Japan. 2007;65(10):959–968. [Google Scholar]
- 290.Kanekiyo Y, Sato H, Tao H. Saccharide Sensing Based on Saccharide-Induced Conformational Changes in Fluorescent Boronic Acid Polymersa. Macromol Rapid Commun. 2005;26:1542–1546. [Google Scholar]
- 291.Elmas B, Senel S, Tuncel A. A New Thermosensitive Fluorescent Probe for Diol Sensing: Poly(N-isopropylacrylamide-co-vinylphenylboronic acid)-alizarin Red S Complex. React Funct Polym. 2007;67(2):87–96. [Google Scholar]
- 292.Suzuki I, Yamauchi A, Sakashita Y, Hirose K, Miura T, Hayashita T. Fluorescence Response Mechanism of D-glucose Selectivity for Supramolecular Probes Composed of Phenylboronic-acid-modified Beta-cyclodextrin and Styrylpyridinium Dyes. Anal Sci. 2007;23(10):1167–1171. doi: 10.2116/analsci.23.1167. [DOI] [PubMed] [Google Scholar]
- 293.Ivanov AE, Galaev IY, Mattiasson B. Interaction of Sugars, Polysaccharides and Cells with Boronate-containing Copolymers: From Solution to Polymer Brushes. J Mol Recognit. 2006;19(4):322–331. doi: 10.1002/jmr.792. [DOI] [PubMed] [Google Scholar]
- 294.Deore BA, Braun MD, Freund MS. pH Dependent Equilibria of Poly(anilineboronic acid)-saccharide Complexation in Thin Films. Macromolecular Chemistry and Physics. 2006;207(7):660–664. [Google Scholar]
- 295.Kato D, Iijima S, Kurita R, Sato Y, Jia JB, Yabuki S, Mizutani F, Niwa O. Electrochemically Amplified Detection for Lipopolysaccharide using Ferrocenylboronic Acid. Biosen Bioelectron. 2007;22(7):1527–1531. doi: 10.1016/j.bios.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 296.Takahashi S, Anzai J. Voltammetric Response of Phenylboronic Acid Polymer-modified Gold Electrode to Sugars. Sensor Lett. 2005;3(3):244–247. doi: 10.2116/analsci.20.757. [DOI] [PubMed] [Google Scholar]
- 297.Chen H, Lee M, Lee J, Kim J-H, Gal Y-S, Hwang Y-H, An WG, Koh K. Formation and Characterization of Self-Assembled Phenylboronic Acid Derivative Monolayers toward Developing Monosaccharide Sensing-Interface. Sensors. 2007;7:1480–1495. [Google Scholar]
- 298.Liu H, Gao ZB, Yao Z, Zheng S, Li Y, Zhu W, Tan X, Luo X, Shen J, Chen K, Hu GY, Jiang H. Discovering Potassium Channel Blockers from Synthetic Compound Database by using Structure-Based Virtual Screening in Conjunction with Electrophysiological Assay. J Med Chem. 2007;50(1):83–93. doi: 10.1021/jm060414o. [DOI] [PubMed] [Google Scholar]
- 299.Iwasawa N, Takahagi H. Boronic Esters as a System for Crystallization-induced Dynamic Self-assembly Equipped with an “On-off” Switch for Equilibration. J Am Chem Soc. 2007;129(25):7754. doi: 10.1021/ja072319q. [DOI] [PubMed] [Google Scholar]
- 300.Kim Y, Hilderbrand SA, Weissleder R, Tung CH. Sugar Sensing Based on Induced pH Changes. Chem Commun. 2007(22):2299–2301. doi: 10.1039/b700741h. [DOI] [PubMed] [Google Scholar]
- 301.Barriet D, Yam CM, Shmakova OE, Jamison AC, Lee TR. 4-Mercaptophenylboronic Acid SAMs on Gold: Comparison with SAMs derived from Thiophenol, 4-mercaptophenol, and 4-mercaptobenzoic Acid. Langmuir. 2007;23(17):8866–8875. doi: 10.1021/la7007733. [DOI] [PubMed] [Google Scholar]
- 302.Ma Y, Qian L, Huang HZ, Yang XR. Buildup of Gold Nanoparticle Multilayer Thin Films Based on the Covalent-bonding Interaction between Boronic Acids and Polyols. J Colloid Interf Sci. 2006;295(2):583–588. doi: 10.1016/j.jcis.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 303.Kitano H, Anraku Y, Shinohara H. Sensing Capabilities of Colloidal Gold Monolayer Modified with a Phenylboronic Acid-carrying Polymer Brush. Biomacromolecule. 2006;7(4):1065–1071. doi: 10.1021/bm050782u. [DOI] [PubMed] [Google Scholar]
- 304.Gardiner SJ, Smith BD, Duggan PJ, Karpa MJ, Griffin GJ. Selective Fructose Transport Through Supported Liquid Membranes Containing Diboronic Acid or Conjugated Monoboronic Acid- Quaternary Ammonium Carriers. Tetrahedron. 1999;55(10):2857–2864. [Google Scholar]
- 305.Duggan PJ. Fructose-permeable Liquid Membranes Containing Boronic Acid Carriers. Aust J Chem. 2004;57(4):291–299. [Google Scholar]
- 306.Draffin SP, Duggan PJ, Duggana SAM, Norrild JC. Highly Selective Lipophilic Diboronic Acid that Transports Fructose as the Trisdentate 2,3,6-β-D-Fructofuranose Ester. Tetrahedron. 2003;59:9075–9082. [Google Scholar]
