Abstract
Background
During phase 1B of acute regional ischemia, the subepi- and subendocardial layers coupled to the inexcitable midmyocardium remain viable.
Objective
The goal of this study is to examine how the degree of hyperkalemia in the surviving layers, the lateral width of border zone between the normal tissue and the central ischemic zone and the degree of cellular uncoupling between the surviving layers and the midmyocardium contribute to the initiation of reentry.
Methods
Simulations were conducted on the state-of-the-art model of rabbit ventricles with realistic representation of the spatial distribution of the ischemic insult.
Results
Hyperkalemia in the surviving layers led to induction of reentry by increasing refractoriness and slowing conduction in the layers. Such reentries were formed solely in the subepicardium. A minimal level of hyperkalemia was required for induction of reentry. Progress increase in hyperkalemia led to a biphasic change in vulnerability to reentry. For each level of hyperkalemia, increased cellular uncoupling between subepicardium and midmyocardium increased inducibility of reentry by restoring subepicardial tissue excitibility via blocking midmyocardial electrotonic effect. In addition, increased lateral width of the border zone prevented inducibility of reentry as conduction block occurred in the central ischemic zone when the wave propagated across the border zone from the normal zone.
Conclusions
The degree of hyperkalemia in the surviving subepicardium, the lateral width of border zone and cellular uncoupling between the subepicardium and midmyocardium determine dispersion of refractoriness, conduction velocity and excitability, and therefore the inducibility of reentry during phase 1B.
Keywords: acute regional ischemia, arrhythmias, phase 1B, reentry, hyperkalemia, cellular uncoupling
Introduction
Acute regional ischemia is the most common factor triggering lethal arrhythmias.1 The incidence of arrhythmias during acute regional ischemia occurs in two phases, termed early (phase 1A, 2–10min post-occlusion) and delayed (phase 1B, 15–45min post-occlusion) respectively, separated by an arrhythmias-scarce period.2 The mechanisms of initiation of reentry during the delayed phase remain, however, rather elusive.
Regional ischemia phase 1B arrhythmias are characterized by a pattern of quasi-planar activation in the surviving subepicardium with a few breakthrough activations from the inexcitable midmyocardium.3 A similar activation pattern might exist in the surviving subendocardium; however, this is difficult to ascertain experimentally. Research has demonstrated that during phase 1B the severity of cellular damage in the subepi- and subendocardium increases progressively,4 while the total amount of excitable subepicardial tissue decreases.3 Furthermore, ischemically damaged but viable subepicardium has been found in the ventricles during subacute ischemia phase (2 to 6.5h post-occlusion).5 Thus, during the progression of acute ischemia, the surviving subepi- and subendocardium within the ischemic region (composed of a central ischemic zone, CIZ, and a border zone, BZ, a transition zone between CIZ and the normal zone, NZ) are also subjected to ischemic damage. Yet, the temporal and spatial distribution of electrophysiological properties in these surviving layers during ischemia phase 1B has not been fully characterized. The presence of lactate dehydrogenase and glycogen found in these two layers3 suggests that these layers were fully oxygenated and not acidotic. Since hyperkalemia (elevation in extracellular potassium concentration [K+]o) is the acute ischemia component most closely associated with decreased excitability,6 decreased excitability in the surviving subepi- and subendocardium is likely to be the result of hyperkalemia. In addition, since a gradient of decreasing excitability can lead to conduction failure,7 the BZ width might be a factor influencing inducibility of reentry. Furthermore, it has been suggested that cellular uncoupling between the inexcitable midmyocardium and the surviving subepi- and subendocardium occurs during acute regional ischemia phase 1B.3 Our recent simulation study8 showed that electrotonic influences of the depolarized inexcitable midmyocardial layer on a normal subepicardial layer can shorten the refractoriness in the latter.
It remains unclear whether and how these three factors, i.e. ischemic damage in the surviving layers, BZ characteristics (particularly its width), and cellular uncoupling between the surviving layers and the inexcitable midmyocardium, underlie initiation of reentry during ischemia phase 1B. The goal of this study is to examine the individual contributions of these three factors and their interplay. We hypothesize that dispersion in refractoriness, conduction velocity (CV), and excitability, determined by the level of hyperkalemia in the viable subepi- and subendocardium, the width of BZ, and the degree of cellular uncoupling (DOU) between the viable layers and the midmyocardium, sets the stage for initiation of reentry in ischemia phase 1B. In order to capture the effects of ventricular geometry and fiber orientation on electrical activation, this study utilized a state-of-the-art anatomically accurate model of the rabbit ventricles, which also incorporated realistic representation of the spatial distribution of the ischemic insult.
