Abstract
Deep brain stimulation (DBS) has been increasingly utilized for the therapeutic treatment of movement disorders, and with the advent of this therapy more postoperative urgencies and emergencies have emerged. In this paper, we will review, identify, and suggest management strategies for both intra- and postoperative urgencies and emergencies. We have separated the scenarios into 1- surgery/procedure related, 2- hardware related, 3- stimulation induced difficulties, and 4- others. We have included ten illustrative (and actual) case vignettes to augment the discussion of each issue.
Keywords: deep brain stimulation, movement disorders, emergency, differential diagnosis, adverse event
Introduction
Neurosurgical procedures for basal ganglia disorders may result in urgent or emergent management issues. Postoperative urgencies and/or emergencies should be identified and treated in an expeditious manner. Deep brain stimulation (DBS) has been increasingly utilized for addressing neurologic and neuropsychiatric disorders [1,2], and with the increasing number of DBS cases being performed each year, there has been a commensurate increase in the number of issues relating to the surgical, the procedural, and to the stimulation related phenomena. Some of these issues have manifested themselves as movement disorders (e.g. dyskinesia, ballism, dystonia), although the majority have presented in other ways [3–5]. In this paper, we have separated the potential scenarios into 1- surgery/procedure related, 2- hardware related, and 3- stimulation induced phenomena. The discussion has been augmented by the use of clinical vignettes which illustrate the diagnosis and management of both urgent and emergent situations. Complications of DBS have unique manifestations, and diagnostic criteria and management have not been fully established in some cases. Therefore, it is possible that clinicians may overlook DBS-induced complications, and delay of the appropriate management. This delay may unnecessarily result in secondary complications. The aim of this paper is to review urgent and emergent DBS-associated situations to provide recommendations for appropriate management.
Methods
Complications with DBS-specific manifestations have been specifically selected for this review, and a PubMed based literature search was performed for each issue. We queried the Institutional Review Board (IRB) approved DBS database of University of Florida Movement Diorders Center (UFMDC) for the illustrative cases from the period between July 2002 and June 2009.
Surgery/procedure related urgencies/emergencies
Intracranial hemorrhage
Case 1. A 73 year-old man with a 16 year-history of Parkinson’s disease (PD) underwent unilateral subthalamic nucleus (STN) lead implantation. There was no history of hypertension, diabetes mellitus (DM), or coronary artery disease (CAD). Following the procedure, he became somnolent, and a postoperative computed tomography (CT) scan revealed a hematoma in the left lateral ventricle (Figure 1B). There was involvement of the third ventricle and the Sylvian aqueduct. The patient developed acute obstructive hydrocephalus that necessitated emergent ventriculostomy. He convalesced for one week postoperatively, and then developed a deep venous thrombosis, an aspiration pneumonia, atrial fibrillation, a urinary tract infection, and sepsis. The total hospitalization was extended to 40 postoperative days. Following eight months of rehabilitation and anticoagurant therapy he has recovered, and implantation of Implantable Pulse Generator (IPG) was scheduled.
Hemorrhage is an emergent adverse event that may be seen following DBS, and may result in significant morbidity or rarely even death. Hemorrhages following DBS may include intracerebral (ICH), intraventricular (IVH), subdural (SDH), subararchnoid (SAH) and epidural (EDH). (Figure 1) Hemorrhagic complications have been assumed to be due to damage to the blood vessels by the microelectrode recordings (MER) and/or macrostimulation passes, and it has been discussed that multiple MERs and/or macrostimulation passes may increase the incidence of hemorrhagic complications (debated but generally accepted among the experts). [6–8] Intracranial hemorrhage can be diagnosed by CT scan which may be sought in the post-operative period and is usually performed as a result of a mental status change and/or a focal neurological deficit. The incidence of hemorrhage varies from 0.6–3.3% [7,9–13]. Intracranial hemorrhage can precipitate secondary complications such as pneumonia, pulmonary embolus, and urinary tract infection. A recent German multicenter study revealed an overall 30-day postoperative mortality rate of 0.4% (5 of 1,183 patients), and mortality due to hemorrhage in 2 of 5 patients [14]. Delay of identification and management of ICH can result in significant morbidity, therefore emergent care should be employed to prevent both primary and secondary complications. When ICH is encountered it mandates immediate neurosurgical consultation, preferably by the neurosurgeon who implanted the DBS system, although this is not always possible. Most patients can be managed conservatively, however if operative intervention for evacuation is deemed necessary every attempt should be made not to remove the DBS hardware (Table 1). Patients requiring evacuation as well as those not requiring surgery have the potential for good recovery.
Figure 1. Computed tomography (CT) scan images of the many types of hemorrhage that may be encountered following DBS lead implantation.
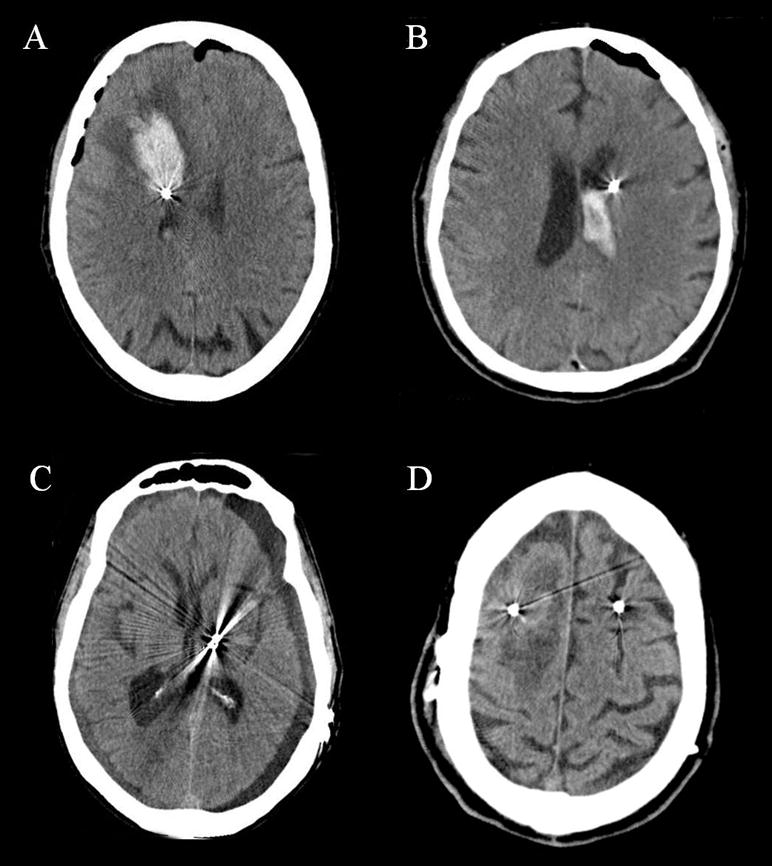
This panel of CT images reveals examples of intracerebral hemorrhage (A), intraventricular hemorrhage (B), subdural hemorrhage (C), and venous infarction (D).
Table 1.