- 307.Altamore TM, Duggan PJ, Krippner GY. Improving the Membrane Permeability of Sialic Acid Derivatives. Bioorg Med Chem Lett. 2006;14(4):1126–1133. doi: 10.1016/j.bmc.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 308.Iwatsuki S, Nakajima S, Inamo M, Takagi HD, Ishihara K. Which is Reactive in Alkaline Solution, Boronate Ion or Boronic Acid? Kinetic Evidence for Reactive Trigonal Boronic Acid in an Alkaline Solution . Inorg Chem. 2007;46(2):354–356. doi: 10.1021/ic0615372. [DOI] [PubMed] [Google Scholar]
- 309.Matsumoto M, Sugimoto T, Kusumoto K, Kondo K. Adsorption of Diols on Silica Gel modified by Phenylboronic Acid. J Chem Eng Jpn. 2007;40(1):26–30. [Google Scholar]
- 310.Tsukagoshi K, Ueno F, Nakajima R, Araki K. Enhancing Effect of Phenylboronic Acid Compounds and their Interactions with the Diol Groups of Saccharides in a Capillary Electrophoresis-Chemiluminescence Detection System. Anal Sci. 2007;23(2):227–230. doi: 10.2116/analsci.23.227. [DOI] [PubMed] [Google Scholar]
- 311.Zhang C, Suslick KS. Syntheses of Boronic-acid-appended Metal Loporphyrins as Potential Colorimetric Sensors for Sugars and Carbohydrates. J Porphyrins Phthalocyanines. 2005;9(9):659–666. [Google Scholar]
- 312.Klis T, Lulinski S, Serwatowski J. 2,3-Difluoro-4-formylphenylboronic Acid. Acta Crystallogr C. 2007;63(Pt 3):o145–146. doi: 10.1107/S0108270107001576. [DOI] [PubMed] [Google Scholar]
- 313.Nicolini J, Testoni FM, Schuhmacher SM, Machado VG. Use of the Interaction of a Boronic Acid with a Merocyanine to Develop an Anionic Colorimetric Assay. Tetrahedron Lett. 2007;48(19):3467–3470. [Google Scholar]
- 314.Bresner C, Day JK, Coombs ND, Fallis IA, Aldridge S, Coles SJ, Hursthouse MB. Fluoride Anion Binding by Cyclic Boronic Esters: Influence of Backbone Chelate on Receptor Integrity. Dalton Trans. 2006(30):3660–3667. doi: 10.1039/b605031j. [DOI] [PubMed] [Google Scholar]
- 315.Ali SR, Ma Y, Parajuli RR, Balogun Y, Lai WYC, He H. A Nonoxidative Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine. Anal Chem. 2007;79:2583–2587. doi: 10.1021/ac062068o. [DOI] [PubMed] [Google Scholar]
- 316.Lin X, Zhang Y, Chen W, Wu P. Electrocatalytic Oxidation and Determination of Dopamine in the Presence of Ascorbic Acid and Uric Acid at a Poly (p-nitrobenzenazo resorcinol) Modified Glassy Carbon Electrode. Sens Actuators, B . 2007;122:309–314. [Google Scholar]
- 317.Zucolotto V, Ferreira M, Cordeiro MR, Constantino CJL, Moreira WC, Oliveira ON. Nanoscale Processing of Polyaniline and Phthalocyanines for Sensing Applications. Sens Actuators, B . 2006;113:809–815. [Google Scholar]
- 318.Jang YJ, Jun JH, Swamy KMK, Nakamura K, Koh HS, Yoon YJ, Yoon J. Fluorescence Sensing of Dopamine. Bull Korean Chem Soc. 2005;26:2041–2043. [Google Scholar]
- 319.Secor KE, Glass TE. Selective Amine Recognition: Development of a Chemosensor for Dopamine and Norepinephrine. Org Lett. 2004;6(21):3727–3730. doi: 10.1021/ol048625f. [DOI] [PubMed] [Google Scholar]
- 320.Jin S, Li M, Zhu C, Tran V, Wang B. Computer-Based De Novo Design, Synthesis, and Evaluationof Boronic Acid-Based Artificial Receptors for Selective Recognition of Dopamine. ChemBioChem. 2008;9:1431–1438. doi: 10.1002/cbic.200700663. [DOI] [PubMed] [Google Scholar]
- 321.Michael M, Thomas S. A Color Sensor for Catecholamines. Angew Chem Int Ed. 2005;44(15):2265–2270. doi: 10.1002/anie.200462702. [DOI] [PubMed] [Google Scholar]
- 322.Wu W, Zhu HR, Fan LZ, Liu DF, Renneberg R, Yang SH. Sensitive Dopamine Recognition by Boronic Acid Functionalized Multi-walled Carbon Nanotubes. Chem Commun. 2007(23):2345–2347. doi: 10.1039/b701254c. [DOI] [PubMed] [Google Scholar]
- 323.Li FL, Zhao XJ, Xu GW. Synthesis and Application of a Nitroboronic Acid-substituated Silica for Affinity Chromatography. Chinese J Anal Chem. 2006;34(10):1366–1370. [Google Scholar]
- 324.Luvino D, Smietana M, Vasseur JJ. Selective Fluorescence-based Detection of Dihydrouridine with Boronic Acids. Tetrahedron Lett. 2006;47(52):9253–9256. [Google Scholar]
- 325.Ishizuka K, Takahashi S, Anzai J. Phenylboronic Acid Monolayer-modified Electrodes Sensitive to Ribonucleosides. Electrochem. 2006;74(8):688–690. [Google Scholar]
- 326.Rogowska P, Cyranski MK, Sporzynski A, Ciesielski A. Evidence for Strong Heterodimeric Interactions of Phenylboronic Acids with Amino Acids. Tetrahedron Lett. 2006;47:1389–1393. [Google Scholar]
