Abstract
Epidemiological and experimental studies provide supportive evidence that lycopene (LY), a major carotenoid from tomatoes and tomato products, may act as a chemopreventive agent against certain types of cancers. We recently showed that high-fat diet (HFD)-induced nonalcoholic steatohepatitis (NASH) promoted diethylnitrosamine (DEN)-initiated hepatocarcinogenesis in a rat model. Using this model, we investigated the efficacy of an equivalent dosage of dietary LY from either a pure compound or a tomato extract (TE) against NASH-promoted hepatocarcinogenesis. Six groups of rats were injected with DEN and then fed either Lieber-DeCarli control diet or HFD with or without LY or TE for 6 weeks. Results showed that both LY and TE supplementations significantly decreased the number of altered hepatic foci expressing the placental form of glutathione-S transferase in the livers of HFD-fed rats. This was associated with significantly lower proliferating cell nuclear antigen positive hepatocytes and cyclinD1 protein, as well as decreased activation of ERK and nuclear NF-κB. Although both LY and TE supplementations reduced HFD-induced lipid peroxidation in the livers, we observed significantly decreased cytochrome P450 2E1, inflammatory foci and mRNA expression of proinflammatory cytokines (TNF-α, IL-1β and IL-12) in the HFD+TE fed group but increased nuclear NF-E2-related factor-2 and heme oxygenase-1 proteins in the HFD+LY fed group, relative to HFD feeding alone. These data indicate that LY and TE can inhibit NASH-promoted hepatocarcinogenesis mainly as a result of reduced oxidative stress, which could be fulfilled through different mechanisms.
Keywords: lycopene, tomato extract, hepatocarcinogenesis, nonalcoholic steatohepatitis
INTRODUCTION
Primary liver cancer has become the fifth commonest malignancy worldwide, accounting for 5.6% of all human cancers 1. Although surgical resection is the major therapeutic option, the lack of early detection or screening biomarkers usually leads to its diagnosis at an advanced stage. Thus, the identification of potential risk factors for early hepatocarcinogenesis and the exploration of preventive or protective measures against them at an early stage are immensely needed. Over the past decade, growing evidence has revealed a close association between hepatocellular carcinoma and nonalcoholic steatohepatitis (NASH), a chronic and progressive liver disease characterized by the concurrence of fat accumulation and infiltration of inflammatory cells in the liver 2–4. Moreover, patients with NASH have a high risk for proceeding to cirrhosis 5 and eventually hepatocellular carcinoma. Recently, we provided experimental evidence that the altered hepatic foci (AHF) expressing placental form of glutathione-S transferase (P-GST), as initiated by hepatic carcinogen diethylnitrosamine (DEN), were significantly increased in the context of high-fat diet induced NASH 6, in which increased oxidative stress could act as a key component contributing to this preneoplastic process. Since NASH has become one of the most common causes of advanced liver diseases in both developed and developing countries 7 and the effective treatment options for NASH patients are still lacking, it is necessary and more attractive to explore effective preventive measures especially from nutritional means.
Lycopene (LY), a non-provitamin A carotenoid, is the most prevalent carotenoid in the western diet and has been reported to possess a strong antioxidant potency 8–10. Recent epidemiological and experimental studies also provide supportive evidence that LY may act as a chemopreventive agent against certain types of cancers such as prostate cancer, gastric cancer, breast cancer and lung cancer 11–13. Although experimental evidence from in vitro studies has suggested a beneficial effect of LY against hepatocarcinogenesis 14–15, data from in vivo studies are inconsistent. For example, administration of LY (300 mg/kg diet) to male rats significantly decreased the size of diethylnitrosamine (DEN)-induced AHF by 65% 16; similarly, fewer P-GST positive hepatic preneoplastic lesions were found in rats after giving them 70 mg LY/kg BW on alternate days for 8 weeks 17. In contrast, LY supplementation (50 mg/kg diet) for 70 weeks did not affect the mean size of AHF in a rat model with spontaneous development of liver cancer 18. These discrepant observations could be related with different models or lycopene dosages used in these studies. However, it is difficult to reconcile these discrepant results, in particular, due to the lack of information regarding hepatic concentrations of LY in these in vivo studies. Moreover, no investigation has been conducted to evaluate whether lycopene can inhibit NASH-promoted hepatocarcinogenesis. Furthermore, a comparative investigation using prostate cancer model found a significantly lower risk of death from prostate cancer in rats fed tomato powder but little effect from pure LY in rats 19, which suggests that LY itself might not be as effective against carcinogenic process as tomatoes, which contains not only lycopene but also other antioxidant nutrients and phytochemicals. Clearly, more evidence supporting this hypothesis and exploration of the possible mechanisms involved are also needed.