Postoperative surgery and hardware related urgencies and emergencies
| Issue | Routine/urgent/emergent | Management |
|---|---|---|
| Intracerebral hemorrhage | Emergent | If the hemorrhage is very large, an emergent craniotomy may need to be performed. |
| Intraventricular hemorrhage | Emergent | Ventriculostomy if necessary may be performed for obstructive hydrocephalus. |
| Subdural hematoma | Emergent | If acute and symptomatic, an emergent craniotomy may be performed in select cases. If chronic, a burr hole irrigation may be performed, or it may be watched conservatively. |
| Epidural hematoma | Emergent | An emergent craniotomy should be performed immediately in severe cases. |
| Venous infarction | Urgent/emergent | Conservative therapy. An emergent craniotomy may be performed if hemorrhage is life threatening. |
| Dyskinetic storm | Urgent | Sedative agents may be administered in select cases. Reducing the dopaminergic medication may help. In some cases ICU care is necessary. |
| Behavioral/cognitive issues | Urgent/emergent | Identify and treat the underlying issues. Selective dopamine blockers (e.g. clozapine, quetiapine) may be used, but nonselective blockers should be avoided if possible. |
| Suicide ideation/attempt | Emergent | Evaluation for an underlying issue such as battery life and/or unintended on/off. Admit the patient to the hospital for multidisciplinary care including behavioral therapy, counseling, medication adjustment and/or stimulation adjustment. |
| Air embolus | Emergent | Wax edges of the burr hole, lower patient’s head, jugular venous compression, administer oxygen |
| Infection-UTI | Routine/urgent | Hydration and appropriate antibiotics, case should be taken to adjust PD medications if necessary as levels may be altered by antibiotics. |
| Infection-lead | Emergent | The lead should be removed and appropriate antibiotics should be administered. |
| Infection-IPG | Urgent | The IPG and usually the extension cable should be removed and appropriate antibiotics should be administered. |
| Lead fracture | Urgent | Lead replacement, if an appropriate candidate. |
| Lead electrical short | Urgent | Lead replacement, or potentially reprogramming at a different contact |
| Lead migration | Urgent | Lead replacement, or surgical alteration of lead position. |
| Lead misplacement | Urgent | Lead replacement. |
| IPG malfunction | Urgent | IPG replacement, manage potential rebound symptoms. |
Venous infarction
Case 2. A 60 year-old man with PD underwent a staged unilateral globus pallidus interna (GPi) DBS. He was discharged on postoperative day #1 following an uncomplicated hospital course, but later that day he began to develop left-sided weakness, lethargy, and confusion which peaked on postoperative day #2. He presented to the emergency room (ER) on postoperative day #4. A head CT scan revealed hemorrhage spreading from the center of the DBS lead. The region was surrounded by edema. (Figure 1D) The diagnosis of venous infarction was made and he was conservatively managed. Following several months, his neurological status returned to baseline, and his DBS was effectively programmed to address both motor fluctuations and parkinsonism.
Venous infarction has been associated with damage to a cortical vein that may occur during DBS surgery. Cerebral edema and hemorrhage may slowly develop and are usually the result of venous stasis or venous hypertension. These phenomena are thought to occur as a result of venous obstruction which usually traces to the damaged cortical vein. Venous infarction can be characterized by a delayed clinical onset with edema and possibly hemorrhage along the path of the DBS lead [15]. These features may be absent in some cases. Importantly, the head CT may not reveal an obvious lesion in the immediate postoperative period, and a repeat CT may be necessary to confirm diagnosis. The prognosis is usually positive in venous infarction occurring post-DBS [7,16]. Careful pre-operative targeting using a high quality contrasted MRI will aid in avoiding cortical veins, and may prevent this complication [7]. If the diagnosis of venous infarction is made postoperatively, the management should include optimizing the venous return (e.g. elevate the head of the bed), managing blood pressure, avoiding dehydration, and initiating early rehabilitation (Table 1).
Dyskinetic storm
Postoperative dyskinesia is a relatively common phenomenon associated with STN DBS, especially in PD patients who preoperatively suffered from severe medication-induced dyskinesia [4]. Microelectrode recording, cannula placement and/or lead placement may all induce dyskinesias especially in patients with PD. Acute and severe exacerbation of dyskinesias (dyskinetic storm) in the operative setting has been previously reported and may be a feature associated with a positive prognosis [3]. In severe cases, dyskinesia may be associated with dyspnea and rhabdomyolysis [17,18], and emergent administration of sedative agents (such as IV propofol) may be required [3] (Table 1). Dyskinesia may also be induced by DBS placement in a delayed fashion [19], and dyskinetic storm may be encountered in the clinical setting following DBS programming. Management of dyskinesia in the clinical settings will also be discussed in the “Stimulation-related motor symptoms” section of this review.
Postoperative behavioral and cognitive problems
Postoperative cognitive and behavioral decline is a common DBS related adverse event. It is usually temporary, but in patients with pre-operative cognitive dysfunction it may persist. The incidence of confusion has been recently reported as 5% in a large single center study [20], but rates may vary depending on preoperative comorbidities, target site, and whether staging of operative sides is employed (i.e., as opposed to same-day simultaneous bilateral DBS implantation) [4,21–23]. The incidence of behavioral and cognitive problems may be higher in STN DBS when compared to other targets [4,23–25]. A recent randomized double-blind study revealed a higher incidence of cognitive adverse events in patients with STN DBS when compared to GPi DBS [23]. Also verbal fluency seemed worse in STN and the change was reported as more surgery related rather than a stimulation related effect (i.e. it occurred in the off STN condition as well as the on STN DBS condition during blinded testing) [23]. Anticholinergics (including not only anti-Parkinsonian medications but also medications for neurogenic bladder) may also increase the risk of postoperative cognitive problems, and may need to be discontinued [26]. It is important for clinicians to keep in mind that advanced age and/or pre-existing neurological compromise may predispose patients to mental status changes following DBS, and this is a compelling reason for centers to perform pre-operative neuropsychological screening [21].
The management of postoperative behavioral and cognitive problems should include a diagnostic workup of potential underlying causes that may exacerbate and/or contribute to the clinical condition. These may include urinary tract infections, hemorrhage or medications related phenomena. If no underlying treatable cause can be identified, the clinician should utilize pharmacotherapy and a multi/interdisciplinary approach to manage the behavioral change(s). This approach may be facilitated in select cases by an inpatient admission.
Patients may become restless and violent postoperatively due to hallucinations/delusions, and this situation may require urgent/emergent care. In these cases, conventional neuroleptics are usually contraindicated, however using selective dopaminergic blockers such as quetiapine or clozapine may be useful [27] (Table 1). Non-selective dopaminergic blockers (e.g. olanzapine, risperidone, haloperidol, etc.) that have been commonly employed for the treatment of behavioral emergencies have also been observed to lead to drug-induced parkinsonism as well as other movement disorders [28,29].
Suicide attempt or ideation
Case 3. A 54 year-old woman with PD and depression who had a left DBS implantation two years prior to presentation was brought to the emergency room following a suicide attempt by drug overdose. Passive suicidal ideation was noted on her psychiatric evaluation prior to DBS.
Several reports have revealed cases of attempted and completed suicide occurring following DBS [30–32]. DBS may increase the risk of suicide when compared to the general population, but not necessarily when compared to a PD population (without DBS) [31,33]. A recent multicenter study revealed that preoperative history of impulse control disorders or compulsive medication use, postoperative depression, postoperative apathy, and being single were strongly associated with suicide attempts [33]. Previous suicide attempts, younger age of the patient, and younger onset of PD were also revealed to be associated with suicide attempt within the same study cohort. Therefore, screening for suicidal ideation following DBS should be routine, and if discovered, it should be treated as an emergency. Clinicians should admit patients to the hospital for multi/interdisciplinary care, which may include cognitive behavioral therapy, counseling, and/or medication/stimulation adjustment(s) (Table 1). Whether DBS results in disinhibition or impulsiveness and ultimately contributes to suicidal ideation or suicide remains controversial [34]. Vigilant pre- and post-operative screening for depression and suicidal ideation are recommended. Preoperative neuropsychological and psychiatric evaluation is highly recommended as a preventative measure [21,32]. Advanced PD patients are likely to have cognitive and/or mood disturbances, and neuropsychologists can play an important role for screening out these issues. Recently, there have been several reports of suicide in dystonia DBS highlighting that this issue may not be solely related to PD [30,31,35].