- 327.Smoum R, Rubinstein A, Srebnik M. Chitosan-pentaglycine-phenylboronic Acid Conjugate: A Potential Colon-specific Platform for Calcitonin. Bioconjugate Chem. 2006;17(4):1000–1007. doi: 10.1021/bc050357y. [DOI] [PubMed] [Google Scholar]
- 328.Luthje S, Bornholdt C, Luning U. Concave Reagents, 46. Polyols as Templates for the Synthesis of Macrocycles from Boronic Acid Building Blocks. Eur J Org Chem. 2006(4):909–915. [Google Scholar]
- 329.Robertson DL, Joyce GF. Selection in Vitro of an RNA Enzyme that Specifically Cleaves Single-Stranded DNA. Nature. 1990;344(6265):467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 330.Tuerk C, Gold L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science. 1990;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 331.Betat H, Vogel S, Struhalla M, Forster HH, Famulok M, Welzel P, Hahn U. Aptamers that Recognize the Lipid Moiety of the Antibiotic Moenomycin A. Biol Chem. 2003;384:10–11. 1497–1500. doi: 10.1515/BC.2003.165. [DOI] [PubMed] [Google Scholar]
- 332.de-los-Santos-Alvarez N, Lobo-Castanon MJ, Miranda-Ordieres AJ, Tunon-Blanco P. Modified-RNA Aptamer-Based Sensor for Competitive Impedimetric Assay of Neomycin B. J Am Chem Soc. 2007;129(13):3808. doi: 10.1021/ja0689482. [DOI] [PubMed] [Google Scholar]
- 333.Fickert H, Fransson IG, Hahn U. In: Aptamers to Small Molecules. Klussmann S, editor. Wiley-VCH Verlag GmbH & Co. KGaA; Weinhein: 2006. pp. 95–115. [Google Scholar]
- 334.Larsen GR, Sako D, Ahern TJ, Shaffer M, Erban J, Sajer SA, Gibson RM, Wagner DD, Furie BC, Furie B. P-selectin and E-selectin - Distinct But Overlapping Leukocyte Ligand Specificities. J Biol Chem. 1992;267(16):11104–11110. [PubMed] [Google Scholar]
- 335.Lasky LA. Selectins - Interpreters of Cell-Specific Carbohydrate Information during Inflammation. Science. 1992;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 336.Knecht BG, Strasser A, Dietrich R, Martlbauer E, Niessner R, Weller MG. Automated Microarray System for the Simultaneous Detection of Antibiotics in Milk. Anal Chem. 2004;76(3):646–654. doi: 10.1021/ac035028i. [DOI] [PubMed] [Google Scholar]
- 337.Serrano JM, Silva M. Trace Analysis of Aminoglycoside Antibiotics in Bovine Milk by MEKC with LIF Detection. Electrophoresis. 2006;27(23):4703. doi: 10.1002/elps.200600184. [DOI] [PubMed] [Google Scholar]
- 338.Stead DA. Current Methodologies for the Analysis of Aminoglycosides. J Chromatogr B. 2000;747(1–2):69. doi: 10.1016/s0378-4347(00)00133-x. [DOI] [PubMed] [Google Scholar]
- 339.Zawilla NH, Diana J, Hoogmartens J, Adams E. Analysis of Neomycin Using an Improved Liquid Chromatographic Method Combined with Pulsed Electrochemical Detection. J Chromatogr B. 2006;833(2):191. doi: 10.1016/j.jchromb.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 340.Cowan JA, Ohyama T, Wang DQ, Natarajan K. Recognition of a Cognate RNA and Aptamer by Neomycin B. Quantitative Evaluation of Hydrogen Bonding and Electrostatic Interactions. Nucleic Acids Res. 2000;28(15):2935–2942. doi: 10.1093/nar/28.15.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wallis MG, Vonahsen U, Schroeder R, Famulok M. A Novel RNA Motif for Neomycin Recognition. Chem Biol. 1995;2(8):543–552. doi: 10.1016/1074-5521(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 342.Keppler OT, Horstkorte R, Pawlita M, Schmidts C, Reutter W. Biochemical Engineering of the N-acyl Side Chain of Sialic Acid: Biological Implications. Glycobiology. 2001;11(2):11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- 343.Lis H, Sharon N. Lectins: Carbohydrate-Specific Proteins that Mediate Cellular Recognition. Chem Rev. 1998;98(2):637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 344.Masud MM, Kuwahara M, Ozaki H, Sawai H. Sialyllactose-binding Modified DNA Aptamer Bearing Additional Functionality by SELEX. Bioorg Med Chem. 2004;12(5):1111–1120. doi: 10.1016/j.bmc.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 345.Ritzeler O, Hennig L, Findeisen M, Welzel P, Muller D, Markus A, Lemoine G, Lampilas M, vanHeijenoort J. Synthesis of a Trisaccharide Analogue of Moenomycin A(12) Implications of New Moenomycin Structure-Activity Relationships. Tetrahedron. 1997;53(5):1675–1694. [Google Scholar]