In the present study, using our established rat model with precancerous lesions developing in the context of NASH 6, we investigated the efficacy of supplementing an equivalent dosage of dietary LY from either purified LY or tomato extract (TE) on the development of NASH-promoted AHF development and explored the underlying mechanisms involved.
MATERIALS AND METHODS
Animals, diets and groups
The maintenance and husbandry of rats as well as the procedure of the in vivo study were described in detail elsewhere 6. Briefly, eight-week old male Sprague-Dawley rats (Charles River Co., Wilmington, MA) were given a single i.p. injection of 30 mg DEN/kg body weight. After 3-day recovery from DEN injection, all rats were randomly assigned to 6 groups (n=8) and fed ad libitum either Lieber-DeCarli control diet (CD, 35% energy from fat) or HFD (71% energy from fat) with or without pure lycopene or tomato extract supplements for 6 weeks (Dyets Inc., Bethlehem, PA). The formulas of both diets have been described before 20. The detailed grouping for rats are as follows: 1) CD; 2) CD+LY; 3) CD+TE; 4) HFD; 5) HFD+LY; and 6) HFD+TE. LY and TE were weighed and mixed evenly with the liquid diet. Animal growth and daily dietary intake were closely monitored and body weight was measured weekly. After killing, the liver was promptly excised. Two small portions of the right lobe of the liver were fixed in 10% neutral buffered formalin for histological examination, and the remaining liver was snap frozen in liquid nitrogen and stored at −80°C for subsequent analysis. The Institutional Animal Care and Use Committee at the USDA Human Nutrition Research Center on Aging, Tufts University, approved the animal protocol.
Dietary supplements
Pure LY (BASF Chemical Company, Germany), as 10% (wt: wt) water-soluble beadlets, contained 95% all-trans isomers by HPLC analysis. Based on the previous study in our lab showing a poor absorption of LY in rats from the liquid diet 21, a higher LY dosage (15 mg LY/kg BW per day) was used in this study to reach a physiological relevant concentration of lycopene, as seen in humans. A TE containing 6% lycopene as well as other natural tomato phytonutrients (~1.5% natural tocopherols, ~1% phytoene & phytofluene and ~0.2% beta-carotene) was kindly provided by LycoRed Ltd. (Beer-Sheva, Israel). The dosage of TE (250 mg TE/kg BW per day) was chosen to contain an equivalent amount of lycopene to that from pure LY used (15 mg LY/kg BW per day). The LY and TE supplements for each group were weighed and homogenized with liquid diet that was shown to be sufficiently consumed by rats from our previous studies 6, 22. The diets were prepared twice a week and were stored at −4°C in opaque bottles to prevent degradation of the lycopene.
HPLC analysis
The HPLC analysis for liver and plasma concentrations of lycopene were described in our previous publications 23. Briefly, a separation system (Waters Corporate, Milford, MA) fitted with a C30 column was used to separate lycopene. Liver tissue (100 mg) or plasma (1 ml) was homogenized in 3 ml of saline and ethanol (1:2 ratio). Lycopene was then extracted from the samples using 5 ml of hexane and ether (1:1 ratio) by vortexing for 3 min, centrifuging at 20003g for 10 min at 4°C, and collecting the upper layer. Samples were extracted 3 times and were evaporated under nitrogen gas, after which they were reconstituted with 100 ml of ethanol and ether (2:1, v:v). A 50-ml sample of the final extract was injected into the system to measure lycopene concentrations. Echinenone was used as the internal control to determine the efficiency of extraction. All procedures were conducted under red light.
Immunohistochemical assay
AHF expressing P-GST and proliferating cell nuclear antigen (PCNA) positive hepatocytes were detected and analyzed by immunohistochemical assay, as described previously 6.
Histological examination of inflammatory foci
Formalin-fixed and paraffin-embedded liver tissues were processed routinely for hematoxylin and eosin (H&E) staining. The numbers of hepatic lobular inflammatory foci were counted at 20× magnification from 10 randomly selected fields under light microscope by two independent investigators, as previously described 21. The mean number of the foci for each group was determined and then compared.
Lipid peroxidation assay
Lipid peroxidation end products malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) in the liver were assessed by colorimetric assay (Oxford Biochemical Research Inc, Oxford, MI) in the liver as reported previously 6.
