Abstract
Although age- and degeneration-related changes in the morphology and biochemistry of the annulus fibrosus have been extensively reported, studies of tensile strength changes show only a weak correlation with maturity. Given that the disc is a tissue system in which significant levels of deformation occur with normal physiological loading, there may be structure-related properties that provide a better indicator of the influence of ageing on its function. This study is a morphological investigation of lamellar interfibre cohesivity with respect to maturity. Anterior segments of ovine lumbar discs in two age groups were cut at one of two section angles to generate intralamellar and interlamellar slices. These slices of hydrated annular tissue were subjected separately to microtensile and swelling forces, and examined using differential interference contrast microscopy. There were distinct differences in microstructural responses to transverse extension between the immature and mature intralamellar slices. The immature tissue exhibited a diffuse expansion of the array to form a fine fibrous net. In contrast, the mature tissue displayed a discontinuous expansion with the development of clefts and localized fibre buckling. A difference was also observed in the free-swelling response; the immature slices remained planar, whereas the cropped lamellar fibres in the mature slices exhibited a folded, buckled morphology. Morphological evidence from these experiments infers differences in fibre cohesivity between the immature and mature tissues, consistent with biochemical and histological studies. More extreme levels of deformation in the mature tissue could result in discontinuous opening of the fibrous arrays, which might have the potential to lead to cleft formation. These clefts may, in turn, provide micropaths through which nuclear material could extrude. Importantly, with many animal studies carried out on immature discs, the results here suggest that some caution is required with respect to extrapolating annular behaviour beyond this age group.
Keywords: differential interference contrast optical microscopy, lamellar fibre cohesivity, micromechanical response, ovine lumbar disc
Introduction
Age- and degeneration-related changes in the morphology and biochemistry of the annulus fibrosus have been extensively reported (Bernick et al. 1991; Buckwalter, 1995; Marchand & Ahmed, 1990; Roughley, 2004; Urban & Roberts, 1995), indicating numerous changes such as lamellae thickening, decrease in water and proteoglycan content with fragmentation and alterations in cross-linking of the collagen fibres. It has been suggested that such age-related changes will reduce the mechanical strength of the tissue (Roughley, 2004), decrease the disc's structural integrity (Buckwalter, 1995), influence failure mechanisms (Marchand & Ahmed, 1990) and lead to an unstable annulus (Bernick et al. 1991).
However, mechanical property studies of the ageing annulus reveal a more ambiguous picture. Fujita et al. (1997) examined the radial tensile properties of 1-mm-thick transverse slices and reported a 30% decrease in yield and ultimate stress for degenerated discs. Iatridis et al. (1998) studied the compressive properties of the outer annular wall and showed increased elastic stiffening of the degenerate tissue. Gu et al. (1999) found that the hydraulic permeability coefficient of the annulus fibrosus depended significantly on the degree of disc degeneration. Only for the non-degenerate grade I tissue was the hydraulic permeability significantly anisotropic (direction dependent) with the greatest permeability being in the radial direction.
In contrast to the above, several other studies have shown only poor correlation between the mechanical properties of the annulus and age. Acaroglu et al. (1995) investigated the circumferential tensile behaviour of single lamellae and noted only small changes, with the degenerated annulus failing at a lower stress and requiring less energy to induce failure than the non-degenerate annulus. Holzapfel et al. (2005) performed tension tests on single lamellae but parallel to the fibre direction. They also found only weak correlation between age and tensile strength. Ebara et al. (1996) studied the circumferential tensile behaviour of multiple-layer annulus samples and reported only weak correlations between tensile properties and age.
These reports of insignificant alterations in mechanical properties with degeneration are not surprising in view of the fact that most testing was conducted on small samples removed from their context of a highly complex and anisotropic disc architecture (Marchand & Ahmed, 1990; Pezowicz et al. 2005, 2006a; Schollum et al. 2008, 2009). Further, there may be structure-related properties apart from tensile strength that might provide a more meaningful indicator of the influence of ageing on disc function. This is a potentially important consideration given that we are dealing with a tissue system in which significant levels of deformation occur with normal physiological loading.
