Abstract
Collagen fibres in tendons and ligaments run straight but in some regions they show crimps which disappear or appear more flattened during the initial elongation of tissues. Each crimp is formed of collagen fibrils showing knots or fibrillar crimps at the crimp top angle. The present study analyzes by polarized light microscopy, scanning electron microscopy, transmission electron microscopy the 3D morphology of fibrillar crimp in tendons and ligaments of rat demonstrating that each fibril in the fibrillar region always twists leftwards changing the plane of running and sharply bends modifying the course on a new plane. The morphology of fibrillar crimp in stretched tendons fulfills the mechanical role of the fibrillar crimp acting as a particular knot/biological hinge in absorbing tension forces during fibril strengthening and recoiling collagen fibres when stretching is removed. The left-handed path of fibrils in the fibrillar crimp region gives rise to left-handed fibril helices observed both in isolated fibrils and sections of different tendons and ligaments (flexor digitorum profundus muscle tendon, Achilles tendon, tail tendon, patellar ligament and medial collateral ligament of the knee). The left-handed path of fibrils represents a new final suprafibrillar level of the alternating handedness which was previously described only from the molecular to the microfibrillar level. When the width of the twisting angle in the fibrillar crimp is nearly 180° the fibrils appear as left-handed flattened helices forming crimped collagen fibres previously described as planar crimps. When fibrils twist with different subsequent rotational angles (< 180°) they always assume a left-helical course but, running in many different nonplanar planes, they form wider helical crimped fibres.
Keywords: collagen handedness, fibrillar crimp, helical crimps, ligament, planar crimps, tendon
Introduction
The structure of tendons and ligaments relates to their function, so that the extracellular matrix array always reflects the biomechanical roles of these tissues. Tendons ensure the transmission of muscle contraction strength to bone, whereas ligaments transmit forces from bone to bone and limit/guide joint motions. Extracellular matrix proteoglycans like decorin have been suggested to enhance the tensile properties of collagen and play a role in transmitting and resisting tensile stresses, namely facilitating fibrillar slippage during tissue deformation (Cribb & Scott, 1995; Scott, 1996; Fratzl et al. 1997; Pins et al. 1997; Puxkandl et al. 2002; Robinson et al. 2004). More recently it was demonstrated that the quasi-static elastic material properties of the human medial collateral ligament of the knee were unchanged after dermatan sulphate removal, thereby challenging the theory that decorin proteoglycans contribute to the elastic behaviour of the ligament (Lujan et al. 2007). In addition, the complete removal of all glycosaminoglycans in the medial collateral ligament failed to change ligament viscoelasticity (Lujan et al. 2009). Viscous sliding of collagen fibrils seems to occur only in embryonic tissues, whereas after birth, when locomotion begins, the mechanical responses appear to be dominated by the elastic stretching of cross-linked collagen molecules. In particular, kinks occurring within the gap region of the collagen fibril might be straightened at low strains (toe region) because of the greater flexibility of collagen molecules related to lower levels of proline and hydroxyproline and the reduced packing density compared with the overlap region (Fraser et al. 1986; Fraser & Trus, 1986; Fratzl et al. 1997; Silver et al. 2003). Among extracellular matrix structures collagen fibres play a key role in transmitting tensional forces, as supported by many reports that the in-vivo mechanical properties of tendons and ligaments are strictly related to the fibre collagen arrangement, collagen fibril diameter and fibril length (Lanir, 1978; Parry et al. 1978; Doillon et al. 1985; Hart et al. 1992; Woo et al. 1999; Silver et al. 2003; Lo et al. 2004). The collagen fibrils opposing high unidirectional tensional forces in tendons and ligaments have a wide diameter and are less flexible or more rigid than the small collagen fibrils resisting multidirectional forces like those in skin and vessel walls (Ruggeri et al. 1979; Reale et al. 1981; Ottani et al. 2001, 2002). For this reason, collagen fibres run straight and are mainly oriented along the stress direction in tendons, although fibres in some regions show irregular undulations described as crimps. Tendon and ligament crimps disappear or appear more flattened during the initial elongation of tissues (corresponding to the toe region of the stress/strain curve) both in vitro and in vivo (Diamant et al. 1972; Atkinson et al. 1999; Screen et al. 2004a,b;). This event suggests that crimps form a mechanical buffer system protecting collagen fibres during tendon elongation (Elliott, 1965; Viidik & Ekholm, 1968; Diamant et al. 1972; Viidik, 1972; Kastelic et al. 1980; Niven et al. 1982; Rowe, 1985a,b; Hess et al. 1989; Gathercole & Keller, 1991; Jozsa et al. 1991; de Campos Vidal, 1995; Viidik et al. 1996; Jozsa & Kannus, 1997; Hansen et al. 2002; Franchi et al. 2007). However, when stretching forces are removed the tendon crimping pattern reforms, suggesting that crimps may also act like a recoiling system after muscle relaxation in tendons or ensure joint stability during posture in ligaments (Rigby et al. 1959; Franchi et al. 2007; Benjamin et al. 2008).
