Abstract
Background
While reentry within the ventricular myocardium (VM) is responsible for the maintenance of short duration ventricular fibrillation (SDVF, VF duration < 1 min), Purkinje fibers (PFs) are important in the maintenance of long duration ventricular fibrillation (LDVF, VF duration > 1 min).
Objective
We hypothesized that the mechanisms of defibrillation may also be different for SDVF and LDVF.
Methods
A multielectrode basket catheter was deployed in the left ventricle of 8 beagles. External defibrillation shocks were delivered with a ramp-up protocol following SDVF (20 s) and LDVF (150 s). Earliest VM and PF activations were identified following the highest energy shock that failed to terminate VF and the successful shock.
Results
Defibrillation was successful after 36±12 s and 181±14 s for SDVF and LDVF, respectively. The time after shock delivery until earliest activation was detected for failed shocks and was significantly longer following LDVF (138.7±24.1 ms) than SDVF (75.6±8.7 ms). Earliest postshock activation following SDVF typically initiated in the VM (14 of 16 episodes) while it always initiated in the PF (16 of 16 episodes) following LDVF. Sites of earliest activity during sinus rhythm correlated with sites of earliest postshock activation for PF-led cycles but not VM-led cycles.
Conclusion
Earliest recorded postshock activation is in the Purkinje system following LDVF but not SDVF. This difference raises the possibility that the optimal defibrillation strategy is different for SDVF and LDVF.
Keywords: Defibrillation, Purkinje fibers, Long duration ventricular fibrillation, Cardiac mapping
Introduction
Patients with sudden cardiac arrest due to ventricular fibrillation (VF) typically are not defibrillated for several minutes, even in areas with the shortest first response times.1 However, most studies of the mechanism of ventricular defibrillation have been conducted following VF lasting less than 1 min (SDVF).2-7 VF evolves as it continues8, 9 so that the mechanisms of maintenance for SDVF and for VF lasting longer than 1 min (long duration VF, LDVF) may differ.7, 10, 11 For several decades, it has been thought that the primary mechanism of VF maintenance, whether SDVF or LDVF, is reentrant activity in the ventricular myocardium (VM).7, 11 However, recent evidence indicates that while intramural reentry is the dominant driving force in SDVF, Purkinje fiber (PF) activation plays a critical role in LDVF. 7, 10, 11Since the mechanisms of VF maintenance change over time, the mechanisms of defibrillation and the optimal defibrillation treatments may differ for SDVF and LDVF as well.
Methods
Animal Preparation
Eight beagles (9.5 ± 0.8 kg, mean ± SD) from Marshall Bioresources (North Rose, NY) were fasted overnight and anesthetized with sodium thiopental (25 mg/kg iv), intubated, and mechanically ventilated with 2–3% isoflurane in 100% oxygen. ECG Lead II, core body temperature, arterial blood gases, arterial blood pressure, and serum electrolytes were monitored and maintained within normal levels. Hair was removed from the chest and pediatric defibrillation pads were applied.
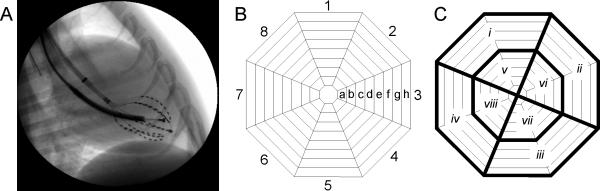
A 31-mm multielectrode basket (Constellation Catheter, model US8031U, Boston Scientific, Natick, MA) was introduced through a femoral artery into the left ventricle (LV) (Fig. 1A). The catheter contained eight splines each with eight electrodes approximately 2-mm apart. Fig. 1 B and C show the basket orientation and the division of the basket electrodes into regions for statistical analysis. An Endotak defibrillation catheter with a pacing tip (Boston Scientific, Natick, MA) was inserted into the right ventricular (RV) apex through a jugular vein.
Figure 1.
A) Fluoroscopic image of a lateral view of the LV basket catheter and the RV catheter used for VF induction. B) Display of the basket orientation in the LV: 1 = anterior free wall, 3 = lateral free wall, 5 = posterior free wall, 7 = septum. Apical electrodes are towards the center of the display (a) and basal electrodes are towards the periphery (h). C) For statistical analysis, first activation locations during sinus rhythm and during the first postshock activation cycle following shocks were grouped into regions i-viii.
Mapping System Configuration
As described previously, the electrodes were connected to a data acquisition system such that the 64 unipolar signals were recorded simultaneously with 56 bipolar signals (7 pairs per spline i.e., electrodes 1-2, 2-3, 3-4, etc.).3 Signals were bandpass filtered between 0.5 Hz and 4 KHz, sampled at 8 KHz, and digitized for offline analysis.
