Abstract
Background
Important functional connections within the “default mode network” (DMN) are disrupted in Alzheimer’s disease (AD), likely from amyloid-beta (Aβ) plaque-associated neuronal toxicity. Here we sought to determine if pathological effects of Aβ amyloid plaques could be seen even in the absence of a task by examining functional connectivity in cognitively normal participants with and without preclinical amyloid deposition.
Methods
Participants with Alzheimer’s disease (AD) (n= 35) were compared with 68 cognitively normal participants who were further subdivided by PET PIB imaging into those without evidence of brain amyloid (PIB−) and those with brain amyloid (PIB+) deposition.
Results
Resting state fMRI demonstrated that, compared with the PIB− group, the PIB+ group differed significantly in functional connectivity of the precuneus to hippocampus, parahippocampus, anterior cingulate, dorsal cingulate, gyrus rectus, superior precuneus and visual cortex. These differences were in the same regions, and in the same direction, as differences found in the AD group.
Conclusions
Thus, prior to any manifestations of cognitive or behavioral changes there were differences in resting state connectivity in cognitively normal subjects with brain amyloid deposition, suggesting that early manifestation of Aβ toxicity can be detected using resting state fMRI.
Keywords: PIB, fMRI, amyloid, Alzheimers Disease, resting state, precuneus, hippocampus
Introduction
At rest, important brain areas constitute a network with correlated spontaneous brain activity, the “default mode network” (DMN) (1). These regions, including the precuneus, are among the earliest regions affected in Alzheimer’s disease (AD) (2). Several groups have demonstrated that AD is associated with DMN resting state fMRI disruptions compared to cognitively normal individuals (3–5). Recent studies (2) demonstrated that approximately 25% of older nondemented individuals have beta amyloid (Aβ) deposition by PIB PET, similar to post-mortem work (6). Thus, both neuropathology and clinical PIB imaging studies support the existence of a pre-clinical AD state with elevated Aβ burden in cognitively normal individuals. Based on the suggested neuronal toxicity of Aβ (7), we hypothesized that the presence of Aβ plaques in non-demented people has functional consequences, perhaps detectable by functional connectivity imaging. In the current investigation we sought to determine if the putative toxic effects of Aβ plaques extend to differences in functional connectivity between cognitively normal individuals with (PIB+) and without (PIB−) Aβ deposition.
Methods
Community-living volunteers enrolled in longitudinal studies of memory and aging at the Washington University Alzheimer’s Disease Research Center (ADRC). Participants (n=35) meeting Clinical Dementia Rating (CDR) criteria for very mild (CDR=0.5) or mild (CDR 1) demenxtia of the Alzheimer type (AD) were matched for age, gender and education with 68 individuals without cognitive impairment (CDR = 0). (Table 1). All participants in the ADRC underwent an extensive battery of neuropsychological testing to establish cross-sectional and longitudinal neuropsychological performance measures (8). CDR 0 cognitively normal participants were divided into two groups based upon PET imaging status: 48 had minimal to no Aβ deposition (PIB−), mean MCBP=0.003. Subjects with MCBP > 0.18, were considered PIB+ (9) (mean MCBP = 0.53; n=20). See Supplement. Detailed information on PIB PET imaging and analysis has been reported (2). Briefly, a 60 min. dynamic PET scan was obtained after injection of approximately 12 mCi of [11C]PIB. Specific MRI-derived regions-of-interest were used to calculate the mean cortical binding potential (MCBP) (2). PIB imaging was not obtained on AD subjects.
Table 1.
Demographics
| PIB − (N = 48) | PIB + N = 20) | AD N = 35) | |
|---|---|---|---|
| Gender (M, F) | 16, 32 | 8, 12 * | 13, 22 |
| Age Mean (SD) | 73.6 (6.6) | 72.7 (6.5) * | 75.3 (6.6) |
| Education | 15.2 (2.7) | 16.5 (3.8) * | 14.8 (4.0) |
| MMSE Mean(SD) | 28.7 (1.2) | 28.9 (1.1)* | 24.7 (3.5)** |
| CDR | 0 | 0 | 0.5 (n=22) |
| 1.0 (n=13) |
No significant differences between PIB+ vs PIB− values >.05.)