Methods
Model Description
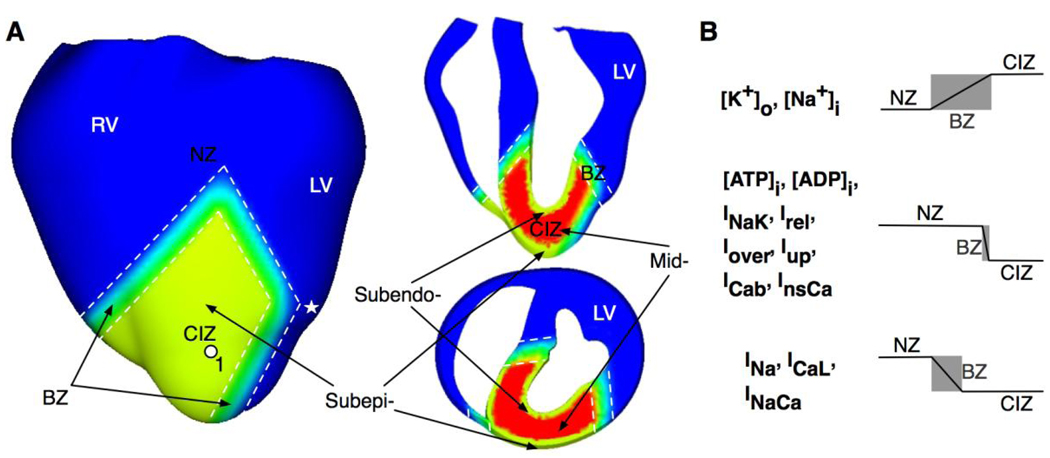
The model employed the anatomically accurate UCSD rabbit ventricular geometry.9 Mathematical description of the electrical behavior of the tissue was based on the bidomain representation with ion channel kinetics described by the Luo-Rudy dynamic model as done previously.10 The distribution of the ischemic lesion is depicted in Fig.1A, which was incorporated in the model based on distribution following occlusion of the left anterior descending artery.11 The ischemic lesion was composed of CIZ and BZ, encompassing part of the anterior myocardial walls in the left and right ventricles (LV and RV) and the septum near the apical region. Transmurally, CIZ encompassed the entire wall thickness, i.e. it included both the inexcitable midmyocardium and the surviving subepi- and subendocardium, except in the septum, where it consisted of inexcitable midmyocardium and the surviving subendocardial layers in LV and RV. The surviving subepi- and subendocardium were modeled as having a uniform 1-mm transmural thickness.4,5,12,13 The width of the BZ was assumed to be different in respect to the different electrophysiological parameters defining ischemic injury (Fig.1B). The width of the BZ for extracellular potassium concentration [K+]o was assumed the same as that for the intracellular sodium concentration ([Na+]i). The latter was ten times the width of the BZ for intracellular ATP and ADP concentrations ([ATP]i and [ADP]i), sodium-potassium pump current (INaK), calcium release from the sarcoplasmic reticulum (Irel), calcium release under calsequestrin buffer overload (Iover), calcium uptake into the sarcoplasmic reticulum (Iup), background calcium current (ICab), and calcium-sensitive nonselective current (InsCa). It was also twice the width of the BZ for the fast sodium current (INa), L-type calcium current (ICaL), and sodium-calcium exchange current (INaCa).14–17 The experimentally reported BZ width for [K+]o has been found to vary between 4 to 10mm during acute ischemia and 1 to 2mm during subacute ischemia.5,11,18–21 In order to investigate how variations in BZ width could affect the inducibility of reentry, two sets of simulations with different BZ widths were conducted. In the control set, the BZ width for [K+]o was assumed to be 5.5mm. In the other set of simulations, it was reduced to ¼ of their control widths. For simplicity, the term BZ used herein referred to that for [K+]o unless otherwise stated.
Fig.1.
A. Anterior and cross-sectional views of the rabbit ventricular model showing demarcation of the normal zone (NZ), central ischemic zone (CIZ) and border zone (BZ) following occlusion of the left anterior descending artery. Colors were used to distinguish the three zones. White dashed lines outline BZ. Asterisk indicates the stimulus site. LV-left ventricle; RV- right ventricle; Subepi-, subepicardium; Subendo-, subendocardium; Mid-, midmyocardium. B. Border zones for different ischemic parameters.
Similar to previous studies,22,23 parameters describing membrane and calcium dynamics were modified to represent the electrophysiological properties of the midmyocardium within CIZ, as listed in Table 1. In order to assure midmyocardial inexcitability within the CIZ, [K+]o there was set to 15mM,24 resulting in a resting membrane potential (RMP) of −60.6mV in this region. Varying degrees of ischemic damage in the subepi- and subendocardium within the CIZ were simulated by varying [K+]o in these layers simultaneously from 10 to 14mM in steps of 1mM. The transition in electrophysiological parameters between NZ and CIZ (i.e. within BZ) in the subepi-, subendocardium, and midmyocardium was assumed linear as done previously.
Table 1.
Model parameters in the normal zone (NZ) and the midmyocardium (Mid), including extracellular potassium concentration ([K+]o), intracellular sodium concentration ([Na+]i), intracellular ATP and ADP concentrations ([ATP]i and [ADP]i), and scaling factors of the maximum conductances of the fast sodium current (INa), L-type calcium current (ICaL), sodium-calcium exchange current (INaCa), sodium-potassium pump current (INaK), calcium release from the sarcoplasmic reticulum (Irel), calcium release under calsequestrin buffer overload (Iover), calcium uptake into the sarcoplasmic reticulum (Iup), background calcium current (ICab), and calcium-sensitive nonselective current (InsCa).
| NZ | Mid | |
|---|---|---|
| [K+]o (mM) | 5.4 | 15.0 |
| [Na+]i (mM) | 10.0 | 15.0 |
| [ATP]i (mM) | 6.8 | 5.0 |
| [ADP]i (µM) | 15.0 | 82.0 |
| SF_INa† | 1.0 | 0.5 |
| SF_ICaL† | 1.0 | 0.5 |
| SF_INaCa† | 1.0 | 0.2 |
| SF_INaK† | 1.0 | 0.3 |
| SF_Iover† | 1.0 | 0.65 |
| SF_Irel† | 1.0 | 0.05 |
| SF_Iup† | 1.0 | 0.9 |
| SF_ICab† | 1.0 | 1.3 |
| SF_InsCa† | 1.0 | 1.7 |
The normal ventricular tissue was assigned a set of standard bidomain conductivities.25 To represent different levels of cellular uncoupling, the intracellular conductivities, , were varied in the fiber and cross-fiber directions at the interfaces between the midmyocardium and each of the subepi- and subendocardium in the ischemic region, where the electrotonic loading of the subepi- and subendocardium by the midmyocardium takes place. Specifically, the parameter DOU was defined as:
Here refers to the standard bidomain intracellular conductivities.25 Three values of DOU were simulated, 0%, 50% and 100%, to investigate changes in inducibility of reentry during transition from normal coupling (0% DOU) to full uncoupling (100% DOU).
