Abstract
Nano/microfluidic technologies are emerging as powerful enabling tools for diagnosis and monitoring of infectious diseases in both developed and developing countries. Miniaturized nano/microfluidic platforms that precisely manipulate small fluid volumes can be used to enable medical diagnosis in a more rapid and accurate manner. In particular, these nano/microfluidic diagnostic technologies are potentially applicable to global health applications, because they are disposable, inexpensive, portable, and easy-to-use for detection of infectious diseases. In this paper, we review recent developments in nano/microfluidic technologies for clinical point-of-care applications at resource-limited settings in developing countries.
Keywords: Nano/microfluidics, Infectious diseases, HIV/AIDS, Point-of-care, Diagnostics, Global health
1. Introduction
Infectious diseases are a leading cause of death in developing countries [1,2]. Over 95 % of these deaths are caused by the lack of proper diagnosis and treatment, such as difficulty in accessing adequate health care infrastructure [3]. To develop diagnostic tools for infectious diseases at resource-limited settings, the World Health Organization (WHO) has established a set of guidelines: (i) affordable, (ii) sensitive, (iii) specific, (iv) user-friendly, (v) rapid and robust, (vi) equipment-free, and (vii) delivery to those who need it, leading to the acronym “ASSURED” [2,4]. These guidelines can be used to develop more suitable diagnostic approaches for low-resource settings to enhance the overall quality of life of global population [5–7].
Point-of-care (POC) diagnostics offer great potential to detect and monitor infectious diseases at resource-limited settings, because POC diagnostics can be taken to remote locations, decreasing the need for large decentralized diagnostics facilities. Desired characteristics of POC diagnostic technologies include (i) disposability, (ii) cost-effectiveness, (iii) ease of use and (iv) portability [8]. POC diagnostics should be able to analyze small volumes of bodily fluids, e.g., blood, saliva and urine. Given the contagious nature of these samples, the devices should be disposable to protect the end-users from exposure to biohazardous waste. In addition, disposable POC devices eliminate unnecessary steps, such as washing processes between sample preparations, which can make the devices easier to use even in regions poorly supplied with water. The cost of diagnostics is also one of the important parameters for global health applications [9]. To decrease the cost of POC diagnosis, several aspects should be considered: (i) minimal use of expensive reagents, (ii) inexpensive manufacturing for mass-production, (iii) quality control, and (iv) miniaturization [3]. In addition, for clinical use of medical diagnostic devices in resource-limited settings, environmental conditions, such as insufficient water, unreliable electricity, high temperatures (35~45°C), and humidity need to be considered [10].
Nano/microfluidic technologies are emerging as powerful methods which could address the challenges imposed by conventional diagnostic devices [11,12]. These approaches enable on-chip POC diagnosis and real-time monitoring of infectious diseases from a small volume of bodily fluids [13]. These technologies can be used to integrate various assays into a single device [14–17] and to deliver target samples to specific reaction chambers in a controlled manner [16,18–23]. Among these technologies, nanofluidics has been highlighted by the recent advent of nanoscience and nanotechnology since the rise of microfluidics in 1990s [24]. Generally, nanofluidics can be defined as the field of studying fluid flow in and around nanoscale objects [24,25]. For its applications to POC diagnostic devices, nanofluidics can be used to enhance microfluidic functions, such as temporal and spatial control of nanosized samples (e.g., HIV virus ~100 nm) in lab-on-a-chip (LOC) devices with nanostructures and nanotech materials [26,27]. Given these features, nano/microfluidic devices have been used for sample preparations, such as continuous blood flow fractionation [28–31], nucleic acid extraction [32], and purification of small molecules [33].
The cost of micro/nanofluidic technologies can be minimized by mass production through simple plastic fabrication techniques. For example, nanostructures that can enhance capillary-driven microflow in plastic microfluidic devices can be easily scaled-up for mass production using nanomolding techniques. Thus, microfluidic devices can enable on-chip POC diagnosis of blood-related infectious diseases in a disposable and mass-producible format [34]. Nano/microfluidic devices can also be built at a low-cost by using other materials, such as paper in a process called “fast lithographic activation of sheet (FLASH)”, without high-end microfabrication facilities [35]. These devices are disposable, cheap and useful for on-chip processes and involve sample pre-treatment in a rapid and high-throughput manner.
In this review, we provide a broad overview of recent advancements in nano/microfluidic technologies for POC diagnostics targeting infectious diseases at resource-limited settings. We also highlight the applications of on-chip POC diagnostics focusing on detection, imaging, and counting techniques. Furthermore, we provide current trends and perspectives for nano/microfluidic POC applications of infectious diseases, such as (i) HIV/AIDS, (ii) malaria, and (iii) tuberculosis (TB).
2. Recent developments in nano/microfluidic technologies targeting infectious diseases at resource-limited settings
In this section, we will specify key examples of recent advances in nano/microfluidic technologies that can enhance the development of a platform for global POC diagnosis and monitoring of infectious diseases at resource-limited settings.
On-chip detection and imaging
On-chip detection and imaging techniques play an important role to detect infectious diseases in a fast and accurate manner. Among them, optical microscopy is a powerful method for detection and imaging of various diseases at molecular and cellular level. Optical microscopy can be combined with other techniques to enhance on-chip detection of blood-related diseases by using erythrocyte deformability [36,37]. Recently the optical microscopy field has significantly evolved through an emergence of optofluidic technologies which fuses optics and microfluidics [38]. The optical microfluidic technologies have been used to develop POC diagnostics with enhanced detection techniques, such as absorbance, fluorescence, chemiluminescence, surface plasmon resonance (SPR), and interferometric detection [12]. However, optical microscopy has several limitations, such as lack of portability, high costs due to use of expensive optics, and regular maintenance. To overcome these limitations, lensless and portable charge-coupled device (CCD)-based platforms have been recently developed [22,39]. This approach can be used to detect cells and particles by shadow imaging, enabling a lensless and ultra-wide field monitoring. Thus, the captured microscale targets (i.e., cells or beads) in a microfluidic device are detected and counted by their shadow images without fluorescence labeling [22]. This approach can be useful for developing a handheld, battery-supplied, imaging platform which will be cheap to maintain and to operate.