Defibrillation Protocol
The SDVF defibrillation threshold (DFT) was determined initially with a 3 crossing bracketing protocol.4 VF was induced by passing a 60 Hz AC current through the pacing tip in the RV, and following 10 s of VF a test shock was delivered with an initial value of 10 J. Shocks were deemed successful if a shockable rhythm was absent 5 s after the shock. Shocks were delivered with a LifePak 20 defibrillator (Physio-Control, Redmond, WA) at the following levels: 2, 3, 4, 5, 6, 8, 10, 15, 20, 30 J. If the test shock succeeded, the energy for the next test shock was decreased by one level, while if the test shock failed, the energy level was increased one level. After a 5 min recovery period, VF was again induced and a shock was delivered at the new level. This process was repeated until we achieved 3 crossings (i.e. a successful shock followed by a failed shock or a failed shock followed by a successful shock). The mean of the crossing point energies was determined to be the DFT.
Next, the SDVF DFT was determined with a ramp-up protocol for comparison with the bracketing protocol. The first shock was delivered after 10 s of VF with an energy <50% of the bracketing protocol DFT. About every 10 s, a shock of the next higher energy would be delivered until successful defibrillation. The highest energy failed shock was called the near DFT shock failure. Following 150 s of VF, the LDVF DFT was determined with a similar ramp-up protocol starting with an energy 50% of the ramp-up SDVF.
Signal Processing and Activation Picking
Each unipolar electrogram was normalized such that the largest recorded activation during the 2 s following a shock had an amplitude of 10 mV. VM activation times were picked with a computer algorithm that selected the most negative peak in the temporal derivative that was more negative than −0.5 V/s and were verified by manual over-reading of displays of the interleaved, temporally aligned, unipolar and bipolar recordings. Purkinje fiber (PF) activations were identified manually as rapid, short-duration activations, typically 1–2 ms in duration, as described previously.3, 12, 13
Activation times were determined during 1) several cycles of sinus rhythm before the SDVF ramp-up DFT protocol was performed, 2) the last 5 cycles before successful shocks, 3) the first 5 cycles following near DFT failed shock during the ramp-up protocols, and 4) the first 5 cycles following successful shocks during the ramp-up protocols.
Statistical Analysis
Data are reported as mean ± standard deviation. Statistically significant differences were determined if p<0.05. Differences in DFTs were tested with ANOVA. A repeated measures ANOVA analysis was conducted to determine significant differences in activation times. Post-hoc analysis (a paired or unpaired t-test, as appropriate) was conducted on values determined to be significant with the repeated measures ANOVA.
First activation locations were binned into regions as shown in Fig 1C. The frequency of PF and VM-led first postshock activations within each region were compared to the frequency of first activations in each region during sinus rhythm by computing Pearson correlation coefficients (r). First activation location frequency was compared for electrodes closer to the LV base (regions i-iv in Fig 1C) and for electrodes closer to the LV apex (regions v-viii) with a chi square distribution.
Results
DFTs and Isoelectric Windows
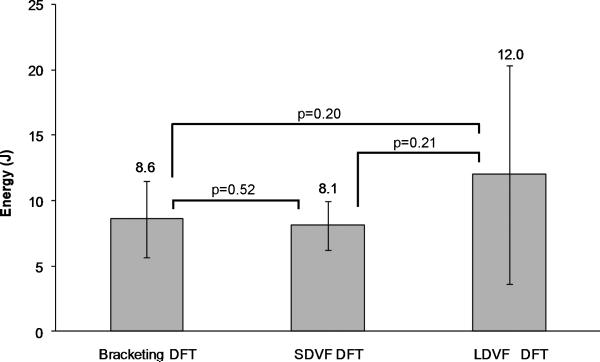
The three DFTs were not significantly different (Fig. 2). The number of shocks delivered in the ramp-up protocols was 3.7±1.3 (range, 2-5) and 4.6±1.8 (range, 2-8) for SDVF and LDVF, respectively (p=0.40). The total VF duration before defibrillation was significantly shorter for SDVF (36±13 s, range 20-55 s) than LDVF (181±14 s, range 160-203 s, p<0.001). As expected from previous studies,8, 9, 14 the mean cycle length of the last 5 cycles before SDVF successful shocks (108 ± 14 ms) was shorter than before LDVF successful shocks (149 ± 25 ms, p<0.05).
Figure 2.
DFTs for the 3 different protocols. Mean values are shown above the standard deviation bars. Brackets indicate paired t-tests performed and the p-values are shown above the brackets.
After near DFT failed and successful shocks, an isoelectric window was present,5 i.e., a period during which no activation was recorded. Two types of postshock activity were observed: (1) Type A successes, immediate termination as indicated by a long isoelectric window (>130 ms) followed by a non-shockable rhythm (i.e., sinus rhythm, asystole, or bradycardia), and (2) Type B successes, postshock repetitive responses before termination, as indicated by a short isoelectric window (≤ 130 ms) followed by 1-5 rapid ectopic cycles that are followed by longer pauses and a non-shockable rhythm.5 Although both types were observed following SDVF, only Type A successes were observed following LDVF (Table 1). The total VF duration was longer for the 2 SDVF Type A successes (53±2.8 s) than for the 6 Type B SDVF successes (30±8.6 s, p=0.002).
