Abstract
Although acetylcholine (ACh) is well known for its neurotransmitter function, recent studies have indicated that it also functions as an immune cytokine that prevents macrophage activation through a ‘cholinergic (nicotinic) anti-inflammatory pathway’. In this study, we used the macrophage-like U937 cells to elucidate the mechanisms of the physiologic control of cytokine production by auto/paracrine ACh through the nicotinic class of ACh receptors (nAChRs) expressed in these cells. Stimulation of cells with lipopolysaccharide upregulated expression of α1, α4, α5, α7, α10, β1 and β3 subunits, downregulated α6 and β2 subunits, and did not alter the relative quantity of α9 and β4 mRNAs. Distinct nAChR subtypes showed differential regulation of the production of pro- and anti-inflammatory cytokines. While inhibition of the expression of the TNF-α gene was mediated predominantly by the α-bungarotoxin sensitive nAChRs, that of the IL-6 and IL-18 genes—by the mecamylamine-sensitive nAChRs. Both the Mec- and αBtx-sensitive nAChRs regulated expression of the IL-1β gene equally efficiently. Upregulation of IL-10 production by auto/paracrine ACh was mediated predominantly through α7 nAChR. These findings offer a new insight on how nicotinic agonists control inflammation, thus laying a groundwork for the development of novel immunomodulatory therapies based on the nAChR subtype selectivity of nicotinic agonists.
Keywords: U937 cells, nicotinic acetylcholine receptors, TNF-α, IL-1β, IL-6, IL-10, IL-18
INTRODUCTION
Although acetylcholine (ACh) is well known for its neurotransmitter function, recent studies have indicated that it also functions as an immune cytokine that prevents macrophage activation through a ‘cholinergic (nicotinic) anti-inflammatory pathway’ [1,2]. The nicotinic ACh receptor (nAChR) agonists have been shown to prevent or treat experimentally induced endotoxemic shock [3–5], sepsis [5–7], hemorrhagic shock [8,9], ischemia-reperfusion [10], subcutaneous inflammation [11], postoperative ileus [12], pancreatitis [13], allergic lung inflammation [14,15], and acute lung injury [16]. The agonist of nAChRs nicotine has been used in clinical trials, but its clinical potential is limited by its collateral toxicity [17]. Appreciations of an important role of α7 nAChR in regulation of the immune inflammation urged a search for selective nicotinic agonists that avoid the undesired side effects of nicotine [2]. Further elucidation of the nAChR-mediated regulation of inflammation should help develop novel treatments allowing to regulate specific types of immune reactions by selectively activating or blocking particular nAChR subtypes expressed in monocytes/macrophages.
Various immune cells possess diverse repertoires of nAChRs and, therefore, respond differently to the nicotinic agonists that exhibit varying affinities to distinct nAChR subtypes. The pharmacologic subtype of the ACh-gated ion channel is determined by a specific combination of the nAChR subunits forming the channel. The “muscle”-type nAChRs can be comprised by α1, β1, γ, δ, and ε subunits, and the “neuronal”-type nAChRs—by α2–α10 and β2–β4 subunits [18–21]. The α7, and α9 subunits can form homomeric nAChR channels sensitive to α-bungarotoxin (αBtx). The heteromeric channels can be composed of α2, α3, α4, α5, α6, β2, β3 and β4 subunits, e.g., α3(β2/β4)±α5, and α9 can also form a heteromeric channel with α10 [21]. The signal transduction pathways downstream of different nAChRs may be activated by both ionic events, such as Ca2+ influx, and changes of the stoichiometry of a multiprotein complex formed by the nAChR subunit(s) [22,23]. Therefore, a net biologic effect of ACh in a particular type of immune cell depends on the subunit composition of the major nAChR subtypes expressed by the cell at a given stage of its development and activation.
The presence of nAChRs in human monocyte/macrophages was suggested by the inhibitory effect of αBtx on monocyte activation [24] and nicotine binding to the human monocytic THP-1 cell line [25]. By now, it has been documented that human, murine and monkey macrophages express classic nAChR subunits [4,26,27]. Expression of α1, α7, and α10 mRNAs has been detected in human macrophages [4], whereas both bone marrow-derived dendritic cells and macrophages from C57BL/6J mice possess mRNAs encoding the nAChR subunits α2, α5, α6, α7, α10 and β2 [28]. Macrophages also express the muscarinic class of ACh receptors [29,30] that can modify the cell response to auto/paracrine ACh.
The human monoblastoid tumor cell line U937 [31] that can be differentiated into macrophage-like cells by treatment with phorbol-12-myristate 13-acetate (PMA) exhibits ACh synthesizing activity of choline acetyltransferase and contains approximately 0.02 pmol/106 of ACh [32]. Although, to the best of our knowledge, the subunit composition of nAChRs expressed in U937 cells have not been established, it has been reported that these cells respond to nicotine [33,34]. Therefore, U937 cells provide a useful model for studying basic mechanisms of macrophage regulation by auto/paracrine ACh through nAChRs.
In this study, we characterized the profile of nAChR subunits expressed in the macrophage-like differentiated U937 cells and demonstrated how the receptor repertoire changes upon cell activation with lipopolysaccharide (LPS). We also established relative contributions of α7- and non-α7 nAChR subtypes expressed in these cells to regulation of the pro- and anti-inflammatory cytokine production. The obtained results indicated that the macrophage nAChR subtypes are differentially coupled to regulation of production of distinct cytokines by auto/paracrine ACh. These findings offer a new insight on how nicotinic agonists control inflammation, thus laying a groundwork for the development of novel immunomodulatory therapies based on the nAChR subtype selectivity of nicotinic agonists.