Simulation Protocols and Data Analysis
To ensure steady-state propagation, for each degree of hyperkalemia in the subepi- and subendocardium, each DOU and each BZ width, the ventricles were paced (S1) epicardially eight times at a basic cycle length of 250ms after the ionic model state variables were given the single-cell steady-state values. Steady-state of propagation was verified by measuring action potential duration (APD) at randomly selected ventricular sites and checking whether APD differences between the last two pacing beats at these sites were less than 1%. In order to facilitate understanding of mechanisms underlying inducibility of reentry, the effects of hyperkalemia in the subepi- and subendocardium and of DOU between these two layers and the inexcitable midmyocardium on the electrophysiological properties of the layers during the last pacing beat were first examined. Activation maps were constructed to analyze propagation. Local activation time was defined as the time of maximum upstroke velocity of the action potential. CV in the direction of the normal to the propagating wavefront was measured by dividing the distance traveled in direction normal to activation isochrones by the conduction time. Local APD was defined as the interval between time of maximum upstroke velocity and time of 90% repolarization at the given site. Effective refractory period (ERP) was determined as the longest S1–S2 interval that failed to produce a propagated wavefront. In order to determine ERP at a site other than the pacing site, addition sets of simulations were conducted with S1 and S2 stimuli applied locally at the site of interest.
To evaluate inducibility of reentry, a vulnerable window (VW) was constructed for each degree of hyperkalemia, each DOU, and each BZ width. A premature stimulus (S2) was applied in NZ close to the BZ (Fig.1), where earliest epicardial activation was documented to typically occur in phase 1B arrhythmias,26 to attempt reentry induction. The stimulus magnitude was 2× the normal diastolic threshold. It was given at various S1–S2 coupling intervals in the range of 135 to 250ms in steps of 5ms. In this manner the limits of VW were determined, defined as the range of coupling intervals for which at least one reentrant beat was induced.
Results
We found that the effects of DOU of 50% on the subepi- and subendocardial electrophysiological properties and reentry induction propensity during phase 1B (as evaluated by the VW) were always intermediate between those of DOU of 0% and 100%. Similar were the findings in a previous study,8 which also used several DOU values. Thus, for brevity, below we present results under the conditions of normal coupling (DOU of 0%) and full uncoupling (DOU of 100%) only.
Electrophysiological Characteristics of Surviving Subepi- and Subendocardium
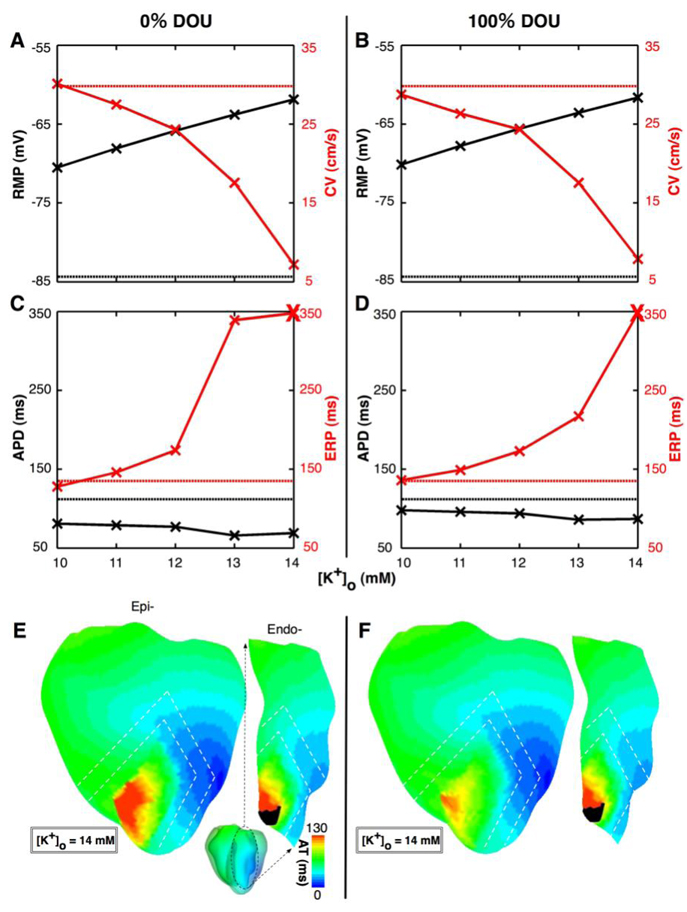
To quantify the effects of hyperkalemia and uncoupling on the electrophysiological properties of the surviving subepi- and subendocardium during the last paced (S1) beat, panels A and B in Fig.2 present RMP (at the site marked 1 in Fig.1) and CV (calculated from isochrones near site 1), while panels C and D present APD and ERP (both at site 1), for varying degrees of hyperkalemia and for DOU of 0% and 100% respectively with control BZ width. The normal values (control) for RMP, CV, APD and ERP were −84.4mV, 29.8cm/s, 112ms, and 135ms respectively (straight dotted lines).
Fig.2.
Changes in subepicardial electrophysiological parameters for varying degrees of hyperkalemia: resting membrane potential (RMP in black, at site 1 marked in Fig.1) and conduction velocity (CV in red, calculated from isochrones near site 1) for DOU of 0% and 100% in panels A and B, respectively, and action potential duration (APD in black, at site 1) and effective refractory period (ERP in red, at site 1) for DOU of 0% and 100% in panels C and D, respectively. Straight dotted lines correspond to normal values. Bold crosses in panels C and D indicate inexcitability. Panels E and F represent activation maps recorded on the anterior epicardial and LV endocardial surfaces for [K+]o of 14mM with DOU of 0% and 100% respectively. White dashed lines outline the BZ. Area in black indicates conduction block.