Table 1.
First postshock activation cycle characteristics
| Sinus | SDVF | LDVF | ||||
|---|---|---|---|---|---|---|
| Failure | Success | Failure | Success | |||
| Type A | Type B | Type A | ||||
| Number of occurrences |
8 |
8 |
2 |
6 |
8 |
8 |
| Earliest postshock activation type |
PF |
VM |
PF |
VM |
PF |
PF |
| Mean earliest postshock PF activation time (ms) |
No shock |
75.6 ± 8.7 |
1235.0 ± 113.8 |
73.3 ± 10.1 |
24.1 |
2941.0 ± 3184.3 |
| Mean earliest postshock VM activation time (ms) |
12.5 ± 3.3 after 1st P |
46.0 ± 14.3*‡ |
1246.3 ± 114.1§ |
53.3 ± 9.9‡ |
152.6 ± 24.1†§ |
2952.3 ± 3181.6§ |
| Total PF activation time (ms) |
20.1 ± 7.0 |
29.6 ± 12.0 |
16.4 ± 1.3 |
28.4 ± 9.9 |
23.9 ± 5.8 |
20.3 ± 3.9 |
| Total VM activation time (ms) |
18.5 ± 3.9¶ |
65.8 ± 12.8# |
18.6 ± 8.3¶ |
65.0 ± 8.8# |
33.0 ± 14.7¶ |
23.9 ± 5.8¶ |
| # of electrodes with both PF and VM activations |
29.8 ± 14.5 |
22.1 ± 7.5 |
37.0 ± 4.0 |
22.3 ± 7.2 |
31.9 ± 9.1 |
30.5 ± 9.7 |
| Mean PF-V delay (ms) |
9.2 ± 2.1 |
−0.5 ± 3.6 |
8.8 ± 1.9 |
5.5 ± 2.3 |
13.8 ± 2.8 |
9.9 ± 2.2 |
| # of electrodes with positive |
29.1 ± 14.7 |
16.5 ± 9.9 |
37.0 ± 4.0 |
18.3 ± 7.6 |
31.9 ± 9.1 |
30.5 ± 9.7 |
| Mean PF-V delay of positive delays |
9.8 ± 1.9 |
8.8 ± 2.3 |
8.8 ± 1.9 |
9.2 ± 1.9 |
13.6 ± 2.8 |
9.9 ± 2.2 |
| # of electrodes with negative PF-V delay |
0.6 ± 0.9 |
5.6 ± 3.5 |
0 |
4.0 ± 2.9 |
0 |
0 |
| Mean PF-V delay of negative delays | −10.0 ± 0.9 | −16.3 ± 4.1 | N/A | −8.1 ± 2.1 | N/A | N/A |
p<0.05 comparing * to †; ‡ to §; and ¶ to #
PF Activation Following Shocks
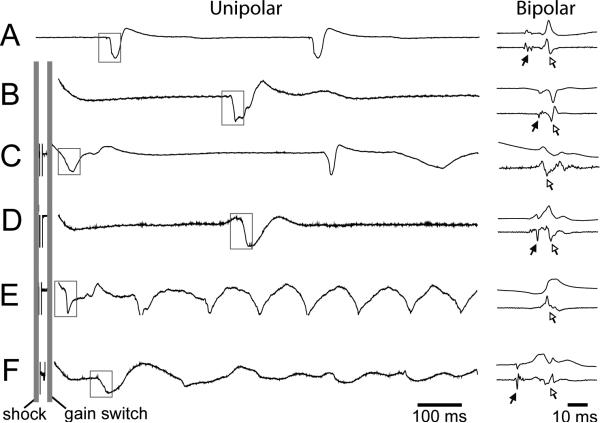
Fig. 3 shows examples of electrograms at the sites of earliest activation in sinus rhythm and after failed and successful shocks. PF activations were more easily detected in bipolar than in unipolar electrograms. PF activations were detected in every case before the earliest VM activations for sinus beats, SDVF Type A successes, LDVF successes, and LDVF failures. VM activations preceded the earliest detected PF activations following all SDVF failures and SDVF Type B successes.
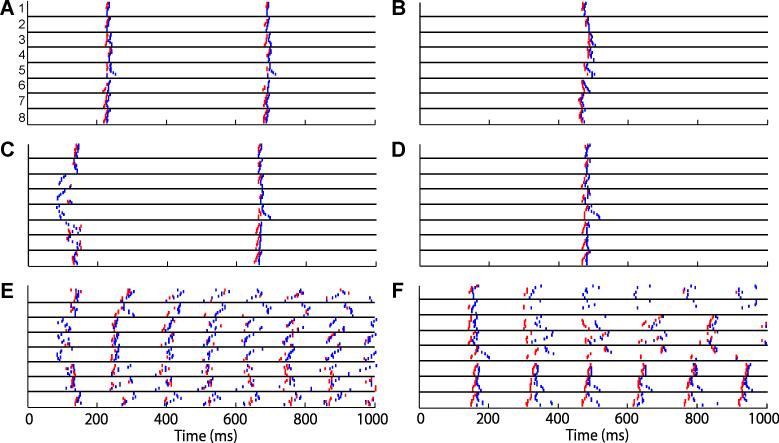
Figure 3.