Gene expression by Real-time PCR
Total RNA was isolated from the liver by TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN) according to the instructions. cDNA was then prepared from the RNA samples using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and an automated thermal cycler (Bio-Rad Laboratories, Hercules, CA). PCR reaction was carried out as described previously 6. Gene expression was analyzed using the following pairs of primers: TNF-α (forward, 5′-CCAGACCCTCACACTCAGATCA-3′; reverse, 5′-TCCGCTTGGTGGTTTGCTA-3′), IL1β (forward, 5′-CTCTCCAGTCAGGCTTCCTTGT-3′ reverse, 5′-CAGGTCATTCTCCTCACTGTCG-3′), IL12 (forward, 5′-ACATCATCAAACCGGACCCA-3′ reverse, 5′-TGCGGACGAAGAACTTGAGG-3′) and GAPDH (forward, AGTGCCAGCCTCGTCTCATAG; reverse, CCTTGACTGTGCCGTTGAACT).
Western Blotting
Liver homogenates and nuclear proteins were prepared from liver samples as previously described 24. Primary antibodies against total and phosphorylated ERK1/2, JNK, cleaved caspase-3, (Cell Signaling Technology, Inc, MA), cyclinD1 and nuclear Nrf2, NF-κB p65 subunit (Santa Cruz Biotechnology, Inc, CA), CYP2E1 (Chemicon International, Temecula, CA), heme oxygenase (HO-1) (Stressgen Biotechnologies Inc. MI) were applied. Anti-GAPDH antibody (Chemicon International, Inc, CA) was used as the internal control for equal loading of proteins.
Statistical analysis
Two-way ANOVA followed by a Tukey-Kramer post-hoc analysis was performed for all continuous variables. Cochran-Mantel-Haenszel followed by Fisher’s exact test was applied for AHF incidence. Kruskal-Wallis overall test was used and followed by Wilcoxon rank-sum test to compare the number of AHF and inflammatory foci. Values were presented as mean ± SEM. A difference was considered to be significant at P < 0.05.
RESULTS
General appearance and lycopene concentrations in plasma and liver
Six-week supplementation of LY or TE did not cause any adverse effects on animal growth or food intake. The body weight and liver weight showed no significant changes among all 6 groups (Table 1). No lycopene was detected in the non-supplemental CD and HFD groups. The mean lycopene concentration in the liver from the HFD-fed groups (HFD+LY, HFD+TE) was significantly greater than that from the CD-fed groups (CD+LY, CD+TE) (P < 0.05), while no significant difference was found between the HFD+LY and HFD+TE fed groups or between the CD+LY and CD+TE fed groups. In contrast, plasma lycopene concentration did not show any significant difference between either different diets (CD vs. HFD) or different supplements (LY vs. TE).
Table 1.
Body weight, liver weight and LY concentrations in plasma and liver
| Body weight (g) |
Liver weight (g) | Lycopene concentration |
|||
|---|---|---|---|---|---|
| Initial | Final | Plasma (nmol/mL) | Liver (nmol/g tissue) | ||
| CD | 229.5 ± 25.1 | 472.3 ± 28.3 | 13.5 ± 2.5 | -- | -- |
| CD+LY | 232.7 ± 15.6 | 474.7 ± 18.5 | 13.5 ± 2.3 | 0.09 ± 0.02 | 7.5 ± 1.0 |
| CD+TE | 234.2 ± 14.4 | 485.1 ± 20.7 | 13.8 ± 1.3 | 0.11 ± 0.03 | 8.3 ± 1.5 |
| HFD | 229.0 ± 27.4 | 479.8 ± 25.4 | 13.7 ± 1.1 | -- | -- |
| HFD+LY | 229.4 ± 26.5 | 473.0 ± 22.2 | 14.7 ± 1.0 | 0.11 ± 0.02 | 17.6 ± 1.5 * |
| HFD+TE | 231.7 ± 20.7 | 493.8 ± 25.9 | 14.9 ± 2.1 | 0.14 ± 0.05 | 14.1 ± 1.4 * |
Values are presented as means ± SEM, n=8.
By two-way ANOVA analysis, liver LY concentration was significantly higher in the HFD-fed groups than in the CD-fed groups (P < 0.0001); no significant difference was found between LY and TE supplemented groups in each dietary category (P = 0.3318).
Precancerous lesions and cell growth
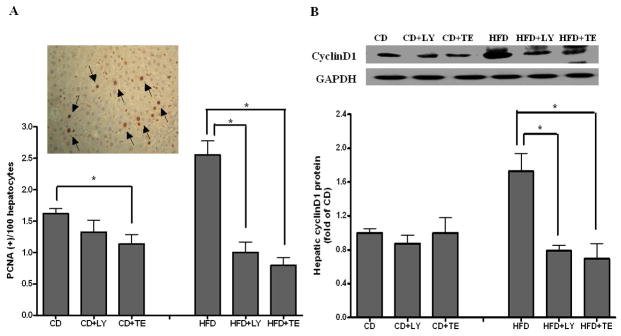
Both TE and LY showed no effect on the incidence of P-GST positive AHF although a decreasing trend was observed between HFD and HFD+LY or HFD+TE fed groups (5/8 vs. 1/8) (Table 2). However, the number of AHF was significantly inhibited up to 80% by dietary supplementations of both LY and TE fed groups as compared with that of HFD alone (P < 0.01). The increase in PCNA positive hepatocytes, as in the HFD group, was significantly prevented by supplementation with either LY or TE to a similar extent (Figure 1A). Fewer PCNA positive hepatocytes were also observed in the CD+TE than that in the CD alone fed group (P < 0.05) (Figure 1A). The decreased PCNA expressions in the HFD+LY and HFD+TE fed groups were associated with significant decreases of hepatic cyclinD1 protein concentrations in both groups as compared with the HFD alone fed group (Figure 1B). We did not detect any significant changes in cleaved caspase-3 among all of the groups (data not shown).