MMSE scores differed between cognitively normal PIB+ and AD (p <.001) and between cognitively normal PIB− and AD p <.001).
fMRI images were acquired on a 3T MRI scanner and processed as previously described (10). Two resting state 6 min. fMRI runs of 164 continuously acquired whole brain volumes were obtained using a gradient spin-echo sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (T2* weighting). High-resolution sagittal 3D T1-weighted structural images were also obtained. All fMRI data were corrected for movement and transformed to a common atlas space. Following preprocessing, correlation maps were obtained by selecting a seed region in the combined bilateral precuneus (+/−7, −60, 21) and creating an image map of correlations using Pearson product-moment correlation and the voxel-by-voxel BOLD time-course (10, 11). Group difference significance maps were created by combining results across subjects using a random effects analysis of the Fischer z transformed correlation maps (see Supplement for more detail). There were no group differences in quality assurance measures, e.g., head movement; (see Supplement).
Results
PIB (−) vs. AD functional connectivity
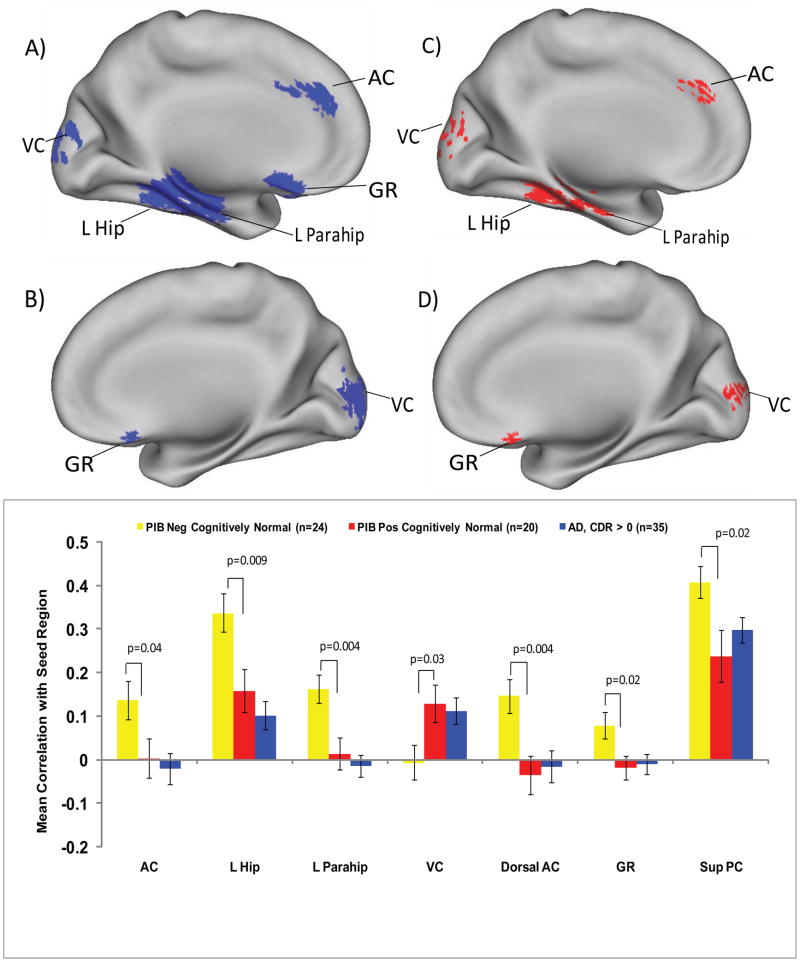
We first examined the functional connectivity differences in patients with early AD (n=35) vs half of the sample without amyloid plaques (PIB−) (n=24; randomly selected from the original 48 PIB− subjects) in order to generate ROIs for hypothesis testing. The group difference significance map of PIB− vs AD functional connectivity was then used for hypothesis testing in a second independent sample of 24 PIB− participants compared with PIB+ participants. As expected, significant differences were found between early AD subjects and (PIB−) age matched cognitively normal elderly in the functional connectivity of the precuneus seed region (+/− 7, −60, 21) (See Figure 1; and Figure S1 in the Supplement). Relative to (PIB−) cognitive normals, individuals with AD had decreased connectivity between precuneus and left hippocampus (−21, −37, −13), parahippocampus (−27, −26, −22), anterior cingulate (−17, +25, +30), gyrus rectus (−05, +14, −23), dorsal cingulate (−23, −08, +43) and superior precuneus (−07, −42, +44), whereas there was increased connectivity with visual cortex (+06, −98, +04). The resulting significance map was projected onto a standard brain surface for display (12) shown in Figure 1. See also Figure S1 in the Supplement.