In this new study we have used a combination of micromechanical manipulation and microstructural analysis of fully hydrated annular samples to gain insights into the alteration in interfibre cohesivity within individual lamellae with respect to maturity.
Materials and methods
Tissue
Sixteen ovine lumbar spines in two age categories (eight lamb and eight ewe spines) were collected fresh and stored frozen at −20 °C until required. The immature lamb spines were from animals aged between 4 and 7 months old, and the mature ewe spines were from animals aged between 3 and 5 years old. All discs exhibited both clear annular/nuclear demarcation and discrete fibrous lamellae, consistent with Thompson grade I/II tissue (Thompson et al. 1990). Typical examples of the L4–5 ovine discs employed in this study are pictured in Fig. 1; the approximate lateral width of these discs is 30 mm. Four of the discs from each cohort were used for the experiments outlined below exploring lamellar cohesivity. The remaining four discs from each cohort were used to obtain an average value of the outer lamellar thickness as follows: the distance encompassing the 10 outermost lamellae (representing the outer third of the annular wall at this location by number of lamellae) was measured in five consecutive 200-μm-thick bone/disc/bone slices per anterior quadrant (generating a total of 20 measurements per age group). The average outer lamellar thickness was 184 μm for the immature and 320 μm for the mature tissue with SDs of 49 and 58 μm, respectively. This is in agreement with published data for the human disc, with the younger disc reportedly having a thickness of 50% of the older specimen (Marchand & Ahmed, 1990) (absolute values of 140 and 280 μm as dehydrated tissue). Macroscopically, the most obvious difference between the two age groups was the extension of defined lamellae into the transition zone of the mature discs. (Note, only the outermost lamellae were examined in this study.) Further detail of ovine disc geometry compared with the human disc has been published by O’Connell et al. (2007).
Fig. 1.
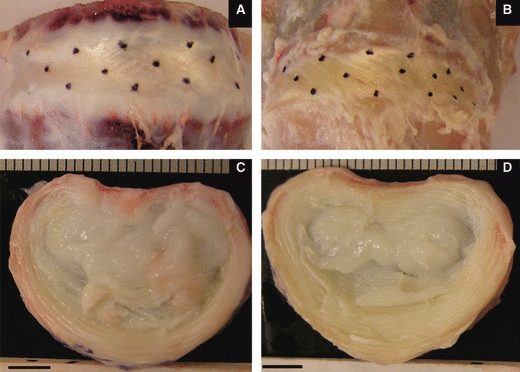
Typical macro views of ovine discs. Anterior views at top (with oblique linking lines between dots indicating fibre angles) and transverse views below. (A and C) Immature disc; (B and D) mature disc. Scale bars = 5 mm.
Methods
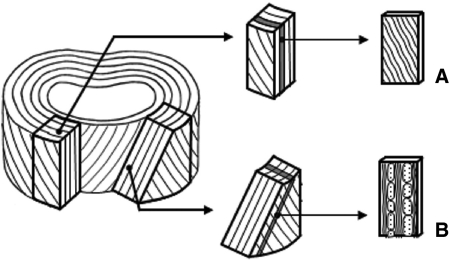
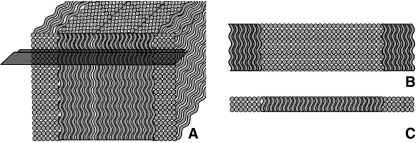
Experiments were restricted to the anterior half of the L4–5 disc. A freezing sledging microtome was used initially to obtain 30-μm-thick slices at one of two section angles – in the plane of the lamellae (intralamellar) for microtensile experiments and oblique (interlamellar) for the swelling experiments (see Fig. 2). In building a better microstructural understanding of the disc architecture, traditional section planes have the disadvantage of chopping the lamellae into non-functional pieces, making relationships between components of the annular wall difficult to discern. The two types of structurally relevant section planes adopted in this research were labelled intralamellar and interlamellar sections by Pezowicz et al. (2006a) and have been utilized in other published studies by these authors (Pezowicz et al. 2006b; Schollum et al. 2008, 2009; Veres et al. 2008). An intralamellar slice yields fibres of parallel orientation from within a single lamella (see Fig. 2A). An interlamellar section cuts into the face of the disc at 45° (oblique angle), close to one of the fibre angles (see Fig. 2B). With successive lamellae aligned approximately 30° above and then 30° below the horizontal plane, the oblique cutting plane contains successive lamellae alternately near in-plane and near cross-section. The cross-sectional bundles are thus cropped to the thickness of the section making them potentially more susceptible to swelling than the adjacent intact in-plane fibres (see Fig. 3).