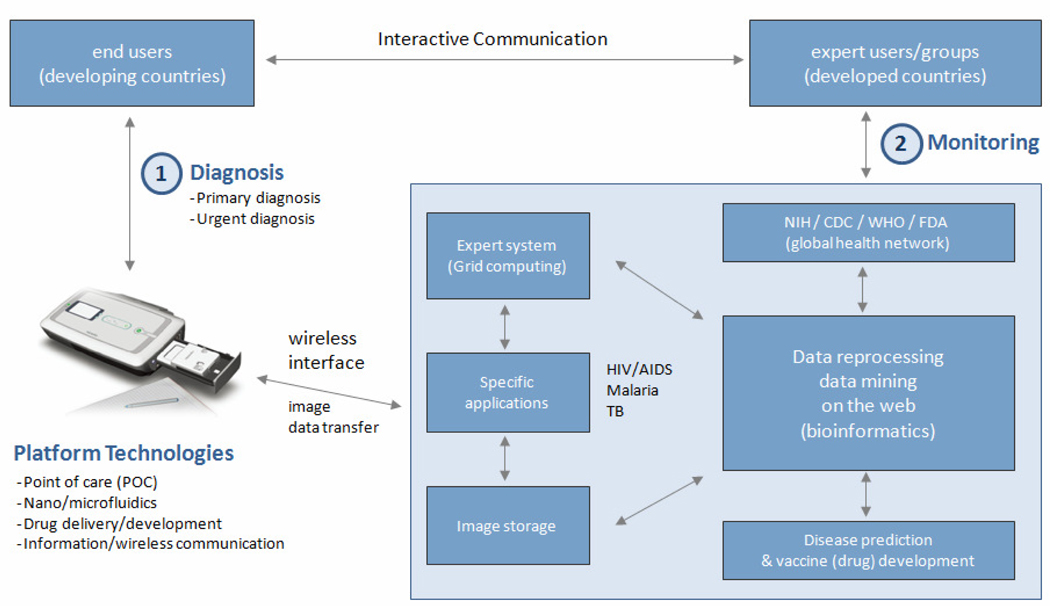
More recently, a new imaging platform, called the “mobile-based clinical microscopy”, has been developed for the development of an integrated and portable mobile phone microscopy system for global health [40,41]. Generally, most regions of developing countries have either insufficient infrastructural resources, such as lack of health care facilities and manufacturers. For the same regions, however, mobile phone or wireless networks are often available, where the camera-equipped mobile phones can also be used at a lower cost, compared to microscopy-related cameras. Recently the mobile phone network, especially available in developing countries, was envisioned as an alternative route to achieve diagnostic imaging and telemedicine [41]. The clinical feasibility of this technique was also demonstrated by conducting successful detection of malaria and tuberculosis by bright field and fluorescence imaging, respectively [41]. This technique is potentially useful to develop a portable platform that can be combined with disposable nano/microfluidic POC devices for global health applications (Figure 1).
Figure 1.
A schematic of a platform for POC diagnostics in developing and developed countries. The device shown above was selected as a representative example of a wireless data-reading platform for global health. The image of the device was reprinted from Ref [3] by permission of Annual Reviews.
On-chip flow cytometry
The ability to rapidly count and analyze cells by using techniques, such as flow cytometry, is important for diagnosis of a number of infectious diseases, such as HIV [42]. Despite this need, conventional flow cytometers are often too expensive to use in resource-limited settings. Thus, for the past decade, there has been interest to miniaturize the flow cytometry instruments [43,44]. To develop miniaturized flow cytometers, several desired features include: (i) label-free detection, (ii) lensless imaging or minimal use of expensive optics, (iii) simple channel geometry, and (iv) sheath-free focusing.
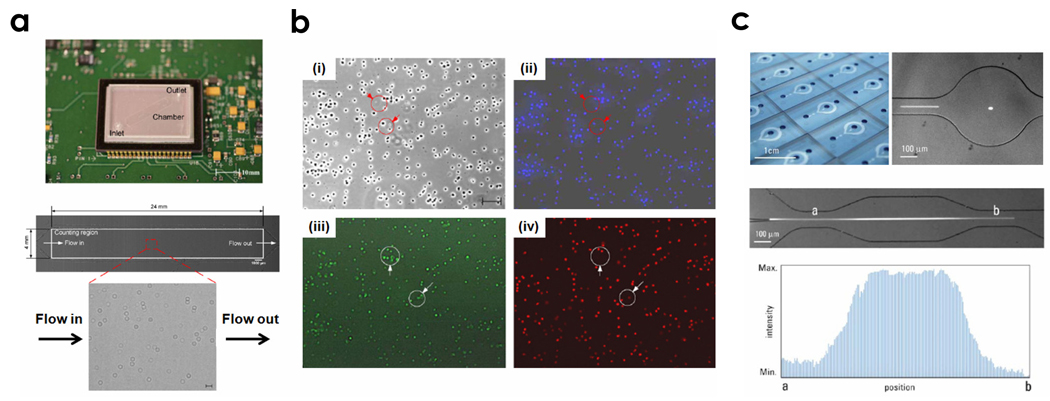
Recently, a microfluidic device was developed to perform a simple, rapid, and affordable counting of CD4 lymphocytes, especially for HIV monitoring in resource-limited settings [45]. This approach enabled the capturing and imaging of CD4 cells within a miniaturized flow chamber by microscope optics and digital camera technology. More recently, a label-free detection method was also developed for counting label-free CD4+ T-lymphocytes in resource-limited settings [29,30]. In this approach, 10 µL of whole blood was injected into a poly(dimethylsiloxane) (PDMS)-based microfluidic chip that was functionalized with antibodies to capture CD4+ cells. Another approach that eliminates the need for expensive optics by using a portable CCD platform has been also used for lensless imaging of CD4+ T-lymphocytes (Figure 2a–c) [22]. We describe this concept in detail in the HIV/AIDS section.
Figure 2.
On-chip microfluidic approaches. (a) CCD-based imaging platform with a disposable microfluidic device (top) and the CCD shadow image of CD4+ T-lymphocytes captured in the microfluidic device (bottom). Scale bar is 100 μm. (b) Phase contrast and fluorescent images to identify captured cells: (i) Phase contrast image of cells, (ii) DAPI stained cells, (iii) CD4+/AF488 stained cells, (iv) CD3+/AF647 stained cells. Modified from Ref [22] with permission from the Royal Society of Chemistry. (c) Photograph of a mass-producible device and a bright field image of the expansion channel at the detection zone (top and middle) and intensity profile of fluorescence of particles flowing through the expansion geometry (bottom). Reprinted from Ref [46] with permission from the Royal Society of Chemistry.