Table 2.
Incidence and multiplicity of P-GST positive AHF (n=8/group)
| P-GST (+) altered hepatic foci |
||
|---|---|---|
| Group | Incidence1 | Multiplicity2 |
| CD | 1/8 | 0.10 ± 0.03 |
| CD+LY | 1/8 | 0.07 ± 0.02 |
| CD+TE | 1/8 | 0.15 ± 0.07 |
| HFD | 5/8 | 1.25 ± 0.05b |
| HFD+LY | 1/8 | 0.17 ± 0.06a |
| HFD+TE | 1/8 | 0.16 ± 0.04a |
The overall P = 0.83 indicating no significant difference among all groups by using Cochran-Mantel-Haenszel Statistic test
Values are presented as means ± SEM, n=8. Kruskal-Wallis overall test followed by Wilcoxon rank-sum test was used for multiple comparisons within either CD-fed groups or HFD-fed groups; values bearing different letter superscripts were significantly different (P < 0.01)
FIGURE 1.
Assessment of Cell proliferation. (A) Hepatocytes expressing PCNA were detected by immunohistochemical assay and then counted from 20 randomly selected fields at ×200 magnification (Insert: typical picture of PCNA positive hepatocytes). (B) Hepatic cyclin D1 protein concentration was measured from whole liver homogenates by using Western blotting. Bars represent means ± SEM, n = 8. * P < 0.05 between groups.
Assessment of hepatic inflammation
HFD feeding resulted in a remarkable presence of inflammatory foci in the liver as compared with CD feeding. These HFD-induced inflammatory foci were significantly reduced by supplementation of TE (> 40% reduction) but not by LY (Figure 2A). No changes of inflammatory foci numbers were observed among all CD-fed groups. Further analysis of mRNA expressions of inflammatory cytokines in the livers found that the induction of TNF-α, IL-1β and IL-12 by HFD feeding was reduced by TE supplementation at a significant level, while no effect was observed from LY supplemented with HFD (Figure 2B-D). Hepatic TNF-α was also significantly increased in both CD+LY and CD+TE groups as compared with that in CD alone (Figure 2B).
FIGURE 2.
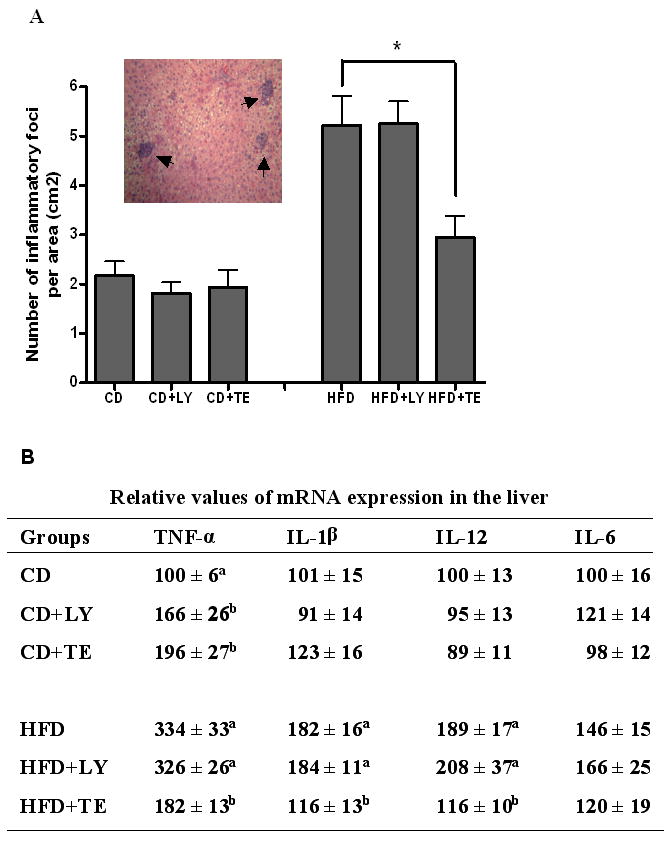
Hepatic inflammation. (A) The number of inflammatory foci was counted in 20 randomly selected fields at ×200 magnification and expressed as average number per area (cm2) (Insert: typical picture of inflammatory foci with clustering of mainly mononuclear cells). (B) The mRNA expression of multiple proinflammatory cytokines in liver after real-time PCR analysis. Quantification of transcript was relative to the GAPDH mRNA levels. Bars represent means ± SEM, n = 8. * P < 0.05 between groups.