Fig. 2.
Anterior blocks of ovine lumbar annulus sectioned: (A) parallel to outer surface (intralamellar) or (B) obliquely (interlamellar) (Pezowicz et al. 2006b, used with permission.).
Fig. 3.
Schematic illustration of lamellar fibre alignment in a block of annular wall and an oblique slice. (A) The front face of the block is an oblique surface like that shown in Fig. 2B. The section plane (see black blade) crops the fibres of one lamellar orientation. (B) Plan view of a slice, as seen in micrographs, with alternating in-plane and cross-sectional lamellae. (C) Front elevation of a slice, illustrating the cropped fibres of the middle lamella.
The intralamellar slices for microtensile testing (n = 15 immature slices and 15 mature slices) were trimmed with the aid of a low-power microscope and small fabric tabs were attached with cyanoacrylate tissue gel. The tabbed slice of annulus in its fully hydrated state (in 0.15 m saline) was then placed in a microtensile device (Pezowicz et al. 2005) mounted on the stage of a differential interference contrast (DIC) microscope. Transverse extension perpendicular to the longitudinal fibre direction was applied discontinuously and still images were captured digitally. The degree of extension was expressed as a ratio of the stretched to the unstretched width of the sample (this is the normal convention for expressing large strain behaviour).
The free-swelling behaviour was studied by placing 30-μm-thick interlamellar slices of immature (n = 12) and mature (n = 12) tissue on a glass slide in a pool of 0.15 m saline and then examining with DIC optics. For an additional set of 30-μm-thick mature slices (n = 6), the physiological saline was substituted for distilled water to provide a higher swelling environment and these slices were then re-examined with DIC optics. Two further sets of mature slices, microtomed at 25 μm (n = 6) and 45 μm (n = 6) respectively, together with the remaining 30-μm-thick slices, were used to investigate how slice thickness influenced swelling behaviour.
Finally, two blocks of annulus from each age group were first chemically fixed in 10% formalin overnight prior to interlamellar sectioning in order to capture the in-situ microstructure and thus provide a reference framework for comparing swelling behaviour in the abstracted slices (n = 12 slices).
Results
Transverse unravelling behaviour of intralamellar slices
In terms of their microstructural response to transverse tensile stretching there were distinct differences between the immature and mature intralamellar slices consistent across all samples in each cohort. Consider first the low-magnification images in Fig. 4A,B, captured at a transverse extension ratio of 1.6. In the immature tissue (Fig. 4A) there was a generally uniform expansion of the collagenous array with the pre-existing faint crimp becoming more distinct (see boxed area in Fig. 4A enlarged in C). By contrast, the mature tissue (Fig. 4B,D) consistently displayed discontinuous fibre array expansion. In this typical example (Fig. 4B) a major cleft has developed accompanied by some localized buckling of the still coherent fibre array as indicated by the ‘creased’ morphology (see black arrows in Fig. 4B with one crease enlarged in D).
Fig. 4.
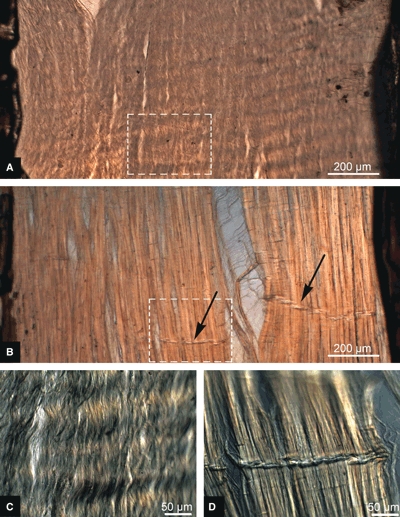
Transverse microtensile deconstruction of intralamellar slices with an extension ratio of 1.6. (A) Macro view, immature. (B) Macro view, mature. Black arrows indicate localized fibre buckling. Fibrous details of the boxed areas are shown in micrographs (C) and (D) for the immature and mature slices, respectively.