Electrograms from the site of earliest recorded postshock for A) normal sinus rhythm, B) a SDVF Type A success, C) a SDVF Type B success, D) a LDVF success, E) a SDVF failure, and F) a LDVF failure. In each panel, the top left trace is the unipolar electrogram at the site of earliest activity, and the lower left trace is its temporal derivative. A time-expanded section of the first activation enclosed in the box is shown for an adjacent bipolar electrogram and its derivative. PF activations are marked with black arrows while VM activations are marked with white-filled arrows. Vertical gray lines in Panels B through F mark the timing of the shock and the timing of the gain switch (from low gain to high gain) of the mapping system.
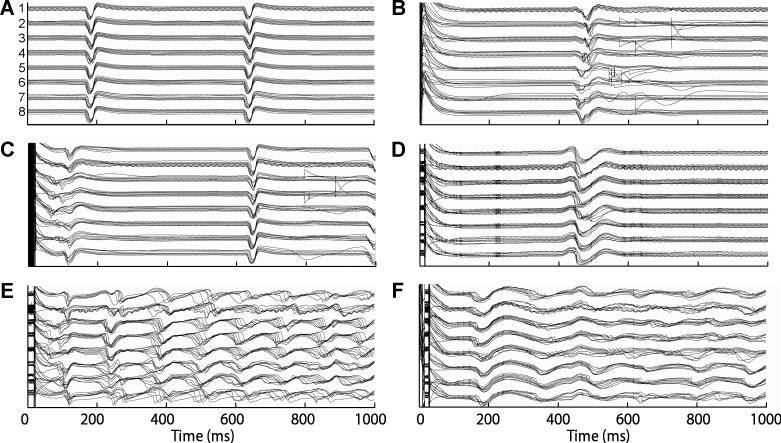
Fig. 4 shows examples of all 64 basket electrograms during sinus rhythm and shocks. Fig. 5 indicates the picked PF and VM activation times for the electrograms shown in Fig. 4. When earliest postshock activation was detected in the VM, a PF activation was rarely detected in that same electrogram during that cycle. The total VM activation time (time from the first VM to the last VM for a cycle) for PF-led cycles was significantly shorter than for cycles in which a VM activation was detected before any PF activations (Table 1).
Figure 4.
The 64 unipolar basket electrograms for the same shock episodes shown in Fig. 3. The trace order is the same as in Fig. 1B (1a at the top, 8h at the bottom). The spline number for each group of traces is shown in Panel A. Electrograms are shown for the first second following the shock.
Figure 5.
Times of PF (red) and VM (blue) activations during the same shock episodes as shown in Fig. 3-4. The trace order is the same as in Fig. 1B (1a at the top, 8h at the bottom). Panels AF are for the same episodes shown in Fig. 3 and 4.
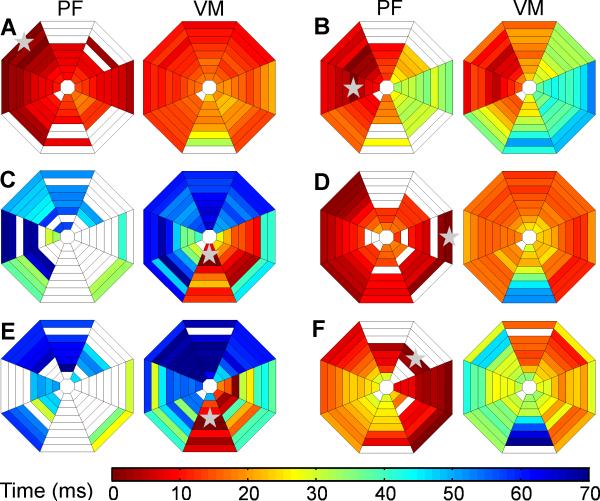
Fig. 5 shows examples of PF and VM activations for a sinus beat and for the first postshock activation cycles. Macro-reentry was not apparent in any of the postshock activation cycles. However, micro-reentry could neither be confirmed nor excluded due to the large inter-electrode distances between the basket electrodes. PF-led cycles exhibited early rapid spread of activation through the PFs followed by relatively rapid spread of activation through the VM with slow conduction through a limited portion of the VM (Fig. 6 A, B, D, and F). VM-led cycles frequently exhibited slow conduction in the VM for 20-30 ms, followed by activation that spread quickly through the PFs and then spread rapidly through the remainder of the VM in a similar activation sequence as the PFs (Fig. 6 C and E).
Figure 6.