Assessment of oxidative stress
Dietary supplementation of either LY or TE significantly decreased HFD induced generation of MDA plus 4-HNE (Figure 3A). Hepatic CYP2E1 protein concentration was decreased by more than 50% in HFD+TE, but not in HFD+LY group, as compared with that of HFD alone (Figure 3B). In contrast, there was a 2.4-fold increase of nuclear protein concentration of NF-E2-related factor-2 (Nrf2) in HFD+LY group but not in HFD+TE group, as compared with HFD alone group (Figure 3C). The increase of Nrf2 in HFD+LY group was also associated with higher expression of hepatic heme oxygenase (HO)-1 protein (Figure 3D). A similarly higher nuclear Nrf2 was also found in CD+LY group but not in CD+TE group, as compared with that in CD alone.
FIGURE 3.
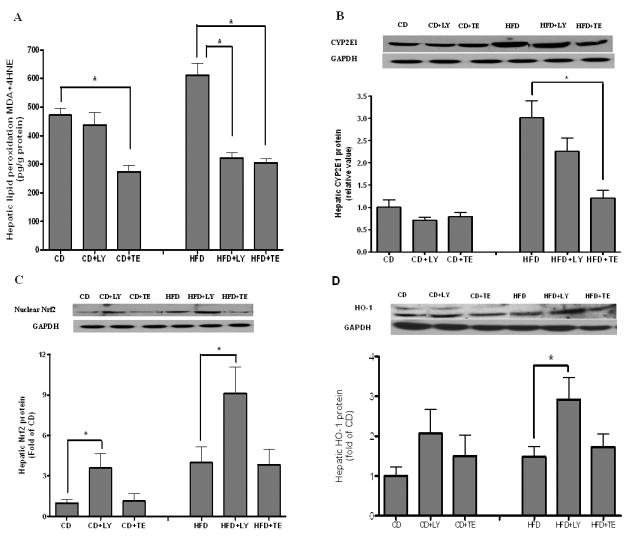
Oxidative stress and regulatory mechanisms. (A) End products of lipid peroxidation (MDA plus 4-HNE) were measured by using the LPO 586 assay kit; (B–D) Hepatic protein concentrations of CYP2E1, nuclear Nrf2 and HO-1 were measured by using Western blotting. Bars represent means ± SEM, n = 8. * P < 0.05 between groups.
Intracellular signals for cell growth
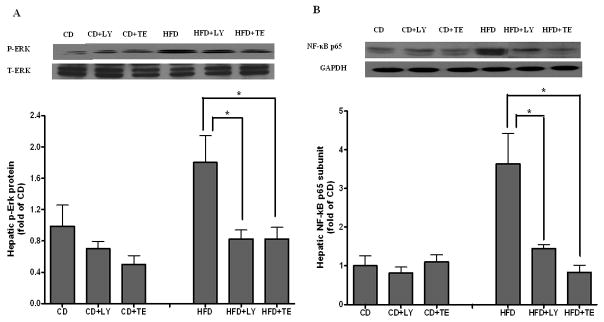
The protein concentrations of total and phosphorylated forms of extracellular signal-regulated kinase (ERK) 1/2 and c-Jun N-terminal kinase (JNK) were measured in the liver. Although no change was found for the total ERK1/2 protein levels, phosphorylated ERK1/2 induced by HFD feeding was significantly reduced by dietary supplementation of either LY or TE (Figure 4A). Neither total nor phosphorylated JNK showed significant changes among all 6 groups (data not shown). In addition, the nuclear concentration of NF-κB p65 subunit induced by HFD (3.1-fold of CD) in the liver was significantly decreased by both LY and TE supplementation (Figure 4B).
FIGURE 4.
ERK and NF-κB activation. (A) Both total ERK and phosphorylated ERK in liver were measured by western blotting with use of specific antibodies. The values represent a relative ratio between the phosphorylated form and the total form. (B) The nuclear concentration of active p65 subunit of NF-κB was measured from liver nuclear extracts by using Western blotting. Bars represent means ± SEM, n = 8. * P < 0.05 between groups.