- 346.van Heijenoort J. Formation of the Glycan Chains in the Synthesis of Bacterial Peptidoglycan. Glycobiology. 2001;11(3):25R–36R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- 347.Schurer H, Buchynskyy A, Korn K, Famulok M, Welzel P, Hahn U. Fluorescence Correlation Spectroscopy as a New Method for the Investigation of Aptamer/Target Interactions. Biol Chem. 2001;382(3):479–481. doi: 10.1515/BC.2001.058. [DOI] [PubMed] [Google Scholar]
- 348.Gilbert BA, Sha M, Wathen ST, Rando RR. RNA Aptamers that Specifically Bind to a K Ras-Derived Farnesylated Peptide. Bioorg Med Chem. 1997;5(6):1115–1122. doi: 10.1016/s0968-0896(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 349.Li M, Lin N, Huang Z, Du L, Fang H, Altier C, Wang B. Selecting Aptamers for a Glycoprotein through the Incorporation of the Boronic Acid Moiety. J Am Chem Soc. 2008 doi: 10.1021/ja801510d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Dirks AJT, van Berkel SS, Hatzakis NS, Opsteen JA, van Delft FL, Cornelissen J, Rowan AE, van Hest JCM, Rutjes F, Nolte RJM. Preparation of Biohybrid Amphiphiles via the Copper Catalysed Huisgen [3+2] Dipolar Cycloaddition Reaction. Chem Commun. 2005(33):4172–4174. doi: 10.1039/b508428h. [DOI] [PubMed] [Google Scholar]
- 351.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed . 2001;40(11):2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 352.van Steenis D, David ORP, van Strijdonck GPF, van Maarseveen JH, Reek JNH. Click-Chemistry as an Efficient Synthetic Tool for the Preparation of Novel Conjugated Polymers. Chem Commun. 2005(34):4333–4335. doi: 10.1039/b507776a. [DOI] [PubMed] [Google Scholar]
- 353.Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Efficiency and Fidelity in a Click-chemistry Route to Triazole Dendrimers by The Copper(I)-catalyzed Ligation of Azides and Alkynes. Angew Chem Int Ed. 2004;43(30):3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 354.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A Stepwise Huisgen Cycloaddition Process: Copper(I)-catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew Chem-Int Edit . 2002;41(14):2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 355.Meldal M, Tornoe CW. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem Rev. 2008;108(8):2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 356.Liu Y, Diaz DD, Accurso AA, Sharpless KB, Fokin VV, Finn MG. Click Chemistry in Materials Synthesis. III. Metal-adhesive Polymers from Cu(I)-catalyzed Azide-alkyne Cycloaddition. J Polym Sci Pol Chem. 2007;45(22):5182–5189. [Google Scholar]
- 357.Kalisiak J, Sharpless KB, Fokin VV. Efficient Synthesis of 2-Substituted-1,2,3-triazoles. Org Lett. 2008;10(15):3171–3174. doi: 10.1021/ol8006748. [DOI] [PubMed] [Google Scholar]
- 358.Huisgen R. 1,3-Dipolar Cycloadditions. Past and Future. Angew Chem-Int Edit . 1963;2(10):565–598. [Google Scholar]
- 359.Finn MG, Kolb HC, Fokin VV, Sharpless KB. Click Chemistry - Definition and Aims. Prog Chem. 2008;20(1):1–4. [Google Scholar]
- 360.Diaz DD, Punna S, Holzer P, McPherson AK, Sharpless KB, Fokin VV, Finn MG. Click Chemistry in Materials Synthesis. 1. Adhesive Polymers From Copper-catalyzed Azide-alkyne Cycloaddition. J Polym Sci Pol Chem. 2004;42(17):4392–4403. [Google Scholar]
- 361.Danesh J, Lewington S, Thompson SG, Lowe GDO, Collins R. Plasma Fibrinogen Level and the Risk of Major Cardiovascular Diseases and Nonvascular Mortality - An Individual Participant Meta-Analysis. JAMA-J Am Med Assoc . 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 362.Wiskur SL, Floriano PN, Anslyn EV, McDevitt JT. A Multicomponent Sensing Ensemble in Solution: Differentiation between Structurally Similar Analytes. Angew Chem Int Ed. 2003;42(18):2070–2072. doi: 10.1002/anie.200351058. [DOI] [PubMed] [Google Scholar]
- 363.Davis AP, Wareham RS. Carbohydrate Recognition through Noncovalent Interactions: A Challenge for Biomimetic and Supramolecular Chemistry. Angew Chem Int Ed. 1999;38(20):2978–2996. [PubMed] [Google Scholar]
- 364.Weis WI, Drickamer K. Structural Basis of Lectin-Carbohydrate Recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 365.Mazik M, Bandmann H, Sicking W. Molecular Recognition of Carbohydrates by Artificial Polypyridine and Polypyrimidine Receptors. Angew Chem Int Ed. 2000;39(3):551. doi: 10.1002/(sici)1521-3773(20000204)39:3<551::aid-anie551>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 366.Mazik M, Cavga H. Carboxylate-Based Receptors for the Recognition of Carbohydrates in Organic and Aqueous Media. J Org Chem. 2006;71(8):2957–2963. doi: 10.1021/jo052479p. [DOI] [PubMed] [Google Scholar]