Morphological studies (transmission electron microscopy, scanning electron microscopy and atomic force microscopy) on Achilles tendon crimps reported that they are composed of single parallel collagen fibrils that, suddenly changing direction at the top angle of each crimp, show a kink. The fibrils in these kinks or fibrillar crimps appeared squeezed, bent and twisted, sometimes lacking the D-banding with a swollen microfibrillar array (Gutsmann et al. 2003; Raspanti et al. 2005). When the tendon is stretched in vivo, the crimps disappear but the fibrillar crimps are still recognizable (Franchi et al. 2007). Fibrillar crimps have been exclusively described in fibrils with a broad diameter, an almost straight microfibril array (5° helical microfibrillar arrangement) and submitted to unidirectional forces as in tendons or some ligaments. Fibrillar crimps look like a deformation of the more rigid large fibrils when they have to flex suddenly forming fibrillar knots in tendon/ligament crimps. Gutsmann et al. (2003) suggested that the large collagen fibrils of the rat tail tendon may behave mechanically like rigid tubes rather than rods, showing flat or squeezed fibrillar kinks when the fibrils change direction. However, whether the fibrillar crimp is a morphological deformation resulting from local flexion in a rigid collagen fibril or is structured to act as a more flexible region of a rigid fibril remains an open question.
Moreover, in-depth knowledge of the fibrillar crimp structure could help to understand the suprafibrillar arrangement in tendons. The collagen fibre array in tendon was described as helical (Learch, 1950; Verzar, 1964; Jozsa et al. 1991) but also as planar and sinusoidal (Rigby et al. 1959; Diamant et al. 1972; Yannas & Huang, 1972). Evans & Barbenel (1975) suggested that the collagen arrangement may be sinusoidal in the tail tendon and helical in others. In addition, crimped fibrils and fibres of different connective tissues were postulated to have a helical or spiral array (de Campos Vidal, 1995, 2003; Freed & Doehring, 2005; de Campos Vidal & Mello, 2009; Grytz & Meschke, 2009) or a planar arrangement (Diamant et al. 1972; Yannas & Huang, 1972; Lanir, 1978; Kastelic et al. 1980; Nicholls et al. 1984; Rowe, 1985b; Gathercole & Keller, 1991; Stolinski, 1995; Jozsa & Kannus, 1997; Kannus, 2000; Silver et al. 2003).
The present study analysed the 3D morphology of fibrillar crimp demonstrating that it gives rise to left-handed helices of collagen fibrils that assemble forming both planar and helical crimps in different tendons and ligaments.
Materials and methods
Twelve female Sprague–Dawley rats (90 days old) were anaesthetized with an intraperitoneal injection of 87 mg kg−1 ketamine (Ketavet; Farmaceutici Gellini Spa, Italy) and 13 mg kg−1 i.m. xylazine (Rompun; Bayer Italia SPA, Italy). Under anaesthesia, the flexor digitorum profundus muscle tendon, Achilles tendon of the gastrocnemius muscle, tail tendon, patellar ligament and medial collateral ligament of the knee were surgically exposed, isolated and fixed in situ (still connected to the muscle–tendon junction and to the bone for tendons and to bones for ligaments) in Karnovsky's solution for scanning electron microscope (SEM) and transmission electron microscope (TEM) analysis. The rats were then killed via an intracardiac injection of Tanax (Hoechst, Frankfurt am Main, Germany). Specimens for scanning electron microscopy were immersed in Karnovsky's solution for 24 h, post-fixed in 1% osmium tetroxide, dehydrated in a graded series of alcohol, sectioned following a frontal or a sagittal plane and finally dehydrated in hexamethyldisilazane. The specimens were then mounted on metal stubs and coated with 5-nm gold in an Emitech K550 sputter-coater to be observed under a SEM (Philips 515, Eindhoven, The Netherlands) operating in secondary-electron mode. All of the procedures were performed strictly according to Italian and European law on animal experimentation.
Specimens for transmission electron microscopy were fixed in Karnovsky's solution for 24 h, post-fixed in 1% osmium tetroxide, dehydrated through a graded series of alcohol and then embedded in Araldite resin. The ultrathin sections were stained with uranyl acetate and lead citrate and examined under a TEM (Philips CM-10).