The vertical sequences of images shown in Fig. 5 provide a more detailed view of the differing modes of progressive fibrous unravelling between the immature and mature tissues. Figure 5A–C illustrates, in three steps, the typical array expansion of an immature slice and Fig. 5D–F illustrates an equivalent series for a typical mature slice. Note how, in the immature tissue, there is a diffuse expansion of the array to form a fine fibrous net, a structural response that is consistent with the earlier study of Pezowicz et al. (2005) of young bovine caudal discs. By contrast, the mature tissue sequence (Fig. 5D–F) typically begins with the formation of a split, which then widens. Deconstructed tissue is shown again in Fig. 6A,B but at higher magnification. There is diffuse separation of the immature fibres (Fig. 6A), whereas the mature specimens (Fig. 6B) show more dramatic separation. The mature collagen fibres bridging the opening are realigned into near-horizontal orientations (see white arrow in Fig. 6B) with minimal participation from the neighbouring collagen fibres. The secondary vertical contraction that is induced in the sample as the transverse extension progresses causes the collagen fibres in the mature section to undergo localized compressive collapse, as demonstrated by the creases traversing the array (see large white arrowhead in Fig. 6B).
Fig. 5.
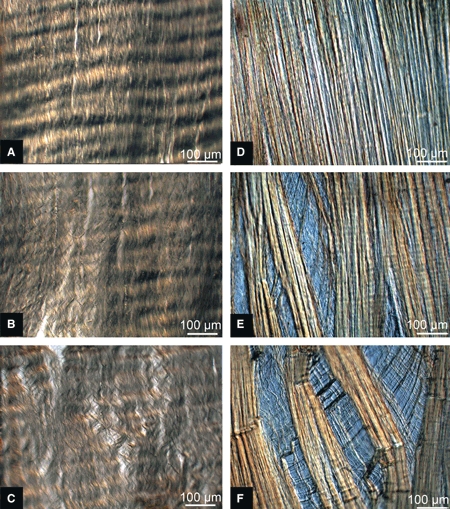
Differential interference contrast micrographs of typical progressive fibre unravelling shown for transverse extension ratios of 1, 1.5 and 2. (A–C) Immature sequence, uniform expansion of array as the transverse tensile load is applied. (D–F) Mature sequence, discontinuous fibre array expansion.
Fig. 6.
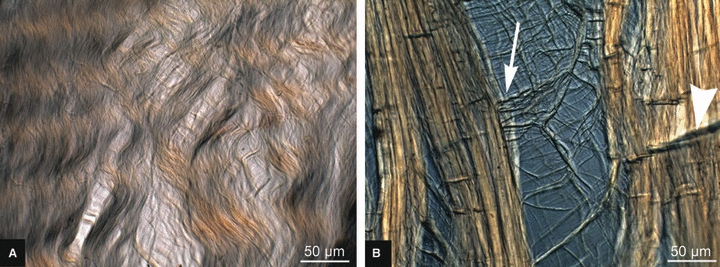
Fibrous detail of expanded tissue array at a transverse extension ratio of 1.5. (A) Immature and (B) mature. The white arrow indicates fibres bridging a cleft realigned to near-horizontal. The white arrowhead indicates localized fibre buckling.
In the slices from both the immature and mature discs, once deconstruction had been induced by stretching and the load removed, there was little or no tendency for the expanded fibrous meshwork to revert to its original unextended configuration, i.e. the stretching had, in fact, permanently disrupted interfibre cohesion.
