Abstract
BACKGROUND
Intra-hepatic arterial 90Yttrium (Y90) microspheres (Theraspheres) have been proposed as a less toxic, invasive therapeutic option to trans-hepatic arterial chemoembolization (TACE) for surgically unresectable hepatocellular carcinoma (HCC). TACE has been shown to prolong survival. However, long term survival remains uncertain.
METHODS
A 2 cohort experience of the treatment of advanced, unresectable and biopsy-proven HCC in North American patients is presented. 691 patients received repetitive cisplatin-based chemoembolization and a following 99 patient cohort with similar treatment criteria, received a planned single dose of Y90. Over this time period, an additional 142 patients were followed without treatment (total: 932 patients).
RESULTS
Overall survival was slightly better in the Y90 group compared to TACE, median of 11.5 vs. 8.5 months. However, selection criteria indicated a small but significant bias towards milder disease in the Y90 group. Using stratification in a 3 tier model, with cases dichotomized by bilirubin of less than 1.5 mg/dL, patients without PVT or with low alpha-fetoprotein plasma levels of less than 25 units/dL, analysis of survival in clinical subgroups showed that the 2 treatments resulted in similar survival. Similarly, patients with PVT or a high alpha-fetoprotein also had similar survival in the 2 treatment groups.
CONCLUSION
Given the present evidence of therapeutic equivalence in survival, Y90 and TACE seem to be equivalent regional therapies for patients with unresectable, non-metastatic HCC.
Keywords: HCC, hepatoma, chemotherapy, Yttrium, internal radiation
INTRODUCTION
The management of primary hepatocellular cancer (HCC) is evolving, as new modalities of therapy are being evaluated. Advances have taken place for patients who present at early stages of tumor extent, with more aggressive approaches to surgical resection, radiofrequency ablation (RFA) and liver transplantation. However the majority of patients who present with HCC in the absence of a surveillance program, have surgically unresectable disease, as a result of their tumor extent or severity of their cirrhosis or both, and in these patients the prognosis is poor.
There is now large experience with hepatic artery chemotherapy, with or without some form of embolization (TACE) to slow tumor growth1,2 However, even though only two randomized controlled trials have shown a survival advantage3,4 In comparison to no treatment controls, this approach appears to confer some palliative benefit to many patients, with tumor shrinkage (partial response) rates in the 10–60% range1,2,14
More recently, internal hepatic irradiation with microspheres carrying 90Yttrium5–8 or other radionuclides9–11, that are injected intra-arterially, has been proposed as an alternative approach to regional chemotherapy, that often only requires single dose administration and appears to be generally safe5,6 At the present time, limited information is available on the efficacy of this approach. We have recently reported short-term acceptability of this approach in a phase II open label trial of 65 patients using this strategy, but did not report on long term survival7. Our clinical hypothesis was that if survival after Y90 therapy was either equivalent or superior to chemotherapy in patients treated with TACE, then outpatient Y90 might be a primary regional treatment of choice in patients with non-metastatic HCC.
We now report our extended open label experience of 90Yttrium (Theraspheres) in a 99 patient cohort with biopsy-proven, unresectable HCC and compare it to 691 similar patients in a preceding cohort of HCC patients treated with a cisplatin-based TACE chemotherapy regimen, as well as to an additional 142 patients who could not receive any treatment. The focus of this report is on survival. In this open label comparison of 2 sequential patient cohorts, we have matched subgroups of HCC based on comparable baseline pre-treatment criteria to evaluate tumor extension and residual hepatic function. Our objective was to determine if there is a difference in survival advantage between therapeutic alternatives.
MATERIALS AND METHODS
Clinical Selection
This report includes consecutive series of all biopsy-proven HCC patients seen at a single medical center who were not candidates for surgical resection, RFA or hepatic transplantation, who received either conventional cisplatin-TACE from 1992 to 2000, or 90Yttrium (Y90) microspheres (Therasphere) from 2000 through 2005.
Baseline evaluation
All patients had tumor biopsy proof of HCC, as well as random biopsy in non-malignant areas of the underlying liver to assess presence of cirrhosis. Baseline triphasic CT scans of the abdomen, chest CT and laboratory blood tests were obtained, that included complete blood count, prothrombin time, liver function tests, creatinine and alpha fetoprotein measurements. Exclusion criteria for either treatment was a bilirubin >3.0 mg/dL, creatinine >2.0 mg/dL, platelets <60,000/µL, granulocytes <1500 µL, presence of metastases at baseline, uncorrectable shunting of blood to the intestines or lungs, and ECOG performance score of 0 or 1. Specifically for Y90, added exclusion criteria were presence of aberrant vessels on the angiogram, feeding stomach or intestines, and a lung shunt delivering a calculated ≥16.5 mCi or 30 Gy.
Chemoembolization therapy for HCC with cisplatin-TACE
For intrahepatic artery chemoembolization, we have used cisplatin. This is based upon the fact that it has tumor shrinkage ability12–15 and has minimal myelosuppressive activity compared with most other agents. This is a useful property in the setting of portal hypertension and accompanying splenomegaly. It is also relatively well tolerated by the cirrhotic liver. It was typically given at a dose of 125 mg/m2 of body surface area (BSA). The cisplatin was given in 1 mg/ ml of normal saline and infused into the right or left hepatic artery over 30 minutes, together with dexamethasone16 20 mg (in an attempt to limit hepatic inflammation), morphine sulfate 5 mg (for pain), as well as intravenous antibiotics (Ancef or Vancomycin) given prior to TACE. A pressure pump was used to deliver the drug. Complete baseline arteriograms of the superior mesenteric artery and celiac axis were obtained using standard selective percutaneous catheterization techniques to identify all hepatic arterial blood supply including any aberrant vessels. Catheterizations were accomplished using standard percutaneous 5 French catheters such as the Cobra -2 and Sos Omni catheter (Angiodynamics, Queensbury, NY). Angiograms were reviewed to ensure that there was complete visualization of all tumor vessels. If there was any discrepancy and all vessels could not be identified, aortograms were performed as needed to evaluate for any aberrant blood supply. Individual treatment sessions were limited to unilobar or segmental therapy to minimize the risk of TACE-induced hepatic failure. Selective catheterization of the right or left hepatic artery was often achieved using a micro-catheter such as the Renegade catheter (Boston Scientific, Natick MA) as needed.