- 367.Mazik M, Cavga H, Jones PG. Molecular Recognition of Carbohydrates with Artificial Receptors: Mimicking the Binding Motifs Found in the Crystal Structures of Protein-Carbohydrate Complexes. J Am Chem Soc. 2005;127(25):9045–9052. doi: 10.1021/ja043037i. [DOI] [PubMed] [Google Scholar]
- 368.Mazik M, Kuschel M, Sicking W. Crown Ethers as Building Blocks for Carbohydrate Receptors. Org Lett. 2006;8(5):855–858. doi: 10.1021/ol052902g. [DOI] [PubMed] [Google Scholar]
- 369.Mazik M, Radunz W, Boese R. Molecular Recognition of Carbohydrates with Acyclic Pyridine-Based Receptors. J Org Chem. 2004;69(22):7448–7462. doi: 10.1021/jo048979k. [DOI] [PubMed] [Google Scholar]
- 370.Mazik M, Radunz W, Sicking W. High Alpha/Beta-Anomer Selectivity in Molecular Recognition of Carbohydrates by Artificial Receptors. Org Lett. 2002;4(26):4579–4582. doi: 10.1021/ol0201759. [DOI] [PubMed] [Google Scholar]
- 371.Mazik M, Sicking W. Molecular Recognition of Carbohydrates by Artificial Receptors: Systematic Studies towards Recognition Motifs for Carbohydrates. Chem Eur J. 2001;7(3):664–670. doi: 10.1002/1521-3765(20010202)7:3<664::aid-chem664>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 372.Mazik M, Sicking W. Pyridine-Based Receptors with High Affinity for Carbohydrates. Influence of the Degree of Steric Hindrance at Pyridine Nitrogen on the Binding Mode. Tetrahedron Lett. 2004;45(15):3117–3121. [Google Scholar]
- 373.Mazik M, Konig A. Recognition Properties of an Acyclic Biphenyl-Based Receptor toward Carbohydrates. J Org Chem. 2006;71(20):7854–7857. doi: 10.1021/jo0610309. [DOI] [PubMed] [Google Scholar]
- 374.Francesconi O, Ienco A, Moneti G, Nativi C, Roelens S. A Self-Assembled Pyrrolic Cage Receptor Specifically Recognizes Beta-Glucopyranosides. Angew Chem Int Ed. 2006;45(40):6693–6696. doi: 10.1002/anie.200602412. [DOI] [PubMed] [Google Scholar]
- 375.Nativi C, Cacciarini M, Francesconi O, Moneti G, Roelens S. A Beta-Mannoside-Selective Pyrrolic Tripodal Receptor. Org Lett. 2007;9(23):4685–4688. doi: 10.1021/ol701959r. [DOI] [PubMed] [Google Scholar]
- 376.Cacciarini M, Cordiano E, Nativi C, Roelens S. A Tricatecholic Receptor for Carbohydrate Recognition: Synthesis and Binding Studies. J Org Chem. 2007;72(10):3933–3936. doi: 10.1021/jo0702286. [DOI] [PubMed] [Google Scholar]
- 377.Nativi C, Cacciarini M, Francesconi O, Vacca A, Moneti G, Ienco A, Roelens S. Pyrrolic Tripodal Teceptors Effectively Recognizing Monosaccharides Affinity Assessment Through a Generalized Binding Descriptor. J Am Chem Soc. 2007;129(14):4377–4385. doi: 10.1021/ja068754m. [DOI] [PubMed] [Google Scholar]
- 378.Vacca A, Nativi C, Cacciarini M, Pergoli R, Roelens S. A New Tripodal Receptor for Molecular Recognition of Monosaccharides. A Paradigm for Assessing Glycoside Binding Affinities and Selectivities by H-1 NMR Spectroscopy. J Am Chem Soc. 2004;126(50):16456–16465. doi: 10.1021/ja045813s. [DOI] [PubMed] [Google Scholar]
- 379.Waki M, Abe H, Inouye M. Helix Formation in Synthetic Polymers by Hydrogen Bonding with Native Saccharides in Protic Media. Chemistry. 2006;12(30):7839–7847. doi: 10.1002/chem.200600315. [DOI] [PubMed] [Google Scholar]
- 380.Abe H, Aoyagi Y, Inouye M. A Rigid C3v-Symmetrical Host for Saccharide Recognition: 1,3,5-Tris(2-hydroxyaryl)-2,4,6-trimethylbenzenes. Org Lett. 2005;7(1):59–61. doi: 10.1021/ol047907c. [DOI] [PubMed] [Google Scholar]
- 381.Abe H, Masuda N, Waki M, Inouye M. Regulation of Saccharide Binding with Basic Poly(ethynylpyridine)s by H+-Induced Helix Formation. J Am Chem Soc. 2005;127(46):16189–16196. doi: 10.1021/ja054134u. [DOI] [PubMed] [Google Scholar]
- 382.Davis AP, Wareham RS. A Tricyclic Polyamide Receptor for Carbohydrates in Organic Media. Angew Chem Int Ed. 1998;37(16):2270–2273. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2270::AID-ANIE2270>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 383.Lecollinet G, Dominey AP, Velasco T, Davis AP. Highly Selective Disaccharide Recognition by a Tricyclic Octaamide Cage. Angew Chem Int Ed. 2002;41(21):4093–4096. doi: 10.1002/1521-3773(20021104)41:21<4093::AID-ANIE4093>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 384.Bissell RA, Bryan AJ, de Silva AP, McCoy CP, Fluorescent PET. (Photoinduced Electron Transfer) Sensors with Targeting/Anchoring Modules as Molecular Versions of Submarine Periscopes for Mapping Membrane-bounded Protons. J Chem Soc, Chem Commun . 1994:405–407. [Google Scholar]