The microfluidic channel geometry is also important for controlling the detection sensitivity. For instance, a flow focusing channel was used to decrease the complexity of channel geometries, resulting in high detection sensitivity and throughput [46] (Figure 2c). This microchannel had a channel width of 100 µm at the inlet and 500 µm at the detection region. The fluorescently-labeled samples were hydrodynamically focused at the entrance of the focusing channel and passed through the expansion channel at the detection region. Since the flow velocity of the samples was decreased at the detection zone inside the expansion channel, the detection sensitivity was enhanced an order of magnitude, compared to normal channels, while preserving the detection throughput since the flow rate remained the same. For the clinical use of the concept, this geometry can be modified with sheathless focusing techniques associated with fluorescent nanoparticles [47]. All these approaches are potentially useful to overcome the optical and geometric constraints of on-chip flow cytometers.
On-chip immunoassay
Nano/microfluidic technologies enable the generation of on-chip immunoassays for POC diagnostic applications. The conventional enzyme-linked immunosorbent assay (ELISA) approach has a number of limitations for its use in resource-limited settings, because it requires long assay times, cumbersome liquid handling, and need for large amounts of expensive reagents and equipment [23]. To address these limitations, recently Kitamori and colleagues developed a practical micro-ELISA system for sensitive and rapid diagnosis. This approach showed potential for development of a POC platform that enables the use of small samples (10 times smaller) and fast analysis (20 times faster) compared to the conventional method [48]. For immunoassay-based diagnosis, rapid diagnostic tests (RDTs) have been developed for detection of various entities. For example, immunoassays can be performed by lateral-flow methods to provide rapid results at a low cost, similar to portable pregnancy tests [49]. To enhance the quantitative results of these RDTs, the immunoassay can be combined with optical, electrical, and mechanical transducers in an integrative format [49–53].
Microfluidics-based immunoassays are potentially beneficial to maximize sensitivity, minimize sample volume requirements, and produce fast and accurate results [54–56]. For example, Yager and colleagues developed an on-chip diffusion immunoassay to measure the concentration of small molecules within microfluidic channels [57]. This assay is based on characterizing the distribution of a labeled probe molecule after it diffuses from one region into another region with antigen-specific antibodies in the microfluidic T-sensor. Thus, microfluidic diffusion-based studies can be desirable for high-throughput screening and analyzing blood samples. Recently, the research group developed a microfluidic immunoassay with on-card dry reagent storage for malaria detection, showing possibility of quantitative analysis of multi-analytes such as human blood samples [53]. A dry cantilever assay has also been developed to increase the sensitivity of immunoassays. This assay with its data tracking and recording functions may be potentially useful for POC-based immunoassay applications [58].
Nanosensors
Today’s POC diagnostics can be improved by modifying conventional detection techniques associated with nano/microfluidic interfaces with lateral flow [59] and diffusion [60], such as surface plasmon resonance (SPR) and atomic force microscopy (AFM). For instance, Yager and colleagues developed a portable SPR system for label-free POC diagnostics [61]. This prototype diagnostic chip consists of a near-infrared light-emitting diode (LED) source and stationary wide-field imaging optics containing a compact liquid crystal polarizer that can be used to electronically switch the source polarization of images. Given the powerful SPR imaging-based reader, this device can be used for rapid detection of the antiepileptic drug (AED) phenytoin in saliva. This system can also be easily combined with micro/nanofluidic channels [62,63], enabling real-time label-free protein detection and separation. However, for the portable POC diagnostic applications, miniaturization of the optics and electronics still remains a challenge for SPR systems.
Nanosensors, such as nanotubes, nanowires, and cantilevers can improve the sensitivity, reduce production costs, and characterize various analytes that have previously been difficult to detect by conventional technologies. For example, carbon nanotube–based sensors for monitoring respiratory gas and biomolecules, such as DNA, protein, and glucose, have been developed [64,65]. These sensors, which detect picomolar concentrations of label-free DNA, show a high sensitivity of molecular detection. In addition, an AFM-based approach was used to detect viruses for diagnosis of viral infections. This platform consists of a silicon chip functionalized with a microarray of antibodies and an AFM-based detector [66]. This technique was used to detect and identify nanoparticles that interacted with tip of the AFM as the tips were scanned on the microarrays [67]. To detect virus particles, a ViriChip assay integrated with microarray, antibody capture, and label-free readout systems was used. The viruses captured on antibody coated surface were analyzed by using AFM. Thus, the glass-based ViriChip can directly detect infectious viral antibody-specific particles within 30 min. In addition, nanosensor technologies hold great potential to develop clinically relevant platforms with high detection sensitivity. These technologies are potentially beneficial to develop nano/microfluidic POC devices that enable label-free detection and identification of actual virus particles in resource-limited settings [68].
3. Current challenges and future perspectives
In this section, we will discuss current challenges and perspectives of nano/microfluidic technologies for POC diagnosis and monitoring of infectious diseases, such as HIV/AIDS, malaria, and tuberculosis.
HIV/AIDS
The World Health Organization emphasized the need to develop POC devices for HIV/AIDS diagnosis and monitoring in resource-limited settings [69]. These POC devices can be developed as an accurate, inexpensive, and disposable platform which enables the enumeration of CD4+ T-lymphocytes and HIV viral load [70–72]. For clinical applications, the POC device needs to detect and count less than 200 CD4+ cells µL−1 and 400 copies mL−1 of HIV from whole blood [73]. ART is targeted at repressing virus replication suppressing the progression of the HIV infection toward the disease (AIDS), resulting in gradual increases in the number of CD4+ T-lymphocytes [74,75]. Therefore, CD4+ T-lymphocyte counting and viral load quantification have been used to monitor HIV. However, the conventional methods, such as expensive flow cytometry and quantitative PCR, still have several limitations such as a long diagnostic time and the need for a well-trained technician [76].
The number of CD4+ T-lymphocyte per microliter of HIV-infected blood has prognostic and therapeutic indications and it has been critically used to monitor disease states and to decide ART treatment [75]. CD4+ T-lymphocytes act as host cells [77]. HIV binds to the CD4 receptor via HIV gp120 envelop glycoprotein, which allows the virus to infect and damage the cells during the process. Currently, the CD4+ T-lymphocyte count has been performed four times per year in developed countries, but only twice a year in developing countries using conventional flow cytometers [78].
For global health applications, a number of studies have been conducted to miniaturize the flow cytometers as a portable POC device [79]. However, a number of challenges, such as the cost and complexity have to be addressed in resource-limited settings. Therefore, simple and mass-producible microfluidic devices can be useful for POC diagnostic applications for HIV/AIDS. For instance, recently a simple and reliable method for quantifying anti-HIV-1 antibodies in the sera of HIV-1 infected patients was developed using the microfluidic immunoassay, called “POCKET (portable and cost-effective)” [50]. In this approach, HIV-enveloped antigen was patterned on a polystyrene surface as a stripe and a slab of PDMS with microchannels was placed orthogonally to the stripe. The HIV-1 infected patient sera were directly introduced into the microchannels to quantify anti-HIV-1 antibodies. Although the microfluidic device allows detecting easily the highly increased anti-HIV-1 antibodies in patient sera, it was insufficient to make a correlation with HIV disease states.