MATERIALS AND METHODS
Cells and Reagents
The human monoblastoid tumor cell line U937 was purchased from ATCC (Catalog #CRL-2367; Manassas, VA) and grown in the ATCC complete growth medium (Catalog #30-2001) at 37°C in a humid, 5% CO2 incubator. To differentiate into macrophages, the U937 cells were treated with 200 nM PMA (Sigma-Aldrich Corporation, St Louis, MO) and allowed to adhere to tissue culture plate for 3 days [35]. The nicotinic ligands epibatidine (Epi), mecamylamine (Mec), methyllycaconitine (MLA) and αBtx, the inhibitor of ACh synthesis hemicholinium-3 (HC-3), and LPS were from Sigma-Aldrich Corporation. AR-R17779 was a gift from AstraZeneca Pharmaceuticals (Wilmington, DE). Particular doses of all drugs were selected based on the pilot dose-response experiments.
Characterization of nAChRs Expressed in U937 Cells
The profile of nAChR subunits expressed in differentiated, macrophage-like U937 cells was determined in a standard reverse-transcription PCR (RT-PCR) assay using the published primer sets for human α1-α7, α9, α10, β1-β4, γ, δ and ε nAChR subunits (Operon, Alameda, CA) and the amplification conditions shown in Table 1. All primers were tested using normal human muscle and brain PCR ready first strand cDNAs purchased from BioChain Institute Inc. (Hayward, CA) [36]. To control for contamination of DNase-treated samples with residual genomic DNA, the reverse transcription step was omitted.
Table 1.
Primers used for RT-PCR analysis of nAChRs in differentiated U937 cells*
| Target mRNA | Forward/reverse primers | Product size, bp | Anneal temperature | Sources |
|---|---|---|---|---|
| α1 | CGT CTG GTG GCA AAG CT CCG CTC TCC ATG AAG TT |
580 | 55 | [86] |
| α2 | GGA GCT CTG CCA CCC CCT AC AAC ATA CTT CCA GTC CTC |
327 | 64 | [87] |
| α3 | CTG GTG AAG GTG GAT GAA GT CTC GCA GCA GTT GTA CTT GA |
464 | 58 | [86] |
| α4 | GGA TGA GAA GAA CCA GAT GA CTC GTA CTT CCT GGT GTT GT |
444 | 58 | [86] |
| α5 | TCA ACA CAT AAT GCC ATG GC CCT CAC GGA CAT CAT TTT CC |
219 | 64 | [87] |
| α6 | GTG GCC TCT GGA CAA GAC AA AAT TAT AAA TAC CCA AAG A |
372 | 58 | [86] |
| α7 | CTT CAC CAT CAT CTG CAC CAT C GGT ACG GAT GTG CCA AGG ATA T |
308 | 55 | [87] |
| α9 | GTC CAG GGT CTT GTT TGT ATC CGC TCT TGC TAT GAT |
403 | 58 | [87] |
| α10 | CTC TCA AGC TGT TCC GTG ACC AAG GCT GCT ACA TCC ACG C |
394 | 64 | [88] |
| β1 | TGT ACC TGC GTC TAA AAA GG GCA GGT TGA GAA CCA CGA CA |
455 | 60 | [87] |
| β2 | CAG CTC ATC AGT GTG CA GTG CGG TCG TAG GTC CA |
347 | 58 | [89] |
| β3 | AGA GGC TCT TTC TGC AGA GCC ACA TCT TCA AAG CAG |
354 | 60 | [89] |
| β4 | GTG AAT GAG CGA GAG CAG AT GGG ATG ATG AGG TTG ATG GT |
524 | 58 | [86] |
| δ | CAG ATC TCC TAC TCC TGC AA CCA CTG ATG TCT TCT CAC CA |
471 | 58 | [86] |
| γ | CGC CTG CTC TAT CTC AGT CA GGA GAC ATT GAG CAC AAC CA |
546 | 56 | [86] |
| ε | GTA ACC CTG ACG AAT CTC AT GTC GAT GTC GAT CTT GTT GA |
432 | 55 | [86] |
All products were sequenced by the designers of the PCR primer used in this study.
Real-time Quantitative Polymerases Chain Reaction (qPCR) Experiments
Total RNA was extracted from U937 cells at the end of exposure experiments with the RNeasy Mini Kit (Qiagen, Valencia, CA) and used in the qPCR assay detailed elsewhere [37]. All qPCR primers were designed with the assistance of the Primer Express software version 2.0 computer program (Applied Biosystems, Foster City, CA) and the service Assays-on-Demand provided by Applied Biosystems. The qPCR reactions were performed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems) and the TaqMan Universal Master Mix reagent (Applied Biosystems) in accordance to the manufacturer’s protocol, as described by us in detail elsewhere [38]. To correct for minor variations in mRNA extraction and reverse transcription, the gene expression values were normalized using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. The data were analyzed with a sequence detector software (Applied Biosystems) and expressed as mean ± standard deviation of mRNA in question relative to that of control.
In-cell Western (ICW) Assay
The ICW assay was performed as described by us in detail elsewhere [39], using the reagents and equipment from LI-COR Biotechnology (Lincoln, NE). After incubation of 3×104 U937 cells/well of a 96-well plate in the growth medium with or without test agents for 16 h, the experimental and control U937 cells were fixed in situ, washed, permeabilized with Triton solution, incubated with the LI-COR Odyssey Blocking Buffer for 1.5 h and then treated overnight at 4°C with a primary mouse antibody to human IL1-β, IL-6, IL-10 or IL-18, TNF-α (R&D Systems, Minneapolis, MN). After that, the cells were washed, and stained for 1 h at room temperature with a secondary LI-COR IRDye 800CW anti-mouse antibody diluted 1:800. Sapphire700 (1:1000) was used to normalize for cell number/well. The protein expression was then quantitated using the LI-COR Odyssey Imaging System.