Myocardial infarction
Case 4. A 58 year-old male with PD, coronary artery disease (CAD) (previously treated with angioplasty), hypertension, diabetes mellitus (DM), and hyperlipidemia underwent unilateral STN DBS placement. An implantable pulse generator (IPG) was placed four weeks following the DBS lead. Following IPG implantation the patient died in his sleep on postoperative day one from a myocardial infarction.
Assessment of medical comorbidities must be performed on all patients undergoing DBS surgery [21]. Clinicians should be aware that patients taking medications that can affect the cardiovascular system such as bromocriptine, and tricyclic antidepressants (TCAs) may be at increased risk when undergoing general anesthesia [36]. The incidence of angina and arrhythmia following DBS surgery has been reported as 0.3% in a recent large single center study [20]. A history of CAD may increase the perioperative risk of MI and angina. High risk patients should be carefully monitored (pre- and postoperatively), and they may even require extra monitoring following general anesthesia. Cardiac or pulmonary symptoms in the postoperative period should be treated as an urgent, and possibly even emergent complications. Although this complication can be encountered following any surgical procedure, chest pain in the region surrounding the IPG DBS skin incision may be erroneously attributed to a DBS related issue rather than to a cardiac related issue [37]. Clinicians should be aware that the risk of comorbidities and medications, and that postoperative chest pain following DBS/IPG implantation can present or evolve into urgent or emergent issues.
Air Embolus
Air embolus a relatively uncommon complication of neurosurgical procedures. However, clinicians should be aware that the incidence of air embolus during DBS surgeries has been reported to be as high as 3% in a recent report, and DBS-related air embolus may manifest differently from other neurosurgical procedures, since during DBS the procedure is performed awake rather than under general anaesthesia [38,39]. When the neurosurgeon is preparing the burr hole for microelectrode recording and/or macrostimulation, air embolus may occur with tachycardia, a rise in the end tidal CO2, and cough. This reaction is typical of entrainment of air into the venous system. It is important to pre-operatively position the patient’s head as close to supine as possible to minimize this complication. In a recent series, Hooper et. al. reported the potential use of an external Doppler device to enhance monitoring for air embolus during DBS [39]. These authors also noted that the cough was the best clinical indicator to pick up an air embolus. When encountered, the head position should be adjusted (lower the patient’s head), bone edges of the burr hole should be waxed, the surgical field rigorously irrigated, and the patient vigorously supported from a cardio-pulmonary standpoint. Additionally, having the neurologist, nurse or physiologist temporarily compress the neck (to inhibit venous return) may aid the neurosurgeon in identifying the problematic region, and in quickly correcting the situation (Table 1).
Hardware-related urgencies/emergencies
Hardware infection
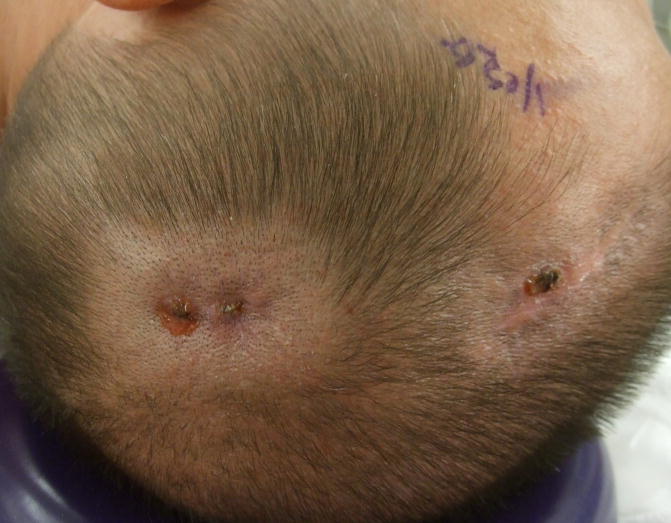
Case 5. A 43 year-old man with a nine year history of PD underwent unilateral STN-DBS. He arrived for a routine clinic appointment and staple removal on postoperative day seventeen. Following the staple removal there was purulent drainage from the cranial incision site, and the pectoral incision revealed tender erythema. (Figure 2) He was admitted to the hospital urgently, and the IPG and the extension wire were both removed. A course of intravenous antibiotics was completed prior to re-implantation.
Case 6. A 71 year-old man with a history of medically refractory essential tremor (ET) underwent a unilateral thalamic DBS implantation. Four weeks following surgery, the patient reported to the clinic for routine follow up care. Headache and progressive dysphagia were the chief complaints, and a head CT scan revealed a brain abscess along the DBS lead tract. The CT scan demonstrated an edematous lesion surrounding the DBS lead which was enhanced with contrast media. (Figure 3) He was admitted for emergent craniotomy, DBS lead removal and abscess drainage.
Hardware related infections are not uncommon in DBS. The incidence of infection and/or erosion following device implantations has been reported to range between 0–15.2 % [10,40–46]. Even the most vigilant surgical technique cannot guarantee the absence of postoperative infectious complications. The devices in these scenarios may require emergent removal, in contrast to the management of ICH, which may not require lead removal (Table 1). Cultures should be sent anytime hardware is removed or a potentially infectious pocket aspirated. Although several factors may be related to infection rate, pre- and postoperative prophylactic antibiotics may prevent hardware infection, however the evidence base for their use is weak [44,46]. One recent study did however report a reduction in the infection rate by locally injecting anti-staphylococcal antibiotics (e.g. neomycin, polymixin) directly into the operative wound [46].
There are several factors that may impact the management of a DBS infection. These include 1- whether the infection is deep or superficial, 2- whether the brain lead is involved, and 3- whether there are single or multiple sites of involvement [40]. A superficial infection may be managed in select cases in a non-operative and conservative fashion, however a deep infection may require emergent hardware removal. When the brain lead and/or multiple sites are involved, many DBS teams elect to remove all hardware (lead, extension, and IPG). In cases where only the IPG or extension cable appears infected, there is an option to remove only the purported infected hardware and to attempt to preserve the brain lead. Following a course of 6–8 weeks of IV antibiotic therapy, device(s) re-implantation may be possible.
Hardware malfunction
Case 7. A 76 year-old woman with a history of essential tremor (ET) who underwent a left thalamic DBS implantation three years prior presented to clinic with sudden tremor recurrence. A few days before her presentation, following a mammogram she experienced a tingling sensation in the right upper extremity with an abrupt loss of benefit in her right upper extremity tremor. When the device was checked the impedance was discovered to be greater than 2000 ohms, and a chest x-ray revealed a flipped IPG and a twisted extension cable. (Figure 4) A fracture of the extension cable was suspected, and replacement of the cable resolved the issue.
When a DBS patient reports sudden loss of efficacy, the clinician should consider hardware malfunction [4,47]. Mechanical stress to the device may result in lead fracture, a break in the extension cable, or an IPG failure. Blomstedt et al. reported 7 of 8 broken electrodes in their cases were encountered in patients with ET, and they speculated head tremor may have contributed to the adverse event(s) [44]. Compulsive manipulation of the IPG device, referred to as “Twiddler’s syndrome”, has also been reported to result in extension cable fractures [48].