Lee and colleagues have also developed a disposable polymer-based RT-PCR chip containing pinched microvalves for POC diagnostics [80]. For early diagnosis of HIV infection, this device has been used to detect the HIV p24 and gp120 that are major capsid and envelop proteins encoded by the HIV gag and envelop gene. For practical POC applications, however, this approach requires optimization for HIV-infected whole blood processing and simplified POC operation at resource-limited settings.
For the clinical use of POC devices in resource-limited settings, a number of challenges should be addressed, such as (i) CD4+ T-lymphocytes captured from unprocessed HIV-infected whole blood and (ii) simple, rapid, and accurate counting of the captured cells. Previously, the devices that enable label-free CD4+ T-lymphocyte capture and imaging have been developed [29,30]. Although these devices are disposable, they still require expensive optical microscope equipments to count the captured CD4+ T-lymphocytes. To overcome this limitation, Demirci and colleagues have recently developed a handheld CCD-based microfluidic platform for HIV monitoring [22]. The microfluidic CD4+ T-lymphocyte counting devices were fabricated out of poly(methylmethacrylate) (PMMA), glass slides and double-sided adhesive film without using high-cost equipment (Figure 2a). Therefore, the device material and production costs were significantly decreased for use in resource-limited settings. In addition, the captured label-free CD4+ T-lymphocytes from fingerprick whole blood were detected by CCD sensor by lensless shadow imaging techniques and were counted by automatic cell counting software in a few seconds, without any fluorescent labeling or an optical microscope [22,39]. This approach enables the microscale miniaturization of handheld devices which uses fingerprick whole blood (10 µL) for CD4+ T-lymphocyte counts. Furthermore, this device can be of great interest for performing amplification-free HIV viral load quantification. Most recently, a microfluidic device was developed for HIV capture and imaging by quantum dots (QDs) from an HIV-infected patient whole blood [68]. Quantum dots with two different colors were used to detect the captured HIV by dual-staining of the envelope gp120 glycoprotein and its high-mannose glycans. HIVs were successfully captured from unprocessed HIV-infected patient whole blood onto microfluidic devices and were directly imaged. However, a successful design of nanofluidic device for an ultimate HIV viral load monitoring platform will be critical to increase the accuracy and possibility that the nanoscale virus particles (~120 nm) bind onto the chamber surface and to simultaneously discard micrometer sized-cells from HIV-infected patient whole blood.
Malaria
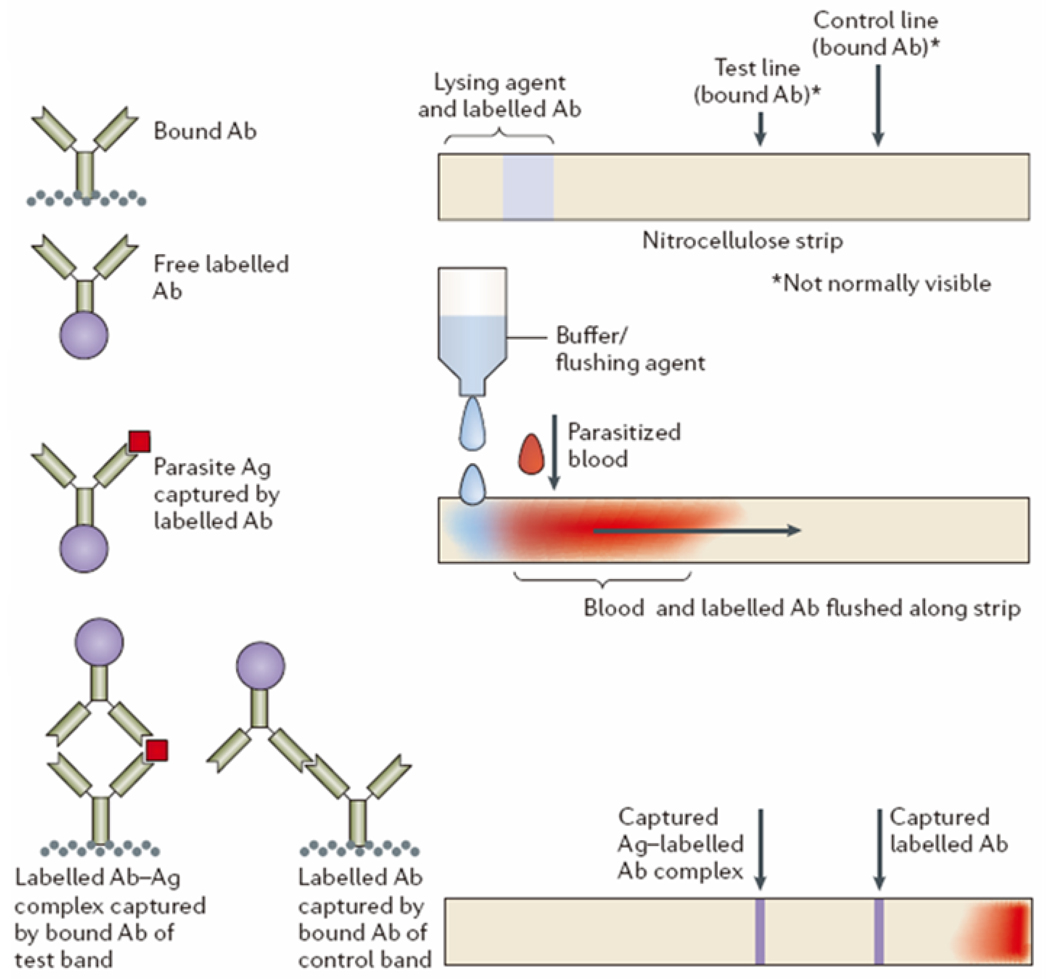
Rapid detection and treatment plays an important role in preventing the spread of malaria. In addition, improper diagnosis and treatment may also result in drug abuse or unexpected side effects [81]. Therefore, simple yet highly accurate diagnostic devices are required to minimize improper use of anti-malarial drugs or antibiotics. There are several approaches to diagnose malaria infection in resource-limited settings, such as lateral-flow RDTs [82,83]. The first observation of malaria infection in human blood was in the 1880s [84] and microscopy has been considered as the gold standard for diagnosis of malaria. This method to detect malaria infection and parasites in resource settings requires well-trained researchers [85]. To overcome the need for well-trained experts and equipment, nano/microfluidic technologies can be used to develop POC devices for fast and accurate malaria diagnosis (Figure 3). Figure 3 shows the schematic of the lateral flow strip chip for diagnosis of malaria infection [86]. For example, the RDT strip chip can detect proteins derived from the blood of malaria parasites in a microfluidic format and can also be realized as commercialized products (not shown here). This chip enables the generation of a series of visible lines to indicate the presence of specific antigens in blood that is clearly visible to the naked eye when antibody is accumulated at the test line.