Free-swelling behaviour
A difference was observed between the immature and mature tissues in their free-swelling response in 0.15 m saline bathing solution. Whereas the immature slices lay flat in the plane of the slide (Fig. 7A), the cropped lamellar fibres of the mature slices exhibited a raised, convoluted morphology (see Figs 7B and 8A). When cover-slipped for microscopy, the convolutions were flattened into what appears to be a localized fold (see Figs 7D and 8B). High-magnification viewing of these folds combined with a careful through-section focussing (see Fig. 9A) showed that the fibres traversing the fold were in the plane of the section, consistent with the tilting of the cropped fibres to accommodate the fold-back as shown schematically in Fig. 9B (see region 2). Note how the tilted cropped fibres merge gradually into the unperturbed region on one side of the fold (see region 3 in Fig. 9A,B) with a distinct change in focal depth on the other side (see region 1 in Fig. 9A,B).
Fig. 7.
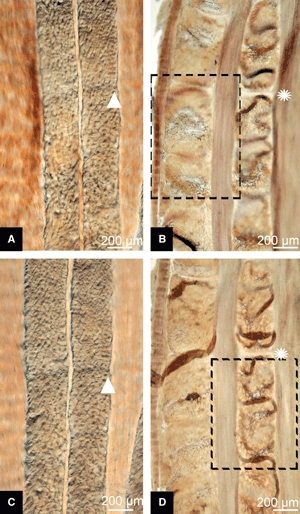
Differential interference contrast micrographs of free-swelling behaviour of interlamellar slices in 0.15 m saline. (A) Planar profile of immature slice. (B) Convoluted form of cross-sectional lamellae in mature slice. (C and D) Same pair of slices cover-slipped for improved microscopy (see location tags: white star and triangle). For the boxed area in (B) see Fig. 8A. For the boxed area in (D) see Fig. 8B.
Fig. 8.
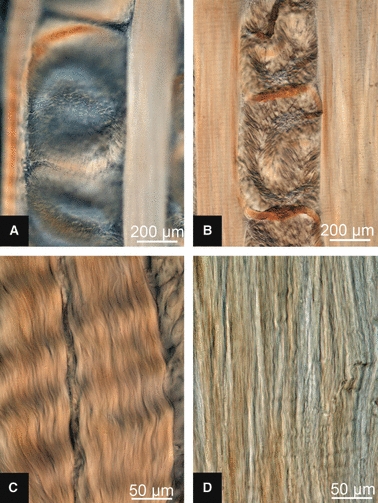
(A) High-magnification micrograph of free-swelling behaviour of mature slice in 0.15 m saline. (B) Suspected creases in mature slice. (C and D) In-plane lamellae of immature and mature slices, respectively.
Fig. 9.
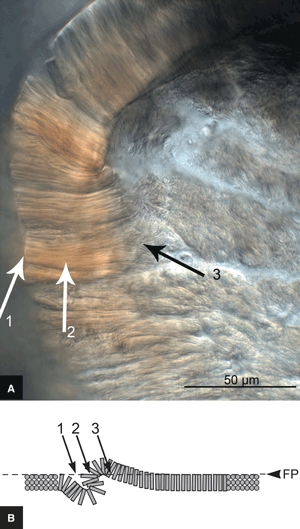
(A) High-magnification micrograph of a suspected crease in a mature slice. (B) Side-view schematic of proposed crease formation, with the dashed line indicating the plane in focus (FP). At site 2 the fibres traversing the crease are in the plane of the section due to their reorientation to accommodate the fold-back. 1, step change in focal depth; 3, gradual reorientation of fibres.
To test the hypothesis that these fold morphologies were an artefact of taking thin slices from a highly anisotropic architecture, rather than a structural feature inherent in the tissue, the fold width was examined in slices of different thickness. It was predicted that a thicker slice would take a greater distance to fold back on itself, which would be reflected in the fold width (see the schematics in Fig. 10A,B). Conversely, if the fold was a structural feature, then altering the section depth would not affect its width. Consistent with the proposed hypothesis, the 25-μm-thick slices (see Fig. 10A) resulted in narrower localized folds than those in the 45-μm-thick slices (see Fig. 10B).
Fig. 10.
