Abstract
Fatty acids stored as triglycerides (TG) in the fat body serve as precursor in multiple processes including energy production and synthesis of cellular components. Mobilization of fatty acids from TG depends on the action of lipases. The fat body triglyceride lipase from Manduca sexta, MsTGL, is the only insect lipase that has been purified and characterized, so far. A TGL cDNA from M. sexta fat body encoding a 649 amino acid protein was cloned and its identity confirmed by mass spectrometry and Edman sequencing data of the purified protein. The protein sequence has conserved domains and residues of potential importance for the function and regulation of TGL activity.
The expression of TGL and the lipase activity of fat body homogenates were studied in several developmental stages of M. sexta. TG hydrolase activity of fat body increased as larva grew to the last instar and, then, decreased to minimal levels during pupa stage. Lipase activity was progressively restored in adult insects and reached maximum values at this stage. The fat body lipase activity from adult insects, 1–2 day after emergence, was 9-fold higher than that from 2–3 days old 5th-instar larvae. A good correlation was found between the abundance of TGL protein and the lipase activity of fat body homogenates. This correlation and the expression pattern of TGL throughout development are consistent with the notion that TGL is the main fat body TG lipase of M. sexta.
Keywords: Lipase, TG-lipase, TGL, AKH, Fat body, Lipolysis, Manduca sexta, adipocytes
INTRODUCTION
Neutral lipids in the form of triglycerides (TG) are the predominant form of storage of fatty acids and comprise the main energy reserve in all animals (Wolins et al. 2006). Insects accumulate TG as lipid droplets (LDs) within the cytoplasm of fat body cells during feeding periods, and rely on these reserves to support the energy requirements associated to non-feeding periods, sustained flight and embryo development (Beenakkers et al. 1985, Canavoso et al. 2001, Arrese and Soulages 2010). Storage of fatty acids is essential in insects for other functions as well. Fatty acids serve as precursors in the synthesis of waxes and pheromones, and as components of cuticular lipids in addition to participate in phospholipid synthesis for membrane biogenesis and synthesis of eicosanoids (Downer et al 1976)
Mobilization of TG stores from LDs is catalyzed by lipases. The fat body of M. sexta has a cytosolic lipase (TGL) that represents the only insect TG-hydrolase purified and characterized (Arrese and Wells 1994). TGL is a polypeptide with a relative mass of 74-76kDa that has been identified as the homolog of D. melanogaster CG8552 (Arrese et al. 2006). In addition to the TG hydrolase activity, TGL is also a phospholipase (type A1) with the ability to hydrolyze the phospholipids of the outer layer of the LDs (Arrese et al. 2006). This activity is expected to facilitate the access of TGL to the core of the LDs where TG molecules localize. TG hydrolysis necessarily involves the interaction of the lipase with the lipid droplet. However, TGL does not achieve a tight association with the lipid droplets and experimentally is only found in the cytosol regardless of the lipolytic condition (Patel et al. 2005).
Lipolysis is under hormonal regulation by the neuropeptide adipokinetic hormone (AKH) (Gade and Auerswald 2003), which elicits a glucagon-like action mediated by a G protein-coupled receptor that activates both inositol phosphate and cAMP signaling responses (Gade et al. 1997; Staubli et al. 2002; Van der Horst et al. 2001). Studies in the locust fat body showed that cAMP and/or calcium are involved in mediating the action of AKH mobilizing lipids (Lum and Chino 1990; Spencer and Candy 1976; Wang et al. 1990). In adult M. sexta the lipolytic response induced by AKH is associated with a rapid activation of cAMP-dependent protein kinase A (PKA) and a sustained increase in calcium influx (Arrese et al. 1999). A recent study on Bombyx mori AKH receptor showed that when the receptor was expressed in HEK293 cells the intracellular levels of cAMP and calcium increased upon receptor activation by AKH. In addition, a different kinase -ERK1/2- was also activated by AKH (Zhu et al. 2009). The lipolytic response in insects seems to be mainly controlled trough reversible phosphorylation / dephosphorylation reactions. However, the complete sequence of reactions underlying the AKH signaling mechanism has not been elucidated in any insect system yet.
Studies on the lipolytic activity of cytosolic fractions of fat bodies have shown an AKH-dependent activation of TG hydrolase activity in moth (Arrese and Wells 1997), beetle (Auerswald et al. 2005) and locust (Auerswald and Gade 2006). However, the lipolytic activation was modest and the mechanism of such activation is unknown. Since AKH induces a rapid fourfold increase in PKA activity of M. sexta fat body, PKA mediated protein phosphorylation was considered a major factor in the activation of lipolysis. TGL can be phosphorylated by PKA in vitro (Arrese and Wells, 1994). However, in vitro studies using PKA and TGL from M. sexta fat body showed that TGL phosphorylation does not affect its activity against lipid droplets (Patel et al. 2004; Patel et al. 2005). Furthermore, TGL is constitutively phosphorylated and its phosphorylation level is unchanged by AKH (Patel et al. 2006). But TGL activity was 2.4-fold higher when assayed against lipid droplets isolated from AKH-stimulated fat bodies, suggesting an effect of AKH on the lipid droplets (Patel et al. 2005). Further experiments investigated the AKH-induced changes in the phosphorylation level of lipid droplet proteins: Lsd1 has been identified as the major PKA target and the phosphorylation level of Lsd1 correlated with TGL activity (Patel et al. 2005). Moreover, most of the AKH lipolytic (~70%) response can be accounted by changes induced in the lipid droplets whereas changes in the cytosol are responsible for 30% of the lipolytic response (Patel et al. 2006). The nature of AKH-induced changes in the cytosol including the activation of TGL remains to be elucidated. Therefore activation of lipolysis is a complex process that involves at least the cytosolic TGL and the lipid droplet associated protein, Lsd1. The details of the signals and protein interactions that ultimately lead to an increase in the rate of TG hydrolysis remain to be elucidated. A full understanding of the regulation of TGL activity requires the identification of possible sites and/or domains of the TGL molecule that could interact with other proteins or cofactors. In this study we report the cloning of a cDNA of M. sexta TGL and an analysis of the primary structure that shows the presence of conserved residues and domains of potential functional relevance.