Figure 3.
Schematic of the lateral flow strip chip to diagnose malaria. (Top) Device preparation with nitrocellulose strip, (middle) Operation principle of a lateral flow strip chip, and (bottom) Expected results of a lateral flow strip. Reprinted from Ref [86] with permission from Nature Publishing.
Rathod et al. developed microfluidic channels to study malaria pathogenesis related complex interactions between host cell ligands and parasitized erythrocytes [87]. Because the microfluidic channels successfully mimic the sizes and shapes of small capillary blood vessels, they could observe host-parasite interaction and malaria-infected red blood cells in capillary environment. The malaria diagnostic device is inexpensive and handheld for on-site analysis of patient samples and only requires microliter volumes of samples; therefore, they have the potential to be widely used at field sites for more accurate malaria diagnosis.
Tuberculosis (TB)
TB is one of the main fatal infectious diseases to the HIV-infected patients, increasing death rate and drug resistance of immune suppressed patients [88,89]. Sputum smear microscopy has been used for TB diagnosis with relatively lower sensitivity in resource-limited settings [90,91]. Sheehan et al. reported a polymerase chain reaction (PCR)-based Mycobacterium tuberculosis diagnostic device designed as a microfluidic device for DNA amplification [92]. They described a noncontact thermo-cycling approach using low power halogen lamp for DNA amplification from the pathogen. In addition, sample and reagent consumption is reduced and the lower thermal mass decreases the required time for PCR from hours to minutes. Because of the layout of the microfluidic device, low cost heat source and low power consumption of the system present a possibility to develop a portable field device. More recently, Lee et al. have developed a portable microfluidic nuclear magnetic resonance (NMR) biosensor for rapid, quantitative, and multiplexed detection of biological targets, such as bacteria, cancer biomarkers, and TB [93] (Figure 4). The biosensor containing PMMA-based microfluidic mixers, microcoil arrays, printed circuit board, and a permanent dipole magnet was fabricated by photolithography and electroplating techniques. This sensor is based on a self-amplifying proximity assay and magnetic nanoparticles. In this system, magnetic nanoparticles served as a proximity sensor that bound to target biomolecules and subsequently formed soluble nanoscale clusters, which led to NMR signal changes. The data can be electronically obtained without bulky and expensive optical components. This fast, simple, and high-throughput device is particularly useful in resource-limited settings. They also demonstrated the use of this system for the detection and characterization of infectious agents, such as bacteria, viruses, fungi, and parasites.
Figure 4.
Microfluidic NMR biosensor combined with magnetic nanoparticles for potential applications of TB test in resource-limited settings. (a) Principle of proximity assay using magnetic particles (top) and signal detection (bottom). (b) Schematic diagram of the device. (c) Photograph of an actual microcoil which generates radio frequency (RF) magnetic fields to excite samples and receives the resulting NMR signal. (d) Image of a microfluidic network. (e) Schematic of the NMR electronics. Reprinted from Ref [93] by permission of Nature Publishing Group.
In summary, this review focused recent advances of nano/microfluidic POC devices and its clinical applications at resource-limited settings. Most of them showed great potential to meet clinical and technical requirements for global health care. However, one major challenge still remains about how to network these nano/microfluidic POC diagnostics effectively between developed and developing countries, that can be communicated with an interactive feedback for more enhanced global health. As described before, current mobile-phone networks can be potentially beneficial for developing such a network-based platform that can improve nano/microfluidic POC devices consistently. Another major challenge may be about how to establish a proper regulation and standard that can evaluate nano/microfluidic POC diagnostics for clinical use [94]. The evaluation criteria may also include several processes, especially for developing diagnostic tests, such as identification of the diagnostic target, optimization of test reagents, and development of a prototype. As shown in the literature, nano/microfluidic technologies hold great promise that can establish a standard of detection sensitivity level for POC devices through quantitative, proof-of-principle studies in a fast, controlled, and high-throughput manner. Furthermore, to get approval for legal clinical use of POC devices (e.g., regulatory demands by FDA or NIH), nano/microfluidic technologies should also be capable of satisfying critical evaluation criteria, such as test characteristics and factors: (i) test performance (sensitivity and specificity), (ii) ease of use, (iii) conditions of use and storage, and (iv) shelf life [94,95]. Specifically, for commercialization, the efforts in this field should be made towards non- and minimally instrumented, nano/microfluidic POC diagnostics by developing platforms that can function without any peripherals [96].
4. Concluding remarks
Nano/microfluidic technologies have been successfully integrated with current POC devices for on-chip diagnosis and monitoring of infectious diseases at resource-limited settings. Nano/microfluidic POC diagnostics have significant advantages over conventional diagnostics such as reducing costs and increasing portability and disposability. Future trends in these diagnostics may keep focusing on extending its availability to decentralized hospitals and rural areas, more effectively where wireless networks are available. In this review, we provided an overview of recent advances in nano/microfluidic technologies for POC diagnostics. Here, we specified key requirements, such as disposability, cost-effectiveness, ease of use, and portability that can improve and further advance nano/microfluidic POC diagnostics for a wider clinical use at resource-limited settings. We also discussed current challenges and perspectives of nano/microfluidic POC diagnostics for clinical applications, such as HIV/AIDS, malaria, and tuberculosis. Much progress, however, still remains to be made to further reduce costs and establish standard criteria that can evaluate nano/microfluidic POC devices for use at resource-limited settings.
Acknowledgments
This work was supported by the National Institute of Health by grants (EB007249, DE019024, HL092836, and AI081534). W. G. Lee was partially supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-357-D00035).