The DBS programming/interrogation device should be used to measure the impedance and current drain for each of the four lead contacts. This procedure will assist in verifying the physical integrity of the DBS system. A high impedance along with a low current drain may be consistent with a lead fracture or with an extension cable break. Alternatively a low impedance with possible high current drain may be supporting evidence for a short circuit. In short circuits the patient will frequently complain of a shock-like sensation when palpating the IPG or when pressing along the extension cable tract. A plain film x-ray should be obtained to search for a fracture along the course of the lead or extension wire. (Figure 5) When the location of the problem cannot be precisely identified, the next step is to replace the extension wire and to retest impedances in the operating room setting. This procedure may save replacement of the intracranial lead in select cases [47]. Clinicians should always keep in mind that contacts with normal impedances/current drain values, are potentially programmable. An attempt to reprogram these functioning contacts should be sought prior to recommending replacement (Table 1).
Figure 5.
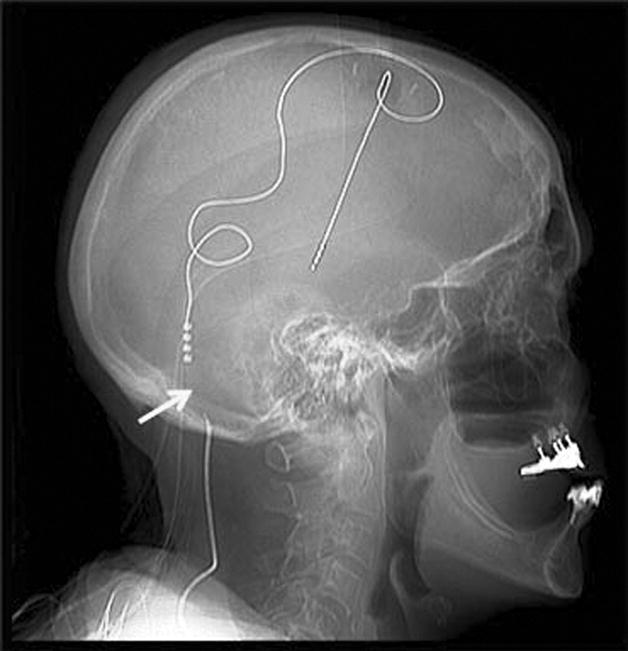
A skull x-ray revealed an extension cable fracture.
Lead migration
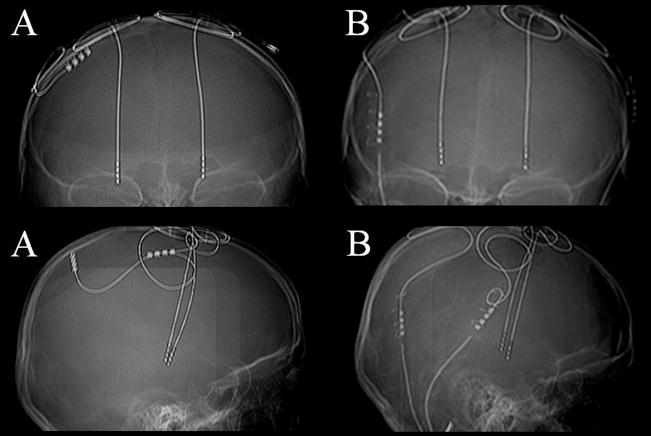
Case 8. A 7 year old boy with DYT-1 positive generalized dystonia underwent bilateral GPi DBS. After an initial dramatic response, his benefit deteriorated over the first year. Measurement and comparison of his DBS leads revealed dorsal lead migration. (Figure 6) His head circumference was measured and found to be 51.5cm preoperatively and 53cm 30 months later. Repositioning the DBS leads recaptured benefits.
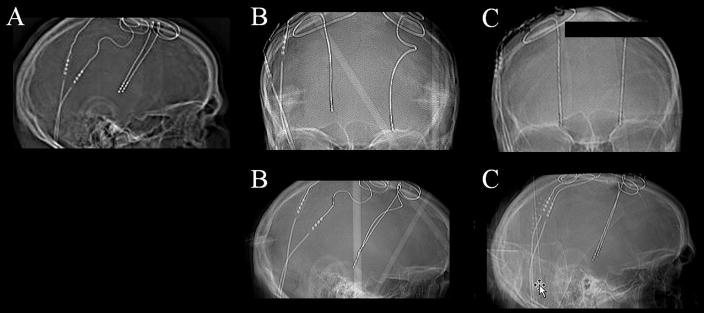
Case 9. A 26 year old man developed tardive dystonia following exposure to a neuroleptic drug used to address his severe depression. He subsequently underwent bilateral GPi-DBS. Preoperatively he suffered severe and painful retrocollic head jerks. Postoperatively his subjective pain and head jerking clinically improved (pain approximately 50+% and movement disorders approximately 40–50%). Six months following the operation the benefits waned, and a CT scan revealed that the left and the right leads had migrated 15.6mm and 4.6mm ventrally from their initial position (Figure 7). The patient underwent successful lead replacements.
Lead migration, either dorsal or ventral, can result from a malfunction of anchoring devices, skull growth, or vigorous head movements [47,49]. Yianni et al. reported that 3 of 133 patients (2.3%) experienced lead migration [49]. All three patients had dystonia, and the authors hypothesized that axial movements contributed to lead migration. When ventral lead migration is noted in a patient with GPi DBS, clinicians should be cautious as the ventral lead migration may result in severe mood changes due to the spread of the stimulation to other regions such as but not limited to the amygdala [50]. Skull growth in children is another cause of lead migration as illustrated by case 7. When lead migration is noted, changing the active contact (deeper or shallower depending on the direction of migration) should be attempted in most cases prior to surgical revision [51](Table 1). This adverse event highlights the importance of examining post-operative imaging.
Lead misplacement
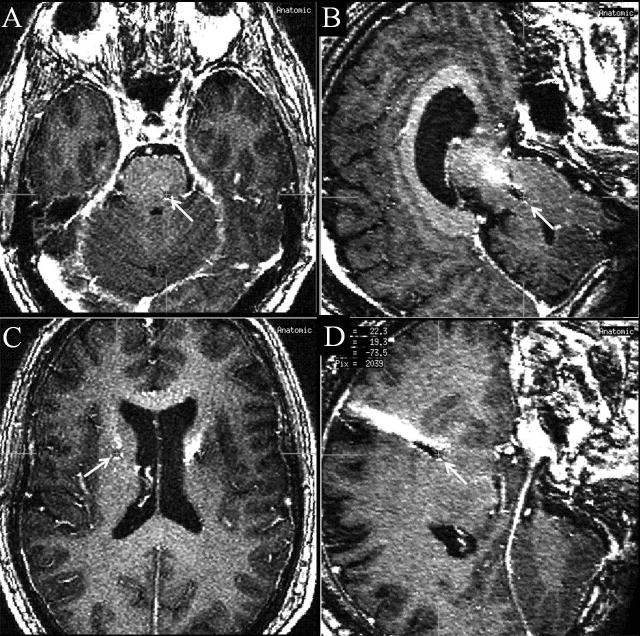
Case 10. A 60 year-old man with a history of PD underwent bilateral STN DBS at an outside institution 5 years prior to presentation to our clinic. He reported a lack of benefit from DBS, and repeated programming in the past had not improved his situation. A MRI scan revealed lead misplacement (Figure 8). The patient was unwilling to undergo a lead replacement because of a combination of claustrophobia and fear of the surgical suite.
Lead misplacement is a not uncommon complication of DBS surgery and has been reported to be associated with technical error, intraoperative brain shift (usually the result of a cerebrospinal fluid (CSF) leak [5]), and/or a failure in devices designed to secure the lead [49]. A suboptimal outcome from DBS surgery and/or low thresholds for stimulation induced side effects (when the device is interrogated) may suggest lead misplacement. DBS leads placed in suboptimal locations may not be able to be corrected by programming [47]. Stimulation-induced side effects can lead to an urgent or an emergent situation, and in extreme cases can result in severe and sometimes unexpected symptoms. Even slight variations (sometimes only millimeters) of the location of a DBS lead may alleviate negative symptoms and lead to a more optimal therapeutic benefit [51](Table 1).