Some specimens, first finely chopped with razor blades, were subjected to gentle mechanical disruption with a Dounce homogenizer. Drops of the suspension containing isolated collagen fibrils were put on Formvar-coated, 300-mesh copper grids. Negative staining was carried out by dripping 1% phosphotungstic acid dissolved in 0.1 m phosphate buffer, pH 7.4. Double-positive staining was performed by putting grids on drops of 1% phosphotungstic acid aqueous solution, pH 3.2, for 10 min and, after brief washing in distilled water, on drops of 1% uranyl acetate aqueous solutions, pH 4.2, for 20 min. The grids were subsequently washed in distilled water and dried with filter paper. Specimens were examined under a TEM (Philips CM-10).
Three other female Sprague–Dawley rats (90 days old) were anaesthetized as above. A resin brace, modified to induce foot dorsal flexion, was applied to one posterior leg and the foot was progressively flexed to a final angle of 55°. The stretching position was maintained for 15 min. At the end of the stretching session, and still under anaesthesia, the tendon of the gastrocnemius muscle still under tension was surgically exposed, isolated and fixed in situ (still connected to the muscle–tendon junction and to the bone) in Karnovsky's solution. The rats were then killed as above and the stretched tendons were excised, sectioned following a frontal plane and processed for SEM analysis as described above (Philips CM-10). All of the procedures were performed strictly according to Italian and European law on animal experimentation.
Results
The sagittal section of the tendon of the flexor digitorum profundus muscle, Achilles tendon of the gastrocnemius muscle, tail tendon and patellar ligament observed on scanning electron microscopy showed collagen fibres running parallel and densely packed interrupted only by periodic crimps. Each collagen fibre was mostly formed by large and few small collagen fibrils coursing straight and densely packed but suddenly changing direction in the top angle of each crimp. Nevertheless, crimps differed in size, top angle and base length among different tendons; all fibrils changing their course showed a particular knot described as a fibrillar crimp. Fibrillar crimps observed at higher magnification showed that the fibrils first twisted leftwards, changing their plane of running, and then bent sharply also changing the direction of coursing. The different planes of coursing of the collagen fibrils were parallel to each other and therefore crimps appeared like planar crimps (Figs 1–4).
Fig. 1.
SEM picture of sagittal section of the tendon of flexor digitorum profundus muscle of rat. Two fibres of mostly large collagen fibrils running parallel and densely packed bend forming crimps. At the top angle of these crimps the fibrils first twist leftwards and then bend forming a fibrillar knot or fibrillar crimp. Bar = 1 μm.
Fig. 4.
SEM picture of a sagittal section of rat patellar ligament. Collagen fibrils forming a fibre twist leftwards and then bend in the fibrillar crimp regions. Bar = 1 μm.
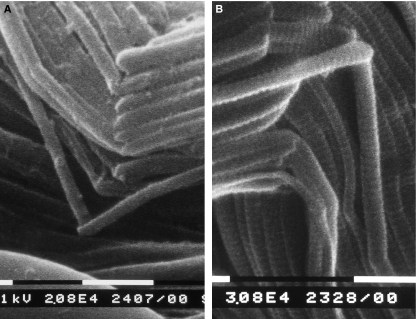
Fig. 2.
(A-B) SEM pictures of sagittal section of Achilles tendon of gastrocnemius muscle of rat. Both A) and B) show a tendon crimp composed of fibrillar crimps. Note that both small and large collagen fibrils first twist leftwards changing the plane of their running and then bend changing their direction in the new plane. Bar = 1 μm.
Fig. 3.
SEM picture of sagittal section of rat tail tendon. Collagen fibrils of a collagen fibre run densely packed and form a crimp. At the top angle of the crimp the collagen fibrils twist leftwards and then bend forming many parallel fibrillar crimps. Bar = 1 μm.
On transmission electron microscopy the collagen fibrils in some fibrillar crimp regions did not clearly show their D-period showing a less regular array or an apparent swelling of their microfibrils (Fig. 5).
Fig. 5.
TEM picture of fibrillar crimps in the tendon of flexor digitorum profundus muscle of rat. Parallel collagen fibrils in the fibrillar crimp region twist leftwards and bend changing both their direction and plane of coursing. No D-period banding is observable in the fibrillar crimps but a microfibril swelling is clearly evident. Bar = 500 nm.