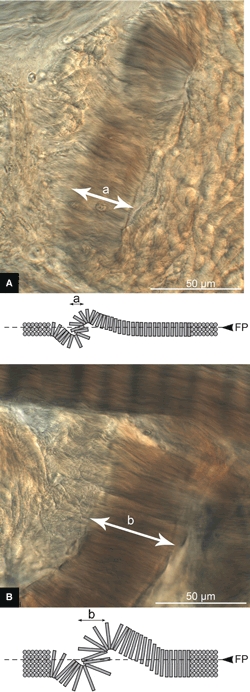
High-magnification micrographs of suspected creases in mature slices with section thickness of (A) 25 μm and (B) 45 μm. Beneath each micrograph is a side-view schematic of the suspected crease formation. FP, Focal Plane. Note the expected increase in crease width with increasing slice thickness, b > a.
Further evidence that this fold morphology was a swelling response could be seen in the response of the mature slices when the physiological saline (0.15 m) was substituted with distilled water to provide a higher swelling tension (see Fig. 11A,B, respectively). The convolutions are more extreme in distilled water than in the 0.15 m saline.
Fig. 11.
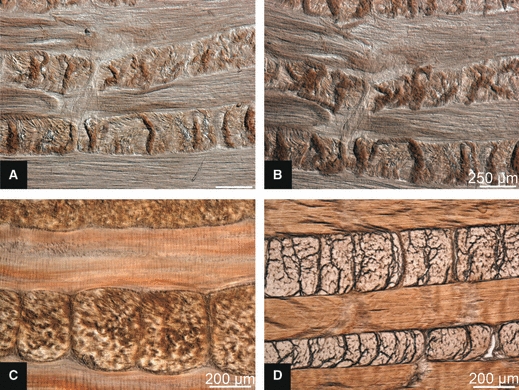
Further evidence that the ‘creases’ are a swelling effect. The same mature slice bathed in (A) 0.15 m saline and (B) distilled water. (C) Fixed mature slice, note absence of creases. (D) Fixed immature slice.
Artificially stabilizing the fibrous structures via formalin fixation of the block prior to sectioning greatly reduced the swelling response in the mature tissue, eliminating the folds altogether (see Fig. 11C). By contrast, fixation had no noticeable effect on the immature tissue (see Fig. 11D).
Aside from the swelling response apparent in the cropped cross-sectional lamellae, of further note is the difference in crimped morphology of the in-plane lamellae in the two age groups. The high-magnification images contained in Fig. 8C,D illustrate the typical in-plane lamellae of the immature and mature tissues, respectively. This morphology difference can also be seen in the lower magnification images contained in Figs 4 and 5.
Discussion
The complex structural integration of the nucleus, endplate and annulus, make it exceptionally difficult to isolate causative factors and sequential events in the complex cascade leading to intervertebral disc degeneration. This study, by using morphologically normal intervertebral discs from immature and mature ovine specimens, allowed the isolation of age as the specific variable in the study of annular structural unravelling behaviour. Gu et al. (1999) pointed to tissue dehydration [strongly linked with increasing age (Urban & McMullin, 1988)] as the cause of reduced radial permeability, probably causing a reduced energy dissipation capability of the disc. Alterations in lamellar unravelling behavior appear to similarly indicate a reduced functionality of the aged annulus and could conceivably be a contributor to the cascade of degeneration. Importantly, with many animal studies carried out on immature discs, the results here suggest that some caution is required with respect to extrapolating annular behaviour beyond this age group.
There is a clear difference in the behaviour of immature and mature annular fibres in response to transversely applied tensile forces. The immature tissue demonstrates graduated separation and widening between the interconnected longitudinal fibres. In the mature tissue the adjacent fibres appear to be firmly attached to one another, largely resisting any more uniform or generalized separation of the array. Instead the arrays split locally and then progressively widen from these same sites. The local creases that form imply an increased stiffness of the fibres and a tendency to eventually buckle as secondary contraction occurs along the primary array direction with transverse stretching. The previously reported increases in both collagen cross-linking and fragmentation associated with ageing (Bernick et al. 1991; Buckwalter, 1995; Roughley, 2004; Urban & Roberts, 1995) are consistent with the responses that we have observed in this new microstructural study.