Stimulation-related urgencies/emergencies
Stimulation-related motor symptoms
Stimulation-related motor symptoms include dyskinesia, chorea/ballism, gait disturbances, motor pulling, verbal fluency problems (verbal fluency has motor and non-motor/cognitive components), dysarthria, and hypophonia (Table 2). Many stimulation-induced motor symptoms resolve following reprogramming of the voltage, pulse width, and/or frequency.
Table 2.
Postoperative stimulation induced urgencies and emergencies
| Issue | Routine/urgent/emergent | Management |
|---|---|---|
| Chorea/ballism | Routine/urgent | Try to program slowly (e.g. slow increase of voltage over many weeks/months). May try dorsal contact. May also reduce dopaminergic medications. |
| Dyskinesia | Routine/urgent | Reducing the dopaminergic medication may help. Try to program slowly (e.g. slow increase of voltage over many weeks/months). May try dorsal contact. |
| Motor pulling | Urgent | Try to reduce voltage or pulse width. Try bipolar stimulation, or possibly another lead contact. Some situations may require lead replacement. Check lead location. |
| Gait disturbance | Routine/urgent | Try another setting (e.g. another contact, reducing pulse width, voltage or frequency). Low frequency (60Hz) with higher voltage or pulse width may help. |
| Verbal fluency problem | Routine | Try another contact, perhaps a more dorsal contact on the DBS lead. Try changing stimulation to bipolar or decrease pulse width, voltage or frequency. |
| Dysarthria/dysphagia | Routine | Try another contact. Try changing stimulation to bipolar or decrease pulse width, voltage or frequency. Check lead location. Prescribe speech therapy. |
| Hypophonia | Routine | Try another contact. Try changing stimulation to bipolar or decrease pulse width, voltage or frequency. Prescribe speech therapy. |
| Cognitive decline | Routine | This problem may be disease progression, surgery-related, or stimulation-related. Seek neuropsychological testing, consider reprogramming to a dorsal contact. Check lead location. |
| Mania/hypomania | Routine/urgent | Adjust medications. Consider discontinuation of dopamine agonist and use of clozapine or quetiapine. Consider moving to a dorsal contact and/or decreasing the pulse width, voltage or frequency. Check lead location. Consider admission for multi/interdisciplinary management. |
| Impulse control | Urgent | Adjust medications. Consider discontinuation of dopamine agonist and addition of clozapine or quetiapine. Consider moving to a dorsal contact and/or decreasing the pulse width, voltage or frequency. Check lead location. Consider admission for multi/interdisciplinary management. |
| Suicide ideation/attempt | Emergent | Admit the patient to the hospital for multi/interdisciplinary care, and treat underlying cause. May need both medication adjustment and programming. Check lead location. |
| Anxiety/fear | Urgent | Consider more frequent and higher doses of dopaminergics, and altering DBS contacts, perhaps moving more dorsal. Check lead location. Consider admission for multi/interdisciplinary management. |
| Severe depression | Emergent | Behavioral therapy, counseling, medication adjustment and/or stimulation adjustment. Check lead location. Consider admission for multi/interdisciplinary management. |
| Postoperative mania | Urgent | Behavioral therapy, counseling, medication adjustment and/or stimulation adjustment. Check lead location. Consider admission for multi/interdisciplinary management. |
| Pseudobulbar cry/laughter | Urgent | SSRI, TCA or dextromethorphan. |
| Autonomic features | Urgent | May habituate on own, try stimulation parameter adjustments, or change contact if continues to be troublesome. |
| Sensory phenomena | Urgent | Try reduce voltage or pulse width. Try bipolar, or possibly other contact. |
| Accidental on/off | Urgent/emergent | Turn on the IPG, keep a diary to identify the problem. |
| Symptom rebound (motor and/or non-motor) | Emergent | DBS hardware workup including impedance check, battery check, x-ray study, and assess for tolerance. |
If dyskinesia or chorea/ballism was induced by stimulation, reducing the amplitude/voltage of stimulation, or reducing the levodopa equivalent dose may alleviate the issue. Severe stimulation-induced dyskinesia/ballism in the clinic setting should alert the DBS programmer that voltage adjustment should be performed very slowly (sometimes in 0.1–0.2 volt increment increases over many weeks). When stimulation induced hyperkinesia is encountered, the ultimate outcome for patients is usually excellent. One important exception is underlying infection (e.g. pneumonia, UTI, etc.) which may exacerbate dyskinesia. We suggest an evaluation for an infectious process should be sought in cases where medical management proves difficult [18].
Although preexisting gait and/or speech problems (e.g. on medication freezing, dysarthria, and hypophonia) do not typically respond to stimulation [23,52], stimulation-induced gait and speech issues may be improved in select cases with reprogramming, sometimes into a bipolar configuration. Patients themselves may discover relief by switching one or both devices (utilizing a remote control) to an off position when speaking. Additionally recent reports have revealed changing high frequency (>100Hz) to lower frequency (<100Hz) programming settings may improve gait, voice and other clinical features [53,54]. More research into DBS settings that may have the potential to improve or enhance clinical symptoms will be required, as some of the current low frequency settings seem to provide only temporary relief [53,54].
Stimulation-related non-motor symptoms
When stimulation spreads to surrounding neuronal regions, and to limbic and associative regions within grey matter structures, symptoms such as unpleasant feelings, paresthesias, behavioral complaints, and cognitive issues may emerge (Table 2). Pseudobulbar laughter and crying (mood incongruent) have been reported with stimulation, and both have been reportedly addressed by the use of antidepressant medications and by DBS reprogramming [55–57]. Two of the most worrisome stimulation-induced issues are depression and mania [32,58–62]. Both may require medication changes, reprogramming, verification of lead locations and potential hospitalization [47]. Depression should be carefully followed as it can result in suicidal ideation or suicidal attempt [32]. Several reports have linked stimulation of the substantia nigra region to acute depression in patients with STN DBS [58,59]. Abrupt cessation or reduction of dopaminergic medications can also result in apathy or depression [52,63]. If depression follows induction of stimulation, reprogramming to a more dorsal contact may be one solution. The lead location should be checked as misplacement into non-motor regions is one explanation for stimulation induced non-motor features. Useful strategies include administration of antidepressants/antipsychotics, titrating neuropsychiatric medications to optimal doses, and completely optimizing dopaminergic medications [52]. Inpatient care including multi/interdisciplinary approaches (including psychiatrists, psychologists, neurologists, neurosurgeons and other health professionals) and behavioral therapies may also prove useful.
Accidental on/off
When the DBS device unpredictably turns off, the clinician must investigate potential environmental triggers (the device has a duty log to assist in documenting these occurrences). Exposure to magnetic forces (e.g., a magnetized ice freezer or store security devices) is the most commonly reported etiology [47]. Prescribing “rechecking” of the DBS device on a regular or semi-regular schedule may prove useful (utilizing the patient issued remote control). Additionally, having the patient document and describe activities and relevant environments may yield the source of the problem. The patient should be educated to avoid strong magnetic fields, and to have their remote device with them at all times in order to recheck on/off status, and to learn prevention strategies for accidental on/off’s. Most patients who undergo DBS surgery do not have any issues with accidental on/off’s during the lifetime of their devices.