With normal coupling, the increase in [K+]o was accompanied with a concomitant decrease in RMP, CV and APD, and an increase in ERP. Moreover, every 1mM increase in [K+]o was associated with approximately 2.2mV decrease in RMP, whereas changes in CV, APD and ERP were non-monotonic. For each degree of hyperkalemia, the difference in both RMP and CV between the cases of normal coupling and full uncoupling was no more than 3.6% of control. These results demonstrate that the electrotonic interaction between the inexcitable midmyocardium and the subepicardium influenced RMP and CV in the latter only minimally. It, however, had a much larger influence on APD and ERP. Specifically, APD for normal coupling was always shorter than that for full uncoupling, by 15.9±1.3% of that in control. Additionally, for [K+]o of 10mM, ERP was 5.2% shorter for normal coupling than for full uncoupling. For [K+]o of 13mM, however, ERP was 151.1% more than control for normal coupling, while it was only 60.7% more for full uncoupling.
For [K+]o of 14mM, excitation at site 1 failed (indicated by bold crosses in panels C and D) for both DOUs even with S1–S2 intervals of above 600ms and stimulus amplitudes of 10× the normal diastolic threshold. Panels E and F in Fig.2 depict isochronal activation maps recorded on the anterior epicardial and LV endocardial surfaces for [K+]o of 14mM for normal coupling and full uncoupling, respectively. Conduction block (area in black) occurred in 4.0% of the ischemic subendocardium, near the apical anterior interventricular groove. Such block, however, did not occur in the subepicardium, despite the fact that the same electrophysiological changes were implemented in these two layers. Note that the activation time (Fig.2E–F) did not vary across the ischemic borders (dashed lines), which indicates the effect of electrotonic interaction due to cellular coupling at the border.
Inducibility of Reentry
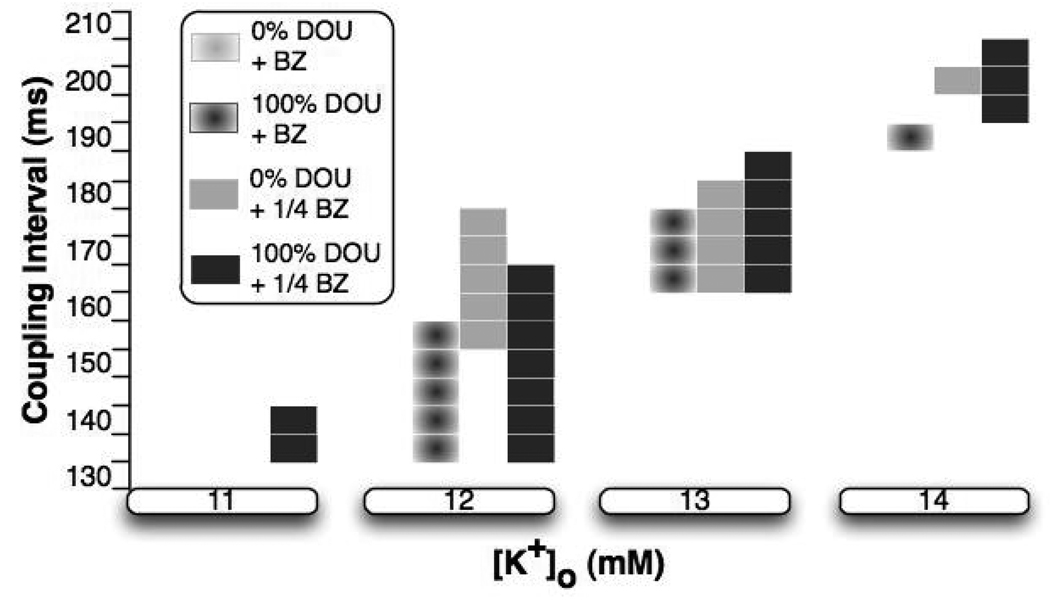
Not unexpectedly, for [K+]o of 11 to 14mM, extended ERP and slower CV in the surviving subepi- and subendocardium caused either by hyperkalemia, or by a combination of hyperkalemia and electrotonic effects exerted by the inexcitable midmyocardium, set the stage for induction of reentry. The duration of the VW for reentry induction, however, varied depending on the degree of hyperkalemia, DOU values, and BZ widths, as portrayed by Fig.3. The following sections analyze the mechanisms underlying these differences in VW duration.
Fig.3.
Vulnerable window durations for different degrees of hyperkalemia, DOU values, and BZ widths.