Statistical Analysis
All experiments were performed in duplicates or triplicates, and the results were expressed as mean ± standard deviation. Statistical significance was determined using Student’s t-test. Differences were deemed significant if the calculated p value was <0.05.
RESULTS
The nAChR Subunits Expressed in U937 Cells
The RT-PCR experiments using previously characterized human nAChR subunit gene-specific primers (Table 1) and PMA-differentiated U937 cells revealed the expression of α1, α4, α5, α6, α7, α9, α10, β1, β2, β3 and β4 subunits (Fig. 1A). The products were not amplified from putative contaminating DNA. These results indicated that the macrophage-like U937 cells express both the muscle (i.e., α1 and β1 containing)- and the neuronal (i.e., α4-, α6-, α9- and α7-made) subtypes of nAChRs. The homomeric ACh-gated ion channels in these cells can be comprised by several α7, and α9 subunits, and the heteromeric channels—by α9 plus α10 subunits as well as a combination of several other nAChR subunits found in these cells.
Figure 1. PCR analysis of nAChR subunit expression in differentiated U937 cells.
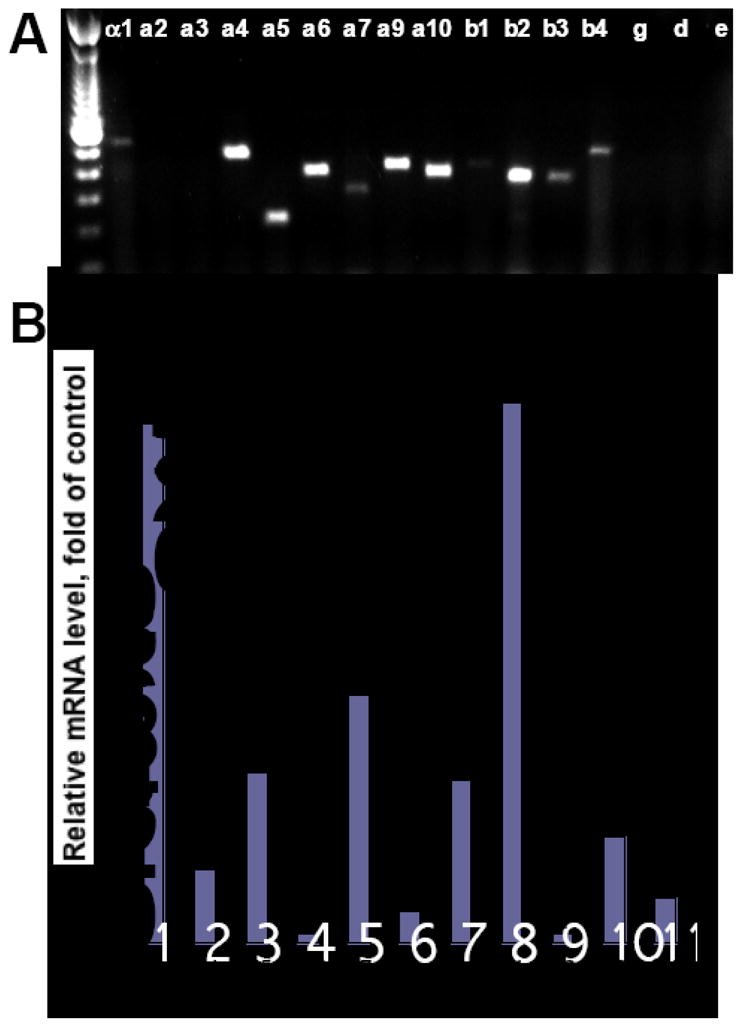
A. Expression of nAChR subunits in the U937 cells pretreated with 200 nM PMA was analyzed by RT-PCR using published primers (Table 1). Left lane is a 100 bp molecular weight ladder.
B. qPCR was performed after 16 h of incubation of differentiated U937 cells with 200 ng/ml LPS in a humid, 5% CO2 incubator at 37°C, as detailed in the Materials and Methods section. The results are expressed as fold of control determined in control PMA-differentiated U937 cells, and taken as 1. Asterisk = p<0.05 compared to control.
Activation of Differentiated U937 Cells Alters the Profile of their nAChR Subunits
Using qPCR, we found that stimulation of PMA-pretreated U937 cells for 16 h with 200 ng/ml LPS altered expression of the genes encoding different nAChR subunits, suggesting reciprocal changes in the structure and function of the channels formed. Compared to unstimulated U937 cells, the relative amount of mRNA encoding α1, α4, α5, α7, α10 and β1 subunits increased, that encoding α6 and β2 subunits decreased, and the relative quantity of α9, β3 and β4 mRNAs did not change significantly (Fig. 1B). Notably, the major increase was observed for α1 and β1 mRNAs (approximately 15-fold), as well as α5 and α7 mRNAs (approximately 7-fold). Because the initial levels of α1, β1 and α7 subunits appeared to be rather low (Fig. 1A), both the muscle-type and the α7-containing nAChR subtypes seem to be the most sensitive to induction by LPS. An increase of α5 and α10 subunits indicates that the subunit composition of constitutively expressed nAChRs becomes enriched with the non-ACh-binding subunits forming α4β3α5 and α9α10 nAChR channels. Activation of U937 cells appeared to be associated with a decrease of channels comprised by α6 and β2 subunits.