Differences in TGL expression could also be part of the regulation of lipolysis, especially during developmental stages in M. sexta. For instance, a single AKH peptide is responsible for the mobilization of glycogen and lipids in M. sexta (Ziegler et al. 1990). However, the effect of AKH on the mobilization of energy reserves is dependent on the developmental stage. In larval stage, AKH activates glycogenolysis whereas stimulates lipolysis in adult insects (Ziegler 1991). No information about the developmental pattern of expression of TGL in insects is available. In the present study we investigated the expression of TGL at the protein level and its correlation with the lipolytic activity of fat body in different developmental stages of M. sexta.
MATERIALS AND METHODS
Materials
[tri-9,10-3H(N)]oleoylglycerol was purchased from Perkin Elmer Life Sciences (Boston, MA). Q Sepharose was purchased from Amersham Biosciences (Piscataway, NJ). Trypsin sequencing grade was purchased from Promega (Madison, WI). Protease inhibitors were purchased from Sigma-Aldrich (St. Louis, MO). Electrophoresis items were from Invitrogen (Carlsbad,CA). All other chemicals were of analytical grade. DNA sequencing was performed by the Core Facility of our department using an ABI Model 3700 DNA Analyzer.
Insects
M. sexta eggs were purchased from Carolina Biological (NC) and larvae were reared at 25°C on artificial diet (Bell and Joachim 1976). At the end of the fourth larval instar the first sign of the molt was identified by the appearance of head capsule slippage. Typically slipped head capsule (HC) lasts about 29 h and insects in the middle of this period were used as HC. The initiation of wandering behavior was detected by the exposure of the dorsal aorta. Adult insects were maintained at room temperature without food.
M. sexta TGL cDNA sequencing
Total RNA was isolated from the fat bodies of adult of M. sexta using Trizol reagent (Invitrogen). From total RNA, poly(A)RNA was subsequently isolated using Poly(A) Purist MAG (Ambion). mRNA was reversed transcribed using oligod(T)18-primer. The resulting cDNA was used as template in PCR reactions using the following primers: YHW (forward), 5'- GTGGTGTACCATTGGTTCTAC-3', SVE (reverse), 5'-AGTCATCCACCACTTCCTCCACG-3' and CYW (reverse), 5'-GCCCCAGTAGCACACATGGCTGCTCAGGGCG-3'. These primers were designed based on two internal amino acid sequence (YHWFYSVDVEDK and SVEEVVDDFR, respectively) obtained by mass spectrometry analysis and Edman degradation of purified MsTGL (Arrese et al. 2006) and a highly conserved region towards the C-term of CG8552 (FALSSHVCYWGSE). A cDNA of approximately 590bp and 1600bp were amplified using primers YHW/SVE and YHW/CYW, respectively. The products were cloned into pCR-II TOPO vector (Invitrogen), and sequenced using the Core Facility ABI Model 3730 DNA Analyzer. To clone the cDNA encoding the MsTGL protein, we designed gene specific primers to determine the 5'-and 3'-ends of the transcript using RACE PCR. The SMART RACE cDNA Amplification kit (BD Biosciences) was used according to manufacturer's instructions. 3'-RACE was performed using the specific forward primer 1730F, 5'-TGTGACGAGAGTAAAGAGGAGTTC-3' and nested primer 1830F, 5'-GCAGGAGGCGCCGCTCGAGC-3' with reverse primers provided with the kit. For 5'-RACE the reverse specific primer 450R, 5'- 5'-GTCCAATACACCGCT GTTCGCAACCTC-3', and nested primers 360R, 5'-CAGTGCTATTGTACGCCT CTTCAAGCGCTC-3', and 310R, 5'-CCGCCATATTGATTTATCTTCGACATCCA-3' were used with the forward primer and nested forward primer supplied with the SMART RACE cDNA Amplification kit. The 5'-and 3'-RACE PCR products were cloned into the pGEM-T Easy Vector (Promega) and sequenced in both directions. The coding region of M. sexta TGL was amplified from cDNA by PCR using the following forward and reverse primers: N-TGL, 5'-ATGAACGATAGTACGGAAAGGA-3, and CTGL, 5'-ATCCCTCAACCAGCCGTGGCGGCACGTGATAA-3', respectively. The 1950bp PCR product was cloned into the pGEM-T Easy Vector and the sequence was confirmed in both directions. Two independent clones were analyzed. The cDNA sequence of MsTGL has been deposited in GenBank (Accession number FJ807781).
Northern blot analysis
One or two micrograms of poly(A)RNA was loaded in a 1 % agarose / 1.2% formaldehyde gel, and transferred to a nylon membrane by capillary blotting. A clone of a 0.6 kb fragment of MsTGL cDNA that was cloned into pCR-II TOPO vector was used as template to generate a radiolabeled antisense RNA probe by in vitro transcription using the Megascript kit (Ambion). The membrane was hybridized with TGL probe in a solution of 0.5M sodium phosphate, 7% SDS, 1mM EDTA pH 7.2 at 68°C, then washed at 68°C with 0.5X SSC, 0.12% SDS for 10 min followed by 0.1X SSC, 0.1%SDS for 50 min. Autoradiography was carried out at −70°C for 12 hr using an intensifying screen.
Purification of TGL
TGL was purified from the cytosolic fraction of M. sexta fat body homogenates as reported previously (Arrese and Wells 1994).
Peptide mass fingerprinting and data analysis
The isolated TGL was separated on 8% SDS-PAGE and in-gel digested with trypsin in 25 mM ammonium bicarbonate at 4°C overnight. Peptides were extracted and analyzed by MALDI-Tof mass spectrometry in the Core Facility using the department ABI Voyager De PRO MALDI-Tof MS and α-cyano-4-hydroxycinnamic acid as matrix. The data obtained from the MALDI-Tof spectra were compared with the theoretical peptides obtained from the deduced amino acid sequence using FindMod at ExPASy (http://ca.expasy.org/tools/dna.html).