Chemoembolization or trans arterial chemoembolization (TACE) therapy with cisplatin, was given on a protocol designed to minimize side effects of nausea, vomiting, pain, ototoxicity, nephrotoxicity and neurotoxicity and be completed over 24 hours. Most of the patients underwent treatment under conscious sedation. A combination of an anxiolytic agent, such as midazolam HCI, and pain medication with fentanyl citrate was used. A diuretic regimen was designed to prevent cisplatin-mediated nephrotoxicity. 250 ml of 3 % saline was given intravenously prior to chemotherapy. In addition, the patients were given aggressive intravenous hydration. This was done using D5/½ normal saline or just normal saline (diabetics) with 20 mEq of KCl per L at 250 ml/hr for a minimum of 3 hr. Once the patient was in the vascular procedure room, the fluid rate was increased to 2 liters over 2 hours immediately prior to the cisplatin infusion, together with immediate intravenous infusion of the diuretics, mannitol 12.5 g and furosemide 40 mg during the cisplatin infusion. We also gave an intravenous bolus of sodium thiosulfate 9 g/m2 immediately before the chemotherapy and a 6 hour intravenous infusion of 1.5 g/m2/hr afterwards. This integrated management has resulted in the essential disappearance of cisplatin-mediated ototoxicity, nephrotoxicity and neurotoxicity. Intravenous hydration at 150 ml/hr was continued post-chemotherapy until the patient was discharged from the hospital the following morning. Prior to cisplatin, a single intravenous dose of Kytril 1 mg (Granisetron) or Zofran 32 mg (Ondansetron), together with dexamethasone (Decadron) 4 mg was given. Aggressive anti-inflammatory and triple anti-emetics over the 24 hours of the protocol consisting of a combination of Dexamethasone, Reglan and Benadryl, or Kytril were all given repetitively over the 24 hours of the protocol. After cisplatin, intravenous Reglan 2 mg/kg (metoclopramide), Benadryl 25 mg and Decadron 4 mg was given every 3 hours for the next 12 hours.
Initially, Gelfoam sponge particles were injected into the hepatic artery at the beginning of the administration of chemotherapy, half way through the cisplatin infusion and again at the end of the cisplatin administration. The objective of this approach was to cause vascular slowing (chemo-occlusion), but not complete occlusion. This partial embolization approach was used in an attempt at a greater safety margin, especially when portal vein thrombus was present. The arterial flow was monitored during the chemotherapy by regular bolus injections of angiographic dye after each insertion of Gelfoam, to check the vascular flow. The cisplatin in 150–250 ml saline was then infused at a rate to allow the entire volume to be administered in 30–45 minutes. Gelfoam aliquots typically were given before, during and at the end of the chemotherapy infusion14
More recently, we substituted biospheres (Embospheres) 100–300 microns in pre-filled syringes for gelfoam, due to convenience and particle size consistency17 The Embospheres were mixed with water soluble contrast material (Optiray 320, Mallinckrodt, Hazelwood, MO), as per the manufacturer’s directions, and injected under fluoroscopic guidance. In most cases, embolization was performed to the point of visibly stagnant blood flow. Only moderate ante-grade slowing was used as the end point in those patients with elevated serum bilirubin levels.
All patients underwent baseline computed tomography (CT) scans for evaluation of tumor burden and location. Baseline liver function tests were obtained in addition to renal function and coagulation studies. Follow-up CT was obtained immediately prior to the next treatment. Thus, post treatment 1, a CT was obtained immediately prior to treatment 2, and post treatment 3, a CT was obtained immediately prior to treatment 4. Complete blood count, prothrombin time, liver function tests, creatinine and alpha fetoprotein measurements, were obtained weekly, throughout the treatment process. The cisplatin-TACE was repeated every 8 to 12 weeks, depending upon the hepatic tolerance, the tumor response and recovery of the WBC, platelets, bilirubin levels and on the time for clinical patient recovery (mainly tiredness).
90Yttrium microspheres protocol
The hepatic artery catheterization and Y90 delivery was performed in the angiography suite, as was the TACE. The potential for radiation exposure to the stomach and intestines, which could result in non-healing radiation ulcers was minimized by a pre-treatment planning celiac and hepatic angiogram. The potential for inducing radiation pneumonitis following portal-systemic shunting of microspheres delivered to the cirrhotic liver, was minimized by pre-treatment 99Tc MAA (99technetium macro-aggregated albumin) scan, after the radiotracer was injected into the hepatic artery followed by SPECT scanning (single photon emission computerized tomography) of the lung at the time of the planning angiogram, and performed typically 2–3 weeks prior to the planned treatment date. The 99Tc MAA scan was used to exclude patients from this procedure who might otherwise have >16.5 mCi of injected Y90 going to their lungs. Therasphere treatment was given by rapid bolus injection over 1–5 minutes into the right or left hepatic artery, with the intent to deliver 135–150 Gy (13,500–15,000 rads) to the treated lobe, as previously described8,18
The protocol plan was to treat the patient only with a single dose of Y90 to the liver lobe having the dominant disease burden and then to monitor the patient only, in subsequent months, unless follow up CT scans showed progressive disease. In this situation, a second treatment was given to the hepatic lobe with tumor progression. Otherwise, patients were just followed with 2-monthly clinic visits with physical examinations, labs and follow-up CAT scans. The reason for this plan, in contrast to the repeated treatments that are typical of TACE (and all other cancer chemotherapeutics) was to minimize the (unknown) toxicities of Y90 to the cirrhotic liver. Therasphere therapy is currently approved for treatment of HCC under a humanitarian device exemption (HDE) approval. An individual IRB-approved informed consent was obtained from each patient in this Y90 treatment group as well as an IRB-approved data collection consent. The cisplatin-TACE group was not considered experimental and patients therefore signed a standard hospital treatment consent form for the hepatic arterial chemotherapy. The data was collected and analyzed under an IRB-approved data collection protocol. Time from patient referral to first treatment was about 2 weeks for TACE patients, but about 4 weeks for Y90 patients, due to the need in the latter for a planning angiogram and the scheduling of Y90 shipments for each patient.