Effect of BZ Width on VW
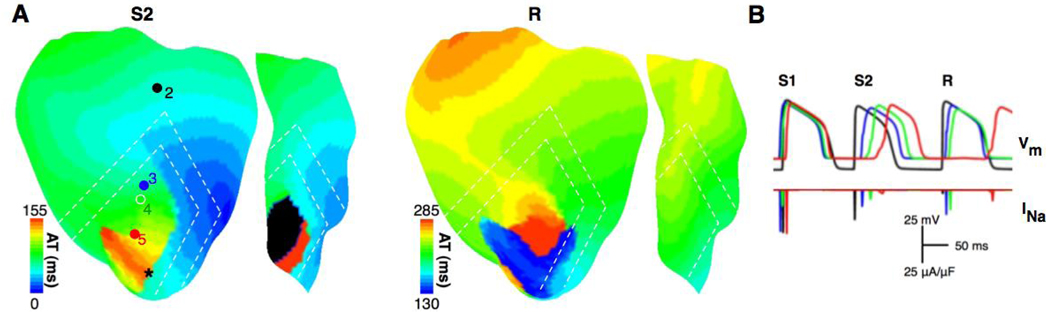
The duration of VW was shorter with control BZ width than with ¼ of it for any level of hyperkalemia and DOU. To determine the cause of this outcome, Panels A and B in Fig.4 present activation maps on the anterior epicardial surface and traces of transmembrane potential (Vm) and INa from sites 2 to 5 (as marked in panel A), respectively, for simulations with control BZ width, while panels C and D present those with ¼ of the control BZ width. For all panels, the coupling was normal, and [K+]o and coupling interval were 12mM and 155 ms, respectively. In panel A, during propagation elicited by S2, conduction was blocked (area in black) in 55.5% of the ischemic subepi- and subendocardium as a result of insufficient excitatory current (see INa at site 5). Decremental conduction occurred as the wavefront propagated from site 2 to 5. With a narrower BZ (panel C), however, conduction block did not occur during S2. A reentry (R) was formed following the slow propagation of S2 in the subepicardium. The earliest activation site of the reentrant beat (marked by the black asterisk in the S2 activation map) was at the anterior LV subepicardium. The results thus demonstrate that the inducibility of reentry was reduced with a wider BZ width as conduction block occurred in CIZ.
Fig.4.
Activation maps on the anterior epicardial surface and traces of transmembrane potential (Vm) and fast sodium current (INa, recorded from sites 2 to 5 marked in panel A) for a simulation with control BZ width in panels A and B, respectively, and those for a simulation with ¼ of control BZ width in panels C and D, respectively. The coupling is normal, and [K+]o and coupling interval are 12mM and 155ms, respectively. S1, S2, and R refer to the last pacing beat, the premature beat and the reentrant beat. Black asterisk indicates the earliest site of reentrant activation. The insets show enlarged views of traces of INa and L-type calcium current (ICaL) at site 4.
It is interesting to note that in panel D, at site 4, the peak magnitude of INa (solid line in the inset) was only half of the peak magnitude of ICaL (dashed line); the latter dominated the upstroke of the action potential. In contrast, for the control BZ width (panel B), despite a slightly larger peak magnitude of INa during propagation elicited by S2, the peak magnitude of ICaL was 56% lower, and therefore only a graded response ensued. This was due to the slower membrane depolarization (encircled by the dashed ellipse in the Vm trace in panel D) at site 4, which provided additional time for the activation gate of ICaL to open, resulting in a larger ICaL 24 and a transition from INa-dominated to ICaL-dominated upstroke.
Effects of Uncoupling on VW
Fig.3 shows that for any degree of hyperkalemia and any BZ width, the duration of VW was always wider for full uncoupling than for normal coupling. To examine the underlying mechanisms, panels A and B in Fig.5 present activation maps on the anterior epicardial and LV endocardial surfaces and traces of Vm and INa from sites 2 to 5 with all conditions the same as those in Fig.4A except that Fig.5 refers to full uncoupling while Fig.4A referred to normal coupling. For both cases, INa at site 4 was activated at the same time (compare Fig.4B to Fig.5B). APD following S1 was longer in the full uncoupling case than for normal coupling. Consequently, prior to S2 activation, the availability of open sodium channels was 19.0% less for full uncoupling than for normal coupling (evaluated by the product of inactivation gates h and j of INa), since there was less time for h and j to recover. Nonetheless, the peak magnitude of INa was 67.0% larger for full uncoupling than for normal coupling. Since the peak magnitude of INa was determined by both the availability of open sodium channels and the magnitude of excitatory current, the less availability of open sodium channels yet larger peak magnitude of INa in the case of full uncoupling indicated that this case was associated with larger excitatory current. This is readily understandable since in the absence of electrotonic load from the inexcitable midmyocardium, the availability of charge for downstream depolarization in the subepicardium increases, thus increasing the inducibility of reentry.
Fig.5.
Activation maps on the anterior epicardial surface and traces of Vm and INa in panels A and B, respectively, for a simulation with all other conditions the same as in Fig.4A, except that DOU is 100%. All notations are the same as in Fig.4.
Reentrant beats were formed only subepicardially. This is shown in Fig.5, where during propagation following S2, conduction was blocked in 15.2% of the ischemic subendocardium, while a reentrant beat (R) was formed subepicardially (earliest site of activation marked with the black asterisk in S2 activation map).
Effect of Hyperkalemia on VW
Fig.3 shows that as the degree of hyperkalemia in the ischemic subepi- and subendocardium increased, for any given DOU value and BZ width, the duration of VW underwent a biphasic change (an increase followed by a decrease), peaking at [K+]o of 12 mM. Specifically, no reentry was formed at [K+]o of 10 mM for any DOU value and BZ width. As [K+]o increased from 10 to 12mM, within the surviving subepicardium ERP was extended by 46 and 37ms and CV decreased by 5.8 and 4.4cm/s for normal coupling and full uncoupling, respectively (Fig.2). The resulting larger differences in ERP and CV between the surviving subepicardium and the surrounding normal tissue allowed the formation of reentry since normal tissue on the right of the ischemic zone (see Fig.4C and Fig.5A) had enough time to fully recover from S2 activation by the time the S2 wavefront traversed the surviving subepicardium from left to right (Fig.4C and Fig.5A). This explains the initial increase in vulnerability to reentry with the increase in [K+]o. Increasing [K+]o from 12 to 14mM doubled ERP in the surviving subepicardium for any DOU value (Fig.2). Such increase in ERP led to S2 conduction failure in the surviving subepicardium at relatively low coupling intervals (between 135 to 160ms) for any DOU value and BZ width, since the surviving subepicardium had not recovered from S1 activation when the S2 wavefront arrived. Thus, the lower limit of VW moved towards the higher coupling intervals as shown in Fig.3, which resulted in an overall decrease in the number of induced reentries and thus in a decrease in the vulnerability to reentry.