Processing of fat body homogenates for the determination of TG-lipase activity and western blotting
Fat bodies from five insects were pooled and homogenized with a Potter-Elvehjem glass homogenizer, using 6 ml of homogenization buffer (50 mM Tris, pH 7.4, 0.25 M sucrose, 2 mM EDTA, 0.2 mM benzamidine, 10mg/l leupeptin, 1mg/l aprotonin, 2 mM dithiothreitol). The homogenate was overlaid with 2 ml of buffer without sucrose, and subjected to ultracentrifugation at 65,000 × g for 1 hr in a Beckman Ti70 rotor. The infranatant –the clear fraction underneath the fat cake- was collected and used to determine lipase activity and protein concentration. All steps were carried out on ice or at 4 °C. The TG-lipase activity of insects in different stages of development was directly determined in the infranatant. The infranatant was also directly used for western analysis of TGL protein levels in larvae. However, for the determination of TGL protein levels in the fat body of adult insects the infranatant was subjected to one chromatographic step to remove storage proteins. Thus, the infranatants from adult insects were passed through a Q-Sepharose column equilibrated with buffer (10 mM Na2HPO4, pH 7.4, 1 mM EDTA, 0.1% 2-mercaptoethanol (v/v), 0.1 mM benzamidine, 0.02 % Triton X-100, 10mg/l leupeptin, 1mg/l aprotinin). After extensive wash with equilibration buffer containing 20–130mM NaCl, TGL was eluted in one fraction with 300 mM NaCl in the same buffer. Lipase activity was measured in all the washes and the 300mM NaCl fractions. Recoveries among different samples were very similar. Typically, this fraction contained ~ 30% of the initial amount of total protein and ~ 60 % of the total lipase activity of fat body homogenate.
Assay of TGL activity
Lipase activity was assayed using micellar TG substrate as described previously (Arrese and Wells 1994). Enzyme activity was determined in aliquots containing 200μg of total protein and the activity was expressed as nmol of FFA/min-mg protein and nmol FFA/min-fat body. Data were statistically analyzed using one-way ANOVA.
Western blotting
Polyclonal antibodies against purified TGL were raised in chicken at Cocalico Biologicals (Reamstown, PA). For western blotting, proteins were separated by SDS-PAGE (8%) and transferred to nitrocellulose membranes. Immunodetection was performed using anti-TGL antibody (Arrese et al. 2006). After incubation of membrane with horseradish peroxidase-conjugated rabbit anti-chicken secondary antibody, peroxidase activity was detected using ECL chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ) and exposed to X-ray films.
Other methods
Protein concentrations were determined by the Bradford dye-binding assay using bovine serum albumin as standard. The deduced amino acid sequence was obtained using the translate tool at ExPASy (http://ca.expasy.org/tools/dna.html). Open reading frame identification was done by using ORF Finder at NCBI (http://www.ncbi.nlm.nih.gov). Predictions of MsTGL phosphorylation sites were obtained as described (Blom et al. 2004) using NetPhosk 1.0 at http://www.cbs.dtu.dk/services/NetPhosK. The secondary structure of TGL was estimated with the program PredictProtein (Rost et al. 2004) at http://www.predictprotein.org.
RESULTS
Cloning and analysis of Manduca sexta TGL cDNA sequence
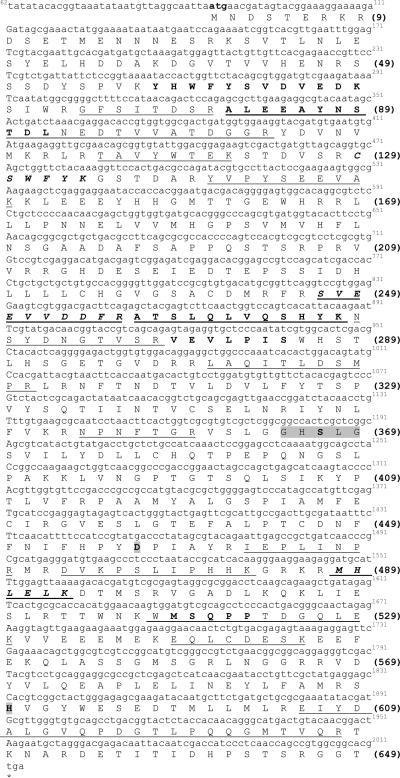
A TGL cDNA from M. sexta was cloned using RT-PCR methods and cDNA synthesized from fat body mRNA. MsTGL cDNA is 2246bp long and contains an open reading frame (ORF) of 1950 bp that encodes a 649 amino acid protein (Fig 1) with a theoretical molecular weight of 73.8 kDa and an isoelectric point of 5.5. The ORF ends at position 2044 and is followed by a 202 -nucleotide 3'-untranslated region including a poly(A) tail of 21 residues. Two copies of the potential polyadenylation signal are found at positions 2200 and 2238, respectively.
Figure 1. Nucleotide and deduced amino acid sequence of TGL from M. sexta.
cDNA nucleotide (62–2044) is shown above the deduced amino acid sequences (1–649). Amino acid residues are aligned with the first nucleotide of each codon. The stop codon TGA is marked by an asterisk. The amino acid sequences underlined represent matched peptides obtained from the MALDI-Tof analysis of the tryptic digest of the gel excised protein. The amino acid microsequence obtained by LC/MS/MS and Edman degradation from MsTGL is in bold. The consensus lipase catalytic motif is highlighted in gray. The cDNA sequence of MsTGL has been deposited in GenBank (Accession number FJ807781).