Tumor Responses
World Health Organization (WHO) tumor response on CT or MRI was determined for measurable lesions (>1 cm) by using the cross-product of perpendicular diameters to assess the need for further cycles of therapy. Corresponding lesions from baseline and post-treatment scans were compared during venous and arterial contrast phases to assess tumor morphology and size changes. “Complete response” was defined as a change in the sum of the cross-products to zero (i.e., a 100% reduction), “partial response” as a decrease in the sum of cross-products by at least 50%, “stable disease” as a decrease in the sum of cross-products by less than 50% or an increase less than 25%, and “progression” as an increase in the sum of cross-products by at least 25%. RECIST criteria came into general use more than half way through these treatments. Therefore, WHO response criteria were used throughout this series, which was started in 1992.
Statistical Methods
Overall survival was the only end point used, defined as the time between the date of first treatment and date of death. A substantial investment of effort was made to identify time of death in each patient to avoid issues involved with censorship with loss to follow up that would introduce a bias to underestimate median values. Data used to relate to survival time were dichotomized. This included demographics, historical risk factors, clinical characteristics, conventional liver function tests and the results of CTs, defined by the largest tumor size, the number of tumors and CT evidence of tumor extension including the presence or absence of portal vein thrombosis (PVT). These parameters were obtained at the time of initial clinical evaluation at this clinic. Univariate survival curves were estimated using the Kaplan-Meier method. Differences in median ± 95% confidence limits for each subgroup analysis in survival rates were compared by log rank test. Hazard ratios were calculated using the Cox proportional regression model. Analyses were carried out using SAS 9.1. Throughout the tables, although the data is shown for TACE, Y90 and no treatment groups, the statistical analyses were applied only to comparison between the 2 treatments, namely TACE and Y90.
RESULTS
Patient Demographics
932 patients with HCC that were considered unsuitable for resection, RFA or liver transplantation were managed with chemoembolization (TACE) (n=691), Y90 microspheres (n=99) or with no treatment (n=142). The no treatment group were those who were referred for treatment, but due to poor liver function or presence of metastases, were considered to be unlikely to be helped by either of these 2 intra-arterially delivered therapies. The demographic distribution of cases for the two treatment types is presented in Table 1A & 1B. Of these, the majority was male. However, there were a similar proportion of males and females in each treatment category. HCV alone was present in 19% of cases in the chemoembolization group and 30% in the Y90 group. HBV alone as well as both HBV and HCV were present in similar proportion in each group (Table 1A). Baseline radiological assessment with abdominal CT scan indicated that there were a similar proportion of cases in each treatment group that had tumor size ≥ 5 cm, number of tumors > 5, bilobar disease and elevated alpha-fetoprotein. However there were a slightly higher proportion of cases with portal vein thrombosis (PVT) in the chemoembolization group as compared to the Y90 group (42% vs. 28%) p < 0.08). There were a higher proportion of cases in the chemoembolization group (25%) who had a bilirubin ≥ 1.5 mg/dL than in the Y90 group (13%), p< 0.044 (Table 1B).
Table 1.
| Table 1A. Demographic variables in patients with unresectable HCC treated with TACE, Y90 or no treatment | |||
|---|---|---|---|
| Variable | TACE pt. number (%) |
Y90 pt. number (%) |
p-value |
| Total group | 691 (100) | 99 (100) | |
| Males | 518 (75) | 70 (70) | ns |
| Females | 173 (25) | 29 (30) | ns |
| Alcohol | 217 (31) | 37 (37) | ns |
| Tobacco | 101 (15) | 19 (19) | ns |
| HBV only | 97 (14) | 9 (9) | ns |
| HCV only | 132 (19) | 30 (30) | 0.057 |
| Cirrhosis | 462 (66) | 80 (80) | ns |
| Table 1B. Clinical variables in patients with unresectable HCC treated with either TACE, Y90 or no treatment | |||
|---|---|---|---|
| Variable | TACE pt. number (%) |
Y90 pt. number (%) |
p-value |
| Total group | 691 (100) | 99 (100) | |
| Tumor Number ≥5 | 257 (37) | 26 (26) | ns |
| Bilobar disease | 354 (51) | 43 (43) | ns |
| Portal Vein Thrombosis (PVT) | 295 (42) | 28 (28) | 0.08 |
| Alpha-fetoprotein ≥25 ng/ml | 465 (67) | 58 (58) | ns |
| Bilirubin ≥1.5 mg/dL | 173 (25) | 13 (13) | 0.044 |
| Album in <3.5 g/dL | 449 (65) | 66 (66) | ns |
Hazard Ratio (HR) analysis of survival between two the treatment groups
In the second step of the analysis, we undertook a hazard ratio (95% Cl) analysis of survival time between risk factors observed in HCC patients treated in each of the two groups separately (TACE and Y90) (Table 2). Males and patients with alcohol intake had a significantly increased HR in the TACE group but not the Y90 group. Cases with tobacco use had a comparatively higher HR in the Y90 group. With respect to concomitant liver disease, the presence of HBV was more hazardous to cases treated with Y90, but less hazardous to cases treated with TACE. The presence of cirrhosis was associated with increased risk in each group. Tumor size did not confer increased HR. However, tumor number ≥ 5 conferred risk only in the Y90 group. The presence of PVT and alpha-fetoprotein was a risk factor in each group. A bilirubin of ≥ 1.5 mg/dl and albumin of < 3.5 g/dl was associated with a significant HR for each treatment group.