Discussion
This study investigated the role of 1) ischemic damage in the surviving subepi- and subendocardium, 2) width of BZ and 3) cellular uncoupling between the surviving layers and the midmyocardium in the inducibility of reentry during acute regional ischemia phase 1B. The present study did not focus on heterogeneities in uncoupling that might take place in the central ischemic zone; the effect of these on arrhythmogenesis was examined in our previous study9, where a tissue slab representing the central ischemic zone only with added heterogeneities in uncoupling was used.
The present study found that the degree of hyperkalemia in the surviving subepi- and subendocardium, the width of BZ, and DOU between the surviving layers and the midmyocardium determined the dispersion of refractoriness, CV and excitability, and therefore the inducibility of reentry. Specifically, hyperkalemia in the surviving layers resulted in the induction of reentry by increasing the refractoriness and decreasing CV in these layers. For a fixed level of subepicardial hyperkalemia, increased DOU between the midmyocardium and the surviving layers led to an increase in the inducibility of reentry by restoring subepi- and subendocardial tissue excitibility as midmyocardial electrotonic effect diminished. In addition, reentries were formed solely in the subepicardial layer. A minimal level of hyperkalemia ([K+]o of 11mM in subepicardial CIZ) was required for the induction of reentry regardless of DOU and BZ width. Further increase in [K+]o led to a biphasic change in VW duration. Increased BZ width, i.e., a less steeper gradient of decreasing excitability, resulted in the inhibition of reentry induction as conduction block occurred in CIZ when the wave propagated across BZ from NZ.
Role of Hyperkalemia in Initiation of Reentry
The degree of hyperkalemia in the surviving subepi- and subendocardium is critical for inducibility of reentry. For [K+]o of 10mM, subepicardial ERP and CV in CIZ were close to that in NZ and no reentry was induced for any DOU and BZ width. As [K+]o increased to 11mM, ERP exceeded that in NZ as PRRP increased due to the slower recovery from inactivation of INa. Meanwhile, CV decreased due to the inactivation of INa as a result of hyperkalemia-induced resting membrane depolarization. Thus, reentry was possible because of the extended refractoriness and slow conduction in the ischemic subepicardium. Further increase in [K+]o to 13mM resulted in a significant reduction in availability of open sodium channels, as the resting membrane depolarization reached 20mV, leading to propagation failure; no reentry was formed. Therefore, VW duration was the longest for [K+]o of 12mM. Interestingly, at this level of hyperkalemia, transition from INa-dominated to ICaL-dominated upstroke took place in the ischemic subepicardium; this represents a compensatory response of tissue with reduced excitability.7
In infarcted hearts (4 to 5 days post-occlusion), where ventricular tachycardia was sustained in the surviving subepicardium overlying the infarct, ERP and CV in the surviving layer were 43ms longer and 22% slower than those in NZ, respectively.27 In our study, where reentry was formed in the surviving subepicardium overlying the severely-damaged midmyocardium, with [K+]o of 12mM, ERP and CV were 38ms longer and 18.5% slower than control for any DOU examined. Therefore, findings from our study may be applicable to the infarcted heart, the electrophysiological properties of which bear a close resemblance to conditions defined herein.
This study is the first to suggest the possible involvement of hyperkalemia in arrhythmogenesis in the setting of phase 1B, while previous experimental or computational studies regarding effects of hyperkalemia focused on arrhythmias during phase 1A or subacute myocardial infarction.28,29 Our study demonstrated a biphasic change in vulnerability with the increase in [K+]o regardless of the presence of cellular uncoupling between midmyocardium and subepi-/subendocardium, which has not been shown previously. In addition, such biphasic change in vulnerability occurred without the involvement of hypoxia and acidosis in the subepi- and subendocardium, while hypoxia and acidosis are essential to arrhythmogenesis in ischemia phase 1A, as found by Trenor et al.28
The BZ width, as investigated in this study, also played a role in arrhythmogenesis. An important insight emerging from this study is that increased BZ width diminishes the inducibility of reentry as conduction block occurs in CIZ. Such conduction failure stems from the progressive reduction in the availability of excitatory current along the gradient of decreasing excitability.7
Role of Cellular Uncoupling in Initiation of Reentry
RMP on the epi- and endocardial surfaces did not change as the subepi- and subendocardium decoupled from the midmyocardium. Since RMP determined the availability of INa, consequently, no changes in CV took place there. A noticeable difference, however, was observed between APD and ERP for each of the DOU values. Specifically, in the full coupling case, the midmyocardial load led to shortened APD and lengthened PRRP of the paced propagation, which in turn reduced inducibility of reentry by blocking the propagation of the premature wavefront.
In this study, coupling was homogeneous throughout the ischemic region. Figure 2 and Figure 3 showed that ERP and inducibility of reentry were different for DOU of 0% and 100%. Thus, increased dispersion in ERP, and therefore larger susceptibility to reentry, may occur with heterogeneities in DOU in the ischemic region.
Pattern of Reentry
The surviving subepi- and subendocardium exhibited the same changes in electrophysiological properties. Conduction block, however, occurred preferentially in the subendocardium during both pacing (Fig.2) and premature stimulation (Fig.5). Consequently, reentry was only formed in the subepicardium under the conditions defined in this study. The decreased tissue excitability in the subendocardium may stem from different electrotonic influences from the neighboring tissue30 due to geometrical factors, including the larger degree of curvature of the subendocardium than of the subepicardium. Moreover, the subendocardial conduction block site was near the apical interventricular groove, consistent with observations in the canine heart one week post-occlusion.31 This is likely due to the sharp curvature near the interventricular groove and/or fibers running predominantly in the base-apex direction (perpendicularly to the direction of propagation) within the surviving subendocardium.