Nicotinic Effects on the LPS-induced Production of Pro-inflammatory Cytokines
The role of nAChRs in regulation of the inflammatory cytokine production was investigated in differentiated U937 cells stimulated with LPS in the presence or absence of nAChR ligands. Previous studies have demonstrated that LPS upregulates production of inflammatory cytokines by PMA-pretreated U937 cells [40], and that nicotine can inhibit this effect [33]. To elucidate the role of individual nAChR subtypes in mediating the reported anti-inflammatory action of nicotinic agonists on monocyte/macrophages, we exposed U937 cells to 200 ng/ml LPS in the absence (control) or presence of 1 μM agonist Epi that can activate all nAChR subtypes expressed in these cells [41,42]. As expected, Epi significantly inhibited expression of the genes encoding the inflammatory cytokines TNF-α, and IL-1β, both at the transcriptional and translational levels, and IL-6 and IL-18—at the protein level (Fig. 2). The dose responses were performed to examine the effects of Epi on the above cytokines at the protein levels. The results demonstrated the dose-dependent inhibition of the levels of TNF-α, IL-1β, IL-6 and IL-18 proteins in differentiated U937 cells stimulated with LPS in the presence of increasing concentrations of Epi (Fig. 2C). To evaluate possible contribution of distinct nAChR subtypes to the inhibitory action of Epi on production of inflammatory cytokines, the cells were exposed to this agonist in the presence of 50 μM of Mec, which inhibits all neuronal nAChR subtypes, or 1 μM of αBtx, which binds to and inhibits both the muscle-type nAChR and the homomeric neuronal-type nAChR channels [41,43–46]. Each antagonist abolished the inhibitory effects of Epi with a slightly distinctive efficacy, and changes in gene expression at the mRNA vs. protein levels somewhat differed (Fig. 2). Both at the mRNA and the protein levels, the expression of the TNF-α gene was altered predominantly through the αBtx-sensitive nAChRs (p<0.05). Both the Mec- and αBtx-sensitive nAChRs regulated expression of the IL-1β mRNA and protein equally efficiently (p<0.05). The alterations of IL-6 and IL-18 expression were observed only at the protein level and were primarily induced through the Mec-sensitive nAChRs (p<0.05).
Figure 2. Nicotinic effects on inflammatory cytokine production by LPS-stimulated U937 cells.
A, B. Differentiated U937 cells, 1×106 cells/well, were incubated for 16 h in a humid, 5% CO2 incubator with 200 ng/ml LPS in the absence or presence of 1 μM Epi ± 50 μM Mec or 1 μM αBtx, after which the expression of the genes encoding TNF-α, IL-1β, IL-6 and IL-18 at the mRNA and protein levels was measured by qPCR (A) and ICW (B), respectively, as detailed in Materials and Methods. Asterisk = p<0.05 compared to LPS given alone; pound sign = p<0.05 compared to Epi given alone.
C. The dose-dependent inhibition of the levels of TNF-α, IL-1β, IL-6 and IL-18 proteins in differentiated U937 cells stimulated with LPS in the presence of increasing concentrations of Epi.
The results are expressed as fold of control determined in control PMA-differentiated U937 cells, and taken as 1.
These results suggested that the macrophage nAChR subtypes are differentially coupled to regulation of production of pro-inflammatory cytokines.
Regulation of IL-10 Production
Since it has been documented that human monocytes and differentiated, macrophage-like U937 cells produce IL-10, which can be upregulated by LPS as well as agonists of cellular receptors to cytotransmitters and endocrine hormones [47–49], we next measured nicotinic effects on the expression of the IL-10 gene in PMA-pretreated U937 cells stimulated by LPS. As expected from previous reports, LPS increased the relative amounts of IL-10 mRNA and protein (Fig. 3A,B). Epi significantly (p<0.05) upregulated expression of the IL-10 gene at the translational level only. The effect of Epi was dose-dependent (Fig. 3C). Both Mec and MLA completely abolished the Epi-depended upregulation of IL-10 (p<0.05).
Figure 3. Nicotinic effects on IL-10 production by LPS-stimulated U937 cells.
A, B. Differentiated U937 cells were stimulated with LPS in the presence or absence of 1 μM Epi ± 50 μM Mec or 100 nM MLA, or 100 μM AR-R17779 (AR) ± 100 nM MLA and used in the qPCR (A) and ICW (B) assays of the IL-10 gene expression as described in the legend to Fig. 2. Asterisk = p<0.05 compared to LPS given alone; pound sign = p<0.05 compared to the relevant agonist given alone.
C. Concentration-dependent effects of Epi and AR-R17779 on the level of IL-10 protein in differentiated U937 cells stimulated with LPS.
The results are expressed as fold of control determined in control PMA-differentiated U937 cells, and taken as 1.
Since it has been previously reported that α7 nAChR expressed in macrophages and dendritic cells predominantly mediates the anti-inflammatory action of ACh [4,16], we sought to identify the role of this receptor in the cholinergic control of IL-10 production. To selectively activate/inactivate the α7-made nAChR we exposed differentiated U937 cells to LPS in the presence of the increasing concentrations of the α7-selective agonist AR-R17779. The IL-10 production was upregulated in a dose-dependent fashion (Fig. 3C). The cells exposed to LPS in the presence of 50–100 μM AR-R17779 showed approximately 2.5-fold increase of IL-10 production that significantly (p<0.05) exceeded Epi-induced upregulation (Fig. 3A,B). To confirm the receptor specificity of AR-R17779 action, we incubated cells with AR-R17779, 100 μM, in the presence or absence of the α7-preferring antagonist MLA, 100 nM [44,50] that completely abolished effect of the agonist (Fig. 3A,B).
These findings indicated that upregulation of IL-10 production in macrophages by auto/paracrine ACh is mediated predominantly through α7 nAChR.