Table 2.
Hazard ratios (±95%CI) between presence or absence of variable of interest Bolded values for comparisons have p<0.05
| Variable | TACE (95% CI) | Y90 (95% CI) |
|---|---|---|
| Demographics | ||
| Males vs. Females | 1.29(1.07–1.55) | 1.63(0.99–2.69) |
| Heavy Alcohol | 1.32(1.08–1.63) | 1.72(0.98–3.01) |
| Heavy Tobacco | 1.20(0.90–1.61) | 2.47(1.17–5.25) |
| Concomitant Liver Disease | ||
| HBV only | 0.77(0.61–0.98) | 2.75(1.31–5.78) |
| HCV only | 1.21(0.98–1.57) | 1.28(0.76–2.16) |
| Cirrhosis (any cause) | 1.50(1.25–1.81) | 1.96(1.02–3.69) |
| Tumor Characteristics | ||
| Tumor Size ≥5 cm | 1.17(0.90–1.38) | 0.78(0.41–1.50) |
| Tumor Number ≥5 | 1.06(0.88–1.3) | 1.8(1.01–3.21) |
| Portal Vein Thrombosis | 1.31(1.11–1.54) | 2.13(1.33–3.39) |
| Alpha-fetoprotein ≥25 ng/ml | 1.56(1.30–1.88) | 1.71(1.09–2.70) |
| Liver Function | ||
| Bilirubin ≥1.5 mg/dL | 1.55(1.29–1.86) | 2.69(1.44–5.04) |
| Albumin <3.5 g/dL | 1.48(1.22–1.78) | 2.03(1.25–3.31) |
Liver function, tumor extent and survival
Overall survival was significantly different between cases treated with TACE as compared to Y90, p < 0.0146 (Figure 1), with a modest sustained difference maintained over the 50 months of observation. Median survival times were 8.5 months for TACE patients and 11.5 months for Y90 patients. Untreated patients had a median survival time of 2 months. The median survival times were compared between subsets of treated patients, based on selected dichotomized variables found to have different HR in Table 3. In these analyses, the median survival in the Y90 group was significantly longer than the TACE group: 11.5 [95%CI 8–16] months vs. 8.5 [95%CI 8–10] months, p <0.05. While there was poorer survival in males than females, females treated with Y90 survived significantly longer compared to females treated with TACE: 19 [95%CI 16–32] months vs. 12 [95%CI 10–15] months, p<0.05.
Figure 1. Overall survival dichotomized by treatment type.
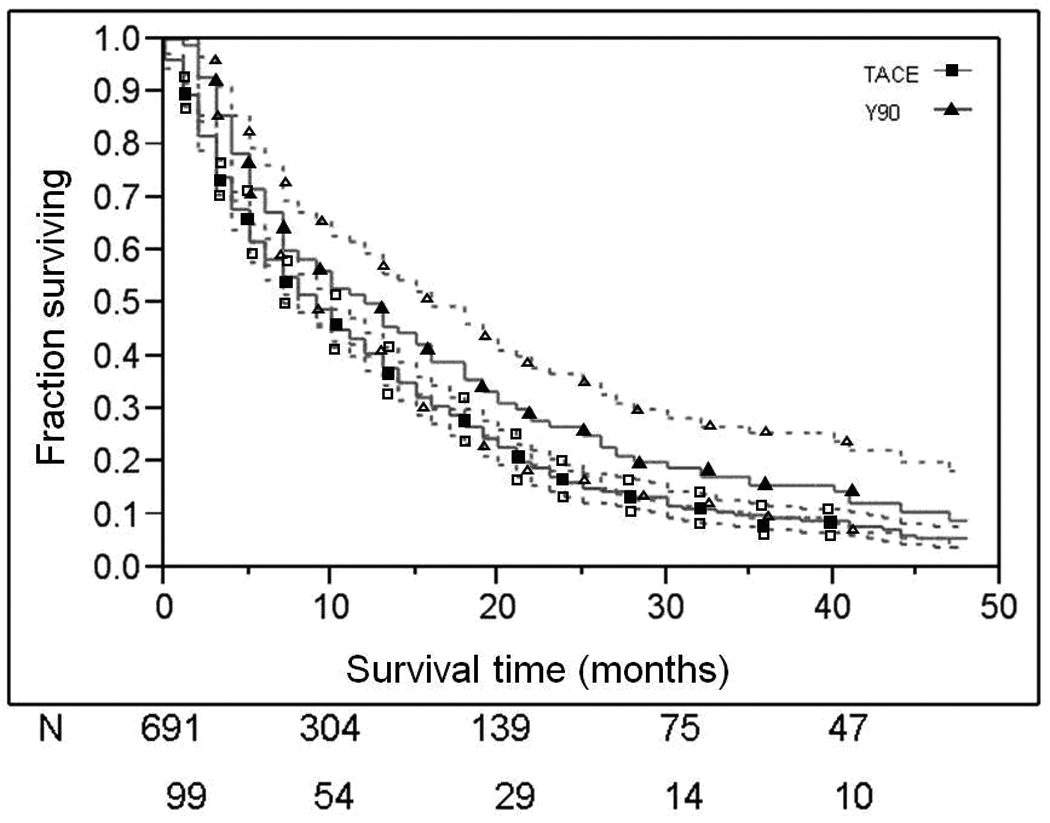
Patient survival curves dichotomized by treatment type. 95% CI are presented as dotted lines. Numbers of patients at risk at each time interval have been provided for TACE and Y90 treatment groups. The top line represents TACE and the bottom Y90.