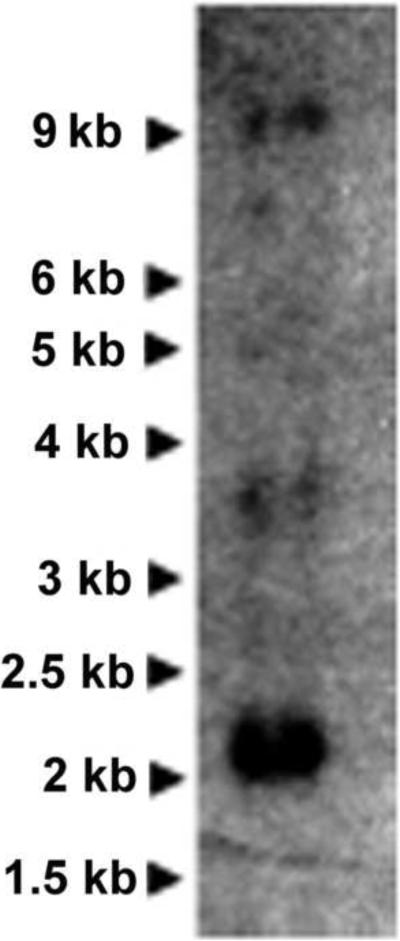
Northern blot analysis of MsTGL using poly(A)-RNA prepared from fat body of adult insects detected a major band corresponding to a transcript of 2.1 kb and two weaker bands corresponding to transcripts of 3.6 and 9 kb, respectively (Fig 2). The 2.1 kb band is close to the size of the transcript shown in Fig 1 (2.2kb). The origin of the other two transcripts is unknown. However, given the high stringency conditions used in the northern blot analysis, the longer transcripts are likely to be products of alternative splicing rather than non-specific hybridizations.
Figure 2. Size of MsTGL transcript.
Northern blot of Manduca sexta RNA extracted from the fat body of 2-day adult male insects. PolyA-RNA was probed with a 0.6kb radiolabeled anti-sense RNA probe.
MALDI-Tof analysis of purified TGL after tryptic digestion identified fourteen matching peptides in the deduced amino acid sequence that are shown underlined in Fig 1. In a previous study we obtained peptide sequences from TGL by LC/MS/MS (CSWFYK, SVEEVVDDFR, MHLELK) and Edman degradation (VEVLPIS, ATSLQLVQSHYK, YHWFYSVDVEDK) that led to the identification of MsTGL as the homolog of Drosophila melanogaster CG8552 (Arrese et al. 2006). The location of those peptide sequences in the deduced amino acid sequence is also shown in Fig 1.
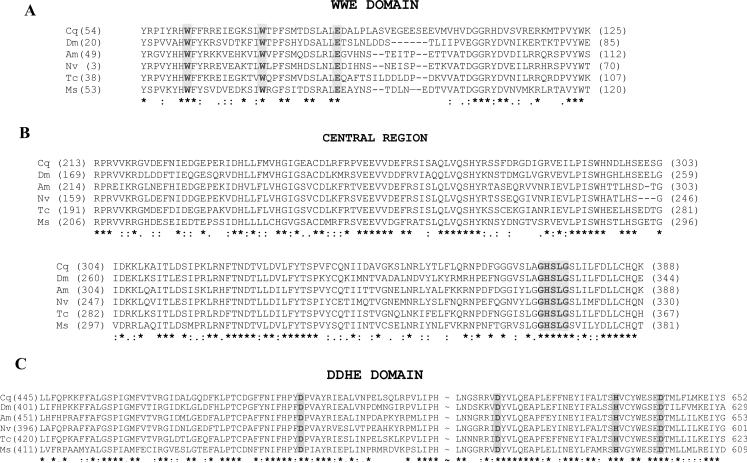
A comparison of the deduced MsTGL protein sequence with the sequences of other putative insect TGLs (CG8552-like) shows significant conservation (~ 53% amino acid identity). The conservation is particularly high in three regions where potential functional domains are found: the N-terminal region that contains a WWE domain (amino acids 46 to 128; Accession number: PF02825), the central region in which the lipase consensus sequence (GHSLG) containing the active site serine (Ser 367) is located, and the C-terminal region that contains the DDHD domain (amino acids 411 to 609; Accession number: PF02862). The DDHD is a long domain named after these four residues that may form a metal binding site. The WWE domain is named after three of its conserved residues and it has been identified in diverse proteins with predicted ubiquitin- and ADP-ribosylation-related functions (Aravind 2001). Fig 3 shows the alignment of deduced protein sequences from five different insects in the three regions indicated above.
Figure 3. Multiple alignment of the three conserved regions identified in MsTGL deduced amino acid sequence and the partial deduced sequences of five representative insects.
Culex quinquefasciatus (Cq, NM_135341.1), Drosophila melanogaster (Dm, XM_001654068.1), Apis mellifera (Am, XM_392149.3), Nasonia vitripennis (Nv, XM_001602690.1), and Tribolium castaneous (Tc, XM_001812847.1). A- Alignment of the N-terminal WWE domains (Pfam: PF02825); B- Alignment of the central regions containing the lipase consensus sequence GHSLG; C- Alignment of the C-terminal domains that are homologous to the DDHD domain (Pfam: PF02862). A central sequence of approximately 80 residues was omitted (~) in the alignment from brevity. Identities in all sequences are indicated with asterisks; fully conservative substitutions are denoted with two dots. Partially conserved substitutions are indicated with a single dot and sequence gaps with dashes.
The predicted secondary structure of TGL shows a 32% α-helix, 20% beta and 48% of non-predicted structure. The arrangement of these predicted secondary structures in the primary sequence is compatible with an α/β hydrolase fold, which is found in numerous lipases and serine proteases (Cygler et al. 1993; Nardini and Dijkstra 1999; Ollis et al. 1992). The catalytic triad of lipases comprises the active site serine, a catalytic acid residue (aspartate or glutamate), and a histidine residue (Cygler et al. 1993). Based on the predicted secondary structure and the sequence alignment of all putative TGL, Asp 457 and His 590 were identified as potential functional active sites together with Ser 367.