If more than one IPG is utilized to power multiple DBS leads, the chest pacemakers must be placed a minimum of six inches apart. Failure to separate the IPG’s in space may result in cross-communication of the devices, and an result in an automatic and unintended reset to the factory default stimulation settings. We have observed this phenomenon in a single case, a boy with generalized dystonia and two chest IPGs (author observations). Interestingly when lying supine this boy’s devices were six inches apart, but when leaning forward in a chair for programming sessions the distance was cut to only four inches. It is therefore important when programming to make sure (especially in children) that patients sit back in the chair, or alternatively lie in a supine position.
Symptom rebound
Several cases of severe symptom rebound following battery failure have been reported following DBS [64,65]. The more beneficial DBS is for clinical symptoms, the more dramatic the rebound symptoms may be. Symptom rebound may include both motor and non-motor manifestations such as tremor, gait problems, stiff legs and suicidal ideation (author observations), as well as severe depression. We have observed rebound of motor symptoms with battery failures in cases of dystonia and Parkinson’s disease, but also rebound of non-motor symptoms including depression and suicidal ideation with battery failure (author observations). Sudden worsening of symptoms should always prompt a battery status check by an experienced DBS programmer. If the device is off, resuming stimulation may be all that is necessary (see section 2.3.3). If the device is on, checking impedances and current drain at each of the four DBS contacts may provide useful information for evaluation of lead integrity (hardware malfunction) as discussed in the “hardware malfunction” section of this paper (Table 2).
Others
Dystonic storm
Dystonic storm or status dystonicus is a rare but possibly life-threatening condition which presents with severe generalized and possibly painful hyperkinetic dystonic spasms. Patients with an underlying history of primary or secondary dystonia are prone to this condition, and stressors such as trauma, infection or surgical intervention can trigger the dystonic spasms. The optimum treatment for this condition is not established, but a reasonable strategy is to use an aggressively increasing approach beginning with oral medications, then graduating to intravenous and intrathecal medications, then switching to deep sedation or anesthesia, and finally culminating with surgery [66]. Dopamine blockade with non-selective agents such as pimozide, risperidone, olanzapine or haloperidol and sedation with propofol and midazolam, have all been reported successful for the short term control of symptoms, and for improving quality of life. Manji et al. has reported that triple therapy with oral tetrabenazine, high-dose benzhexol, and pimozide is effective especially in children [67,68]. However, dystonic storms may be unusually refractory to some oral medications. Patients with dystonic storms should be admitted to the intensive care unit (ICU) due to the possibility of accompanying respiratory compromise, hyperthermia, dehydration, and rhabdomyolysis resulting in potential renal failure. Deep sedation or anesthesia with endotracheal intubation may be required for refractory cases. Additionally, there has been at least one case of a patient with a dystonic storm successfully treated with intrathecal baclofen [69]. DBS or pallidotomy may be an option of last resort in cases where symptoms continue for many weeks/months [70,71]. Clinicians should be aware that postoperative infections and certain medications (dopamine blockers, antiemetics, etc.) can post-operatively induce movement disorders especially in patients with pre-existing basal ganglia damage.
Conclusion
Knowledge of potential DBS urgencies and emergencies can in many cases enhance outcomes. More intra- and postoperative urgencies and emergencies continue to be identified as the DBS field expands. Clinician’s handling DBS in their practices should be versed in the identification and management of surgery/procedure related, hardware related, and stimulation induced issues.
Figure 2. Infection in the cranial skin incision.
The illustration reveals purulent drainage from the cranial incision.
Figure 3. Computed tomography (CT) scan images of a brain abscess following DBS lead implantation.
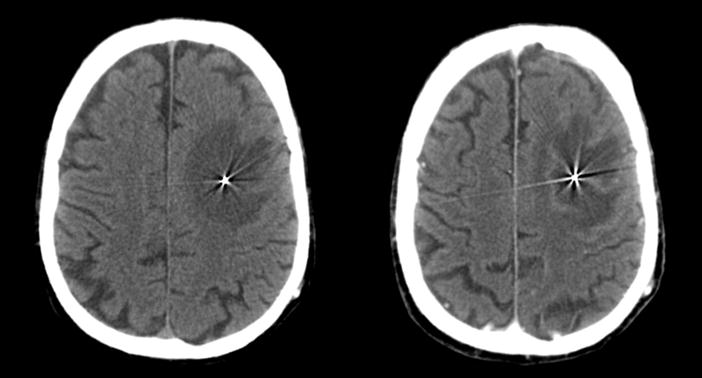
A CT scan image without contrast (left) revealed a low density area which indicated an edematous lesion surrounding the DBS lead. The lesion was enhanced with contrast media (right).
Figure 4. A chest x-ray revealed a twisted extension cable and flipped IPG following a mammography.
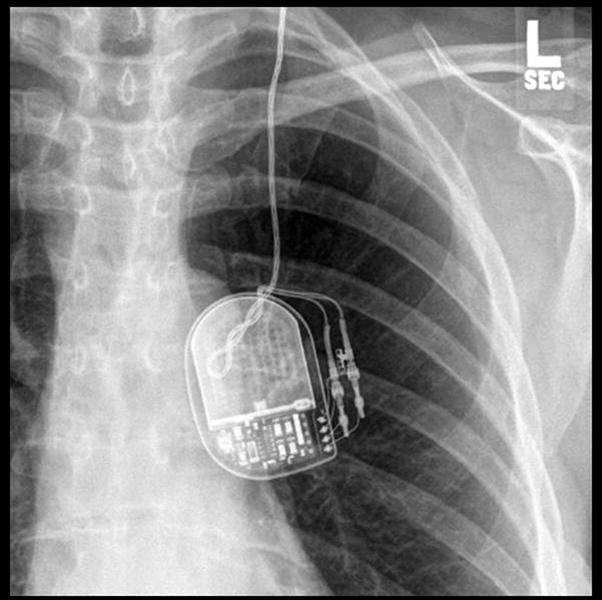
These features have been referred to as the twiddler syndrome.
Figure 6. Dorsal lead migration shown by serial x-rays.
A) A skull x-ray at one month post-implantation. B) A skull x-ray at eight months pre-repeat implantation. Left and right leads had deviated from baseline.
Figure 7. Ventral lead migration shown by serial x-rays.
A) A skull x-ray at one month post-implantation. B) A skull x-ray at fourteen months following first operation. The left and right leads had moved approximately 16mm and 5mm downwards from the initial position, respectively. C) A skull x-ray at one month following lead replacement.
Figure 8. Lead misplacement.