Abbreviations
- AED
antiepileptic drug
- AFM
atomic force microscopy
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- CCD
charge-coupled device
- ELISA
enzyme-linked immunosorbent assay
- FLASH
fast lithographic activation of sheet
- HIV
human immunodeficiency virus
- LED
light-emitting diode
- LOC
lab-on-a-chip
- NMR
nuclear magnetic resonance
- PCR
polymerase chain reaction
- PDMS
poly (dimethylsiloxane)
- PMMA
polymethymetacrylate
- POC
point-of-care
portable and cost-effective
- QD
quantum dot
- RDT
rapid diagnostic test
- RF
radio frequency
- SPR
surface plasmon resonance
- TB
tuberculosis
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 2.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G, Hay DC. Burgess, Requirements for high impact diagnostics in the developing world. Nature. 2006;444 Suppl 1:73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 3.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 4.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Diagnostics for the developing world. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 5.Hay Burgess DC, Wasserman J, Dahl CA. Global health diagnostics. Nature. 2006;444 Suppl 1:1–2. doi: 10.1038/nature05440. [DOI] [PubMed] [Google Scholar]
- 6.Houpt ER, Guerrant RL. Technology in global health: the need for essential diagnostics. Lancet. 2008;372:873–874. doi: 10.1016/S0140-6736(08)61377-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girosi F, Olmsted SS, Keeler E, Hay Burgess DC, Lim YW, Aledort JE, Rafael ME, Ricci KA, Boer R, Hilborne L, Derose KP, Shea MV, Beighley CM, Dahl CA, Wasserman J. Developing and interpreting models to improve diagnostics in developing countries. Nature. 2006;444 Suppl 1:3–8. doi: 10.1038/nature05441. [DOI] [PubMed] [Google Scholar]
- 8.Huckle D. Point-of-care diagnostics: an advancing sector with nontechnical issues. Expert Rev Mol Diagn. 2008;8:679–688. doi: 10.1586/14737159.8.6.679. [DOI] [PubMed] [Google Scholar]
- 9.Huckle D. Point-of-care diagnostics: will the hurdles be overcome this time? Expert Rev Med Devices. 2006;3:421–426. doi: 10.1586/17434440.3.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 11.Chin CD, Linder V, Sia SK. Lab-on-a-chip devices for global health: past studies and future opportunities. Lab Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 12.Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8:2015–2031. doi: 10.1039/b812343h. [DOI] [PubMed] [Google Scholar]
- 13.El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich PS, Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discov. 2006;5:210–218. doi: 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- 15.West J, Becker M, Tombrink S, Manz A. Micro total analysis systems: latest achievements. Anal Chem. 2008;80:4403–4419. doi: 10.1021/ac800680j. [DOI] [PubMed] [Google Scholar]
- 16.Song YS, Moon S, Hulli L, Hasan SK, Kayaalp E, Demirci U. Microfluidics for cryopreservation. Lab Chip. 2009;9:1874–1881. doi: 10.1039/b823062e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagally ET, Scherer JR, Blazej RG, Toriello NM, Diep BA, Ramchandani M, Sensabaugh GF, Riley LW, Mathies RA. Integrated portable genetic analysis microsystem for pathogen/infectious disease detection. Anal Chem. 2004;76:3162–3170. doi: 10.1021/ac035310p. [DOI] [PubMed] [Google Scholar]
- 18.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 19.Dittrich PS, Tachikawa K, Manz A. Micro total analysis systems. Latest advancements and trends. Anal Chem. 2006;78:3887–3908. doi: 10.1021/ac0605602. [DOI] [PubMed] [Google Scholar]
- 20.Schulte TH, Bardell RL, Weigl BH. Microfluidic technologies in clinical diagnostics. Clin Chim Acta. 2002;321:1–10. doi: 10.1016/s0009-8981(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 21.Kang L, Chung BG, Langer R, Khademhosseini A. Microfluidics for drug discovery and development: from target selection to product lifecycle management. Drug Discov Today. 2008;13:1–13. doi: 10.1016/j.drudis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon SJ, Keles HO, Ozcan A, Khademhosseini A, Hæggstrom E, Kuritzkes D, Demirci U. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens Bioelectron. 2009;24:3208–3214. doi: 10.1016/j.bios.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya A, Klapperich CM. Design and testing of a disposable microfluidic chemiluminescent immunoassay for disease biomarkers in human serum samples. Biomed Microdevices. 2007;9:245–251. doi: 10.1007/s10544-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 24.Eijkel JCT, Berg Avd. Nanofluidics: what is it and what can we expect from it? Microfluidics and Nanofluidics. 2005;1:249–267. [Google Scholar]
- 25.van den Berg A, Wessling M. Nanofluidics: silicon for the perfect membrane. Nature. 2007;445:726. doi: 10.1038/445726a. [DOI] [PubMed] [Google Scholar]
- 26.Holtzel A, Tallarek U. Ionic conductance of nanopores in microscale analysis systems: where microfluidics meets nanofluidics. J Sep Sci. 2007;30:1398–1419. doi: 10.1002/jssc.200600427. [DOI] [PubMed] [Google Scholar]
- 27.Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clinical Chemistry. 2007;53:2002–2009. doi: 10.1373/clinchem.2007.090795. [DOI] [PubMed] [Google Scholar]
- 28.Toner M, Irimia D. Blood-on-a-chip. Annu Rev Biomed Eng. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng X, Irimia D, Dixon M, Sekine K, Demirci U, Zamir L, Tompkins RG, Rodriguez W, Toner M. A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip. 2007;7:170–178. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Irimia D, Dixon M, Ziperstein JC, Demirci U, Zamir L, Tompkins RG, Toner M, Rodriguez WR. A microchip approach for practical label-free CD4+ T-cell counting of HIV-infected subjects in resource-poor settings. J Acquir Immune Defic Syndr. 2007;45:257–261. doi: 10.1097/QAI.0b013e3180500303. [DOI] [PubMed] [Google Scholar]
- 31.VanDelinder V, Groisman A. Perfusion in microfluidic cross-flow: separation of white blood cells from whole blood and exchange of medium in a continuous flow. Anal Chem. 2007;79:2023–2030. doi: 10.1021/ac061659b. [DOI] [PubMed] [Google Scholar]