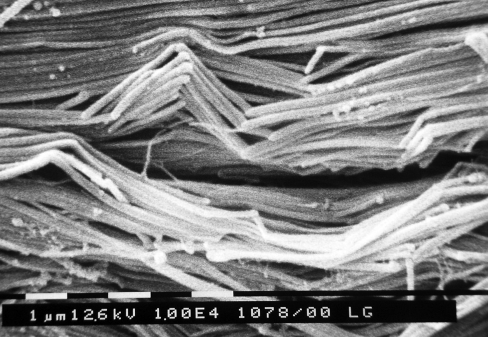
Densely packed collagen fibres ran intertwining each other in the frontal sectioned medial collateral ligament of rat. Each collagen fibre was composed of collagen fibrils running parallel but forming crimps when they changed direction. At the top of each crimp the fibrils formed knots similar to the fibrillar crimps described in tendons. In particular, the fibrils in the fibrillar crimp region firstly twisted leftwards, changing their plane of running, and then bent, modifying their direction of coursing (Fig. 6). These fibrils, running on different but parallel planar planes, appeared like planar crimps (Fig. 7). However, the fibrils in many collagen fibres of the medial collateral ligament twisted leftwards, bending to form fibrillar crimps at the top of each crimp, but coursed in different non-planar planes forming helical crimps (Fig. 8). The morphological aspects of fibrillar crimps did not change in all tissues and animals.
Fig. 6.
SEM picture of a frontal section of rat medial collateral ligament of the knee. Collagen fibres run intertwined and fibrils are not running as parallel and densely packed as in tendons. When fibrils form crimps they always show fibrillar crimps. In fibrillar crimp regions all fibrils first twist leftwards and then bend changing first the plane and then the direction of running. Bar = 1 μm.
Fig. 7.
SEM picture of a frontal section of Achilles tendon of gastrocnemius muscle of rat. Many collagen fibres are composed of fibrils forming fibrillar crimps at the top of each crimp. The collagen fibres and fibrils running in parallel planar planes form “planar crimps”. Bar = 10 μm.
Fig. 8.
SEM picture of a frontal section of rat medial collateral ligament of the knee. Two collagen fibres are composed of fibrils left-twisting and bending to form fibrillar crimps at the top of each crimp. The fibres show a helical array because the fibrils course in different non planar planes. Crimps of these fibres appear as helical crimps. Bar = 1 μm.
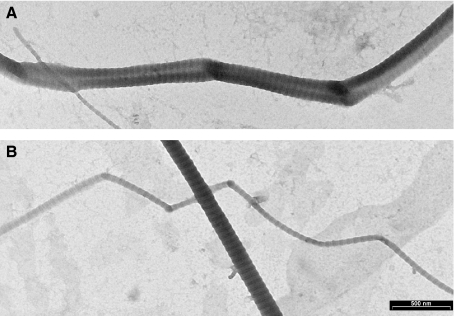
The isolated collagen fibrils observed on transmission electron microscopy showed fibrillar knots characterized by fibril twisting and bending like the described fibrillar crimps. Some isolated fibrils forming many fibrillar crimps showed a flattened helical array (Fig. 9).
Fig. 9.
Isolated collagen fibrils from medial collateral ligament of rat. A) A large collagen fibril forming different fibrillar crimps assumes a flattened helical array (compare it with Fig. 4); B) a large collagen fibril coursing straight intertwines with a smaller fibril showing a flattened helical array (compare it with Fig. 6). Bar = 500 nm.
In frontal sections of the stretched Achilles tendons observed on scanning electron microscopy most of the collagen fibres ran straight and parallel to the long axis of the tendon and few flattened crimps were observed. In completely flattened crimps the stretched collagen fibrils running straight and parallel still showed knots similar to but slightly different from the fibrillar crimps observed in relaxed tendons; the fibrils still twisted leftwards running in two different parallel planes without showing any bending (Fig. 10).
Fig. 10.
In vivo stretched rat Achilles tendon at SEM. In a completely flattened crimp the stretched collagen fibrils run straight and parallel still showing knots similar but different to the fibrillar crimps observed in relaxed tendons: fibrils still twist leftwards, running straight in two different parallel planes, but not bending. Bar = 1 μm.
Discussion
Crimps in tendons and ligaments are composed of parallel densely packed collagen fibrils that suddenly change direction in the region of the top angle of each crimp forming 3D special local arrays described as fibrillar crimps. The fibrillar crimps are thought to be the microscopic structure responsible for the mechanical functions of crimps in absorbing/transmitting loads and recoiling of collagen fibres in tendons and ligaments (Raspanti et al. 2005; Franchi et al. 2007; Benjamin et al. 2008).