Arrows indicate the tip of DBS leads. An axial slice (A) and a sagittal slice (B) of a T1 weighted magnetic resonance imaging (MRI) scan revealed the tip of the left DBS lead located too far posterior and deep for the subthalamic nucleus (STN). An axial slice (C) and a sagittal slice (D) of the same MRI scan revealed too shallow location of the left DBS lead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson WS, Lenz FA. Surgery insight: Deep brain stimulation for movement disorders. Nat Clin Pract Neurol. 2006;2:310–20. doi: 10.1038/ncpneuro0193. [DOI] [PubMed] [Google Scholar]
- 2.Hariz MI. Psychosurgery, deep brain stimulation, and the re-writing of history. Neurosurgery. 2008;63:E820. doi: 10.1227/01.NEU.0000325681.70894.91. author reply E820. [DOI] [PubMed] [Google Scholar]
- 3.Hooper AK, Ellis TM, Foote KD, Zeilman P, Okun MS. Dyskinetic storm induced by intra-operative deep brain stimulator placement. The Open Neurosurgery Journal. 2009;2:1–3. [Google Scholar]
- 4.Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, et al. Deep brain stimulation: Postoperative issues. Mov Disord. 2006;21 (Suppl 14):S219–37. doi: 10.1002/mds.20957. [DOI] [PubMed] [Google Scholar]
- 5.Hariz MI. Complications of deep brain stimulation surgery. Mov Disord. 2002;17 (Suppl 3):S162–6. doi: 10.1002/mds.10159. [DOI] [PubMed] [Google Scholar]
- 6.Hariz MI, Fodstad H. Do microelectrode techniques increase accuracy or decrease risks in pallidotomy and deep brain stimulation? A critical review of the literature. Stereotact Funct Neurosurg. 1999;72:157–69. doi: 10.1159/000029720. [DOI] [PubMed] [Google Scholar]
- 7.Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56:722–732. doi: 10.1227/01.neu.0000156473.57196.7e. discussion 722–32. [DOI] [PubMed] [Google Scholar]
- 8.Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S, et al. Intraoperative micro-and macrostimulation of the subthalamic nucleus in parkinson’s disease. Mov Disord. 2002;17 (Suppl 3):S155–61. doi: 10.1002/mds.10158. [DOI] [PubMed] [Google Scholar]
- 9.Sansur CA, Frysinger RC, Pouratian N, Fu KM, Bittl M, Oskouian RJ, et al. Incidence of symptomatic hemorrhage after stereotactic electrode placement. J Neurosurg. 2007;107:998–1003. doi: 10.3171/JNS-07/11/0998. [DOI] [PubMed] [Google Scholar]
- 10.Beric A, Kelly PJ, Rezai A, Sterio D, Mogilner A, Zonenshayn M, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73–8. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- 11.Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware complications of subthalamic stimulation: A series of 160 procedures. Neurology. 2004;63:612–6. doi: 10.1212/01.wnl.0000134650.91974.1a. [DOI] [PubMed] [Google Scholar]
- 12.Videnovic A, Metman LV. Deep brain stimulation for parkinson’s disease: Prevalence of adverse events and need for standardized reporting. Mov Disord. 2008;23:343–9. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- 13.Gorgulho A, De Salles AA, Frighetto L, Behnke E. Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgery. J Neurosurg. 2005;102:888–96. doi: 10.3171/jns.2005.102.5.0888. [DOI] [PubMed] [Google Scholar]
- 14.Voges J, Hilker R, Botzel K, Kiening KL, Kloss M, Kupsch A, et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord. 2007;22:1486–89. doi: 10.1002/mds.21481. [DOI] [PubMed] [Google Scholar]
- 15.Morishita T, Burdick A, Okun MS, Jacobson C, Foote KD. Cerebral venous infarction: An avoidable complication of deep brain stimulation surgery. 13th International Congress of Parkinson’s Disease and Movement Disorders; Paris. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, et al. Deep brain stimulation for movement disorders: Morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779–84. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- 17.Kipps CM, Fung VS, Grattan-Smith P, de Moore GM, Morris JG. Movement disorder emergencies. Mov Disord. 2005;20:322–34. doi: 10.1002/mds.20325. [DOI] [PubMed] [Google Scholar]
- 18.Factor SA, Molho ES. Emergency department presentations of patients with parkinson’s disease. Am J Emerg Med. 2000;18:209–15. doi: 10.1016/s0735-6757(00)90023-8. [DOI] [PubMed] [Google Scholar]
- 19.Herzog J, Pinsker M, Wasner M, Steigerwald F, Wailke S, Deuschl G, et al. Stimulation of subthalamic fibre tracts reduces dyskinesias in stn-dbs. Mov Disord. 2007;22:679–84. doi: 10.1002/mds.21387. [DOI] [PubMed] [Google Scholar]
- 20.Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106:621–5. doi: 10.3171/jns.2007.106.4.621. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez RL, Fernandez HH, Haq I, Okun MS. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13:253–60. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- 22.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in parkinson disease. Arch Neurol. 2005;62:554–60. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 23.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The compare trial. Ann Neurol. 2009 doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okun MS, Green J, Saben R, Gross R, Foote KD, Vitek JL. Mood changes with deep brain stimulation of stn and gpi: Results of a pilot study. J Neurol Neurosurg Psychiatry. 2003;74:1584–6. doi: 10.1136/jnnp.74.11.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C. Multicenter study on deep brain stimulation in parkinson’s disease: An independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23:416–21. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 26.Tune LE, Damlouji NF, Holland A, Gardner TJ, Folstein MF, Coyle JT. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet. 1981;2:651–3. doi: 10.1016/s0140-6736(81)90994-6. [DOI] [PubMed] [Google Scholar]
- 27.Poewe W. When a parkinson’s disease patient starts to hallucinate. Pract Neurol. 2008;8:238–41. doi: 10.1136/jnnp.2008.152579. [DOI] [PubMed] [Google Scholar]
- 28.Frucht SJ. Movement disorder emergencies in the perioperative period. Neurol Clin. 2004;22:379–87. doi: 10.1016/j.ncl.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Poston KL, Frucht SJ. Movement disorder emergencies. J Neurol. 2008;255 (Suppl 4):2–13. doi: 10.1007/s00415-008-4002-9. [DOI] [PubMed] [Google Scholar]
- 30.Foncke EM, Schuurman PR, Speelman JD. Suicide after deep brain stimulation of the internal globus pallidus for dystonia. Neurology. 2006;66:142–43. doi: 10.1212/01.wnl.0000191328.05752.e2. [DOI] [PubMed] [Google Scholar]
- 31.Burkhard PR, Vingerhoets FJ, Berney A, Bogousslavsky J, Villemure JG, Ghika J. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170–2. doi: 10.1212/01.wnl.0000145603.48221.b5. [DOI] [PubMed] [Google Scholar]
- 32.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide after bilateral subthalamic nucleus stimulation for parkinson’s disease. Mov Disord. 2002;17:1084–5. doi: 10.1002/mds.10198. [DOI] [PubMed] [Google Scholar]
- 33.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for parkinson’s disease. Brain. 2008;131:2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okun MS, Rodriguez RL, Mikos A, Miller K, Kellison I, Kirsch-Darrow L, et al. Deep brain stimulation and the role of the neuropsychologist. Clin Neuropsychol. 2007;21:162–89. doi: 10.1080/13825580601025940. [DOI] [PubMed] [Google Scholar]
- 35.Ghika J, Villemure JG, Miklossy J, Temperli P, Pralong E, Christen-Zaech S, et al. Postanoxic generalized dystonia improved by bilateral voa thalamic deep brain stimulation. Neurology. 2002;58:311–3. doi: 10.1212/wnl.58.2.311. [DOI] [PubMed] [Google Scholar]
- 36.Burton DA, Nicholson G, Hall GM. Anaesthesia in elderly patients with neurodegenerative disorders: Special considerations. Drugs Aging. 2004;21:229–42. doi: 10.2165/00002512-200421040-00002. [DOI] [PubMed] [Google Scholar]