The Role of Auto/paracrine ACh in the Nicotinic Anti-inflammatory Pathway
Since U937 cells synthesize ACh [32], we used these cells as a model-system to investigate relative contribution of auto/paracrine ACh to the nicotinic anti-inflammatory pathway. In this series experiments, 1 h prior to addition of LPS, the cultures of PMA-differentiated U937 cells were fed with the medium containing 20 μM of the metabolic inhibitor of ACh synthesis HC-3 [51,52]. By both qPCR and ICW, the level of IL-10 gene expression in the HC-3 treated cells decreased by approximately 5-fold (Fig. 4). If the HC-3 pretreated cells were stimulated with LPS in the presence of Epi, which could substitute ACh at the nAChR binding site, the IL-10 production increased and approached the control levels determined in the cells exposed to LPS without test drugs. Thus, elevation of IL-10 by Epi in the presence of HC-3 apparently occurred because the exogenously added agonist exhibited an ACh-like effect by activating nAChRs. This effect of Epi could be abolished by both Mec and MLA, with the latter antagonist exhibiting a significantly (p<0.05) higher efficiency (Fig. 4).
Figure 4. Assessment of contribution of auto/paracrine ACh to the nAChR-mediated upregulation of IL-10 production.
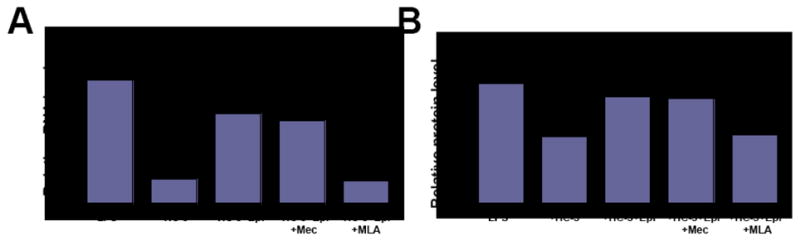
Differentiated U937 cells, 1×106 cells/well, were preincubated for 1 h with 20 μM HC-3 in a humid, 5% CO2 incubator, after which, the cells were exposed without washing to 200 ng/ml LPS and 1 μM Epi ± 50 μM Mec or 100 nM MLA, and used in the qPCR and ICW assays of IL-10 gene expression, as detailed in Materials and Methods. The dose of Epi used in this experiment was chosen based on the dose response curve for Epi shown in the panel “C” of Fig. 3, since in both experiments Epi was used to upregulate IL-10. The results are expressed as fold of control determined in PMA-differentiated U937 cells without stimulation with LPS or test drugs, and taken as 1. Plus sign = p<0.05 compared to control; asterisk = p<0.05 compared to LPS given alone; pound sign = compared to HC-3 given alone.
These results indicated that the cholinergic control of inflammation due to upregulation of IL-10 production through the α7-coupled pathway may represent a common mechanism of anti-inflammatory action of non-neuronal ACh.
DISCUSSION
It has become increasingly clear that in addition to its well known function as a neurotransmitter, ACh plays a much wider role in life being ubiquitously expressed in various cells and organisms and coupled to regulation of a large variety of biological processes [53,54]. The ACh regulatory axis, which is comprised by the cholinergic enzymes and receptors, choline high-affinity transporter and vesicular ACh transporter, has been found to play an important role in mediating host responses to environmental stimuli, including the immune response [55,56]. Recent research has convincingly demonstrated that the nicotinic arm of the regulatory ACh axis in immune cells is coupled to the physiologic control of T- and B-lymphocyte survival and function [57,58].
In this study, we used the macrophage-like U937 cells to elucidate the mechanisms of the physiologic control of cytokine production by auto/paracrine ACh through the nicotinic class of ACh receptors expressed in these cells. Both the muscle- and the neuronal-types of nAChRs were found to be expressed in differentiated U937. The repertoire of the receptors changed upon cell stimulation with LPS. Distinct nAChR subtypes showed differential regulation of the production of pro- and anti-inflammatory cytokines. The IL-10 gene expression was altered due to inhibition of endogeneous ACh production, indicating that the auto/paracrine ACh plays an important role in the physiologic control of macrophage function during the immune inflammation.
The cholinergic (nicotinic) anti-inflammatory pathway [3–5,59,60] is a physiologic (neuro)immune mechanism that regulates innate immune function and controls inflammation. The functional activity of this pathway can be modulated through both neuronal and non-neuronal cholinergic components, such as efferent vagal neurons and macrophage nAChRs, respectively. The outcome of pharmacologic stimulation of the nicotinic anti-inflammatory pathway, however, may be either beneficial and harmful. On the one hand, the nicotinic agonists have already been successfully used to treat various in vitro and in vivo models of inflammation (reviewed in [61]). On the other hand, nicotine and cigarette smoke cause alterations of the innate defense mechanisms and immune surveillance due to changes in local cytokine environment and functional impairment of the monocyte/macrophage and dendritic cell systems [62–68]. It has been documented that nicotine: (i) lowers endocytosis and phagocytosis of human monocyte-derived dendritic cells and decreases the levels of IL-12 [67]; (ii) reduces cytokine release from LPS-stimulated human leukemia peripheral blood monocyte cells [69] and human monocytes [70]; (iii) reduces TNF-α release and expression of TNF-α mRNA in murine alveolar macrophage cell line [14]; (iv) downregulates IL-1β production by human peripheral blood monocytes [71]; and (v) inhibits the LPS-induced IL-1 and IL-8 expression at the transcriptional level in the U937 cells [33]. This cell line was used in this study as an in vitro model system for nicotinic regulation of cytokine production by macrophages. Indeed, experiments with U937 cells have limitations, as the monocytic cell lines do not often mimic data obtained from primary cells and importantly, in vivo situations. Therefore, future studies should determine if the nicotinic effects on cytokine production observed by us in the present study can be replicated with normal macrophages.
Our results demonstrated for the first time the complete profile of nAChRs expressed by U937 cells, and revealed changes in the repertoire of the nAChR subunits upon cell activation of with LPS. The fact that activation of U937 cells with LPS produced a dramatic change in the levels of expression of the nAChR subunit genes is not surprising. It has been documented that mammalian cells change the repertoire of their nAChRs during differentiation and upon environmental stimulation [38,78–82]. Recent analysis of the biologic effects of macrophage nAChRs through microarray analysis of nicotine-induced changes in gene expression in U937 cells revealed that 118 genes are up-regulated and 97 down-regulated [34].