Tendon collagen fibrils bend sharply only in the fibrillar crimp region; for this reason, fibril flexibility has to be related to the fibrillar crimps (Silver et al. 2003). Some have suggested that the fibril diameter could be inversely related to collagen molecular flexibility and that the different sequence of amino acids in tropocollagen could influence the flexibility of the single fibril. The region of the triple helix with the Gly-Pro-Hyp sequence is very rigid, whereas regions of the molecule devoid of proline and hydroxyproline have the highest flexibility (Silver et al. 2003). Gutsmann et al. (2003) suggested that the large collagen fibrils of the rat tail tendon may behave mechanically like tubes rather than rods, showing flat or squeezed fibrillar kinks when fibrils change their direction. In previous research we observed that the fibrils in fibrillar crimp regions suddenly changing direction appeared squeezed and also bent or twisted (Raspanti et al. 2005). A factor that is mechanically related to fibril flexibility is the microfibrillar arrangement. Fibrils in connective tissues like skin, fasciae, wall vessels, nerve and tendon sheaths submitted to multidirectional forces, showing a unimodal small diameter (25–100 nm), a helical microfibrillar array of 17° with respect to the fibril axis and a 64-nm D-periodicity, appear extremely flexible and can accommodate extreme curvatures without harm, never showing fibrillar crimps. By contrast, thicker fibrils (100–300 nm) submitted to unidirectional loadings, as in tendons and some ligaments, showing a microfibrillar winding array of 5° and consequently a 67-nm D-periodicity, are more rigid and cannot suddenly bend without structural deformations like the fibrillar crimps first described as kinks (Franchi et al. 2008). However, the fibrillar crimps in tendons and ligaments submitted to unidirectional forces functionally act more like knots than kinks; when a physiological stretching is applied to the tendon in vivo the fibrillar crimps can still be observed inside partially or completely flattened crimps (Franchi et al. 2007). A structural organization of a fibrillar crimp like a kink would imply a structural resistance to straightening, tension increase and concentration in a subsection of the fibril when stretched. This would result in an overload and possibly fatigue of certain microfibrils with respect to others. In contrast, the structural arrangement of fibrillar crimps like knots, resulting from a local structural release of the helical constraint, appears more reasonable: (i) it is compatible with fibril helical microstructure; (ii) it allows crimps at rest without loading; and (iii) it does not imply load concentration in subsections of the fibril when stretched.
The present study analysed the 3D morphology of the fibrillar crimp also relating its structure to the repeated alternating handedness among successive structural levels, from collagen molecules to fibrils, in different tendons and ligaments. In-depth analysis of the 3D change in direction of collagen fibrils when forming fibrillar crimps revealed that fibrils do not really bend on the same plane, simply forming kinks, but twist and then bend, changing both their plane and course and forming knots. Moreover, it was very surprising to observe that all collagen fibrils forming fibrillar crimps in the examined tendons and ligaments twist leftwards with respect to the main fibril axis. The newly described left-handedness of collagen fibrils in fibrillar crimps can be added to the alternating hierarchical handedness at successive levels of structure in the fibril. In fact, collagen is a trimeric molecule with a repeating Gly-X-Y triplet in which X and Y are usually proline and hydroxyproline, respectively (van der Rest & Garrone, 1991; Canty & Kadler, 2005). All fibrillar collagens are synthesized as soluble precursors or procollagens. The proteolytic processing of N- and C-terminal propeptides leads to the production of mature collagen molecules that spontaneously assemble into fibrils. Collagen consists of three polypeptide left-handed chains that assemble forming a unique triple right-handed tropocollagen helical structure (Kadler et al. 1996; Hulmes, 2002; Brodsky & Persikov, 2005; Canty & Kadler, 2005; Hongo et al. 2005). Tropocollagen molecules linearly and laterally aggregate into microfibrils that laterally aggregate to form fibrils (Kannus, 2000; Silver et al. 2003; Canty & Kadler, 2005). In particular, five tropocollagens are twisted in a left-handed helix forming a single microfibril (Chen et al. 1991; Kure et al. 1995; Wess et al. 1998) and microfibrils arranged in a rightward helix to form a single collagen fibril (Holmes et al. 2001; Orgel et al. 2006). This hierarchical alternating handedness among successive levels supports a rope-like structure model and plays a functional role in increasing the fibril's resistance to elongation (Hulmes, 2002; Bozec et al. 2007).
When large and poorly flexible collagen fibrils with an almost straight microfibrillar arrangement (5° right-handed helix) have to bend in tendons and ligaments they first twist leftwards with respect to the main fibril axis, changing the plane of their running, and then bend, changing the direction of their course in the new plane. This sudden change of direction is possible because the fibrils in the fibrillar crimp region change their microfibrillar arrangement, which presumably assumes a helical array similar to that described in the flexible thin fibrils of other connective tissues (Ottani et al. 2001, 2002). Fibrillar crimps allowing a sharp change of fibril direction represent the more flexible regions of the rigid thick fibrils; in the fibrillar crimp they show a microfibrillar disruption or disorder visible as a local disappearance of the D-period and an apparent microfibrillar swelling (Raspanti et al. 2005; Franchi et al. 2007). The different arrangement of the microfibrils and the local release of the upper hierarchical structural constraint could reproduce a local change in the mechanical characteristics of the fibril. Through the local release of the structural constraint the microfibrils would be allowed to arrange in space without loading, according to their released length, producing a knot. The fibrillar crimps fulfilling local fibril flexibility seem to interrupt the straight course of the rigid fibrils and behave as biological hinges both absorbing tension and guiding the recoil of collagen fibres (Fig. 11).