Activation maps showing PF (left diagram in each panel) and VM (right diagram in each panel) activation times of the first postshock cycle for the shock episodes shown in Figures 2-4. Activation times are indicated by the color bar. White indicates that no activation was detected at that electrode. Gray stars indicate the site of earliest activation in each panel.
Total VM activation time was significantly shorter for the first postshock cycles in which earliest activation was recorded in the PFs rather than in the VM (Table 1). Post hoc analysis did not reveal differences in isoelectric window lengths for SDVF Type B successes and SDVF failures, but the isoelectric window was significantly longer for LDVF failures than SDVF failures. The isoelectric window was significantly longer after LDVF successes, LDVF failures, and SDVF Type A successes than after SDVF failures and SDVF Type B successes.
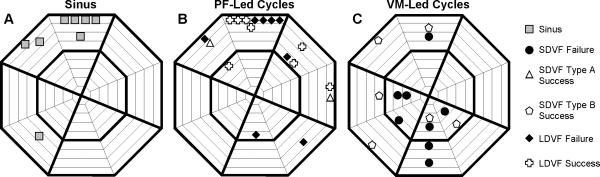
Fig. 7 shows the site of earliest recorded activation during sinus rhythm, following shocks in which the first postshock cycle initiated in the PFs, and following shocks in which the first postshock cycle initiated in the VM for all animals. The location of first activation was similar for sinus rhythm (Fig 7A) and first postshock activation cycles in which earliest activation was recorded in PF (Fig 7B, r = 0.86, p<0.05), but correlation was low for the location of first activation between sinus rhythm (Fig 7A) and the rhythm in which earliest activation was recorded in VM (Fig. 7C, r = 0.11). Correlation also was low for earliest recorded activation sites between first postshock activation cycles in which earliest activation was recorded in PF (Fig. 7B) and in which it was recorded in VM (Fig 7C, r=0.01). Earliest activation was more common in electrodes towards the base than the apex for sinus rhythm and PF-led cycles (Fig 7A and 7B, p<0.05), but not for VM-led cycles (Fig. 7C). A high incidence of earliest postshock activation was in the anterior/anterior septal base electrodes when earliest recorded activation was PF (Region i in Fig. 6D), but was scattered throughout the regions when earliest recorded postshock activation was in VM (Fig. 7C).
Figure 7.
Locations of earliest recorded postshock activation for all 8 animals in A) PF-led sinus rhythm, B) PF-led first postshock activation cycles, and C) VM-led first postshock activation cycles. For statistical analysis, first postshock activation cycles were grouped as shown in Fig. 1C.
Discussion
The primary findings of this study are as follows: 1) With the exception of Type A successes (2 of 16 trials), earliest recorded activation following near DFT strength shocks following SDVF arises in VM (14 of 16 trials) but following LDVF arises in PFs (16 of 16 trials); 2) Type B shock successes are common following SDVF, but do not occur following LDVF; 3) Isoelectric windows following failed near-DFT shocks are longer after LDVF than after SDVF.
PFs in SDVF vs. LDVF
SDVF and LDVF are different electrophysiologically. The rapid activation rate and global ischemia of LDVF cause shortening of action potential duration (APD) and prolongation of post repolarization refractoriness.15, 16 The activation rate slows significantly as LDVF progresses and, in canines, an activation gradient develops in which the endocardium activates more rapidly than the epicardium.14, 17 In normal hearts, spontaneous refibrillation is common after defibrillation following LDVF but not SDVF.17, 18
We previously demonstrated that the PF system is active during the first postshock activation cycle following SDVF.3 Due to the limited number of PF activations detected in that study (a mean of 7.6 per experiment), we were unable to determine whether the first postshock activation cycle initiated in the PFs or initiated in the VM and then propagated retrogradely into the PFs. PF activations did not typically precede VM activations at the earliest postshock activation sites. The previous study was in swine, in which the PF system is present not only near the endocardium but also throughout the VM wall nearly to the epicardium.19 The PF system in canines, as in humans, is limited primarily to the subendocardium,19, 20 and therefore, it can be recorded with endocardial electrodes. The present study demonstrates that in SDVF, the first recorded endocardial postshock activity is in VM, explaining why we did not detect PF activation preceding VM activation at the early sites in the earlier study. However, in the present study earliest recorded postshock activation initiated consistently in PFs after LDVF.
Recent experiments have demonstrated that the PF system may play an increasingly important role in VF maintenance as the duration of VF increases.12, 13 As VF progresses, activation wavefronts propagate antegradely from the PFs into the VM and retrogradely from the VM into the PFs.12 Chemical ablation of the PFs with Lugol's solution in canines eliminates the transmural gradient in activation rate and causes LDVF to spontaneously terminate much earlier than in control hearts.13 PFs may be more active than VM during LDVF because the PFs are more resistant to the effects of the global ischemia caused by VF and the subendocardial PFs are exposed to the oxygenated blood in the ventricular cavity during LDVF. 14, 17, 21 Although the APDs of PFs are longer than those of VM at slow activation rates,22 the PF APDs shorten more rapidly than the VM APDs at rapid activation rates23 so that the APDs may be shorter for PFs than for VM cells during LDVF. The shorter APD and increased triggered activity of the PFs under ischemic conditions24, 25 may be the source of first activation following failed defibrillation shocks.