Figure 1. Altered Functional Connectivity to the Precuneus.
All images are shown in medial sagittal section and identify statistically significant regional differences in functional connectivity of the precuneus between AD and (PIB−) cognitively normal individuals (blue) and between (PIB+) and (PIB−) cognitively normal individuals (red). The top left (1a) and right (1c) images display the left medial surfaces in AD vs PIB− and PIB+ vs PIB− participants, respectively. The second row left (1b) and right (1d) images display the right medial sagittal surfaces in the AD vs PIB− and PIB+ vs PIB− participants, respectively. The regions identified were in visual cortex-(VC) (both L and R), L hippocampus—(L hip), L parahippocampus—(L parahip), L anterior cingulate (AC), L (AD only) and R gyrus rectus (GR)(both groups). For brevity, not shown are the dorsal cingulate (DC) and superior precuneus (sup PC) regions. The graph compares regional correlation magnitudes for AD, PIB+ and PIB− individuals. *p-values denote significant differences in connectivity between (PIB+) and (PIB−) groups.
PIB (−) vs PIB (+) connectivity
Next, the regions of abnormal connectivity derived from the hypothesis generating AD vs. PIB− group analysis were examined in the comparison of the PIB− (n=24) vs PIB+ (n=20) cognitively normal participants. The PIB− group in this comparison was completely separate from the PIB− group used in the analysis of the AD group. (see Supplement). As in the hypothesis generating analysis, resting state functional connectivity was computed using a seed in the identical precuneus region. PIB− vs PIB+ group differences were evaluated by voxel-wise t-tests confined to regions of significant difference in the hypothesis generating analysis. We report here PIB− vs PIB+ group differences thresholded at a voxel-wise significance of 0.05. However, each of these voxels had independently shown a PIB− vs AD p < 0.05 effect, yielding a combined significance of p < 0.0025. In most regions, AD and PIB+ cognitively normal participants both demonstrated decreased functional connectivity compared with PIB− participants. These regions were left hippocampus (−26, −39, −15), parahippocampus (−23, −25, −22), anterior cingulate (−12, +30, +28), gyrus rectus (+04, +20, −21), dorsal cingulate (−22, +03, +43) and superior precuneus (−06, −42, +43). However, in the visual cortex (−02, −95, +10) (shown in Figure 1), significantly increased connectivity was observed.
Connectivity and MCBP
Correlations between precuneus BP and all target ROIs were conducted to determine whether there was a relationship between amyloid burden and connectivity. There were no significant correlations between amyloid deposition in the precuneus and the precuneus-regional functional connectivity in the PIB+ group (see Supplement).