Implications of the Study
The presence of phase 1B arrhythmia is uncertain in man, although it has been speculated that phase 1B arrhythmias occurs earlier in man than in animal models.32 The mechanisms of this delayed phase of arrhythmias have remained unknown. Cellular uncoupling has been suggested to play an important role. Specifically, it was believed be pro-arrhythmic by causing slowed conduction.33 More advanced uncoupling was suggested to be anti-arrhythmic by causing conduction block and thus termination of arrhythmias.32 Our study, however, showed that more advanced uncoupling could be also pro-arrhythmic by blocking midmyocardial electrotonic effects and restoring subepicardial tissue excitability. Since ischemic preconditioning delays cellular uncoupling,34 it could delay the occurrence of phase 1B arrhythmias. Our finding regarding the existence of a minimal degree of hyperkalemia for induction of reentry suggests that therapeutic interventions that inhibit hyperkalemia, such as calcium channel blockers,35 may be beneficial in reducing phase 1B arrhythmias.
Limitations
The transmural thickness of the surviving subepi- and subendocardium was uniformly 1-mm in this study. The thickness has been reported to vary locally, between 0.2 to 1mm, although the variations throughout the ischemic region have not been fully characterized.4,12 In the infarcted heart, the surviving subepicardium has been reported to be of 0.5 to 4mm in thickness.5,13 The resting membrane depolarization on the epi- and endocardial surfaces resulting from midmyocardial electrotonic effects is larger if the layers are thinner. This is readily understood since electrotonic effects diminish with increasing distance. Indeed, RMP of −60mV in the midmyocardium was shown to result in 9mV resting membrane depolarization on the surface of the 0.55-mm surviving layer in our previous study,8 in contrast to the lack of depolarization on the surface of the 1-mm subepicardium in this study. Thus, variations in transmural thickness of the surviving layers are expected to lead to variations in RMP elevation, which can result in dispersion in excitability and therefore in increased inducibility of reentry.
The conclusions drawn here are from one generic heart anatomy and one particular ischemic zone morphology. Different geometries of the organ and the ischemic zone might result in different reentrant patterns. Similarly, only one stimulation site was used; a change in the location of the stimulus site could also alter the specific pattern of the reentry. Finally, because the model is computationally complex, it precludes a thorough analysis of different scenarios, such as different ratios between border zone widths, size of infarct, etc.
Conclusions
This study aimed to investigate the conditions that contribute to the initiation of reentry during acute regional ischemia phase 1B. It showed that dispersion in refractoriness, CV and excitability, determined by subepicardial hyperkalemia, BZ width, and cellular uncoupling between the subepicardium and the midmyocardium, is critical for initiation of reentry during acute regional ischemia phase 1B.
Acknowledgements
The authors wish to thank Dr. Blanca Rodriguez, Dr. Joris R. de Groot and Dr. Ruben Coronel for the useful discussions.
Funding: This study was supported by National Institutes of Health (R01-HL082729 and R01-HL067322 to N.T.) and National Science Foundation (CBET-0601935 to N.T.).
List of Abbreviations
- CIZ
Central ischemic zone
- BZ
Border zone
- NZ
Normal zone
- DOU
Degree of uncoupling
- LV
Left ventricle
- RV
Right ventricle
- CV
Conduction velocity
- ERP
Effective refractory period
- APD
Action potential duration
- RMP
Resting membrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001;345:1473–1148. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Kaplinsky E, Ogawa S, Balke C, et al. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation. 1979;60:397–403. doi: 10.1161/01.cir.60.2.397. [DOI] [PubMed] [Google Scholar]
- 3.de Groot JR, Wilms-Schopman FJ, Opthof T, et al. Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res. 2001;50:362–372. doi: 10.1016/s0008-6363(01)00222-x. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara H, Ashraf M, Sato S, et al. Transmural cellular damage and blood flow distribution in early ischemia in pig hearts. Circ Res. 1982;51:683–693. doi: 10.1161/01.res.51.6.683. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb GJ, Kubo SH, Alonso DR. Ultrastructural characterization of the border zone surrounding early experimental myocardial infarcts in dogs. Am J Pathol. 1981;103:292–303. [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 7.Kléber A, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 8.Jie X, Rodriguez B, de Groot JR, et al. Reentry in survived subepicardium coupled to depolarized and inexcitable midmyocardium: insights into arrhythmogenesis in ischemia phase 1B. Heart Rhythm. 2008;5:1036–1044. doi: 10.1016/j.hrthm.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetter FJ, McCulloch AD. Three-dimensional analysis of regional cardiac function: a model of rabbit ventricular anatomy. Prog Biophys Mol Biol. 1998;69:157–183. doi: 10.1016/s0079-6107(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 10.Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaitsev AV, Guha PK, Sarmast F, et al. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circ Res. 2003;92:546–553. doi: 10.1161/01.RES.0000061917.23107.F7. [DOI] [PubMed] [Google Scholar]