Epicardial pacing in dogs results in an activation sequence in which the wavefronts spread relatively slowly in a quasi-elliptical pattern that follows the VM fiber orientation.26, 27 Once the wavefront reaches the endocardium and is transmitted retrogradely to the PF system, a second pattern of activation with high propagation velocity and irregularly shaped isochrones is observed. When the PF system is removed by Lugol's ablation, this pattern of activation does not occur.27 Our findings are consistent with activation initiating intramurally in the VM following SDVF: slow spread of activation for the first 20-30 ms followed by rapid excitation of the remainder of the endocardium once activation entered the PF system (Fig. 6 C and E). PF-led cycles following defibrillation demonstrated rapid spread of excitation (as evidenced by the significantly shorter total VM activation times), consistent with excitation initiating in and spreading through the endocardium by means of PFs (Fig. 6 A, B, D, and F).
Cardiac models including PFs in realistic reconstructions of cardiac geometries reported that, following shocks, earliest activation appeared in PFs and that PFs helped stabilize early postshock reentry by providing alternative conduction pathways in addition to the VM.28, 29 Our experimental result that first post-shock activation appeared in PFs following LDVF is consistent the predictions of these models. However, we did not typically find first activation in the PF system following SDVF.
Near DFT shock failures following LDVF had an isoelectric window nearly 100 ms longer than following SDVF. One potential explanation for this observation is that the shock was of sufficient strength to terminate reentry during LDVF, but that activity in the PFs led to shock failure and reinitiation of VF. While the reason for the isoelectric window following both successful and failed shocks remains unexplained,30 emergence of the first postshock activity in VM after SDVF and PFs after LDVF may indicate different mechanisms of defibrillation.
Triggered Activity
Previous studies with pinacidil, an early afterdepolarization inhibitor, and flunarizine, a delayed afterdepolarization (DAD) inhibitor suggested that activation following failed near threshold shocks after SDVF is not caused by triggered activity.6 However, our results raise the possibility that triggered activity may play a role in postshock activity following LDVF. In SDVF intramural reentry plays an important role in VF maintenance, but as LDVF progresses, the percentage of wavefronts that originate from focal sources increases while detectable intramural reentry decreases.10 Since the mechanisms of VF maintenance evolve as VF continues, it is possible that the mechanism responsible for the first postshock activation also changes as VF duration increases.
DADs in the PFs during regional ischemia have been shown to cause ventricular tachycardia (VT) and VF.24 Free radical scavengers reduce DADs and the incidence of VT and VF in a similar canine model.31 During LDVF, regional ischemia, the rapid activation rate of VF, and an intracellular calcium overload may lead to an arrhythmogenic substrate in the PFs and subendocardium.32 This pro-arrhythmic substrate has not yet had time to develop in SDVF but may play a critical role in LDVF shock failure.
PFs Response to Shocks
A study in isolated canine papillary muscle by Li et al. demonstrated that large shocks with a potential gradient greater than 21.7 V/cm caused rapid firing in PFs but caused prolonged shock induced refractoriness in VM.33 Allred et al. reported the maximum potential gradient measured with plunge needles within the ventricles for near DFT strength transthoracic shocks in swine was 28.7±17 V/cm and the mean potential gradient was 15.4±8.2 V/cm.34 There were only two failed shocks following LDVF in that study. In both, earliest postshock activation occurred near the endocardium where the potential gradient was more than one standard deviation below the mean. Therefore, the mechanism by which earliest postshock activation arose in the PFs following LDVF in our study may be different than that observed by Li et al.
Conclusions
The different postshock activation patterns and mechanisms of defibrillation failure following SDVF and LDVF may have clinical implications. The vast majority of research dealing with defibrillation shock waveforms and durations, has been conducted in animals and humans following VF episodes lasting less than one minute. However, with the exception of patients with ICDs, most individuals who develop VF are not defibrillated for several minutes.1 If the mechanism of shock failure varies with the duration of VF, there arises the possibility that more effective techniques of defibrillating and resuscitating a patient with LDVF may exist than those developed in models of SDVF. There may be pharmacologic interventions (such as DAD blockers6 or free radical scavengers31) or defibrillation and resuscitation techniques that improve defibrillation following LDVF but not SDVF. This possibility merits additional research efforts.