DISCUSSION
This study clearly demonstrates that both LY and TE containing LY can significantly inhibit DEN-initiated and NASH-promoted early hepatocarcinogenesis. In the present study, LY dosage from either pure LY or TE was well tolerated in our rat model. The LY concentrations in both plasma (0.09~0.14 μM) and liver (7.5~17.6 nmol/g) in the rats were within the range normally seen in humans (i.e. 0.1–0.9 μM in plasma and 0.1–20 nmol/g in liver, respectively) 25, indicating that its effect in this model took place at physiologically relevant concentrations. The higher hepatic LY concentration from all HFD-fed groups than that from CD-related groups could be due to the absorption of lipophylic LY, which involves incorporation into micelles along with fat from the diet 26. Since the rest ingredient of the lycopene beadlet is mainly starch carrier and contains no other functional components, the similar hepatic LY concentrations between LY and TE fed groups allow us to investigate potential functional differences between LY and TE. In addition, plasma LY showed no significant difference among all groups with the supplementation. This could be related to the overnight fasting of the animals since previous study found that the peak accumulation of LY in plasma in rats occurs between 4 to 8 hours after a single gavage 27.
In the present study, dietary supplementations of LY or TE showed a trend toward lower incidence of P-GST positive AHF but this change did not reach a statistical significance. One possibility is that the sample size per group was not large enough to detect a potential difference among the treatments. Another explanation is that both supplements were administered to rats three days after DEN injection, which may make it difficult to see inhibition on DEN mediated cell initiation process. However, the average number of P-GST positive AHF was significantly reduced in HFD+LY and HFD+TE groups as compared with that in HFD alone, suggesting an anti-carcinogenic effect on the tumor promotion stage by both supplements. The mechanistic link between LY and its anti-carcinogenic potential has been proposed to be via multiple mechanisms depending on the specific model used, such as inhibition of cell proliferation, induction of apoptosis and promotion of gap junction communication 13. The lower expression of PCNA and cyclinD1 protein in the liver of rats from either HFD+TE or HFD+LY group as compared with HFD alone suggests that an inhibitory effect on NASH-promoted cellular proliferation, which could contribute to their anti-tumorigenesis activity. Although LY has been reported to induce cell apoptosis in some cancer cell lines 28 and attenuate alcoholic apoptosis in HepG2 cells 29, we did not find an effect on cell apoptosis as evidenced by no significant changes of cleaved caspase-3 by either LY or TE treatment. This observation is in agreement with our previous results that LY supplementation restores down-regulated apoptosis to normal levels in the lung of smoke exposed ferrets23.
Increased oxidative stress, the major “second hit” for NASH pathogenesis, can elicit a wide spectrum of cellular damages and intracellular signaling changes that has been associated with hepatocarcinogenesis 30. The association between decreased lipid peroxidation and reduced foci growth in HFD+LY and HFD+TE suggests that the antioxidant efficacies exhibited by LY and TE are responsible for their protection against carcinogenesis. LY has been demonstrated to be a potent antioxidant from in vitro 31 and in vivo studies 32. However, the inhibition of LY and TE on HFD-induced oxidative stress in our model seems to be mediated via different mechanisms. LY was found to show little effect on HFD-induced CYP2E1 protein expression, the essential enzyme for free radical generation in NASH, whereas CYP2E1 protein was remarkably inhibited by TE supplementation with HFD. Since TE contained other components (e.g., tocopherols, phytoene and phytofluene), it seems that these additional components other than lycopene in TE may contribute to this benefit. For example, a previous study showed that tocopherol, which is contained in TE, inactivated CYP2E1 in the liver 33. Clearly, whether the inhibition of CYP2E1 by TE is due to a combined or synergistic effect of multiple constituents in tomatoes needs further investigation.