Fig. 11.
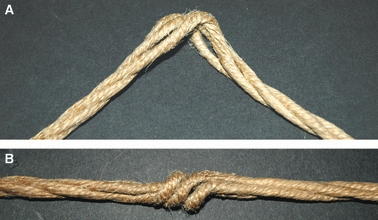
A) Single subfilaments (tropocollagen) arranged in a left helical array form single rope filaments (microfibrils composed by left-handed helices of tropocollagens) which twisting rightwards form a rope (single fibril composed by microfibrils arranged in a rightward helix). When the rope (fibril) twists leftwards of about 180° in a local region, the single rope filaments (microfibrils) appear swelled also changing their handedness. B) When the rope (fibril) is stretched at the ends the portion of the rope, twisting leftward, acts like a knot (fibrillar crimp). (Compare it with tendon fibrils stretched in vivo in Fig. 10).
Such a 3D morphology of fibrillar crimps and the repeated leftward twisting seem to play a role in determining the fibril arrangement; fibrils, always twisting leftwards (changing the running plane) and then bending (changing their direction) in the fibrillar crimp regions, always assume a left-handed helical array along their course. The width of the fibril rotation angle seems to determine different fibrillar helical arrangements. Fibrils, presumably stretched along the same direction by unidirectional tensional forces, show a twisting angle of nearly 180° assuming a flat helical course, each fibril running almost only in two different adjacent planes. The flattened helical fibrils assembling one to each other form flattened helical fibres that are described by some authors as planar crimps (Diamant et al. 1972; Yannas & Huang, 1972; Lanir, 1978; Kastelic et al. 1980; Nicholls et al. 1984; Rowe, 1985b; Gathercole & Keller, 1991; Stolinski, 1995; Jozsa & Kannus, 1997; Kannus, 2000; Silver et al. 2003). When fibrils twist in the fibrillar crimp region with different subsequent rotation angles (< 180°), they assume a left-helical course but, running in many different non-planar planes, they form the wider helical crimped fibres described by other authors (Fig. 12) (Evans & Barbenel, 1975; de Campos Vidal, 1995, 2003; Freed & Doehring, 2005; de Campos Vidal & Mello, 2009; Grytz & Meschke, 2009).
Fig. 12.
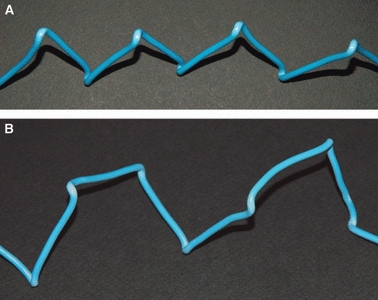
A) Model of a single flattened left-handed helix simulating a flattened collagen fibril helix forming “planar crimps” (compare it with collagen fibrils in Figs 7 and 9B). B) Model of a wider lefthanded helix simulating the collagen fibrils forming helical crimps (compare it with collagen fibrils in Fig. 8).
Biomechanical studies and mathematical measurements of the fibrillar crimp structure influencing the collagen suprafibrillar array behaviour in different functional conditions are in progress.
Concluding remarks
All collagen fibrils in the fibrillar crimp region of both tendons and ligaments show a particular knot; they always twist leftwards first, changing their plane of running, and then sharply bend, changing their course on the new plane. This fibrillar crimp structure may reflect a local increased flexibility of the thick rigid fibrils as a consequence of a local helical arrangement of microfibrils.
The particular knot (leftward twisting and bending) described in the fibrillar crimp region, still persisting in stretched collagen fibres of tendons and some ligaments, suggests that fibrillar crimps act as biological hinges both absorbing tension during fibril strengthening and guiding the recoil of collagen fibres when stretching is removed.
The left-handed path of fibrils in the fibrillar crimp region gives rise to left-handed fibril helices in both tendons and ligaments. It represents a new final suprafibrillar level of alternating handedness that was previously described only from the molecular to the microfibrillar level.
Some authors described planar crimps, whereas others observed only helical crimps in tendon. Morphological data in this study demonstrate that all crimped fibrils, leftward twisting and bending, assume a left-helical arrangement. When the width of the twisting angle of all fibrillar crimps is nearly 180°, the fibrils appear as left-handed flattened helices assembling and forming crimped fibres described by some authors as planar crimps. When fibrils twist with different subsequent rotational angles (< 180°) along their course they always assume a left-helical array but, running in many different non-planar planes, they form the wider helical crimped fibres described by other researchers.
Acknowledgments
We are indebted to Gianfranco Filippini, D.I.S.T.A., University of Bologna, for his technical assistance with scanning electron microscopy. This work was supported by a grant from the University of Bologna (RFO 2008).