Table 3.
Comparison of survival times (mo.) between patient treatment groups.
| Variable | TACE median survival (95% CI) |
Y90 median survival (95% CI) |
p value between treatment groups |
|---|---|---|---|
| Overall groups | 8.5(8–10) | 11.5(8–16) | <0.05 |
| Gender | |||
| Males | 8(7–10) | 10(7–13) | ns |
| Females | 12(10–15) | 19(16–32) | <0.05 |
| P value for gender | p<0.001 | ns | |
| Cirrhosis | |||
| No Cirrhosis | 12(10–35) | 22(16–40) | ns |
| Cirrhosis | 8(8–9) | 10(7–14) | <0.05 |
| P value for cirrhosis | p<0.0001 | p<0.05 | |
| Tumor Size | |||
| <5 cm. | 10(9–12) | 8(5–18) | ns |
| ≥5 cm. | 8(6–10) | 17(7–40) | <0.05 |
| P value for tumor size | ns | ns | |
| Tumor Number | |||
| <5 | 10(8–13) | 14(7–19) | ns |
| ≥5 | 8(6–10) | 9(5–13) | ns |
| P value for tumor number | ns | p<0.05 | |
| Portal Vein Thrombosis | |||
| Absent | 12(10–14) | 16(12–20) | <0.05 |
| Present | 7(5–9) | 5(4–9) | ns |
| P value for presence PVT | p<0.0001 | p<0.0001 | |
| Alpha-fetoprotein | |||
| <25 ng/ml | 15(14–18) | 18(13–28) | ns |
| ≥25 ng/ml | 7(6–8) | 8(6–13) | ns |
| P value for elevated AFP | p<0.0001 | p<0.05 | |
| Bilrubin | |||
| <1.5 mg/dL | 12(10–13) | 13(9–18) | ns |
| ≥1.5 mg/dL | 5(3–5) | 4(2–10) | ns |
| P value for elevated Bilirubin | p<0.0001 | p<0.0001 | |
| Albumin | |||
| ≥3.5 g/dL | 13(10–15) | 22(13–32) | <0.05 |
| <3.5 g/dL | 7(6–9) | 8(6–13) | ns |
| P value for low albumin | p<0.0001 | p<0.001 |
The presence of cirrhosis resulted in a reduction in survival in each group. However, in the subset groups with cirrhosis, patients treated with Y90 had a marginally longer survival than the TACE group: 10 months [95%CI 7–14] vs. 8 months [95%CI 8–9], p < 0.05). Similarly, the presence of PVT was associated with decreased survival in each group. There was a modest increase in survival in the Y90 group in comparison to TACE in the subset without PVT: 16 [95%CI 12–20] months vs. 12 [95%CI 10–14] months, p < 0.05. Abnormal values for each of the biochemical indices identified in the HR analysis in Table 3, were associated with marked reductions in survival in each group, but only in the comparison of subsets with normal serum albumin was survival longer in the Y90 group than the TACE group: 22 [95%CI 13–32] months vs. 13 [95%CI 10–15] months, p < 0.05, as shown in Table 3.
Three tier model
The survival times indicate that each of the variables of tumor extent, liver function, and concomitant cirrhosis need to be simultaneously taken into consideration to create subgroups of comparable baseline characteristics, before inferences of differences in HCC treatment variables can be drawn. Collectively, the univariate and bivariate analysis of individual risk factors such as concomitant liver disease or the radiological characteristics of the tumor did not take into account variation in liver function. Conversely, the analysis using biochemical indices of liver function did not take into account the presence or absence of tumor extension. We considered the use of multivariate regression analysis. However, as the data elements are closely intertwined and non-parametric, we elected to take advantage of the large size of the study population, to build a three tier pathophysiological model, which is based on subgroup analysis that takes each of the dominant variables into account.
As the first tier of this analysis, we have chosen bilirubin <1.5mg/dL as an index of good liver function and ≥ 1.5mg/dL as an index of poor liver function. In this first tier of the 928 patients with data available for evaluation, 655 (70.2%) patients had a normal bilirubin (Figure 2, tier 1). Those patients with elevated bilirubin levels (29.8%) had an extremely poor prognosis with a median survival of 3 [95%CI 2–4] months as contrasted to the 11 [95%CI 10–13] months in those patients with normal bilirubin (p<0.0001). In the second tier, subdividing patients with good bilirubin into those with (n=308) or without (n=347) PVT, resulted in two groups of substantial size, 37% and 33% of the original overall cohort, respectively. Within these two groups, the presence of PVT was associated with a 50% reduction in median survival (P<0.0001) from a median of 15 [95%CI 13–17] to 8 [95%CI 7–10] months (Figure 2, tier 2). In the remaining cohort with elevated baseline bilirubin, the presence of PVT was still associated with a >2- fold reduction in survival (P <0.001) from 5 [95%CI 3–6] months to 2 [95%CI 1–3] months. Having created subgroups in which the heterogeneity of the overall group had been reduced by considering residual hepatic function and presence or absence of PVT, we then evaluated the impact of different types of treatment on survival. This analysis in the third tier showed no significant treatment effect on survival (Figure 2, tier 3). The no-treatment group is excluded from this tier’s analysis, which explains the difference in total patient numbers, between tier 2 and tier 3. . The statistical significances on the right hand column of Figure 2, relate to comparison of the 2 active treatment cohorts only, and not to the no treatment arm. A similar analysis evaluating the impact of elevated AFP is presented in Figure 3. As with the analysis for PVT, high or low AFP had a marked impact on survival (Figure 3, tier 2). However, within the high AFP or low AFP cohorts, there was no statistically significant difference in survival for the 2 treatments being compared (Figure 3, tier 3).