Numerous phosphorylation sites for different kinases including PKA and PKC were predicted in MsTGL. This is consistent with the facts that TGL can be phosphorylated in vitro by PKA and also is constitutively phosphorylated in vivo (Arrese and Wells 1994; Patel et al. 2006). Conserved phosphorylation sites are distributed throughout the sequence. A PKA phosphorylation site (Ser 80) is present in the WWE domain. PKC sites are found in the central region (Ser 130 and 265) as well as in the DDHD domain (Thr 514 and 625).
TGL alignments of all available sequences (Supplementary data) revealed several strictly conserved Cys (Cys 129, 234, 241 and 446). TGL requires reducing conditions for activity and some these Cys residues could be critical for activity.
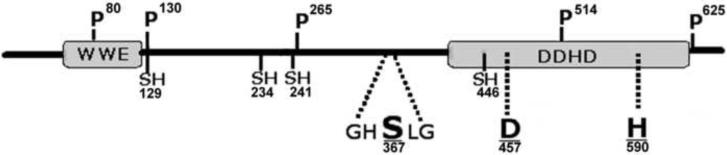
Fig 4 shows a graphic representation of the potential functional elements found in the primary sequence of MsTGL. All these elements (domains, active site, phosphorylation sites, Cys residues) are strictly conserved in all the TGL sequences available suggesting that they may serve an essential function.
Figure 4. Sketch of TGL structure showing key conserved residues and structural regions.
The figure shows the locations of conserved Cys residues and phosphorylation sites present in insect TGLs. It also shows the predicted catalytic triad comprising the serine residue within the GHSLG sequence (366S) and the conserved aspartic (457D) and histidine (590H) located in the DDHD domain.
Fat body TG hydrolase activity during development
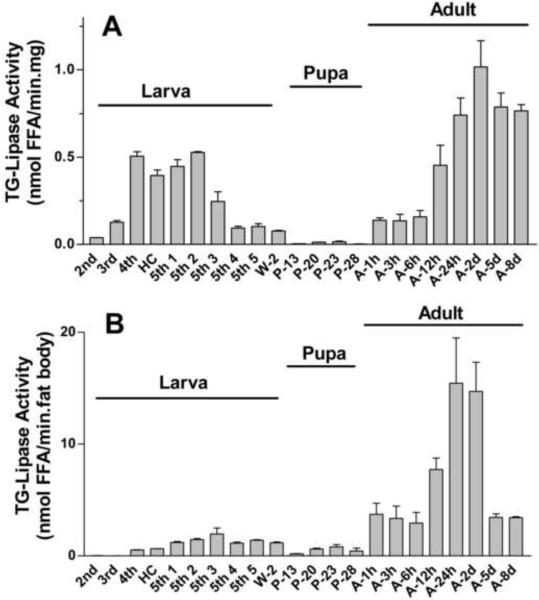
TG hydrolase activity was studied throughout development in the infranatant fraction of fat body homogenates. Fig 5 A shows the changes in lipase activity normalized by protein content (nmol FFA/min.mg of protein). Lipase activity was detected at all developmental stages. However, a broad range of activities, from 0.004 to 1.017 nmol FFA/min/mg, was observed (Fig 5 A). The activity increased during larval development reaching maximum activities between the 4th-instar and the 2nd day 5th-instar. The activity started to decrease in day 3 of the 5th instar and continued decreasing during wandering and pupal stages. The lipase activities during the pupal stages were significantly lower (P<0.05 or lower) than those observed at all other stages with the exception of 2nd-instar. Adult insects emerged with a relatively low fat body lipase activity, but the activity rapidly increased reaching a peak between 24h and 48 h after emergence. At these times the lipase activities of adult insects were significantly higher (P<0.001) than all other fat body TG lipase activities. When the activity is expressed in nmol FFA/min/mg protein the TG hydrolase activity of 48h-adult insects was ~1.9-fold higher (P<0.001) than that observed in 5th-instar (day 2) larvae. The higher TG-lipase activity of adult insects is more evident when the data are expressed per fat body (nmol FFA/min/fat body). Fat bodies from adult insects (1–2 days old) have a total lipase activity nearly 9 times higher (P<0.001) than the average activity of 2 to 3 days old 5th instar larval fat bodies (Fig 5 B).
Figure 5. Fat body lipase activity during development.
The infranatant fractions (200μg protein) of fat body homogenates from larva (L), pupa (P) and adult (A) M. sexta were examined for lipase activity against an emulsion of [3H-triolein] and Triton X-100 as indicated in Methods. Panel A shows the lipase activities normalized by protein content (nmol FFA/min/mg protein); Panel B shows the lipase activities per fat body (nmol FFA/min.fat body). Data that are expressed as means ± SE (n = 4–8). Significant differences (P<0.001) were estimated between A-24 and A-48 and all other fat body TG lipase activities. The lipase activities during the pupal stages were significantly lower (P<0.05 or lower) than those observed at all other stages with the exception of 2nd-instar. Abbreviations: HC, head capsules; W, wanderer; P, pupa; and A, adult (males).
Expression of TGL during development
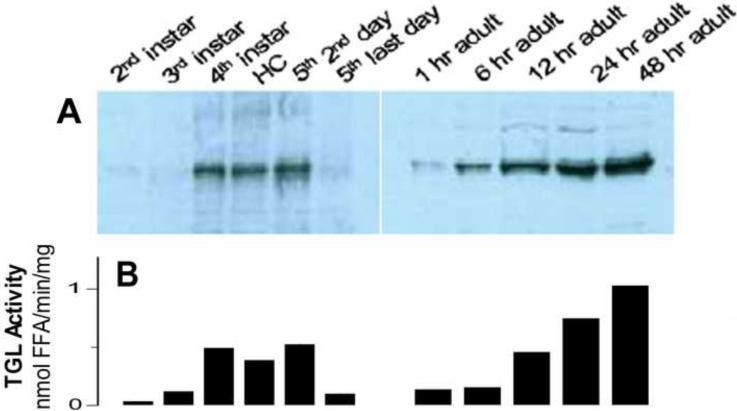
TGL is localized in the cytosolic fraction of fat body (Patel et al. 2005). The protein levels of TGL in larval and adult fat bodies were examined by immunodetection using anti-TGL antibody. The infranatant fractions of fat body homogenates from six different larval stages were directly analyzed by western blotting. As shown in Fig 6A, TGL is present in larval fat bodies and its abundance increases towards the last instar (5th-day 2). The TGL content shows a good correlation with the lipase activity measured in the same samples (r2 = 0.951). The fact that there is a correlation between the amount of TGL and the lipase activity suggests that in the larval stage of M. sexta TGL is the main fat body lipase.