References
- Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure–function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70–75. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–359. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]
- de Campos Vidal B. Crimp as a part of a helical structure. C R Acad Sci III. 1995;318:173–178. [PubMed] [Google Scholar]
- de Campos Vidal B. Image analysis of tendon helical superstructure using interference and polarized light microscopy. Micron. 2003;34:423–432. doi: 10.1016/S0968-4328(03)00039-8. [DOI] [PubMed] [Google Scholar]
- de Campos Vidal B, Mello ML. Structural organization of collagen fibers in chordae tendineae as assessed by optical anisotropic properties and Fast Fourier transform. J Struct Biol. 2009;167:166–175. doi: 10.1016/j.jsb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Canty EC, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Chen JM, Kung CE, Feairheller SH, et al. An energic evaluation of a “Smith” collagen microfibril model. J Protein Chem. 1991;10:535–552. doi: 10.1007/BF01025482. [DOI] [PubMed] [Google Scholar]
- Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen: proteoglycan interactions in stressed tendon. J Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- Diamant J, Keller A, Baer E, et al. Collagen: ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- Doillon CJ, Dunn MG, Bender E, et al. Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res. 1985;5:481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- Elliott DH. Structure and function of mammalian tendon. Biol Rev Camb Philos Soc. 1965;40:392–421. doi: 10.1111/j.1469-185x.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Evans JH, Barbenel JC. Structural and mechanical properties of tendon related to function. Equine Vet J. 1975;7:1–8. doi: 10.1111/j.2042-3306.1975.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Franchi M, Fini M, Quaranta M, et al. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat. 2007;210:1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi M, Raspanti M, Dell’Orbo C, et al. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect Tissue Res. 2008;49:85–91. doi: 10.1080/03008200801913635. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, Trus BL. Molecular mobility in the gap regions of type I collagen fibrils. J Mol Biol. 1986;6:221–226. doi: 10.1007/BF01115010. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, MacRae TP, Miller A, et al. Molecular conformation and packing in collagen fibrils. J Mol Biol. 1986;167:497–521. doi: 10.1016/s0022-2836(83)80347-7. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Misof K, Zizak I, et al. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1997;122:119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- Freed AD, Doehring TC. Elastic model for crimped collagen fibrils. J Biomech Eng. 2005;127:587–593. doi: 10.1115/1.1934145. [DOI] [PubMed] [Google Scholar]
- Gathercole LJ, Keller A. Crimp morphology in the fibreforming collagens. Matrix. 1991;11:214–234. doi: 10.1016/s0934-8832(11)80161-7. [DOI] [PubMed] [Google Scholar]
- Grytz R, Meschke G. Constitutive modelling of crimped collagen fibrils in soft tissues. J Mech Behav Biomed Mater. 2009;2:522–533. doi: 10.1016/j.jmbbm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Gutsmann T, Fantner GE, Venturoni M, et al. Evidence that collagen fibrils in tendons are inhomogenously structures in a tubelike manner. Biophys J. 2003;84:2593–2598. doi: 10.1016/S0006-3495(03)75064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72–77. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- Hart RA, Woo SL, Newton PO. Ultrastructural morphometry of anterior cruciate and medial collateral ligaments: an experimental study in rabbits. J Orthop Res. 1992;10:96–103. doi: 10.1002/jor.1100100112. [DOI] [PubMed] [Google Scholar]
- Hess GP, Cappiello WL, Poole RM, et al. Prevention and treatment of overuse tendon injuries. Sports Med. 1989;8:371–384. doi: 10.2165/00007256-198908060-00005. [DOI] [PubMed] [Google Scholar]
- Holmes DF, Gilpin CJ, Baldock C, et al. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci U S A. 2001;98:7307–7312. doi: 10.1073/pnas.111150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo C, Noguchi K, Okuyama K, et al. Repetitive interactions observed in the crystal structure of a collagen-model peptide, [(Pro-Pro-Gly)9]3. J Biochem. 2005;138:135–144. doi: 10.1093/jb/mvi108. [DOI] [PubMed] [Google Scholar]
- Hulmes DJS. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol. 2002;137:2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, et al. Three-dimensional ultrastructure of human tendons. Acta Anat. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, et al. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Palley I, Baer E. A structural mechanical model for tendon crimping. J Biomech. 1980;13:887–893. doi: 10.1016/0021-9290(80)90177-3. [DOI] [PubMed] [Google Scholar]