Figure 2. Three tier model of survival with stratification based on liver function and PVT.
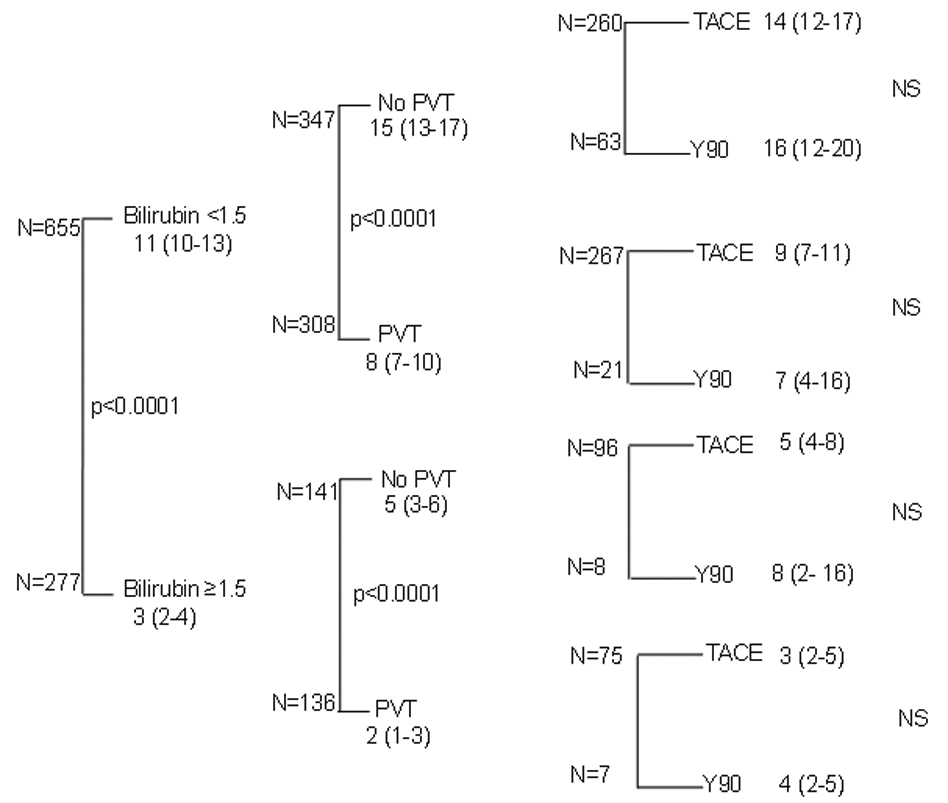
Three tier model with cases dichotomized by bilirubin, PVT and treatment. (Median ± 95CI), Final column of statistical analysis is between TACE and Y90 groups)
Figure 3. Three tier model of survival with stratification based on liver function and AFP.
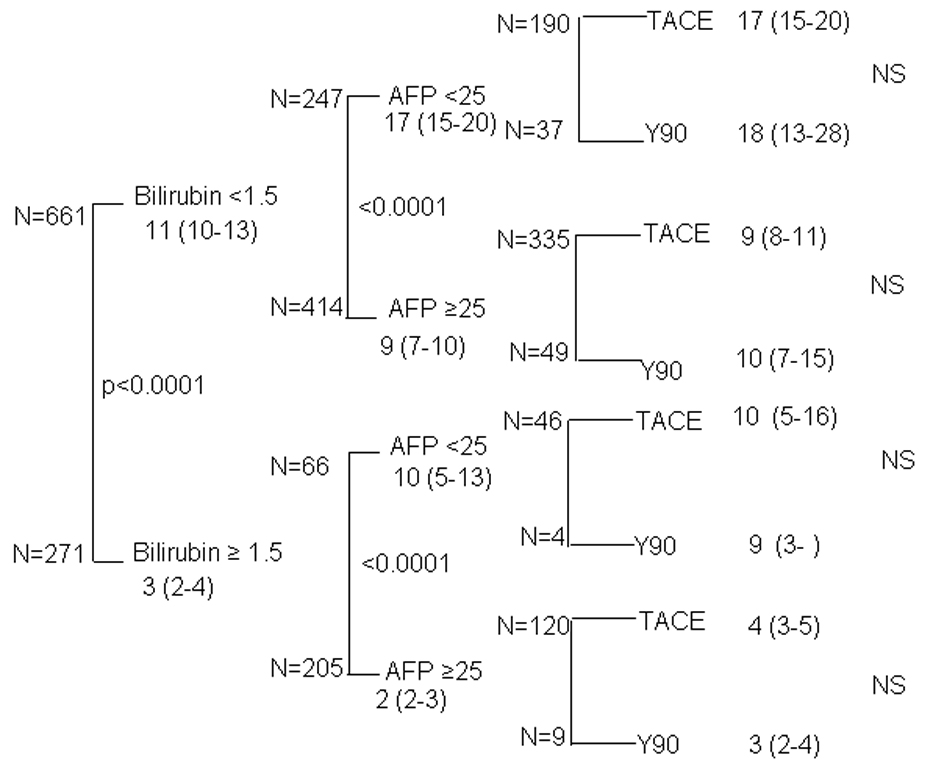
Three tier model with cases dichotomized by bilirubin, AFP and treatment (Median ± 95CI in months, final column of statistical comparison between TACE and Y90 groups).