Figure 6. Changes in TGL protein level during development.
A- TGL protein levels were determined by western blotting in the infranatants of larval fat body homogenates (40μg of protein). The infranatants of adult fat body homogenates were first ran through a single step of Q-Sepharose chromatography and then subjected to western blotting using 20μg of protein. The proteins were separated on SDS-PAGE, transferred to nitrocellulose, and analyzed using anti-lipase antibody; Pooled samples, 4 to 8 insects per developmental stage, were used for western blotting B- The figure shows the average lipase activities corresponding to the developmental stages analyzed by western blotting.
Due to the interference of storage proteins, the abundance of TGL in fat bodies from adult insects cannot be directly analyzed by western blotting. Storage proteins are very abundant and their electrophoretic mobility is very close to that of TGL. Therefore, estimation of the abundance of TGL in adult insects required a chromatographic step to remove the storage proteins from the cytosolic fractions. For this purpose a simple anion exchange chromatography of the cytosol fractions was enough to provide a sample suitable for western blotting. TGL was eluted as a single fraction and the recoveries of protein and lipase activity were similar among different samples. Fig 6A shows a western blot of samples obtained from fat bodies isolated from adult insects at 1h, 6h, 12h, 24h, and 48h after the imaginal molt. TGL abundance increases gradually after emergence (6-A) and this is accompanied by a corresponding increase in lipase activity (6-B). A good linear correlation (r2 =0.945) was found between TGL protein levels, which were estimated by densitometry of the western blots, and the lipase activity (Fig 6A–B). As observed along the larval stages, the correlation observed between total lipase activity of fat body homogenates from adult insects and the levels of TGL estimated by western blotting suggests that TGL is the main fat body lipase.
DISCUSSION
Fatty acids stored as TG within the lipid droplets of fat body adipocytes are mobilized for a number of purposes, including the provision of metabolic fuel to flight muscles, the provision of lipids to the ovaries, and the overall maintenance of the metabolic activity of other tissues, including the fat body (Beenakkers et al. 1985, Canavoso et al. 2001, Arrese and Soulages 2010) Diglyceride, trehalose and proline are the most common metabolic fuel among insects. Diglyceride is directly produced from TG lipolysis (Arrese and Wells, 1997). However, TG lipolysis is also required for the production of fatty acids that undergo β-oxidation for the generation of ATP and acetyl-CoA used for the synthesis and export of trehalose and proline (Gade and Auerswald 2003, Arrese and Soulages 2010). Moreover, large amounts of ATP are needed to support protein synthesis. Given the central role of lipolysis in insect physiology, the rate of fat body TG hydrolysis must be regulated by multiple signals.
The first step of fatty acid mobilization consists in the action of fat body triglyceride lipases to catalyze the hydrolysis of TG molecules contained in the lipid droplets. Since the rate of lipolysis depends on the activity of fat body lipases, understanding the mechanisms of regulation of TGL activity is critical for the understanding of lipolysis in insects. A full understanding of the acute regulation of TGL activity requires the identification of possible sites and/or domains of the TGL molecule that could interact with other proteins. To this end, cloning of M. sexta TGL constitutes an important step towards the understanding of TGL function. Analysis of the primary structure of M. sexta TGL shows several conserved elements that could play a role in the regulation of the lipolytic rate.
A common structural characteristic of lipases is the α/β hydrolase fold and a catalytic triad formed by the nucleophilic serine, a negatively charged residue (Asp or Glu) and a histidine. These residues are always present in loops and the linear order Ser-(Asp/Glu)-His is absolutely preserved (Cygler et al. 1993; Hide et al. 1992). Taking into account that information, the catalytic triad of TGL is predicted to be constituted by Ser367, contained in the consensus lipase sequence GHSLG, and the residues Asp457 and His590 that are predicted to be located in loops. The catalytic triad and the predicted secondary structure of TGL suggest that it belongs to the family of lipases that have a characteristic α/β hydrolase fold. These lipases have a conserved short α-helix (“lid”) covering the active site (Derewenda 1994). In the primary sequence, flanking the lid region, Cys residues are typically found as well as a set of hydrophobic amino acids (Hide et al. 1992). This lid needs to rotate to allow interaction of the substrate with the active site. Two Cys residues flanking the lid region are typically present in lipases of the α/β hydrolase fold (Hide et al. 1992). Among the conserved Cys residues present in MsTGL, Cys 130 and 234 are predicted to be located in loops. These two Cys residues could play a role in the regulation of the lipase activity.
As mentioned above, TG hydrolysis must be regulated by multiple signals including AKH. The lipolytic response induced by AKH in M. sexta involves a rapid increase of Ca2+ influx and an increase in the intracellular concentration of cAMP, which leads to the activation of PKA (Arrese et al. 1999, Van der Horst et al. 2001, Gade and Auerswald 2003). Whether these two pathways are activated simultaneously by the AKH receptor or are the result of a cascade effect remains to be determined. Five conserved phosphorylation sites were identified in TGL. Only one of these sites is specific for PKA, whereas the remaining four are PKC specific. This in interesting because the fat body has relatively high levels of sn-1,2-DG, which are activators of PKC, and these levels increase when lipolysis is stimulated by AKH (Arrese and Wells 1997). The role of PKC in regulating lipolysis has not been studied. However, the presence of conserved PKC sites in TGL suggests that this kinase could be part of the mechanism regulating lipolysis.