On the other hand, the inhibition of lipid peroxidation without an effect on CYP2E1 by LY supplementation also suggests the presence of alternative mechanisms. It has been shown that one of the chemopreventive effects of LY is its ability to induce antioxidant enzymes 34–36 that results in neutralization of reactive oxygen species (ROS). The transcription factor Nrf2 (nuclear factor E2-related factor 2), a key regulator of the cellular response to oxidative stress in multiple tissue and cell types, is a primary factor involved in induction of antioxidant/detoxifying enzymes. Nrf2 has been reported to be induced by various natural antioxidant and chemopreventive agents including lycopene resulting in the trans-activation of multiple antioxidant enzymes once it translocates from the cytosol to the nucleus 36. Heme oxygenase (HO-1), one of the major targets of Nrf2 regulation, is a rate-limiting enzyme in the degradation of heme to produce bilirubin, which behaves as a potent antioxidant by scavenging free radicals. In our model, both nuclear Nrf2 and HO-1 protein concentrations were significantly induced by LY supplementation with HFD relative to HFD alone, suggesting that LY could mediate its protective effects against carcinogenesis by upregulating inherent antioxidant systems to neutralize excess free radicals. This is supported by previous observations of increased nuclear Nrf2 and its regulated antioxidant/enzymes including HO-1 by lycopene or its metabolites 37, 38. We also observed increased levels of nuclear Nrf2 from HFD alone group as compared with CD, which is in agreement with previous findings that Nrf2 is activated in rodent models of chemical-initiated hepatotoxicity and hepatic carcinogenesis 39, 40. It seems that increased nuclear Nrf2 by HFD feeding acts as an adaptive response to increased oxidative stress, but is not sufficient to counteract the generation of free radicals. It is noteworthy that supplementation of TE, which contains an equivalent amount of LY, showed no effect on Nrf2 activation. We speculate that since increased hepatic CYP2E1 has been closely associated with upregulation of Nrf2 mRNA and protein expression as a result of ROS generation 41, 42, the inhibition of CYP2E1 and its mediated oxidative stress by TE at the first place may make it no need for Nrf2 activation in the absence of extra oxidant stimulus. The exact mechanism underlying this phenomenon may need further investigation.
It should be pointed out that there was a discrepant observation of hepatic CYP2E1 in response to LY treatment between this study and our previous study 21, in which we found that the co-administration of alcohol and LY, even at a dosage less than that of the present study, significantly increased hepatic CYP2E1 protein in the alcohol-fed rats, suggesting a need for caution among individuals consuming both alcohol and LY. Since we did not observe any effect of LY on hepatic CYP2E1 expression in the rats fed with HFD in the present study, therefore, the discrepancy could be due to the interactions of LY supplementation with heavy alcoholic intake. For example, we have detected several unidentified polar metabolites of lycopene in the livers of the rats fed with excessive alcohol consumption (unpublished data) but not in the livers of rats fed with HFD, suggesting that excess formation of lycopene metabolites by alcohol may affect hepatic CYP2E1 expression. This area may need further investigation.
One of the interesting findings in this study is that TE, not LY, displayed an efficacy to protect against HFD-induced liver inflammation by decreasing both the number of inflammatory foci and mRNA expression of multiple proinflammatory cytokines. Although the potential mechanism underlying the anti-inflammatory efficacy manifested by TE, possible due to a combined or synergistic effect of multiple constituents in tomatoes, this function does not enable us to see a more potent inhibition on AHF by TE supplement than that by pure lycopene in this animal model. Since previous evidence has shown that the inflammatory cytokines can mediate its cytotoxicity by causing mitochondria dysfunction and exaggerating the generation of free radicals 43, we hypothesized that the HFD-induced inflammation in NASH could promote AHF development via an oxidant-dependent manner. This speculation was further supported by the similar inhibitions of Erk and NF-κB activation by dietary LY or TE supplement. It has been shown that both Erk and NF-κB can be activated in response to the increased oxidative stress 44 and promote the mutagenic growth in hepatocarcinogenesis 45, 46. These data support our notion that the antioxidant efficacy manifested by either LY or TE may account for their major benefits against NASH-promoted early carcinogenesis in this model.
Acknowledgments
The authors thank Dr. Donald E. Smith for his assistance on animal care and handling.
Funding
The work was supported by NIH grant R01CA104932, Tufts Cancer Center pilot project award 004-08PP and US Department of Agriculture grant 1950-51000-064S. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of National Institute of Health and the U.S. Department of Agriculture.
Abbreviations used are
- CD
control diet
- DEN
diethylnitrosamine
- ERK
extracellular signal-regulated kinase
- 4-HNE
4-hydroxynonenal
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- HFD
high-fat diet
- HO-1
heme oxygenase-1
- JNK
c-Jun N-terminal kinase
- MDA
malondialdehyde
- NASH
nonalcoholic steatohepatitis
- Nrf2
NF-E2-related factor 2
- P-GST
placental form of glutathione S-transferase
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of interest statements
Author Disclosures: Yan Wang, Lynne M. Ausman, Andrew S. Greenberg, Robert M. Russell and Xiang-Dong Wang have no conflicts of interest.