Tumor responses
Tumor responses (Table 4) were measured radiologically after 3 chemoembolization cycles or 6 months from the first treatment, or at 6 months for Y90, in all patients. Complete response rates were similar and low for both modalities. Partial responses were 55% and 33% for chemoembolization and Y90 respectively, but stable disease was found for 29% vs. 35% of patients treated with chemoembolization or Y90, respectively. Thus, over-all tumor control (CR+PR+stable disease) was 89% for chemoembolization and 76% for Y90 patients. However, this likely under-estimated the Y90 tumor response benefits, as the pace of radiological change differed for the 2 treatment modalities. Chemoembolization responses, when they occurred, were typically soon after the first treatment, with vascular responses occurring before size responses. Y90 responses occurred much more slowly, with vascular responses occurring within 2 months of treatment, but size responses (which were used for all response assessments in Table 4) often followed after many (6–18) months and for some patients, they continued for greater than 24 months after a single treatment. Chemoembolization patients had an average of 2.5 treatments (range 2–12). Y90 patients had a planned single treatment, but 30% required a second treatment due to late-appearing new lesions.
Table 4.
Tumor responses to treatment
| TACE n(%) |
Y90 n (%) |
|
|---|---|---|
| Total | 691 | 99 |
| Complete response | 37 (5) | 3 (3) |
| Partial response | 380 (55) | 38 (38) |
| Stable disease | 199 (29) | 35 (35) |
| Progressive disease | 75 (11) | 23 (23) |
DISCUSSION
The management of HCC that is considered too extensive for surgical resection or RFA, poses a difficult and unresolved dilemma in the role of therapeutic choices to assist in palliative care1,2,19,20 In recent years, a strategy of choice is the direct delivery of chemotherapy via the hepatic artery directly to the tumor vasculature2,14,15,20 The present clinical experience was obtained in 2 sequential cohorts of patients with unresectable, advanced HCC. In the first group, we describe our overall experience of patients treated with a structured protocol of cisplatin infusion, accompanied by gelfoam or Embospheres arterial embolization, which was designed to reduce but not completely obstruct (embolize)14 the hepatic arterial blood flow and to minimize side effects (TACE group). This has recently undergone a suggested change in terminology, from TACE to ‘chemoembolization’21 TACE is used here merely as a convenience. In the second group, patients were treated with an intrahepatic arterial rapid injection of 90Yttrium microspheres (Y90 group), designed to deliver 135–150 Gy (13,500–15,000 rads) to the tumor site.
Our transition in elective choice of first line therapy was a clinically based impression that the palliative benefit of the Y90 was greater than TACE and in particular, required a smaller number of treatment hospitalizations6–8 However, there was no prior evidence to compare the duration of survival from the time of the procedure. The observation in this cohort comparison is that there was a small but statistically significant difference of Y90 longer survival in the overall group (Figure 1). This difference was observed immediately following the procedure with differences being sustained over time. We consider, however, that this difference could be explained by the selection of patients with milder disease in the Y90 group. When a tiered approach was used to stratify subsets, there was no residual difference in survival between the 2 therapeutic alternatives. Clearly, this sub-set strategy reduces the power of each observation, but even if trends were supported in a larger series, the differences in medians would imply marginal differences.
The main toxicity of chemoembolization was tiredness and loss of appetite for 7 to 10 days post each treatment. The major inconvenience to the patients was the need for multiple treatments. Similar loss of appetite occurred in some Y90 patients. The identification of therapeutic equivalence in survival is of help in clinical decision making, particularly as the cisplatin TACE combination used here has previously been shown to confer survival advantage in a randomized controlled study4. We have recently reported in detail the early clinical consequences of Y90 in a subset of 65 patients in this cohort7. In that series, two had complete responses, 18 had partial responses, 27 had tumor stabilization and 19 had tumor progression. The overall clinical appraisal in that report, was that the efficacy data for tumor stabilization was encouraging, and the toxicity profile qualitatively less than prior experience with chemotherapy. We, therefore, conclude that in a risk benefit analysis between the two treatment options; that Y90 offers a superior option for palliative care and suggest that a randomized comparative trial with or without a control group may not be merited at this time.
We have previously used this three tier strategy to identify elevated bilirubin as the strongest predictor of poor survival in this patient group with PVT, elevated alpha-fetoprotein levels, being at high risk22 and being female being low risk23. An analogous tiered strategy has previously used PVT to indicate differences in outcome to Y908 This observation raises the question of whether any intrahepatic arterial procedure should even be offered in patients with a bilirubin even as modestly elevated as 1.5 mg/dl if they have either a PVT or an alpha-fetoprotein level as their prognosis is so limited.
The study reported here, which was based on clinical outcome studies of two sequential patient treatment cohorts, raises the possibility of selection bias and changes that would be expected to occur over time that would not be present in a conventional randomized clinical trial. We argue that by using a tiered stratification, there is evidence of differences in selection with a bias towards less severe disease in the Y90 group. In considering change in management over time, the details of each therapeutic protocol are presented in detail as they indicate a thoughtful sustained attention to the overall process of each strategy. Even if the general care did improve at later times, the time related bias would be expected to be an advantage to the Y90 group. However, the modest differences between groups could already be accounted for. Even if Y90 had a lesser response than TACE, the effect would be marginal and not impact the overall risk benefit ratio. The patients who received no treatment are included for perspective only. They were different from the treated patients, usually because of extra-hepatic cancer, or because of the severity of their liver failure or poor performance status. Thus, comparison statistics were not performed for this group. However, the group was clearly heterogeneous. Even though all patients died, not all patients had an equally bad prognosis, as seen in those patients with normal bilirubin, low alpha-fetoprotein or absence of cirrhosis (Table 3).