- 32.Kokoris M, Nabavi M, Lancaster C, Clemmens J, Maloney P, Capadanno J, Gerdes J, Battrell CF. Rare cancer cell analyzer for whole blood applications: automated nucleic acid purification in a microfluidic disposable card. Methods. 2005;37:114–119. doi: 10.1016/j.ymeth.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Helton KL, Yager P. Interfacial instabilities affect microfluidic extraction of small molecules from non-Newtonian fluids. Lab Chip. 2007;7:1581–1588. doi: 10.1039/b709585f. [DOI] [PubMed] [Google Scholar]
- 34.Chung S, Yun H, Kamm RD. Nanointerstice-driven microflow. Small. 2009;5:609–613. doi: 10.1002/smll.200800748. [DOI] [PubMed] [Google Scholar]
- 35.Martinez AW, Phillips ST, Wiley BJ, Gupta M, Whitesides GM. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip. 2008;8:2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 2003;100:14618–14622. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WG, Bang H, Yun H, Lee J, Park J, Kim JK, Chung S, Cho K, Chung C, Han DC, Chang JK. On-chip erythrocyte deformability test under optical pressure. Lab Chip. 2007;7:516–519. doi: 10.1039/b614912j. [DOI] [PubMed] [Google Scholar]
- 38.Psaltis D, Quake SR, Yang C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature. 2006;442:381–386. doi: 10.1038/nature05060. [DOI] [PubMed] [Google Scholar]
- 39.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8:98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 40.Granot Y, Ivorra A, Rubinsky B. A new concept for medical imaging centered on cellular phone technology. PLoS ONE. 2008;3:e2075. doi: 10.1371/journal.pone.0002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS ONE. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandy FF. Twenty-five years of clinical flow cytometry: AIDS accelerated global instrument distribution. Cytometry A. 2004;58:55–56. doi: 10.1002/cyto.a.10102. [DOI] [PubMed] [Google Scholar]
- 43.Fu AY, Spence C, Scherer A, Arnold FH, Quake SR. A microfabricated fluorescenceactivated cell sorter. Nat Biotechnol. 1999;17:1109–1111. doi: 10.1038/15095. [DOI] [PubMed] [Google Scholar]
- 44.Goddard GR, Sanders CK, Martin JC, Kaduchak G, Graves SW. Analytical performance of an ultrasonic particle focusing flow cytometer. Anal Chem. 2007;79:8740–8746. doi: 10.1021/ac071402t. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez WR, Christodoulides N, Floriano PN, Graham S, Mohanty S, Dixon M, Hsiang M, Peter T, Zavahir S, Thior I, Romanovicz D, Bernard B, Goodey AP, Walker BD, McDevitt JT. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med 2. 2005;2:e182. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bang H, Yun H, Lee WG, Park J, Lee J, Chung S, Cho K, Chung C, Han DC, Chang JK. Expansion channel for microchip flow cytometers. Lab Chip. 2006;6:1381–1383. doi: 10.1039/b604578b. [DOI] [PubMed] [Google Scholar]
- 47.Yun H, Min J, Lee WG, Bang H, Park J, Chung C, Chang JK, Han D-C. Microchip flowcytometer using fluorescent silica Nanoparticles for HIV Screening. 11th International Conference on Miniaturized Systems for Chemistry and Life Sciences (µTAS2007); 2007. pp. 1258–1260. [Google Scholar]
- 48.Ohashi T, Mawatari K, Sato K, Tokeshi M, Kitamori T. A micro-ELISA system for the rapid and sensitive measurement of total and specific immunoglobulin E and clinical application to allergy diagnosis. Lab Chip. 2009;9:991–995. doi: 10.1039/b815475a. [DOI] [PubMed] [Google Scholar]
- 49.Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci U S A. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sia SK, Linder V, Parviz BA, Siegel A, Whitesides GM. An integrated approach to a portable and low-cost immunoassay for resource-poor settings. Angew Chem Int Ed Engl. 2004;43:498–502. doi: 10.1002/anie.200353016. [DOI] [PubMed] [Google Scholar]
- 51.Morozov VN, Groves S, Turell MJ, Bailey C. Three minutes-long electrophoretically assisted zeptomolar microfluidic immunoassay with magnetic-beads detection. J Am Chem Soc. 2007;129:12628–12629. doi: 10.1021/ja075069m. [DOI] [PubMed] [Google Scholar]
- 52.Yacoub-George E, Hell W, Meixner L, Wenninger F, Bock K, Lindner P, Wolf H, Kloth T, Feller KA. Automated 10-channel capillary chip immunodetector for biological agents detection. Biosens Bioelectron. 2007;22:1368–1375. doi: 10.1016/j.bios.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Stevens DY, Petri CR, Osborn JL, Spicar-Mihalic P, McKenzie KG, Yager P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip. 2008;8:2038–2045. doi: 10.1039/b811158h. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann M, Delamarche E, Wolf M, Hunziker P. Modeling and optimization of highsensitivity, low-volume microfluidic-based surface immunoassays. Biomed Microdevices. 2005;7:99–110. doi: 10.1007/s10544-005-1587-y. [DOI] [PubMed] [Google Scholar]
- 55.Cho JH, Han SM, Paek EH, Cho IH, Paek SH. Plastic ELISA-on-a-chip based on sequential cross-flow chromatography. Anal Chem. 2006;78:793–800. doi: 10.1021/ac051453v. [DOI] [PubMed] [Google Scholar]
- 56.Parsa H, Chin CD, Mongkolwisetwara P, Lee BW, Wang JJ, Sia SK. Effect of volume- and time-based constraints on capture of analytes in microfluidic heterogeneous immunoassays. Lab Chip. 2008;8:2062–2070. doi: 10.1039/b813350f. [DOI] [PubMed] [Google Scholar]
- 57.Hatch A, Kamholz AE, Hawkins KR, Munson MS, Schilling EA, Weigl BH, Yager P. A rapid diffusion immunoassay in a T-sensor. Nat Biotechnol. 2001;19:461–465. doi: 10.1038/88135. [DOI] [PubMed] [Google Scholar]
- 58.Burg TP, Godin M, Knudsen SM, Shen W, Carlson G, Foster JS, Babcock K, Manalis SR. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature. 2007;446:1066–1069. doi: 10.1038/nature05741. [DOI] [PubMed] [Google Scholar]
- 59.Carter DJ, Cary RB. Lateral flow microarrays: a novel platform for rapid nucleic acid detection based on miniaturized lateral flow chromatography. Nucleic Acids Res. 2007;35:e74. doi: 10.1093/nar/gkm269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson KE, Foley JO, Yager P. Concentration gradient immunoassay. 1. An immunoassay based on interdiffusion and surface binding in a microchannel. Anal Chem. 2007;79:3542–3548. doi: 10.1021/ac062349w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu E, Chinowsky T, Nelson K, Johnston K, Edwards T, Helton K, Grow M, Miller JW, Yager P. SPR imaging-based salivary diagnostics system for the detection of small molecule analytes. Ann N Y Acad Sci. 2007;1098:335–344. doi: 10.1196/annals.1384.026. [DOI] [PubMed] [Google Scholar]