References
- 1.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Mori S, Yamasaki T, Sakaida I, Takami T, Sakaguchi E, Kimura T, Kurokawa F, Maeyama S, Okita K. Hepatocellular carcinoma with nonalcoholic steatohepatitis. J Gastroenterol. 2004;39:391–6. doi: 10.1007/s00535-003-1308-3. [DOI] [PubMed] [Google Scholar]
- 3.Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–60. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–31. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 5.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–6. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;12:1048–58. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 8.Unlu NZ, Bohn T, Francis DM, Nagaraja HN, Clinton SK, Schwartz SJ. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br J Nutr. 2007;98:140–6. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 9.Britton G. Carotenoids 1: structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. [PubMed] [Google Scholar]
- 10.Krinsky NI. Mechanisms of action of biological antioxidants. Proc Soc Exp Biol Med. 1992;200:248–254. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- 11.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–41. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 13.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implication for cancer prevention. Nutr Rev. 2008;66:667–83. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B human hepatoma cells. Biofactors. 2005;23:129–39. doi: 10.1002/biof.5520230302. [DOI] [PubMed] [Google Scholar]
- 15.Burgess LC, Rice E, Fischer T, Seekins JR, Burgess TP, Sticka SJ, Klatt K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range. Toxicol In Vitro. 2008;22:1297–300. doi: 10.1016/j.tiv.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astorg P, Gradelet S, Berges R, Suschetet M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr Cancer. 1997;29:60–8. doi: 10.1080/01635589709514603. [DOI] [PubMed] [Google Scholar]
- 17.Toledo LP, Ong TP, Pinho AL, Jordao A, Jr, Vanucchi H, Moreno FS. Inhibitory effects of lutein and lycopene on placental glutathione S-transferase-positive preneoplastic lesions and DNA strand breakage induced in Wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr Cancer. 2003;47:62–9. doi: 10.1207/s15327914nc4701_8. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Kitade Y, Masaki T, Nishioka M, Satoh K, Nishino H. Effects of lycopene and Sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutr Cancer. 2001;39:96–101. doi: 10.1207/S15327914nc391_13. [DOI] [PubMed] [Google Scholar]
- 19.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 20.Lieber Charles S, Leo Maria A, et al. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–9. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 21.Veeramachaneni S, Ausman LM, Choi SW, Russell RM, Wang XD. High dose lycopene supplementation increase hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J Nutr. 2008;138:1329–35. doi: 10.1093/jn/138.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr. 2008;138:1866–71. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induce apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferret. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 24.Chung J, Chavez PR, Russell RM, Wang XD. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene. 2002;21:1539–47. doi: 10.1038/sj.onc.1205023. [DOI] [PubMed] [Google Scholar]
- 25.Boileau TW, Boileau AC, Erdman JW. Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood) 2002;227:914–9. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 26.Van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000;130:503–6. doi: 10.1093/jn/130.3.503. [DOI] [PubMed] [Google Scholar]
- 27.Mathews-Roth MM, Welankiwar S, Sehgal PK, Lausen NGC, Russett M, Krinsky NI. Distribution of [14C] canthaxanthin and [14C] lycopene in rats and monkeys. J Nutr. 1990;120:1205–13. doi: 10.1093/jn/120.10.1205. [DOI] [PubMed] [Google Scholar]
- 28.Hantz HL, Young LF, Martin KR. Physiologically attainable concentration of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med. 2005;230:171–9. doi: 10.1177/153537020523000303. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Leo MA, Lieber CS. Lycopene attenuates alcoholic apoptosis in HepG2 cells expressing CYP2E1. Biochem Biophys Res Commun. 2003;308:614–8. doi: 10.1016/s0006-291x(03)01435-9. [DOI] [PubMed] [Google Scholar]
- 30.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 31.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–8. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 32.Moselhy SS, Al Mslmani MA. Chemopreventive effect of lycopene alone or with melatonin against the genesis of oxidative stress and mammary tumors induced by 7,12 dimethyl(a)benzanthracene in sprague dawely female rats. Mol Cell Biochem. 2008;319:175–80. doi: 10.1007/s11010-008-9890-6. [DOI] [PubMed] [Google Scholar]
- 33.Tirmenstein MA, Ge X, Elkins CR, Fariss MW. Administration of the tris salt of alpha-tocopheryl hemisuccinate inactivates CYP2E1, enhances microsomal alpha-tocopherol levels and protects against carbon tetrachloride-induced hepatotoxicity. Free Radic Biol Med. 1999;26:825–35. doi: 10.1016/s0891-5849(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 34.Breinholt V, Lauridsen ST, Daneshvar B, Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000;154:201–10. doi: 10.1016/s0304-3835(00)00401-8. [DOI] [PubMed] [Google Scholar]
- 35.Velmurugan B, Bhuvaneswari V, Burra UK, Nagini S. Prevention of N-methyl-N0-nitro-N-nitrosoguanidine and saturated sodium chlorideinduced gastric carcinogenesis in Wistar rats by lycopene. Eur J Cancer Prev. 2002;11:19–26. doi: 10.1097/00008469-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–86. [PubMed] [Google Scholar]
- 38.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–8. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–6. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 41.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16:992–6. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 42.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–53. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 43.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–6. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 44.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling fro suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 45.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72:30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 46.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]