While expression of both α7 and non-α7 nAChRs was affected by LPS stimulation, the present study was focused on the anti-inflammatory function of ACh mediated by the α7-coupled pathway, in keeping with the notion that the ACh-gated ion channels comprised by α7 subunits are coupled to suppression of inflammation [4,70,72–74]. Noteworthy, activated U937 cells overexpressed α7. This observation, taken together with the results of pharmacologic experiments with the α7 ligands AR-R17779 and MLA, indicated that the major contribution of the macrophage α7 nAChR to the anti-inflammatory function of auto/paracrine ACh is upregulation of IL-10 production. Hence, our rationale for measuring the effect of HC-3 on production of only IL-10, but not other cytokines, was elucidation of the role of α7 nAChR in regulation of anti-inflammatory cytokine production by ACh.
The observed changes of the repertoire of nAChRs indicates that activated U937 cells express pharmacologically different receptors compared to the resting cells. It is well documented that inclusion of α5 subunits alters the properties of heteromeric nAChRs. In particular, channels containing the α5 subunits can be activated and desensitized by much lower (nanomolar) concentrations of nicotine than their non-α5-containing analogues [75]. Moreover, α5 increases the channel permeability to Ca2+ and its sensitivity to intracellular Ca2+, which makes heteromeric receptors formed similar to α7 nAChR homomers [76,77]. Similarly, addition of α10 subunit to α9-made homomers alters the resulting receptor sensitivity to extracellular Ca2+ and increases its agonist-mediated desensitization [21]. Therefore, activated U937 cells appear to not only overexpress α7 nAChRs but also re-organize other nAChR subtypes, thus synergizing with the α7 function.
The ability of the nicotinic agonist epibatidine to decrease pro-inflammatory cytokines is in keeping with known immunosuppressive action of nicotine [83], and with our previous reports about negative effect of nAChR activation on T- and B-cell functions, and antibody production [57,58,74,84]. The Mec-sensitive nAChRs coupled to downregulation of IL-6 and IL-18 production can be the α5 containing α4 receptors, because previous studies have demonstrated that activation of α4 nAChR in a mouse macrophage cell line produces an anti-inflammatory response by inhibiting TNF-α, IL-6, and IL-12 production [26]. On the other hand, since upregulation of the genes encoding α1 and β1 subunits by LPS considerably exceeded that of α7, one may speculate that the muscle-type macrophage nAChR also plays an important role in the anti-inflammatory action of nicotinic agonists mediated by downregulation of pro-inflammatory cytokines.
The knowledge about coupling of distinct nAChR subtypes to selective regulation of production of pro- and anti-inflammatory cytokines may be exploited for the development of novel therapeutic strategies allowing to achieve either immunosuppression or immunostimulation, depending on the receptor selectivity of the nicotinic agonist used. In addition to being pro-inflammatory, the studied cytokines can exert substantial effects on innate and adaptive immunity. In particular, IL-1, IL-6 and TNF-α are co-stimulatory for T and B lymphocytes; IL-6 drives proliferation and differentiation of B-cells into antibody-secreting cells, whereas IL-18 is a potent activator of natural killer cells and a stimulator of the Th2 response [85].
In conclusion, results of the present study identified the major macrophage nAChR subtypes that can mediate the physiologic control of cytokine production by auto/paracrine ACh, and pointed out an important role of the macrophage ACh regulatory axis in the immune inflammation. Our observations of the differential control of inflammation by ACh through α7 and non-α7 nAChRs expand the current knowledge about the “cholinergic anti-inflammatory pathway”. The unique coupling of macrophage nAChR subtypes to regulation of specific pro- and anti-inflammatory cytokines can diversify the immunoregulatory effects of ACh, thus allowing this auto/paracrine cytotransmitter to coordinate the immune response to a specific environmental stimulus. The muscle- and the neuronal-types of the macrophage nAChRs that regulate cytokine production are potential targets for the pharmacologic regulation of inflammation. The pharmacologic regulation of inflammation outside the central nervous system may be achieved by using nicotinic agents with poor or no permeability of the blood-brain barrier. Future studies should be focused on the nAChR subtypes that appear to mediate the immunomodulatory effects of ACh on monocyte/macrophages.