- 37.Resnick AS, Foote KD, Rodriguez RL, Malaty IA, Moll JL, Carden DL, et al. The number and nature of emergency department encounters in patients with deep brain stimulators. J Neurol. 2009 doi: 10.1007/s00415-009-5343-8. in press. [DOI] [PubMed] [Google Scholar]
- 38.Deogaonkar A, Avitsian R, Henderson JM, Schubert A. Venous air embolism during deep brain stimulation surgery in an awake supine patient. Stereotact Funct Neurosurg. 2005;83:32–5. doi: 10.1159/000085024. [DOI] [PubMed] [Google Scholar]
- 39.Hooper AK, Okun MS, Foote KD, Haq IU, Fernandez HH, Hegland D, et al. Venous air embolism in deep brain stimulation. Stereotact Funct Neurosurg. 2009;87:25–30. doi: 10.1159/000177625. [DOI] [PubMed] [Google Scholar]
- 40.Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: Incidence and management in a large series. Neurosurgery. 2008;62:360–366. doi: 10.1227/01.neu.0000316002.03765.33. discussion 366–7. [DOI] [PubMed] [Google Scholar]
- 41.Joint C, Nandi D, Parkin S, Gregory R, Aziz T. Hardware-related problems of deep brain stimulation. Mov Disord. 2002;17 (Suppl 3):S175–80. doi: 10.1002/mds.10161. [DOI] [PubMed] [Google Scholar]
- 42.Kondziolka D, Whiting D, Germanwala A, Oh M. Hardware-related complications after placement of thalamic deep brain stimulator systems. Stereotact Funct Neurosurg. 2002;79:228–33. doi: 10.1159/000070836. [DOI] [PubMed] [Google Scholar]
- 43.Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1268–1274. doi: 10.1097/00006123-200206000-00017. discussion 1274–6. [DOI] [PubMed] [Google Scholar]
- 44.Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: A ten year experience. Acta Neurochir (Wien) 2005;147:1061–4. doi: 10.1007/s00701-005-0576-5. discussion 1064. [DOI] [PubMed] [Google Scholar]
- 45.Koller W, Pahwa R, Busenbark K, Hubble J, Wilkinson S, Lang A, et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997;42:292–9. doi: 10.1002/ana.410420304. [DOI] [PubMed] [Google Scholar]
- 46.Miller JP, Acar F, Burchiel KJ. Significant reduction in stereotactic and functional neurosurgical hardware infection after local neomycin/polymyxin application. J Neurosurg. 2009;110:247–50. doi: 10.3171/2008.6.17605. [DOI] [PubMed] [Google Scholar]
- 47.Okun MS, Rodriguez RL, Foote KD, Sudhyadhom A, Bova F, Jacobson C, Bello B, Zeilman P, Fernandez HH. A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat Disord. 2008;14:532–8. doi: 10.1016/j.parkreldis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Geissinger G, Neal JH. Spontaneous twiddler’s syndrome in a patient with a deep brain stimulator. Surg Neurol. 2007;68:454–6. doi: 10.1016/j.surneu.2006.10.062. discussion 456. [DOI] [PubMed] [Google Scholar]
- 49.Yianni J, Nandi D, Shad A, Bain P, Gregory R, Aziz T. Increased risk of lead fracture and migration in dystonia compared with other movement disorders following deep brain stimulation. J Clin Neurosci. 2004;11:243–5. doi: 10.1016/j.jocn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Piacentini S, Romito L, Franzini A, Granato A, Broggi G, Albanese A. Mood disorder following dbs of the left amygdaloid region in a dystonia patient with a dislodged electrode. Mov Disord. 2008;23:147–50. doi: 10.1002/mds.21805. [DOI] [PubMed] [Google Scholar]
- 51.Ellis TM, Foote KD, Fernandez HH, Sudhyadhom A, Rodriguez RL, Zeilman P, et al. Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery. 2008;63:754–60. doi: 10.1227/01.NEU.0000325492.58799.35. discussion 760–1. [DOI] [PubMed] [Google Scholar]
- 52.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced parkinson’s disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 53.Moreau C, Defebvre L, Destee A, Bleuse S, Clement F, Blatt JL, et al. Stn-dbs frequency effects on freezing of gait in advanced parkinson disease. Neurology. 2008;71:80–4. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- 54.Brozova H, Barnaure I, Alterman RL, Tagliati M. Stn-dbs frequency effects on freezing of gait in advanced parkinson disease. Neurology. 2009;72:770. doi: 10.1212/01.wnl.0000339385.187472.7d. author reply 770–1. [DOI] [PubMed] [Google Scholar]
- 55.Low HL, Sayer FT, Honey CR. Pathological crying caused by high-frequency stimulation in the region of the caudal internal capsule. Arch Neurol. 2008;65:264–6. doi: 10.1001/archneurol.2007.53. [DOI] [PubMed] [Google Scholar]
- 56.Okun MS, Raju DV, Walter BL, Juncos JL, DeLong MR, Heilman K, et al. Pseudobulbar crying induced by stimulation in the region of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2004;75:921–3. doi: 10.1136/jnnp.2003.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar R, Krack P, Pollak P. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;341:1003–4. author reply 1004. [PubMed] [Google Scholar]
- 58.Blomstedt P, Hariz MI, Lees A, Silberstein P, Limousin P, Yelnik J, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: A case report. Parkinsonism Relat Disord. 2008;14:253–6. doi: 10.1016/j.parkreldis.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 60.Miyawaki E, Perlmutter JS, Troster AI, Videen TO, Koller WC. The behavioral complications of pallidal stimulation: A case report. Brain Cogn. 2000;42:417–34. doi: 10.1006/brcg.1999.1113. [DOI] [PubMed] [Google Scholar]
- 61.Romito LM, Raja M, Daniele A, Contarino MF, Bentivoglio AR, Barbier A, et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in parkinson’s disease. Mov Disord. 2002;17:1371–74. doi: 10.1002/mds.10265. [DOI] [PubMed] [Google Scholar]
- 62.Krack P, Kumar R, Ardouin C, Dowsey PL, McVicker JM, Benabid AL, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord. 2001;16:867–75. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- 63.Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, et al. Bilateral subthalamic stimulation for parkinson’s disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000;92:615–25. doi: 10.3171/jns.2000.92.4.0615. [DOI] [PubMed] [Google Scholar]
- 64.Hariz MI, Johansson F. Hardware failure in parkinsonian patients with chronic subthalamic nucleus stimulation is a medical emergency. Mov Disord. 2001;16:166–8. doi: 10.1002/1531-8257(200101)16:1<166::aid-mds1031>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 65.Hariz MI, Shamsgovara P, Johansson F, Hariz G, Fodstad H. Tolerance and tremor rebound following long-term chronic thalamic stimulation for parkinsonian and essential tremor. Stereotact Funct Neurosurg. 1999;72:208–18. doi: 10.1159/000029728. [DOI] [PubMed] [Google Scholar]
- 66.Mariotti P, Fasano A, Contarino MF, Della Marca G, Piastra M, Genovese O, et al. Management of status dystonicus: Our experience and review of the literature. Mov Disord. 2007;22:963–8. doi: 10.1002/mds.21471. [DOI] [PubMed] [Google Scholar]
- 67.Vaamonde J, Narbona J, Weiser R, Garcia MA, Brannan T, Obeso JA. Dystonic storms: A practical management problem. Clin Neuropharmacol. 1994;17:344–7. doi: 10.1097/00002826-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Manji H, Howard RS, Miller DH, Hirsch NP, Carr L, Bhatia K, Quinn N, et al. Status dystonicus: The syndrome and its management. Brain. 1998;121 (Pt 2):243–52. doi: 10.1093/brain/121.2.243. [DOI] [PubMed] [Google Scholar]
- 69.Dalvi A, Fahn S, Ford B. Intrathecal baclofen in the treatment of dystonic storm. Mov Disord. 1998;13:611–2. doi: 10.1002/mds.870130344. [DOI] [PubMed] [Google Scholar]
- 70.Elkay M, Silver K, Penn RD, Dalvi A. Dystonic storm due to batten’s disease treated with pallidotomy and deep brain stimulation. Mov Disord. 2009;24:1048–53. doi: 10.1002/mds.22515. [DOI] [PubMed] [Google Scholar]
- 71.Teive HA, Munhoz RP, Souza MM, Antoniuk SA, Santos ML, Teixeira MJ, et al. Status dystonicus: Study of five cases. Arq Neuropsiquiatr. 2005;63:26–9. doi: 10.1590/s0004-282x2005000100005. [DOI] [PubMed] [Google Scholar]