- 385.Klein E, Crump MP, Davis AP. Carbohydrate Recognition in Water by a Tricyclic Polyamide Receptor. Angew Chem Int Ed. 2005;44(2):298–302. doi: 10.1002/anie.200461409. [DOI] [PubMed] [Google Scholar]
- 386.Ferrand Y, Crump MP, Davis AP. A Synthetic Lectin Analog for Biomimetic Disaccharide Recognition. Science. 2007;318(5850):619–622. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]
- 387.Yi HP, Shao XB, Hou JL, Li C, Jiang XK, Li ZT. Hydrogen bonding-mediated oligobenzamide foldamer receptors that efficiently bind a triol and saccharides in chloroform. New J Chem. 2005;29(9):1213–1218. [Google Scholar]
- 388.Ulevitch RJ, Tobias PS. Recognition of Endotoxin by Cells Leading to Transmembrane Signaling. Curr Opin Immunol. 1994;6(1):125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 389.Mecca T, Cunsolo F. Calix[4]arene-Based Ligands as Endotoxin Receptors. Tetrahedron. 2007;63(44):10764–10767. [Google Scholar]
- 390.Gong HY, Wang DX, Xiang JF, Zheng QY, Wang MX. Highly Selective Recognition of Diols by a Self-Regulating Fine-Tunable Methylazacalix[4]pyridine Cavity: Guest-Dependent Formation of Molecular-Sandwich and Molecular-Capsule Complexes in Solution and the Solid State. Chem-Eur J . 2007;13(27):7791–7802. doi: 10.1002/chem.200700498. [DOI] [PubMed] [Google Scholar]
- 391.Weis WI, Drickamer K, Hendrickson WA. Structure of a C-Type Mannose-Binding Protein Complexed with an Oligosaccharide. Nature. 1992;360(6400):127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 392.Fontana JD. The Presence of Phosphate in Glycogen. FEBS Lett. 1980;109(1):85–92. doi: 10.1016/0014-5793(80)81317-2. [DOI] [PubMed] [Google Scholar]
- 393.Imada T, Kijima H, Takeuchi M, Shinkai S. Discrimination between Glucose-1-Phosphate and Glucose-6-Phosphate with a Boronic-Acid-Appended Metalloporphyrin. Tetrahedron Lett. 1995;36(12):2093–2096. [Google Scholar]
- 394.Horrocks WD, Sudnick DR. Lanthanide Ion Luminescence Probes of the Structure of Biological Macromolecules. Acc Chem Res. 1981;14:384–392. [Google Scholar]
- 395.Gichinga MG, Striegler S. Effect of Water on the Catalytic Oxidation of Catechols. J Am Chem Soc. 2008;130(15):5150–5156. doi: 10.1021/ja078057+. [DOI] [PubMed] [Google Scholar]
- 396.Kilpatrick DC. Handbook of Animal Lectins: Preperties and Biomedical Applications. 2000 [Google Scholar]
- 397.Van Damme EJM, Peumans WJ, Pustzai A, Bardocz S. Handbook of Plant Lectins: Properties and Biological applicaions. 1998 [Google Scholar]
- 398.Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume Lectin Structure. Biochim Biophys Acta-Protein Struct Molec Enzym . 1998;1383(1):9–36. doi: 10.1016/s0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 399.Young NM, Oomen RP. Analysis of Sequence Variation among Legume Lectins -a Ring of Hypervariable Residues Forms the Perimeter of the Carbohydrate-Binding Site. J Mol Biol. 1992;228(3):924–934. doi: 10.1016/0022-2836(92)90875-k. [DOI] [PubMed] [Google Scholar]
- 400.Ambrosi M, Cameron NR, Davis BG. Lectins: Tools for the Molecular Understanding of the Glycocode. Org Biomol Chem. 2005;3(9):1593–1608. doi: 10.1039/b414350g. [DOI] [PubMed] [Google Scholar]
- 401.Chelvanayagam G, Heringa J, Argos P. Anatomy and Evolution of Proteins Displaying the Viral Capsid Jellyroll Topology. J Mol Biol. 1992;228(1):220–242. doi: 10.1016/0022-2836(92)90502-b. [DOI] [PubMed] [Google Scholar]
- 402.Mizuno M, Noguchi M, Imai M, Motoyoshi T, Inazu T. Interaction Assay of Oligosaccharide with Lectins Using Glycosylasparagine. Bioorg Med Chem Lett. 2004;14(2):485–490. doi: 10.1016/j.bmcl.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 403.Nomura K, Takahashi N, Hirose M, Nakamura S, Yagi F. Overall Carbohydrate-Binding Properties of Castanea Crenata Agglutinin (CCA) Carbohydr Res. 2005;340(12):2004–2009. doi: 10.1016/j.carres.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 404.Pedroso MM, Watanabe AM, Roque-Barreira MC, Bueno PR, Faria RC. Quartz Crystal Microbalance Monitoring the Real-Time Binding of Lectin with Carbohydrate with High and Low Molecular Mass. Microchem J. 2008;89(2):153–158. [Google Scholar]