Acknowledgments
This work was supported by the NIH grants GM62136, DE14173 and ES014384, and research grants from the Institute for Science and Health (to S.A.G.), and Philip Morris USA Inc. and Philip Morris International (to M.V.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–84. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Wang P. The neuronal strategy for inflammation. Novartis Found Symp. 2007;280:223–33. discussion 33–7. [PubMed] [Google Scholar]
- 3.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 6.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–8. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 8.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–94. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 9.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–9. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–6. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 11.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 13.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Blanchet MR, Israel-Assayag E, Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med. 2004;169:903–9. doi: 10.1164/rccm.200210-1154OC. [DOI] [PubMed] [Google Scholar]
- 15.LaRosa G, Lamppu D, Tunkey C, Libertine L. α7 nicotinic acetylcholine receptor agonists attenuate allergic lung inflammation. Biochem Pharmacol. 2007;74 SMA49 (5.2) [Google Scholar]
- 16.Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37:186–92. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas GA, Rhodes J, Ingram JR. Mechanisms of disease: nicotine--a review of its actions in the context of gastrointestinal disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:536–44. doi: 10.1038/ncpgasthep0316. [DOI] [PubMed] [Google Scholar]
- 18.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–6. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 19.Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Lena C, Le Novere N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Brain Res Rev. 1998;26:198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 20.Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–23. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- 21.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. α10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–6. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–8. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Chernyavsky AI, Arredondo J, Qian J, Galitovskiy V, Grando SA. Coupling of ionic events to protein kinase signaling cascades upon activation of α7 nicotinic receptor: Cooperative regulation of α2-integrin expression and Rho-kinase activity. J Biol Chem. 2009;284:22140–8. doi: 10.1074/jbc.M109.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whaley K, Lappin D, Barkas T. C2 synthesis by human monocytes is modulated by a nicotinic cholinergic receptor. Nature. 1981;293:580–3. doi: 10.1038/293580a0. [DOI] [PubMed] [Google Scholar]
- 25.Morgan D, Parsons ME, Whelan CJ. Investigation of nicotine binding to THP-1 cells: evidence for a non-cholinergic binding site. Biochem Pharmacol. 2001;61:733–40. doi: 10.1016/s0006-2952(00)00587-6. [DOI] [PubMed] [Google Scholar]
- 26.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–24. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 27.Galvis G, Lips KS, Kummer W. Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J Mol Neurosci. 2006;30:107–8. doi: 10.1385/JMN:30:1:107. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–9. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Sato E, Koyama S, Okubo Y, Kubo K, Sekiguchi M. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am J Physiol. 1998;274:L970–9. doi: 10.1152/ajplung.1998.274.6.L970. [DOI] [PubMed] [Google Scholar]
- 30.Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther. 2007;115:208–22. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–77. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 32.Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 33.Sugano N, Shimada K, Ito K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252:25–8. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- 34.Koshi R, Sugano N, Orii H, Fukuda T, Ito K. Microarray analysis of nicotine-induced changes in gene expression in a macrophage-like human cell line. J Periodontal Res. 2007;42:518–26. doi: 10.1111/j.1600-0765.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson K, Forsbeck K, Gidlund M, Sundstrom C, Totterman T, Sallstrom J, Venge P. Surface characteristics of the U-937 human histiocytic lymphoma cell line: specific changes during inducible morphologic and functional differentiation in vitro. Haematol Blood Transfus. 1981;26:215–21. doi: 10.1007/978-3-642-67984-1_35. [DOI] [PubMed] [Google Scholar]
- 36.Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006;132:653–63. doi: 10.1007/s00432-006-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arredondo J, Chernyavsky AI, Marubio LM, Beaudet AL, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: Regulation of gene expression through α3β2 nicotinic receptor in oral epithelial cells. Am J Pathol. 2005;166:597–613. doi: 10.1016/s0002-9440(10)62281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: acceleration of sequential expression of α5 and α7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 2008;22:1356–68. doi: 10.1096/fj.07-9965.com. [DOI] [PubMed] [Google Scholar]
- 39.Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125:1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 40.Garrelds IM, van Hal PT, Haakmat RC, Hoogsteden HC, Saxena PR, Zijlstra FJ. Time dependent production of cytokines and eicosanoids by human monocytic leukaemia U937 cells; effects of glucocorticosteroids. Mediators Inflamm. 1999;8:229–35. doi: 10.1080/09629359990397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–45. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 42.Lembeck F. Epibatidine: high potency and broad spectrum activity on neuronal and neuromuscular nicotinic acetylcholine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:378–85. doi: 10.1007/pl00005364. [DOI] [PubMed] [Google Scholar]
- 43.Conti-Tronconi BM, McLane KE, Raftery MA, Grando SA, Protti MP. The nicotinic acetylcholine receptor: structure and autoimmune pathology. Crit Rev Biochem Mol Biol. 1994;29:69–123. doi: 10.3109/10409239409086798. [DOI] [PubMed] [Google Scholar]
- 44.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels, 1st edition (2005 revision) Br J Pharmacol. 2005;144 (Suppl 1):S1–128. doi: 10.1038/sj.bjp.0706158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–82. [PubMed] [Google Scholar]
- 46.Rueter LE, Donnelly-Roberts DL, Curzon P, Briggs CA, Anderson DJ, Bitner RS. A-85380: a pharmacological probe for the preclinical and clinical investigation of the alphabeta neuronal nicotinic acetylcholine receptor. CNS Drug Rev. 2006;12:100–12. doi: 10.1111/j.1527-3458.2006.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77:211–6. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 48.Carruba G, D’Agostino P, Miele M, Calabro M, Barbera C, Bella GD, Milano S, Ferlazzo V, Caruso R, Rosa ML, Cocciadiferro L, Campisi I, Castagnetta L, Cillari E. Estrogen regulates cytokine production and apoptosis in PMA-differentiated, macrophage-like U937 cells. J Cell Biochem. 2003;90:187–96. doi: 10.1002/jcb.10607. [DOI] [PubMed] [Google Scholar]