The results of this study should be considered within the context of the US experience. It reflects a marked contrast to Japan, where screening has identified a much greater proportion of early cases where the major consequence of the HCC is related to the location and size of the tumor24. The clinical situation in advanced HCC disease is much more complex as two or more concomitant disease processes progress as independent processes. In addition to the tumor characteristics, such as abnormal production of alpha-fetoprotein or tumor extension into the portal vein, there is the potential for underlying hepatic predisposing disease (hepatitis or cirrhosis) as well as tumor replacement of viable liver. It is our impression that in this late stage of disease, patients often die as a result of hepatic failure, rather than as a consequence of tumor extension25. We have not identified the cause of death in this overall cohort, but it is interesting to observe the differences in survival based on a serum bilirubin of less than 1.5 mg/dl being used to identify conserved function, the presence of either PVT or alpha-fetoprotein a measure of advanced tumor (Table 2 Table 3 and Figure 2 and Figure 3). If these measures are considered surrogate biomarkers for the two processes, loss of hepatic function is the most frequent life limiting process25 The literature on TACE for HCC is confusing, with many non-comparable trials1,2 Thus, it has never been established whether 3 or 2 drugs are superior to one drug, and if so, which combination is optimal15. Although lipiodol is frequently used, it has drawbacks, including difficulty in assessing subsequent CT scans12,15. Many different embolizing agents are also reported, from various institutions. These variables, together with the heterogeneity of patients in any trial, as well as variations in patient selection or liver function in different trials, severely impede comparison of response results or survival results between different institutions.
In conclusion, in comparing the clinical outcomes of chemoembolization (TACE) versus Y90 strategies for palliative care of advanced, surgically unresectable HCC, the risk and inconvenience of repetitive inpatient TACE needs to be weighed against limited treatments in an outpatient setting for Y90, when the two approaches are therapeutically equivalent with respect to survival. Despite the limitations of study design in comparing sequential cohorts, marginal advantage for the Y90 group can be explained on the basis of small but significant differences in selection bias towards milder disease. We also confirm that if serum bilirubin >1.5 mg/dl is used as a biomarker of impaired residual hepatic function, that such patients have a poor survival with either therapy (Fig 2). We consider that the available information shows these to regional treatment strategies to result in similar survival.
Acknowledgements
NIH 5RO1DK05919-05
Reference List
- 1.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J.Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 5.Salem R, Thurston KG, Carr BI, Goin JE, Geschwind JF. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J.Vasc.Interv.Radiol. 2002;13:S223–S229. doi: 10.1016/s1051-0443(07)61790-4. [DOI] [PubMed] [Google Scholar]
- 6.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 8.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Han KH, Lee JT, Paik YH, Ahn SH, Lee JD, et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin.Cancer Res. 2006;12:543–548. doi: 10.1158/1078-0432.CCR-05-1730. [DOI] [PubMed] [Google Scholar]
- 10.Wang XM, Yin ZY, Yu RX, Peng YY, Liu PG, Wu GY. Preventive effect of regional radiotherapy with phosphorus-32 glass microspheres in hepatocellular carcinoma recurrence after hepatectomy. World J.Gastroenterol. 2008;14:518–523. doi: 10.3748/wjg.14.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernal P, Raoul JL, Vidmar G, Sereegotov E, Sundram FX, Kumar A, et al. Intra-arterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int.J.Radiat.Oncol.Biol.Phys. 2007;69:1448–1455. doi: 10.1016/j.ijrobp.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, et al. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042–1050. doi: 10.1002/cncr.11111. [DOI] [PubMed] [Google Scholar]
- 13.Shibata J, Fujiyama S, Sato T, Kishimoto S, Fukushima S, Nakano M. Hepatic arterial injection chemotherapy with cisplatin suspended in an oily lymphographic agent for hepatocellular carcinoma. Cancer. 1989;64:1586–1594. doi: 10.1002/1097-0142(19891015)64:8<1586::aid-cncr2820640805>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Carr BI. Hepatic artery chemoembolization for advanced stage HCC: experience of 650 patients. Hepatogastroenterology. 2002;49:79–86. [PubMed] [Google Scholar]
- 15.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc.Intervent.Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny N, Seiter K, Niedzwiecki D, Chapman D, Sigurdson E, Cohen A, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327–334. doi: 10.1002/1097-0142(19920115)69:2<327::aid-cncr2820690209>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Amesur NB, Zajko AB, Carr BI. Chemo-embolization for unresectable hepatocellular carcinoma with different sizes of embolization particles. Dig.Dis Sci. 2008;53:1400–1404. doi: 10.1007/s10620-007-9995-x. [DOI] [PubMed] [Google Scholar]
- 18.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc.Interv.Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MB, O'Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann.Surg.Oncol. 2008;15:1008–1014. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 20.Williams S, Palmer D, Johnson P. New medical options for liver tumours. Clin.Med. 2007;7:351–356. doi: 10.7861/clinmedicine.7-4-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc.Interv.Radiol. 2007;18:1469–1478. doi: 10.1016/j.jvir.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Carr BI, Buch SC, Kondragunta V, Pancoska P, Branch RA. Tumor and liver determinants of prognosis in unresectable hepatocellular carcinoma: a case cohort study. J.Gastroenterol.Hepatol. 2008;23:1259–1266. doi: 10.1111/j.1440-1746.2008.05487.x. [DOI] [PubMed] [Google Scholar]
- 23.Buch S, Kondragunta V, Branch R, Carr B. Gender-based outcomes differences in unresectable hepatocellular carcinoma. Hepatology Int. 2:95–101. doi: 10.1007/s12072-007-9041-2. 7 A.D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mima S, Sekiya C, Kanagawa H, Kohyama H, Gotoh K, Mizuo H, et al. Mass screening for hepatocellular carcinoma: experience in Hokkaido, Japan. J.Gastroenterol.Hepatol. 1994;9:361–365. doi: 10.1111/j.1440-1746.1994.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 25.Couto OF, Dvorchik I, Carr BI. Causes of death in patients with unresectable hepatocellular carcinoma. Dig.Dis.Sci. 2007;52:3285–3289. doi: 10.1007/s10620-007-9750-3. [DOI] [PubMed] [Google Scholar]