Due to the smallness of the test slice dimensions (including section thickness), there was no possibility of obtaining any correlation between applied force and extension. Rather, we have used the morphological response to transverse stretching to infer potential differences in interfibre bonding relationships between the two tissue groups. The more uniform expansion of the immature array to form a fine, diffuse network of relatively discrete collagen fibres and fibre bundles (see Fig. 6A) clearly implies a higher level of organization than merely an array consisting of largely unconnected parallel fibres. Brinckmann (1986) induced damage to the inside of the disc wall, leaving only the outermost layers intact, prior to applying a load and measuring the resultant radial bulge. There were insufficient samples in the study of Brinckmann (1986) to draw conclusions regarding the effect of degeneration of the discs, although the general trend was for the least degenerate discs to show the most radial bulge. This suggests that the expansion of the mature array as exhibited in this study may require a higher load than the diffuse response seen in the younger tissue.
Further, the more localized separation that we have observed in the aged annulus suggests that a reduced functional performance could be expected in the aged disc. Under more extreme levels of deformation, widening of the array could result in a discontinuous opening of the array that may lead to cleft formation. This, combined with a nucleus still having hydrostatic properties, could be responsible for the report of mechanically induced prolapse occurring most readily in discs aged 40–50 years (Adams & Hutton, 1982; Gallagher, 2002). Such a disruptive scenario is also consistent with the mechanism proposed for disc herniation in adults in which extrusion of mainly nuclear material occurs through pre-existing annular clefts (Moore et al. 1996).
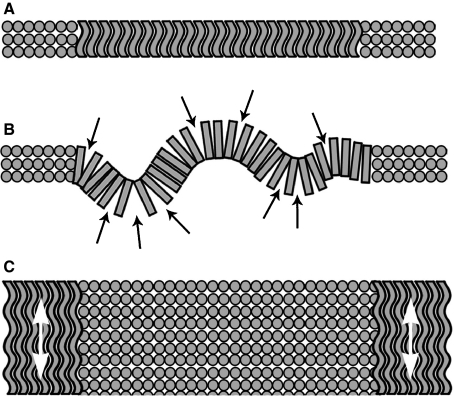
The second component of our study shows a clear difference in the tissue response to swelling pressure between immature and mature lamellae. Two different mechanisms that could produce the distinct swelling responses of immature and mature interlamellar slices are illustrated schematically in Fig. 12. The first relates to the interfibre cohesivity of the cropped cross-sectional lamellae (see Fig. 12A,B). The idealized lamellae are constructed of tightly packed perpendicular fibrils (see Fig. 12A). For this tissue to exhibit anything other than a planar profile would require fibre separation. In the immature tissue the swelling forces are insufficient to overcome the interfibre cohesion and the tissue remains planar. Mature tissue, in contrast, may have (i) a less uniform degree of cohesion between fibres (see Fig. 12B) and/or (ii) some fibres in the plane of the section that are free to flex when exposed to swelling forces, with no fibre separation required.
Fig. 12.
Mechanisms that could produce the distinct swelling responses of mature and immature thin interlamellar slices. (A) The swelling force is insufficient to cause fibre separation between the tightly packed cropped fibres in the immature slice; the resultant planar profile can be seen. (B) Mature tissue, in contrast, may have less uniform cohesion between fibres, hence swelling forces result in a convoluted profile to the cropped lamellae. (C) The crimped in-plane fibres in the immature tissue offer reversible extensibility (see white arrows), thus accommodating expansion of the cropped cross-sectional fibres.
A second possible mechanism affecting the distinct swelling responses observed relates to the stiffening of the mature collagen fibres (see Fig. 12C). We saw earlier in Fig. 8C,D the dramatic loss of crimped morphology in the collagen fibres across this age range. The collagen fibre is a high-strength biomaterial component but when crimped, as in the immature tissue, it can readily undergo low-stress reversible extension. If we consider our interlamellar slice in Fig. 12C, the intact in-plane fibres of the immature slice could extend (see white arrows) to accommodate the free swelling in the adjacent lamellae. Conversely, the stiffened fibres of the mature tissue would be less able to offer extension along their length, thus forcing the cross-sectional lamellae within the thin slice to buckle/convolute as the fibres swell.