Limitations
In this closed-chest model, we recorded only from the LV endocardium. Earliest endocardial activations may have arisen from intramural wavefronts traveling towards the endocardium that appeared focal as they broke through to the endocardial surface.35 Since PFs are limited primarily to the subendocardium in canines,19 VM wavefronts propagating intramurally toward the endocardium should have been detected as endocardial VM activations before spreading retrogradely into the PF system if they had been present. The RV endocardium was not mapped in the present study and the possibility exists that PF led first activation signals could have been transmitted antegradely through the right bundle branch to the His bundle and then down the left bundle branch. Future studies mapping the PF system in both ventricles will be needed to definitively determine the role of the RV PF system in defibrillation.
This study was conducted in healthy canine hearts, while VF usually occurs in diseased hearts. Defibrillation mechanisms may differ between normal hearts and diseased hearts.
Funding Sources
This study was supported by National Heart, Lung, and Blood Institute grants (HL085370, HL028429, and K99HL091138). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Abbreviations
- VF
ventricular fibrillation
- LDVF
long duration ventricular fibrillation
- SDVF
short duration ventricular fibrillation
- LV
left ventricle
- RV
right ventricle
- DFT
defibrillation threshold
- VM
ventricular myocardium
- PF
Purkinje fiber
- VT
ventricular tachycardia
- APD
action potential duration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valenzuela TD, Roe DJ, Nichol G, et al. Outcomes of Rapid Defibrillation by Security Officers after Cardiac Arrest in Casinos. N Engl J Med. 2000;343:1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 2.Dosdall DJ, Fast V, Ideker RE. Mechanisms of defibrillation. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology : from cell to bedside. 5th ed Saunders; Philadelphia: 2009. pp. 499–508. [Google Scholar]
- 3.Dosdall DJ, Cheng KA, Huang J, et al. Transmural and endocardial Purkinje activation in pigs before local myocardial activation after defibrillation shocks. Heart Rhythm. 2007;4:758–65. doi: 10.1016/j.hrthm.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chattipakorn N, Fotuhi PC, Ideker RE. Prediction of defibrillation outcome by epicardial activation patterns following shocks near the defibrillation threshold. J Cardiovasc Electrophysiol. 2000;11:1014–21. doi: 10.1111/j.1540-8167.2000.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen PS, Shibata N, Dixon EG, et al. Activation during ventricular defibrillation in open-chest dogs. Evidence of complete cessation and regeneration of ventricular fibrillation after unsuccessful shocks. J Clin Invest. 1986;77:810–23. doi: 10.1172/JCI112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Walcott GP, Smith WM, Ideker RE. Evidence that activation following failed defibrillation is not caused by triggered activity. J Cardiovasc Electrophysiol. 2005;16:1200–5. doi: 10.1111/j.1540-8167.2005.50045.x. [DOI] [PubMed] [Google Scholar]
- 7.Ideker RE. Ventricular fibrillation: how do we put the genie back in the bottle? Heart Rhythm. 2007;4:665–74. doi: 10.1016/j.hrthm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Rogers JM, Killingsworth CR, et al. Evolution of activation patterns during long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2004;286:H1193–1200. doi: 10.1152/ajpheart.00773.2003. [DOI] [PubMed] [Google Scholar]
- 9.Tovar OH, Jones JL. Electrophysiological deterioration during long-duration ventricular fibrillation. Circulation. 2000;102:2886–2891. doi: 10.1161/01.cir.102.23.2886. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural foci during long duration fibrillation in the pig ventricle. Circ Res. 2008;102:1256–64. doi: 10.1161/CIRCRESAHA.107.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabereaux PB, Dosdall DJ, Ideker RE. Mechanisms of VF maintenance: Wandering wavelets, mother rotors, or foci. Heart Rhythm. 2009;6:405–415. doi: 10.1016/j.hrthm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabereaux PB, Walcott GP, Rogers JM, et al. Activation patterns of Purkinje fibers during long-duration ventricular fibrillation in an isolated canine heart model. Circulation. 2007;116:1113–9. doi: 10.1161/CIRCULATIONAHA.107.699264. [DOI] [PubMed] [Google Scholar]
- 13.Dosdall DJ, Tabereaux PB, Kim JJ, et al. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2008;295:H883–9. doi: 10.1152/ajpheart.00466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worley SJ, Swain JL, Colavita PG, Smith WM, Ideker RE. Development of an endocardial-epicardial gradient of activation rate during electrically induced, sustained ventricular fibrillation in dogs. Am J Cardiol. 1985;55:813–20. doi: 10.1016/0002-9149(85)90162-6. [DOI] [PubMed] [Google Scholar]