- 49.Izeboud CA, Mocking JA, Monshouwer M, van Miert AS, Witkamp RF. Participation of beta-adrenergic receptors on macrophages in modulation of LPS-induced cytokine release. J Recept Signal Transduct Res. 1999;19:191–202. doi: 10.3109/10799899909036645. [DOI] [PubMed] [Google Scholar]
- 50.Levin ED, Bettegowda C, Blosser J, Gordon J. AR- R17779, and α7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–80. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Guyenet P, Lefresne P, Rossier J, Beaujouan JC, Glowinski J. Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol Pharmacol. 1973;9:630–9. [PubMed] [Google Scholar]
- 52.Veldsema-Currie RD, Labruyere WT, Langemeijer MW. Depletion of total acetylcholine by hemicholinium-3 in isolated rat diaphragm is less in the presence of dexamethasone. Brain Res. 1984;324:305–12. doi: 10.1016/0006-8993(84)90041-6. [DOI] [PubMed] [Google Scholar]
- 53.Grando SA, Kawashima K, Wessler I. The non-neuronal cholinergic system in humans. Life Sci. 2003;72:2009–12. doi: 10.1016/s0024-3205(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 54.Grando SA, Kawashima K, Kirkpatrick CJ, Wessler I. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80:2181–5. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008;106:186–92. doi: 10.1254/jphs.fm0070109. [DOI] [PubMed] [Google Scholar]
- 56.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Structure and function of the nicotinic arm of acetylcholine regulatory axis in human leukemic T cells. Int J Immunopathol Pharmacol. 2009;22:461–72. doi: 10.1177/039463200902200223. [DOI] [PubMed] [Google Scholar]
- 58.Arredondo J, Omelchenko DM, Chernyavsky AI, Qian J, Skok M, Grando SA. Functional role of the nicotinic arm of the acetylcholine regulatory axis in human B-cell lines. J Exp Pharmacol. 2009 doi: 10.2147/jep.s7055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257:156–66. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- 60.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonaz B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology. 2007;133:1370–3. doi: 10.1053/j.gastro.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 62.Green GM, Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967;276:421–7. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz SL, Evans DE, Lundin JE, Bond JC. Inhibition of pinocytosis by nicotine. J Pharmacol Exp Ther. 1972;183:370–7. [PubMed] [Google Scholar]
- 64.Schwartz SL. Interaction of nicotine and other amines with the endocytic and exocytic functions of macrophages. Fed Proc. 1976;35:85–8. [PubMed] [Google Scholar]
- 65.Thyberg J, Hedin U, Stenseth K, Nilsson J. Effects of nicotine on the fine structure of cultivated mouse peritoneal macrophages. Acta Pathol Microbiol Immunol Scand [A] 1983;91:23–30. doi: 10.1111/j.1699-0463.1983.tb02722.x. [DOI] [PubMed] [Google Scholar]
- 66.Ortega E, Barriga C, Rodriguez AB. Decline in the phagocytic function of alveolar macrophages from mice exposed to cigarette smoke. Comp Immunol Microbiol Infect Dis. 1994;17:77–84. doi: 10.1016/0147-9571(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 67.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–73. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 69.Sykes AP, Brampton C, Klee S, Chander CL, Whelan C, Parsons ME. An investigation into the effect and mechanisms of action of nicotine in inflammatory bowel disease. Inflamm Res. 2000;49:311–9. doi: 10.1007/s000110050597. [DOI] [PubMed] [Google Scholar]
- 70.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–23. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pabst MJ, Pabst KM, Collier JA, Coleman TC, Lemons-Prince ML, Godat MS, Waring MB, Babu JP. Inhibition of neutrophil and monocyte defensive functions by nicotine. J Periodontol. 1995;66:1047–55. doi: 10.1902/jop.1995.66.12.1047. [DOI] [PubMed] [Google Scholar]
- 72.The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–28. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 73.Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–64. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 74.Fujii YX, Fujigaya H, Moriwaki Y, Misawa H, Kasahara T, Grando SA, Kawashima K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J Neuroimmunol. 2007;189:69–74. doi: 10.1016/j.jneuroim.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 75.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha-5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–51. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 76.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–20. [PubMed] [Google Scholar]
- 77.Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci. 1999;868:578–90. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- 78.Ndoye A, Buchli R, Greenberg B, Nguyen VT, Zia S, Rodriguez JG, Webber RJ, Lawry MA, Grando SA. Identification and mapping of keratinocyte muscarinic acetylcholine receptor subtypes in human epidermis. J Invest Dermatol. 1998;111:410–6. doi: 10.1046/j.1523-1747.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen VT, Ndoye A, Hall LL, Zia S, Arredondo J, Chernyavsky AI, Kist DA, Zelickson BD, Lawry MA, Grando SA. Programmed cell death of keratinocytes culminates in apoptotic secretion of a humectant upon secretagogue action of acetylcholine. J Cell Sci. 2001;114:1189–204. doi: 10.1242/jcs.114.6.1189. [DOI] [PubMed] [Google Scholar]
- 80.Arredondo J, Hall LH, Ndoye A, Nguyen VT, Chernyavsky AI, Bercovich D, Orr-Urtreger A, Beaudet AL, Grando SA. Central role of fibroblast α3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab Invest. 2003;83:207–25. doi: 10.1097/01.lab.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- 81.Kues WA, Sakmann B, Witzemann V. Differential expression patterns of five acetylcholine receptor subunit genes in rat muscle during development. Eur J Neurosci. 1995;7:1376–85. doi: 10.1111/j.1460-9568.1995.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 82.Bairam A, Joseph V, Lajeunesse Y, Kinkead R. Developmental profile of cholinergic and purinergic traits and receptors in peripheral chemoreflex pathway in cats. Neuroscience. 2007;146:1841–53. doi: 10.1016/j.neuroscience.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 83.Kalra R, Singh SP, Kracko D, Matta SG, Sharp BM, Sopori ML. Chronic self-administration of nicotine in rats impairs T cell responsiveness. J Pharmacol Exp Ther. 2002;302:935–9. doi: 10.1124/jpet.302.3.935. [DOI] [PubMed] [Google Scholar]
- 84.Skok M, Grailhe R, Changeux JP. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur J Pharmacol. 2005;517:246–51. doi: 10.1016/j.ejphar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–31. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maus ADJ, Pereira EFR, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol. 1998;54:779–88. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- 87.Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, Goerdt S, Deichmann M. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol. 2004;123:937–49. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- 88.Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, α10, that confers functionality to the α9-subunit. Mol Pharmacol. 2002;61:150–9. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- 89.Carlisle DL, Hopkins TM, Gaither-Davis A, Silhanek MJ, Luketich JD, Christie NA, Siegfried JM. Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respir Res. 2004;5:27. doi: 10.1186/1465-9921-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]