Modulation of TGL activity also involves interactions with other proteins such as Lsd1, and these interactions are controlled by metabolic signals. TGL can be directly modulated by phosphorylation of the lipid droplet associated protein Lsd1 (Arrese et al. 2008b). As suggested for other proteins (Aravind 2001), the WWE domain of TGL could play a role in the interaction of TGL with other proteins. Future studies involving the expression of MsTGL mutants will allow defining the role of key conserved residues and domains in the function of TGL and the lipolytic activity of fat body.
During the purification of MsTGL from adult fat bodies we had previously observed that in every chromatographic step the single major peak of lipase activity correlated with the presence of TGL (Arrese et al. 2006). Based on this observation we inferred that TGL was likely to be the main TG-lipase present in M. sexta. Supporting this hypothesis, the present study showed a strong correlation between the abundance of TGL in the cytosolic fractions, as determined by western blot, and the lipase activity observed throughout larval and adult stages.
This study also showed that the expression of TGL is a factor defining the lipolytic activity of fat body. The highest lipase activity and TGL protein content are observed in fat body of adult insects. Two days after emergence adult insects have a lipase activity (per mg of fat body protein) that is ~1.9-fold higher than that observed in 5th-instar larvae. However, the total fat body TG-lipase activity of adult insects is ~9-fold greater than the lipase activity of 5th-instar larva (Fig 5 B). These observations are consistent with a major role of TGL in the mobilization of lipid stores and also with the physiological differences that distinguish larval and adult stages of M. sexta. This finding is consistent with the fact that larvae eat constantly and accumulate lipid reserves, whereas adult insects consume lipid stores to support the energy demands imposed by reproduction and flight (Ziegler 1985). Moreover, the lipase activity and TGL level do not increase during non-feeding periods, for instance during the head capsule slippage, HC, of the last larval molt (4th to 5th). However, during this transition a rapid activation of glycogen phosphorylase as early as 2–4 h after the animals stopped feeding has been observed (Siegert et al. 1993). Glycogen-phosphorylase is more abundant in the larval stages of M. sexta and glycogen reserves are nearly exhausted by the end of pupal period (Ziegler 1991). Altogether these observations indicate that when glycogen stores are available they are utilized first to support energy metabolism, whereas lipid stores are spared for utilization during the adult stage.
A previous study (Ziegler et al. 1984) showed that the AKH response in adult M. sexta, as judged by the increase in the concentration of hemolymph lipids, develops several hours after the imaginal molt. No adipokinetic response is found during the first 8 h of the adult life. After 16 h the insect becomes responsive to AKH, but the response is even greater on the second day of adult life. The results of that study agree with the gradual increase in TGL that is observed after emergence. Altogether the present study supports the involvement of TGL in the lipolytic response induced by AKH.
A major difference between larva and adult M. sexta resides in the effect of AKH, which in larvae activates glycogen phosphorylase without promoting the secretion of lipids which is observed in moths (Siegert and Ziegler 1983; Siegert 1987; Siegert 1995). In adult M. sexta, AKH promotes lipolysis and a two-fold increase in hemolymph lipid levels (Arrese and Wells 1997). The fat bodies of both larvae and moths have large amounts of TG stored and are responsive to AKH. Therefore, it is possible that AKH promotes lipid mobilization only in adult insects in part because the fat body of adult insects has a 9-fold greater content of TGL than larval fat body. However the lower abundance of TGL in larvae does not explain the lack of lipid mobilization in response to AKH. The lack of lipolytic response in larvae is probably due to the absence of Lsd1 (Arrese et al. 2008a). In vitro and in vivo phosphorylation experiments have shown that AKH-induced phosphorylation of Lsd1 account for nearly 70% of the activation of TGL (Arrese et al. 2008b; Patel et al. 2005). Since Lsd1 is highly expressed in adult insects whereas is undetectable in feeding larvae, the content of Lsd1 in the lipid droplets is probably the major factor limiting the lipolytic response to AKH in larvae.
Part of the lipolytic response (30%) induced by AKH is due to changes that occur in the cytosol, including the activation of TGL (Patel et al, 2006). As mentioned above, AKH-induced activation of TG-lipase activity has been reported in several insects including moth, beetle and locust and this mechanism of activation, which at least in M. sexta is independent of the phosphorylation state of TGL, remains to be elucidated. This type of regulation could be critical to the control of the basal lipolytic activity (AKH independent). The regulation of basal lipolysis is expected to be more relevant in the feeding stages. Further studies are needed to elucidate the mechanism of regulation of TGL activity. The conserved structural elements of TGL identified here will be the focus of future studies leading to advance the understanding of the mechanism of regulation of TGL.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant GM 64677 and Oklahoma Agricultural Experiment Station, Oklahoma State University. The authors are grateful to Elizabeth O'Connell for rearing the insects and providing technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aravind L. The WWE domain: a common interaction module in protein ubiquitination and ADP ribosylation. Trends Biochem Sci. 2001;26:273–5. doi: 10.1016/s0968-0004(01)01787-x. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Flowers MT, Gazard JL, Wells MA. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J Lipid Res. 1999;40:556–64. [PubMed] [Google Scholar]