Conclusions
This study provides a novel view of what are thought to be age-related changes in interfibre cohesivity as inferred from differences in both the unravelling behaviour of lamellae and artefactual morphologies linked to free-swelling behaviour. As the attention of more researchers is drawn to the repair and regenerative therapies of the annulus fibrosus (Bron et al. 2009), our study provides a graphic morphological indication of subtle biomechanical changes occurring in the ageing annulus fibrosus.
With the folded structures in the mature tissue slices confirmed as an artefact of hydrated thin interlamellar slices, we conclude that there is a fundamental change in the level of cohesivity within fibre bundles of the lamellae with ageing. Our identification of these artefacts emphasizes both the importance of thoroughly understanding disc wall morphology and the need to appreciate that significant artefacts of tissue handling can arise, which should not be mistaken for any inherent structural property.
Finally, it is important to emphasize that the deformations that we have employed in this study were not meant to simulate physiologically relevant deformations in the annulus. Rather, the large extension ratios were used to deconstruct the fibrous arrays within the lamellae so as to reveal what we believe are fundamental differences in interfibre cohesion.
Acknowledgments
M.L.S. acknowledges the award of a University of Auckland Doctoral Scholarship. The authors are also grateful for funding provided by the Wishbone Trust (NZ Orthopaedic Association).
References
- Acaroglu ER, Iatridis JC, Setton LA, et al. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury. 1981 Volvo Award in Basic Science. Spine. 1982;7:184–191. [PubMed] [Google Scholar]
- Bernick S, Walker JM, Paule WJ. Age changes to the anulus fibrosus in human intervertebral discs. Spine. 1991;16:520–524. doi: 10.1097/00007632-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Brinckmann P. Injury of the annulus fibrosus and disc protrusions. An in vitro investigation on human lumbar discs. Spine. 1986;11:149–153. doi: 10.1097/00007632-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–313. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Ebara S, Iatridis JC, Setton LA, et al. Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine. 1996;21:452–461. doi: 10.1097/00007632-199602150-00009. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Duncan NA, Lotz JC. Radial tensile properties of the lumbar annulus fibrosus are site and degeneration dependent. J Orthop Res. 1997;15:814–819. doi: 10.1002/jor.1100150605. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Letters. Spine. 2002;27:1378. doi: 10.1097/00007632-200206150-00028. [DOI] [PubMed] [Google Scholar]
- Gu WY, Mao XG, Foster RJ, et al. The anisotropic hydraulic permeability of human lumbar anulus fibrosus. Influence of age, degeneration, direction, and water content. Spine. 1999;24:2449–2455. doi: 10.1097/00007632-199912010-00005. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Schulze-Bauer CA, Feigl G, et al. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005;3:125–140. doi: 10.1007/s10237-004-0053-8. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Setton LA, Foster RJ, et al. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J Biomech. 1998;31:535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006a;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezowicz CA, Schechtman H, Robertson PA, et al. Mechanisms of anular failure resulting from excessive intradiscal pressure: a microstructural-micromechanical investigation. Spine. 2006b;31:2891–2903. doi: 10.1097/01.brs.0000248412.82700.8b. [DOI] [PubMed] [Google Scholar]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part I: a microscopic investigation of the translamellar bridging network. Spine. 2008;33:2702–2710. doi: 10.1097/BRS.0b013e31817bb92c. [DOI] [PubMed] [Google Scholar]
- Schollum ML, Robertson PA, Broom ND. A microstructural investigation of intervertebral disc lamellar connectivity: detailed analysis of the translamellar bridges. J Anat. 2009;214:805–816. doi: 10.1111/j.1469-7580.2009.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Development and degeneration of the intervertebral discs. Mol Med Today. 1995;1:329–335. doi: 10.1016/s1357-4310(95)80032-8. [DOI] [PubMed] [Google Scholar]
- Veres SP, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine. 2008;33:2711–2720. doi: 10.1097/BRS.0b013e31817bb906. [DOI] [PubMed] [Google Scholar]