- 62.Tabak LA. Point-of-care diagnostics enter the mouth. Ann N Y Acad Sci. 2007;1098:7–14. doi: 10.1196/annals.1384.043. [DOI] [PubMed] [Google Scholar]
- 63.Ly N, Foley K, Tao N. Integrated label-free protein detection and separation in real time using confined surface plasmon resonance imaging. Anal Chem. 2007;79:2546–2551. doi: 10.1021/ac061932+. [DOI] [PubMed] [Google Scholar]
- 64.Star A, Han T-R, Joshi V, GabrieL J-CP, Grüner G. Nanoelectronic carbon dioxide sensors. Advanced Materials. 2004;16:2049–2052. [Google Scholar]
- 65.Star A, Tu E, Niemann J, Gabriel JC, Joiner CS, Valcke C. Label-free detection of DNA hybridization using carbon nanotube network field-effect transistors. Proc Natl Acad Sci U S A. 2006;103:921–926. doi: 10.1073/pnas.0504146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nettikadan SR, Johnson JC, Vengasandra SG, Muys J, Henderson E. ViriChip: a solid phase assay for detection and identification of viruses by atomic force microscopy. Nanotechnology. 2004;15:383–389. [Google Scholar]
- 67.Nettikadan SR, Johnson JC, Mosher C, Henderson E. Virus particle detection by solid phase immunocapture and atomic force microscopy. Biochem Biophys Res Commun. 2003;311:540–545. doi: 10.1016/j.bbrc.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Kim YG, Moon S, Kuritzkes DR, Demirci U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosensors and Bioelectronics. 2009;25:253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willyard C. Simpler tests for immune cells could transform AIDS care in Africa. Nat Med. 2007;13:1131. doi: 10.1038/nm1007-1131. [DOI] [PubMed] [Google Scholar]
- 70.Cohen J. Monitoring treatment: at what cost? Science. 2004;304:1936. doi: 10.1126/science.304.5679.1936. [DOI] [PubMed] [Google Scholar]
- 71.Linder V, Sia SK, Whitesides GM. Reagent-loaded cartridges for valveless and automated fluid delivery in microfluidic devices. Analytical Chemistry. 2005;77:64–71. doi: 10.1021/ac049071x. [DOI] [PubMed] [Google Scholar]
- 72.Butte MJ, Wong AP, Sharpe AH, Whitesides GM. Microfluidic device for low-cost screening of newborns for Severe Combined Immune Deficiency. Clinical Immunology. 2005;116:282. [Google Scholar]
- 73.WHO. Patient Monitoring Guidelines for HIV Care and ART. 2005 http://www.who.int/hiv/pub/guidelines/patientmonitoring.pdf.
- 74.Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- 75.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 76.Fiscus SA, Cheng B, Crowe SM, Demeter L, Jennings C, Miller V, Respess R, Stevens W. HIV-1 viral load assays for resource-limited settings. PLoS Med. 2006;3:e417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 78.WHO. Patient Monitoring Guidelines for HIV Care and Antiretroviral Therapy. 2008 http://www.who.int/hiv/
- 79.Spacek LA, Shihab HM, Lutwama F, Summerton J, Mayanja H, Ronald A, Margolick JB, Nilles TL, Quinn TC. Evaluation of a Low-Cost Method, the Guava EasyCD4 Assay, to Enumerate CD4-Positive Lymphocyte Counts in HIV-Infected Patients in the United States and Uganda , JAIDS (Journal of Acquired Immune Deficiency Syndromes) 2006;41:607–610. doi: 10.1097/01.qai.0000214807.98465.a2. [DOI] [PubMed] [Google Scholar]
- 80.Lee SH, Kim SW, Kang JY, Ahn CH. A polymer lab-on-a-chip for reverse transcription (RT)-PCR based point-of-care clinical diagnostics. Lab Chip. 2008;8:2121–2127. doi: 10.1039/b811131f. [DOI] [PubMed] [Google Scholar]
- 81.Evans JA, Adusei A, Timmann C, May J, Mack D, Agbenyega T, Horstmann RD, Frimpong E. High mortality of infant bacteraemia clinically indistinguishable from severe malaria. QJM. 2004;97:591–597. doi: 10.1093/qjmed/hch093. [DOI] [PubMed] [Google Scholar]
- 82.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rafael ME, Taylor T, Magill A, Lim YW, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved. Nature. 2006;444 Suppl 1:39–48. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- 84.Laveran CL. Classics in infectious diseases: A newly discovered parasite in the blood of patients suffering from malaria. Parasitic etiology of attacks of malaria: Charles Louis Alphonse Laveran (1845–1922) Rev Infect Dis. 1982;4:908–911. doi: 10.1093/4.4.908. [DOI] [PubMed] [Google Scholar]
- 85.Jorgensen P, Chanthap L, Rebueno A, Tsuyuoka R, Bell D. Malaria rapid diagnostic tests in tropical climates: the need for a cool chain. Am J Trop Med Hyg. 2006;74:750–754. [PubMed] [Google Scholar]
- 86.Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol. 2006;4:682–695. doi: 10.1038/nrmicro1474. [DOI] [PubMed] [Google Scholar]
- 87.Antia M, Herricks T, Rathod PK. Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathog. 2007;3:e99. doi: 10.1371/journal.ppat.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padayatchi N, Friedland G. Decentralised management of drug-resistant tuberculosis (MDRand XDR-TB) in South Africa: an alternative model of care. Int J Tuberc Lung Dis. 2008;12:978–980. [PubMed] [Google Scholar]
- 89.Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: no time for denial or complacency. PLoS Med. 2007;4:e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ridderhof JC, van Deun A, Kam KM, Narayanan PR, Aziz MA. Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull World Health Organ. 2007;85:354–359. doi: 10.2471/06.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 92.Ke C, Berney H, Mathewson A, Sheehan MM. Rapid amplification for the detection of Mycobacterium tuberculosis using a non-contact heating method in a silicon microreactor based thermal cycler. Sensors and Actuators B. 2004;102:308–314. [Google Scholar]
- 93.Lee H, Sun E, Ham D, Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat Med. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peeling RW, Smith PG, Bossuyt PM. A guide for diagnostic evaluations. Nat Rev Microbiol. 2006;4:S2–S6. doi: 10.1038/nrmicro1522. [DOI] [PubMed] [Google Scholar]
- 95.WHO. Regulation of in vitro diagnostics: a global perspective. http://www.who.int/tdrold/publications/publications/pdf/tbdi/tbdi_annex.pdf.
- 96.Weigl B, Domingo G, Labarre P, Gerlach J. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip. 2008;6:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]