- Arrese EL, Mirza S, Rivera L, Howard AD, Chetty PS, Soulages JL. Expression of lipid storage droplet protein-1 may define the role of AKH as a lipid mobilizing hormone in Manduca sexta. Insect Biochem Mol Biol. 2008a;38:993–1000. doi: 10.1016/j.ibmb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Patel RT, Soulages JL. The main triglyceride-lipase from the insect fat body is an active phospholipase A(1): identification and characterization. J Lipid Res. 2006;47:2656–67. doi: 10.1194/jlr.M600161-JLR200. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Rivera L, Hamada M, Mirza S, Hartson SD, Weintraub S, Soulages JL. Function and structure of lipid storage droplet protein 1 studied in lipoprotein complexes. Arch Biochem Biophys. 2008b;473:42–7. doi: 10.1016/j.abb.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Wells MA. Purification and properties of a phosphorylatable triacylglycerol lipase from the fat body of an insect, Manduca sexta. J Lipid Res. 1994;35:1652–60. [PubMed] [Google Scholar]
- Arrese EL, Wells MA. Adipokinetic hormone-induced lipolysis in the fat body of an insect, Manduca sexta: synthesis of sn-1,2-diacylglycerols. J Lipid Res. 1997;38:68–76. [PubMed] [Google Scholar]
- Auerswald L, Gade G. Endocrine control of TAG lipase in the fat body of the migratory locust, Locusta migratoria. Insect Biochem Mol Biol. 2006;36:759–68. doi: 10.1016/j.ibmb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Auerswald L, Siegert KJ, Gade G. Activation of triacylglycerol lipase in the fat body of a beetle by adipokinetic hormone. Insect Biochem Mol Biol. 2005;35:461–70. doi: 10.1016/j.ibmb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco horn worms and pink bolloworms. Ann Entomol. Soc. Am. 1976;69:365–373. [Google Scholar]
- Beenakkers AM, Van der Horst DJ, Van Marrewijk WJ. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–49. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu. Rev. Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- Cygler M, Schrag JD, Sussman JL, Harel M, Silman I, Gentry MK, Doctor BP. Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins. Protein Sci. 1993;2:366–82. doi: 10.1002/pro.5560020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda ZS. Structure and function of lipases. Adv Protein Chem. 1994;45:1–52. doi: 10.1016/s0065-3233(08)60637-3. [DOI] [PubMed] [Google Scholar]
- Downer RGH, Matthews JR. Patterns of lipid distribution and utilization in insects. Am. Zool. 1976;16:733–45. [Google Scholar]
- Gade G, Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen Comp Endocrinol. 2003;132:10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Gade G, Hoffmann KH, Spring JH. Hormonal regulation in insects: facts, gaps, and future directions. Physiol Rev. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- Hide WA, Chan L, Li WH. Structure and evolution of the lipase superfamily. J Lipid Res. 1992;33:167–78. [PubMed] [Google Scholar]
- Lum PY, Chino H. Primary role of adipokinetic hormone in the formation of low density lipophorin in locusts. J Lipid Res. 1990;31:2039–44. [PubMed] [Google Scholar]
- Nardini M, Dijkstra BW. Alpha/beta hydrolase fold enzymes: the family keeps growing. Curr Opin Struct Biol. 1999;9:732–7. doi: 10.1016/s0959-440x(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Patel RT, Soulages JL, Arrese EL. Adipokinetic hormone-induced mobilization of fat body triglyceride stores in Manduca sexta: role of TG-lipase and lipid droplets. Arch Insect Biochem Physiol. 2006;63:73–81. doi: 10.1002/arch.20143. [DOI] [PubMed] [Google Scholar]
- Patel RT, Soulages JL, Hariharasundaram B, Arrese EL. Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem. 2005;280:22624–31. doi: 10.1074/jbc.M413128200. [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–6. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert K, Ziegler R. A Hormone From The Corpora Cardiaca Controls Fat-Body Glycogen-Phosphorylase During Starvation In Tobacco Hornworm Larvae. Nature. 1983;301:526–527. [Google Scholar]
- Siegert KJ. Carbohydrate-Metabolism In Starved 5th Instar Larvae Of Manduca-Sexta. Archives Of Insect Biochemistry And Physiology. 1987;4:151–160. [Google Scholar]
- Siegert KJ, Speakman JR, Reynolds SE. Carbohydrate and lipid metabolism during the last larval molt of the tobacco hornworm, Manduca sexta. Physiological Entomology. 1993;18:404–408. [Google Scholar]
- Siegert KJ. Carbohydrate-Metabolism During The Pupal Molt Of The Tobacco Hornworm, Manduca-Sexta. Archives Of Insect Biochemistry And Physiology. 1995;28:63–78. [Google Scholar]
- Spencer IM, Candy DJ. HORMONAL-CONTROL OF DIACYL GLYCEROL MOBILIZATION FROM FAT-BODY OF DESERT LOCUST, SCHISTOCERCA-GREGARIA. Insect Biochemistry. 1976;6:289–296. [Google Scholar]
- Staubli F, Jorgensen TJ, Cazzamali G, Williamson M, Lenz C, Sondergaard L, Roepstorff P, Grimmelikhuijzen CJ. Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci U S A. 2002;99:3446–51. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Horst DJ, Van Marrewijk WJ, Diederen JH. Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol. 2001;211:179–240. doi: 10.1016/s0074-7696(01)11019-3. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Hayakawa Y, Downer RGH. FACTORS INFLUENCING CYCLIC-AMP AND DIACYLGLYCEROL LEVELS IN FAT-BODY OF LOCUSTA-MIGRATORIA. Insect Biochemistry. 1990;20:325–330. [Google Scholar]
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–91. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Zhu C, Huang H, Hua R, Li G, Yang D, Luo J, Zhang C, Shi L, Benovic JL, Zhou N. Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett. 2009;583:1463–8. doi: 10.1016/j.febslet.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. Metabolic energy expenditure and its regulation. Springer; Berlin: 1985. pp. 95–118. [Google Scholar]
- Ziegler R. Changes in lipid and carbohydrate metabolism during starvation in adult Manduca sexta. J Comp Physiol [B] 1991;161:125–31. doi: 10.1007/BF00262874. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Eckart K, Law JH. Adipokinetic hormone controls lipid metabolism in adults and carbohydrate metabolism in larvae of Manduca sexta. Peptides. 1990;11:1037–40. doi: 10.1016/0196-9781(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Kegel G, Keller R. Isolation and amino-acid composition of the adipokinetic hormone of Manduca sexta. Hoppe Seylers Z Physiol Chem. 1984;365:1451–6. doi: 10.1515/bchm2.1984.365.2.1451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.