- 15.Robertson PG, Huang J, Chen KA, et al. Increased cycle length during long-duration ventricular fibrillation is caused by decreased upstroke velocity as well as prolonged refractoriness. Heart Rhythm. 2009;6:378–384. doi: 10.1016/j.hrthm.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JL, Tovar OH. Electrophysiology of ventricular fibrillation and defibrillation. Crit Care Med. 2000;28:N219–21. doi: 10.1097/00003246-200011001-00013. [DOI] [PubMed] [Google Scholar]
- 17.Allison JS, Qin H, Dosdall DJ, et al. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18:1306–12. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu TJ, Lin SF, Hsieh YC, Chen PS, Ting CT. Early recurrence of ventricular fibrillation after successful defibrillation during prolonged global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2008;19:203–10. doi: 10.1111/j.1540-8167.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 19.Pak HN, Kim GI, Lim HE, et al. Both Purkinje cells and left ventricular posteroseptal reentry contribute to the maintenance of ventricular fibrillation in open-chest dogs and swine: effects of catheter ablation and the ventricular cut-and-sew operation. Circ J. 2008;72:1185–92. doi: 10.1253/circj.72.1185. [DOI] [PubMed] [Google Scholar]
- 20.Spach MS, Huang S-n, Armstrong SL, Canent RV., Jr Demonstration of peripheral conduction system in human hearts. Circulation. 1963;28:333–338. doi: 10.1161/01.cir.28.3.333. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour RF, Jr., Zipes DP. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1980;46:814–25. doi: 10.1161/01.res.46.6.814. [DOI] [PubMed] [Google Scholar]
- 22.Balati B, Varro A, Papp JG. Comparison of the cellular electrophysiological characteristics of canine left ventricular epicardium, M cells, endocardium and Purkinje fibres. Acta Physiol Scand. 1998;164:181–90. doi: 10.1046/j.1365-201X.1998.00416.x. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RB, Boyden PA, Hoffman BF, Hewett KW. Electrical restitution process in dispersed canine cardiac Purkinje and ventricular cells. Am J Physiol. 1987;253:H1018–25. doi: 10.1152/ajpheart.1987.253.5.H1018. [DOI] [PubMed] [Google Scholar]
- 24.Xing D, Martins JB. Triggered activity due to delayed afterdepolarizations in sites of focal origin of ischemic ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2004;287:H2078–84. doi: 10.1152/ajpheart.00027.2004. [DOI] [PubMed] [Google Scholar]
- 25.Arnar DO, Bullinga JR, Martins JB. Role of the Purkinje system in spontaneous ventricular tachycardia during acute ischemia in a canine model. Circulation. 1997;96:2421–9. doi: 10.1161/01.cir.96.7.2421. [DOI] [PubMed] [Google Scholar]
- 26.Frazier DW, Krassowska W, Chen PS, et al. Transmural activations and stimulus potentials in three-dimensional anisotropic canine myocardium. Circ Res. 1988;63:135–46. doi: 10.1161/01.res.63.1.135. [DOI] [PubMed] [Google Scholar]
- 27.Taccardi B, Punske BB, Macchi E, Macleod RS, Ershler PR. Epicardial and intramural excitation during ventricular pacing: effect of myocardial structure. Am J Physiol Heart Circ Physiol. 2008;294:H1753–66. doi: 10.1152/ajpheart.01400.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deo M, Boyle P, Plank G, Vigmond E. Arrhythmogenic Mechanisms of the Purkinje system during electric shocks: a modelling study. Heart Rhythm. 2009 doi: 10.1016/j.hrthm.2009.08.023. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigmond E, Vadakkumpadan F, Gurev V, et al. Towards predictive modelling of the electrophysiology of the heart. Exp Physiol. 2009;94:563–77. doi: 10.1113/expphysiol.2008.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trayanova N. Drawing the curtain on the isoelectric window? Heart Rhythm. 2007;4:766–7. doi: 10.1016/j.hrthm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing D, Chaudhary AK, Miller FJ, Jr., Martins JB. Free radical scavenger specifically prevents ischemic focal ventricular tachycardia. Heart Rhythm. 2009;6:530–6. doi: 10.1016/j.hrthm.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaugg CE, Wu ST, Barbosa V, et al. Ventricular fibrillation-induced intracellular Ca2+ overload causes failed electrical defibrillation and post-shock reinitiation of fibrillation. J Mol Cell Cardiol. 1998;30:2183–2192. doi: 10.1006/jmcc.1998.0777. [DOI] [PubMed] [Google Scholar]
- 33.Li HG, Jones DL, Yee R, Klein GJ. Defibrillation shocks produce different effects on Purkinje fibers and ventricular muscle: implications for successful defibrillation, refibrillation and postshock arrhythmia. J Am Coll Cardiol. 1993;22:607–14. doi: 10.1016/0735-1097(93)90072-9. [DOI] [PubMed] [Google Scholar]
- 34.Allred JD, Killingsworth CR, Allison JS, et al. Transmural recording of shock potential gradient fields, early postshock activations, and refibrillation episodes associated with external defibrillation of long-duration ventricular fibrillation in swine. Heart Rhythm. 2008;5:1599–606. doi: 10.1016/j.hrthm.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashihara T, Constantino J, Trayanova NA. Tunnel propagation of postshock activations as a hypothesis for fibrillation induction and isoelectric window. Circ Res. 2008;102:737–45. doi: 10.1161/CIRCRESAHA.107.168112. [DOI] [PMC free article] [PubMed] [Google Scholar]