- 405.Lebed K, Kulik AJ, Forro L, Lekka M. Lectin-Carbohydrate Affinity Measured Using a Quartz Crystal Microbalance. J Colloid Interface Sci. 2006;299(1):41–48. doi: 10.1016/j.jcis.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 406.Yonzon CR, Jeoungf E, Zou SL, Schatz GC, Mrksich M, Van Duyne RP. A Comparative Analysis of Localized and Propagating Surface Plasmon Resonance Sensors: the Binding of Concanavalin A to a Monosaccharide Functionalized Self-Assembled Monolayer. J Am Chem Soc. 2004;126(39):12669–12676. doi: 10.1021/ja047118q. [DOI] [PubMed] [Google Scholar]
- 407.Pei Z, Anderson H, Aastrup T, Ramstrom O. Study of Real-Time Lectin-Carbohydrate Interactions on the Surface of a Quartz Crystal Microbalance. Biosens Bioelectron. 2005;21(1):60–66. doi: 10.1016/j.bios.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 408.Gallego RG, Haseley SR, van Miegem VFL, Vliegenthart JFG, Kamerling JP. Identification of Carbohydrates Binding to Lectins by Using Surface Plasmon Resonance in Combination with HPLC Profiling. Glycobiology. 2004;14(5):373–386. doi: 10.1093/glycob/cwh052. [DOI] [PubMed] [Google Scholar]
- 409.Apweiler R, Hermjakob H, Sharon N. On the Frequency of Protein Glycosylation, as Deduced from Analysis of the SWISS-PROT Database. Biochim Biophys Acta, Gen Subj . 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 410.Pochec E, Litynska A, Amoresano A, Casbarra A. Glycosylation Profile of Integrin alpha(3)beta(1) Changes with Melanoma Progression. Biochimica Et Biophysica Acta-Molecular Cell Research . 2003;1643(1–3):113–123. doi: 10.1016/j.bbamcr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 411.Varki A. Biological Roles of Oligosaccharides - All of the Theories Are Correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Che FY, Song JF, Shao XX, Wang KY, Xia QC. Comparative Study on the Distribution of Ovalbumin Glycoforms by Capillary Electrophoresis. J Chromatogr A. 1999;849(2):599–608. doi: 10.1016/s0021-9673(99)00521-x. [DOI] [PubMed] [Google Scholar]
- 413.Hsu K-L, Pilobello KT, Mahal LK. Analyzing the Dynamic Bacterial Glycome with a Lectin Microarray Approach. Nat Chem Biol. 2006;2:153–157. doi: 10.1038/nchembio767. [DOI] [PubMed] [Google Scholar]
- 414.Schmidt MA, Riley LW, Benz I. Sweet New World: Glycoproteins in Bacterial Pathogens. Trends Microbiol. 2003;11(12):554–561. doi: 10.1016/j.tim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 415.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of Microbial Aialic Acid Metabolism. Microbiol Mol Biol Rev. 2004;68(1):132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Lerouge I, Vanderleyden J. O-antigen Structural Variation: Mechanisms and Possible Roles in Animal/plant-microbe Interactions. FEMS Microbiol Rev. 2002;26(1):17–47. doi: 10.1111/j.1574-6976.2002.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 417.Stimpson DI, Hoijer JV, Hsieh WT, Jou C, Gordon J, Theriault T, Gamble R, Baldeschwieler JD. Real-time Detection of DNA Hybridization and Melting on Oligonucleotide Arrays by Using Optical Wave Guides. Proc Natl Acad Sci U S A. 1995;92(14):6379–6383. doi: 10.1073/pnas.92.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Lehr HP, Reimann M, Brandenburg A, Sulz G, Klapproth H. Real-time Detection of Nucleic Acid Interactions by Total Internal Reflection Fluorescence. Anal Chem. 2003;75(10):2414–2420. doi: 10.1021/ac0206519. [DOI] [PubMed] [Google Scholar]
- 419.Ebe Y, Kuno A, Uchiyama N, Koseki-Kuno S, Yamada M, Sato T, Narimatsu H, Hirabayashi J. Application of Lectin Microarray to Crude Samples: Differential Glycan Profiling of Lec Mutants. J Biochem. 2006;139(3):323–327. doi: 10.1093/jb/mvj070. [DOI] [PubMed] [Google Scholar]
- 420.Rosenfeld R, Bangio H, Gerwig GJ, Rosenberg R, Aloni R, Cohen Y, Amor Y, Plaschkes I, Kamerling JP, Maya RBY. A Lectin Array-based Methodology for the Analysis of Protein Glycosylation. J Biochem Biophys Methods. 2007;70(3):415–426. doi: 10.1016/j.jbbm.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 421.Shen WC, Ryser HJ. cis-Aconityl Spacer Between Daunomycin and Macromolecular Carriers: A Model of pH-sensitive Linkage Releasing Drug from a Lysosomotropic Conjugate. Biochem Biophys Res Commun. 1981;102(3):1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 422.Monsigny M, Roche AC, Midoux P. Endogenous Lectins and Drug Targeting. Ann N Y Acad Sci. 1988;551:399–413. doi: 10.1111/j.1749-6632.1988.tb22373.x. discussion 413–394. [DOI] [PubMed] [Google Scholar]