Discussion
The current finding of decreased precuneus resting state functional connectivity with hippocampus, parahippocampus, anterior cingulate, dorsal anterior cingulate, gyrus rectus, and superior precuneus extends findings of disruptions in AD, and highlights the importance of integrity of the default network (3–5), since all these regions fall within the default mode network. We show here that even prior to cognitive impairment, cognitively normal individuals with cerebral Aβ deposits had clear-cut abnormalities in resting state functional connectivity in key regions, suggesting that Aβ deposition is associated with a disrupted functional connectivity within the DMN, even in the absence of a task. Altered resting state functional connectivity between the posterior and anterior portions of the default mode network has been described in AD (3–5). Most fMRI studies in AD to date have examined task-based fMRI responses (13), the interpretation of which is confounded by differences in task performance. In the current study the same regions showed altered resting state functional connectivity in presumptive preclinical AD. Specifically, connectivity between precuneus and hippocampus was significantly lower in individuals with amyloid plaques vs. those without evidence of brain A β plaques, indicating that resting state functional disconnection already had occurred in nondemented aging with Aβ plaques. Because there are extensive anatomical connections between posterior cortical regions and hippocampus and parahippocampus (which includes the entorhinal cortex) (14), it is possible that even without observable Aβ deposits in the hippocampus, the Aβ deposits in the precuneus may alter hippocampal function. The precuneus is known to have very early involvement in PIB deposition (2) and may play a critical role in memory function. Here, decreased resting state hippocampal functional connectivity may explain early disruptions in memory despite minimal early amyloid deposition in AD. Another critically important region for cognitive control, the anterior cingulate, is affected early in the course of PIB deposition (2). Here we also found significant reductions in connectivity in PIB+ vs PIB− subjects in the same regions as in very mild AD.
In contrast, the connectivity between the precuneus and visual cortex was significantly higher in PIB+ non-demented individuals and AD subjects; we note that this region is involved in sensory processing. We speculate that since one role of default mode network appears to be vigilance and modulation of attention across the sensory domain (15), disruptions to this network may result in dysregulation of the normal functional control of sensory processing. Speculatively, regions with increased connectivity following amyloid deposition may reflect impaired function in other involved region(s) that provide inhibitory input or a compensatory effect.
It is likely that these changes indicate a preclinical stage of AD. The “amyloid hypothesis” (16) states that the “accumulation of Aβ in the brain is the primary influence driving AD pathogenesis.” Studies from our group strongly support the concept of pre-clinical AD (17, 18). We now extend this observation to brain activity abnormalities in pre-clinical disease, in individuals with no apparent cognitive abnormalities, but with Aβ plaque deposition associated with resting state differences. How long Aβ pathology needs to be present before disruption of brain circuitry occurs is unknown but is an important topic for future investigation. Using the decreased functional connectivity within the DMN as markers, longitudinal studies will be important in order to determine the time-course of neuronal dysfunction and damage to help plan the optimal timing for potential interventional studies in individuals at risk for symptomatic AD.
Supplementary Material
Acknowledgments
This work was supported by NIH K24MHO79510 (YIS), P50AG05681 (JCM), P01AG03991 (JCM), P01AG026275 (JCM) P30NS048056 (MAM) and P30NS06833 (MAM, MER, AZS). The authors would like to thank all of the Washington University ADRC personnel for their skilled assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited that has been for publication. As a service to our customers we are providing this early version of the. The will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 3.Greicius M, Srivastava G, Reiss A, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supekar K, Menon V, Rubin D, Musen M, Greicius M. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behav Brain Res. 2009;197:103–8. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Price J, Morris J. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe D. Biochemistry and Molecular Biology of Amyloid beta-Protein and the Mechanism of Alzheimer’s Disease. Handb Clin Neurol. 2008;89:245–60. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- 8.Storandt M, Grant E, Miller P, Morris J. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59:1034–41. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 9.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 11.Fox M, Corbetta M, Snyder A, Vincent J, Raichle M. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. PNAS. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Essen D. A Population-Average, Landmark- and Surface-based (PALS) atlas of the human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 13.Sperling R, LaViolette P, O’Keefe K, O’Brien J, Rentz D, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:1–11. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi Y, Amaral D. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 15.Buckner R, Andrews-Hanna J, Schacter D. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Selkoe D. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. Review. [DOI] [PubMed] [Google Scholar]
- 17.Price J, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Morris J, Roe C, Xiong C, Fagan A, Goate A, Holtzman D, Mintun M. APOE Predicts Aâ but not Tau Alzheimer’s Pathology in Cognitively Normal Aging. Ann Neurol. 2009 doi: 10.1002/ana.21843. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.