- Kure M, Araki K, Ogata T. Scanning tunneling microscopic study of osmium-impregnated collagen. J Electron Microsc. 1995;44:207–211. [PubMed] [Google Scholar]
- Lanir Y. Structure–strength relations in mammalian tendon. Biophys J. 1978;24:541–554. doi: 10.1016/S0006-3495(78)85400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learch H. Ueber der aufbau des sehnengewebes. Gegenbaurs Morphol Jahrb. 1950;90:192–205. [Google Scholar]
- Lo IK, Marchuk LL, Leatherbarrow KE, et al. Collagen fibrillogenesis and mRNA levels in the maturing rabbit medial collateral ligament and patellar tendon. Connect Tissue Res. 2004;45:11–22. doi: 10.1080/03008200490278070. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CI, Henninger HB, et al. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Jacobs NT, et al. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol. 2009;106:423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SP, Gathercole J, Shah JS. Morphology of human plamaris longus tendon. Ann Rheum Dis. 1984;43:477–482. doi: 10.1136/ard.43.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven H, Baer E, Hiltner A. Organization of collagen fibers in rat tail tendon at the optical microscope level. Coll Relat Res. 1982;2:131–142. doi: 10.1016/s0174-173x(82)80029-0. [DOI] [PubMed] [Google Scholar]
- Orgel JP, Irving TC, Miller A, et al. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micron. 2001;32:251–260. doi: 10.1016/s0968-4328(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Ottani V, Martini D, Franchi M, et al. Hierarchical structures in fibrillar collagens. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Pins GD, Christiansen DL, Patel R, et al. Self-assembly of collage fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–2172. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, et al. Viscoelastic properties of collagen: synchontron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspanti M, Manelli A, Franchi M, et al. The 3D structure of crimps in the rat Achilles tendon. Matrix Biol. 2005;24:503–507. doi: 10.1016/j.matbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Reale E, Benazzo F, Ruggeri A. Differences in the microfibrillar arrangement of collagen fibrils. Distribution and possible significance. J Submicrosc Cytol. 1981;13:135–143. [PubMed] [Google Scholar]
- van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, et al. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, et al. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Rowe RW. The structure of rat tail tendon. Connect Tissue Res. 1985a;14:9–20. doi: 10.3109/03008208509089839. [DOI] [PubMed] [Google Scholar]
- Rowe RW. The structure of rat tail tendon fascicles. Connect Tissue Res. 1985b;14:21–30. doi: 10.3109/03008208509089840. [DOI] [PubMed] [Google Scholar]
- Ruggeri A, Benazzo F, Reale E. Collagen fibrils with straight and helicoidal microfibrils: a freeze-fracture and thin-section study. J Ultrastruct Res. 1979;68:101–108. doi: 10.1016/s0022-5320(79)90146-1. [DOI] [PubMed] [Google Scholar]
- Scott JE. Proteodermatan and proteokeratan sulphate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagens. Biochemistry (Mosc) 1996;35:8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- Screen HR, Bader DL, Lee DA, et al. Local strain measurement within tendon. Strain. 2004a;40:157–163. [Google Scholar]
- Screen HR, Lee DA, Bader DL, et al. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H. 2004b;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Stolinski C. Disposition of collagen fibrils in human tendons. J Anat. 1995;186:577–583. [PMC free article] [PubMed] [Google Scholar]
- Verzar F. Aging of the collagen fiber. Int Rev Connect Tissue Res. 1964;2:243–300. doi: 10.1016/b978-1-4831-6751-0.50012-4. [DOI] [PubMed] [Google Scholar]
- Viidik A. Simultaneous mechanical and light microscopic studies of collagen fibers. Z Anat Entwicklungsgesch. 1972;136:204–212. doi: 10.1007/BF00519178. [DOI] [PubMed] [Google Scholar]
- Viidik A, Ekholm R. Light and electron microscopic studies of collagen fibers under strain. Z Anat Entwicklungsgesch. 1968;127:154–164. doi: 10.1007/BF00521981. [DOI] [PubMed] [Google Scholar]
- Viidik A, Nielsen HM, Skalicky M. Influence of physical exercise on aging rats. II. Life-long exercise delays aging of tail tendon collagen. Mech Ageing Dev. 1996;88:139–148. doi: 10.1016/0047-6374(96)01729-0. [DOI] [PubMed] [Google Scholar]
- Wess TJ, Hammersley AP, Wess L, et al. A consensus model for molecular packing of type I collagen. J Struct Biol. 1998;122:92–100. doi: 10.1006/jsbi.1998.3991. [DOI] [PubMed] [Google Scholar]
- Woo SLY, Debski RE, Withrow JD, et al. Biomechanics of knee ligaments. Am J Sports Med. 1999;27:533–543. doi: 10.1177/03635465990270042301. [DOI] [PubMed] [Google Scholar]
- Yannas I, Huang C. Fracture of tendon collagen. J Polym Sci A-2 Polym Phys. 1972;10:577–X. [Google Scholar]