- 423.Karlsson KA. Glycobiology: a Growing Field for Drug Design. Trends Pharmacol Sci. 1991;12(7):265–272. doi: 10.1016/0165-6147(91)90568-d. [DOI] [PubMed] [Google Scholar]
- 424.Balzarini J, Schols D, Neyts J, Van Damme E, Peumans W, De Clercq E. Alpha-(1-3)- and Alpha-(1-6)-D-mannose-specific Plant Lectins are Markedly Inhibitory to Human Immunodeficiency Virus and Cytomegalovirus Infections in Vitro. Antimicrob Agents Chemother. 1991;35(3):410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Heinrich EL, Welty LAY, Banner LR, Oppenheimer SB. Direct Targeting of Cancer Cells: A Multiparameter Approach. Acta Histochem. 2005;107(5):335–344. doi: 10.1016/j.acthis.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Welty LAY, Heinrich EL, Garcia K, Banner LR, Summers ML, Baresi L, Metzenberg S, Coyle-Thompson C, Oppenheimer SB. Analysis of Unconventional Approaches for the Rapid Detection of Surface Lectin Binding Ligands on Human Cell Lines. Acta Histochem. 2006;107(6):411–420. doi: 10.1016/j.acthis.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Valentiner U, Fabian S, Schumacher U, Leathem AJ. The Influence of Dietary Lectins on the Cell Proliferation of Human Breast Cancer Cell Lines in vitro. Anticancer Res. 2003;23:1197–1206. [PubMed] [Google Scholar]
- 428.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56(4):425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 429.Damodaran D, Jeyakani J, Chauhan A, Kumar N, Chandra NR, Surolia A. CancerLectinDB: A Database of Lectins Relevant to Cancer. Glycoconj J. 2008;25(3):191–198. doi: 10.1007/s10719-007-9085-5. [DOI] [PubMed] [Google Scholar]
- 430.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, Okeefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, Buckheit RW, Nara PL, Pannell LK, Sowder RC, Henderson LE. Discovery of Cyanovirin-N, a Novel Human Immunodeficiency Virus-inactivating Protein that Binds Viral Surface Envelope Glycoprotein gp120: Potential Applications to Microbicide Development. Antimicrob Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bewley CA. Solution Structure of a Cyanovirin-N : Man Alpha 1-2Man Alpha Complex: Structural Basis for High-affinity Carbohydrate-mediated Binding to gp120. Structure. 2001;9(10):931–940. doi: 10.1016/s0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 432.Bolmstedt AJ, O’Keefe BR, Shenoy SR, McMahon JB, Boyd MR. HIV-inhibitory Natural Products Part 71 - Cyanovirin-N Defines a New Class of Antiviral Agent Targeting N-linked, High-mannose Glycans in an Oligosaccharide-specific Manner. Mol Pharmacol. 2001;59(5):949–954. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- 433.Bokesch HR, O’Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, 2nd, Turpin J, Watson K, Buckheit RW, Jr, Boyd MR. A Potent Novel Anti-HIV Protein from the Cultured Cyanobacterium Scytonema Varium. Biochemistry. 2003;42(9):2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 434.Balzarini J, Neyts J, Schols D, Hosoya M, Vandamme E, Peumans W, Declercq E. The Mannose-Specific Plant-Lectins from Cymbidium Hybrid and Epipactis-Helleborine and the (N-Acetylglucosamine)N-Specific Plant Lectin from Urtica-Dioica Are Potent and Selective Inhibitors of Human-Immunodeficiency-Virus and Cytomegalovirus Replication Invitro. Antiviral Res. 1992;18(2):191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 435.Balzarini J, Vijgen L, Keyaerts E, Van Damme E, Peumans W, De Clercq E, Egberink H, Van Ranst M. Mannose-specific Plant Lectins are Potent Inhibitors of Coronavirus Infection Including the Virus Causing SARS . Antiviral Res. 2004;62(2):A76–76. [Google Scholar]
- 436.Wang JH, Kong J, Li W, Molchanova V, Chikalovets I, Belogortseva N, Luk’yanov P, Zheng YT. A Beta-Galactose-Specific Lectin Isolated from the Marine Worm Chaetopterus Variopedatus Possesses Anti-HIV-1 Activity. Comp Biochem Physiol C: Toxicol Pharmacol . 2006;142(1–2):111–117. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]