- 12.Wilensky RL, Tranum-Jensen J, Coronel R, et al. The subendocardial border zone during acute ischemia of the rabbit heart: an electrophysiologic, metabolic, and morphologic correlative study. Circulation. 1986;74:1137–1146. doi: 10.1161/01.cir.74.5.1137. [DOI] [PubMed] [Google Scholar]
- 13.Peters NS, Coromilas J, Severs NJ, et al. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 14.Coronel R. Heterogeneity in extracellular potassium concentration during early myocardial ischaemia and reperfusion: implications for arrhythmogenesis. Cardiovasc Res. 1994;28:770–777. doi: 10.1093/cvr/28.6.770. [DOI] [PubMed] [Google Scholar]
- 15.Yatani A, Brown A, Akaike N. Effect of extracellular pH on sodium current in isolated, single rat ventricular cells. J Membr Biol. 1984;78:163–168. doi: 10.1007/BF01869203. [DOI] [PubMed] [Google Scholar]
- 16.Irisawa H, Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986;59:348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- 17.Harken A, Barlow C, Harden W, et al. Two and three dimensional display of myocardial ischemic ‘border zone’ in dogs. Am J Cardiol. 1978;42:954–959. doi: 10.1016/0002-9149(78)90681-1. [DOI] [PubMed] [Google Scholar]
- 18.Coronel R, Fiolet JW, Wilms-Schopman FJ, et al. Distribution of extracellular potassium and its relation to electrophysiologic changes during acute myocardial ischemia in the isolated perfused porcine heart. Circulation. 1988;77:1125–1138. doi: 10.1161/01.cir.77.5.1125. [DOI] [PubMed] [Google Scholar]
- 19.Janse MJ, Cinca J, Morena H, et al. The "border zone" in myocardial ischemia. An electrophysiological, metabolic, and histochemical correlation in the pig heart. Circ Res. 1979;44:576–588. doi: 10.1161/01.res.44.4.576. [DOI] [PubMed] [Google Scholar]
- 20.Johnson TA, Engle CL, Boyd LM, et al. Magnitude and time course of extracellular potassium inhomogeneities during acute ischemia in pigs. Effect of verapamil. Circulation. 1991;83:622–634. doi: 10.1161/01.cir.83.2.622. [DOI] [PubMed] [Google Scholar]
- 21.Simson MB, Harden WRr, Barlow CH, et al. Epicardial ischemia as delineated with epicardial S-T segment mapping andnicotinamide adenine dinucleotide (NADH) fluorescence photography. Am J Cardiol. 1979;44:263–269. doi: 10.1016/0002-9149(79)90315-1. [DOI] [PubMed] [Google Scholar]
- 22.Cordeiro JM, Howlett SE, Ferrier GR. Simulated ischaemia and reperfusion in isolated guinea pig ventricular myocytes. Cardiovasc Res. 1994;28:1794–1802. doi: 10.1093/cvr/28.12.1794. [DOI] [PubMed] [Google Scholar]
- 23.Pollard AE, Cascio WE, Fast VG, et al. Modulation of triggered activity by uncoupling in the ischemic border. A model study with phase 1b-like conditions. Cardiovasc Res. 2002;56:381–392. doi: 10.1016/s0008-6363(02)00598-9. [DOI] [PubMed] [Google Scholar]
- 24.Shaw R, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 25.Eason J, Trayanova N. Phase singularities and termination of spiral wave reentry. J Cardiovasc Electrophysiol. 2002;13:672–679. doi: 10.1046/j.1540-8167.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 26.Coronel R, Wilms-Schopman FJ, deGroot JR. Origin of ischemia-induced phase 1b ventricular arrhythmias in pig hearts. J Am Coll Cardiol. 2002;39:166–176. doi: 10.1016/s0735-1097(01)01686-2. [DOI] [PubMed] [Google Scholar]
- 27.Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart C. 2003;284:H372–H384. doi: 10.1152/ajpheart.00512.2002. [DOI] [PubMed] [Google Scholar]
- 28.Trénor B, Romero L, Ferrero JJ, et al. Vulnerability to reentry in a regionally ischemic tissue: a simulation study. Ann Biomed Eng. 2007;35:1756–1770. doi: 10.1007/s10439-007-9353-3. [DOI] [PubMed] [Google Scholar]
- 29.Yin H, el-Sherif N, Caref E, et al. Actions of lidocaine on reentrant ventricular rhythms in the subacute myocardial infarction period in dogs. Am J Physiol. 1997;272:H299–H309. doi: 10.1152/ajpheart.1997.272.1.H299. [DOI] [PubMed] [Google Scholar]
- 30.Kimura S, Bassett AL, Furukawa T, et al. Electrophysiological properties and responses to simulated ischemia in cat ventricular myocytes of endocardial and epicardial origin. Circ Res. 1990;66:469–477. doi: 10.1161/01.res.66.2.469. [DOI] [PubMed] [Google Scholar]
- 31.Helie F, Vinet A, Cardinal R. Cycle length dynamics at the onset of postinfarction ventricular tachycardias induced in canines: dependence on interval-dependent excitation properties of the reentrant substrate. J Cardiovasc Electrophysiol. 2000;11:531–544. doi: 10.1111/j.1540-8167.2000.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 32.de Groot JR, Coronel R. Acute ischemia-induced gap junctional uncoupling and arrhythmogenesis. Cardiovasc Res. 2004;62:323–334. doi: 10.1016/j.cardiores.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Rohr S, Kucera J, Kleber A. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 34.Cinca J, Warren M, Carreño A, et al. Changes in myocardial electrical impedance induced by coronary artery occlusion in pigs with and without preconditioning. Correlation with local ST segment potential and ventricular arrhythmias. Circulation. 1997;96:3079–3086. doi: 10.1161/01.cir.96.9.3079. [DOI] [PubMed] [Google Scholar]
- 35.Sato R, Yamazaki J, Nagao T. Temporal differences in actions of calcium channel blockers on K+ accumulation, cardiac function, and high-energy phosphate levels in ischemic guinea pig hearts. J Pharmacol Exp Ther. 1999;289:831–839. [PubMed] [Google Scholar]