Abstract
The aim of the study was to describe and depict the spatial arrangement of the colon microcirculatory bed as a whole. Various parts of the large intestine and terminal ileum were harvested from either cadaver or section material or gained peroperatively. Samples were then injected with India ink or methylmetacrylate Mercox resin for microdissection and corrosion casting for scanning electron microscopy. The results showed that extramural vasa recta ramified to form the subserous plexus, some of them passing underneath the colon taeniae. Branches of both short and long vasa recta merged in the colon wall, pierced the muscular layer and spread out as the submucous plexus, which extended throughout the whole intestine without any interruption. The muscular layer received blood via both the centrifugal branches of the submucous plexus and the minor branches sent off by the subserous plexus. The mucosa was supplied by the mucous plexus, which sent capillaries into the walls of intestinal glands. The hexagonal arrangement of the intestinal glands reflected their vascular bed. All three presumptive critical points are only gross anatomical points of no physiological relevance in healthy individuals. Neither microscopic weak points nor regional differences were proven within the wall of the whole large intestine. The corrosion casts showed a huge density of capillaries under the mucosa of the large intestine. A regular hexagonal pattern of the vascular bed on the inner surface was revealed. No microvascular critical point proofs were confirmed and a correlation model to various pathological states was created.
Keywords: injection, large intestine, microcirculation, scanning electron microscopy
Introduction
Together with the tunica mucosa, the microcirculatory bed of the colon plays a central role in the absorptive, secretory and protective functions of the colon. Capillary endothelial cells have a low proliferative rate and long turnover time, thus providing a relatively stable structure within most tissues. The two principal functions of the colon appear to be the storage of faeces before evacuation and high-capacity water absorption (Standring, 2005; Kachlik, 2006; Kachlik & Hoch, 2008).
As for the wall of the large intestine, it is composed of five main concentric layers (some of them having several sublayers), which are ordered according to their position from the external surface to the lumen (Wolfram-Gabel et al. 1986): serosa (tunica serosa, serous coat), subserosa (tela subserosa, subserous layer), muscular layer (tunica muscularis, muscular coat), submucosa (tela submucosa) and mucosa (tunica mucosa, mucous membrane). The muscular layer is composed of two layers: longitudinal layer (stratum longitudinale), reduced in the taenia coli (except in the vermiform appendix and rectum), and circular layer (stratum circulare). The mucosa is divided into three parts: muscularis mucosae (lamina muscularis mucosae), propria mucosae (lamina propria mucosae) and epithelium.
A thorough and detailed study summarizing the arrangement of the microcirculatory bed in all layers of the large intestine using injection methods on both the macroscopic and microscopic level has not yet been performed.
Thus, the aim of the study was to describe the microcirculation in the wall of the large intestine in detail by the use of several methods. For study at the macroscopic level, India ink injection was used, whereas at the microscopic level, corrosion casting and histomorphology were applied. Particular attention was paid to the terminology of the branches and plexuses. A model of the arrangement of the blood vessels in the normal (healthy) large intestine was created for possible comparison with pathological states, e.g. Crohn's disease, ulcerative colitis, colorectal cancer, etc.
Materials and methods
Two types of material were used in the study. A total of 142 samples were gathered from 118 cadavers (51 women and 67 men, aged 23–91 years) at the Department of Pathology of the Third Faculty of Medicine, Charles University in Prague and at the Královské Vinohrady Faculty Hospital in Prague. Another 21 samples were harvested during the operations of 20 patients (14 women and 6 men, aged 18–67 years) at the Department of Surgery of both affiliations.
Wedge-shaped specimens were cut out equally from all segments of the large intestine. Each specimen included a 10–15-cm section of the intestine with the mesocolon and supplying blood vessels. The source vessel was dissected, incised and cannulated, rinsed with saline (20–25 °C) and injected. Two injection media were used to replace the transparent saline.
A solution of black India ink (in a ratio of 2 : 3 with distilled water, at 20–25 °C) was successively injected into the vessel by means of hand-controlled pressure. After ligation of the supplying blood vessels, the sample was immersed in an 8% formaldehyde solution to be fixed for at least 10 days. After fixation it was dissected under a Nikon SMZ-U preparation magnifying glass.
A commercial product of the synthetic methylmetacrylate resin, Mercox C1-2B or Mercox C1-2R (Ladd Research, Burlington, VA, USA). A mixture containing 4 mL of prepolymerized resin and 1 mL of the monomer of methylmetacrylic acid containing 62.5 mg of the methylmetacrylate paste as an accelerator was made in the syringe and injected. Following injection and the polymerization of Mercox, a 7.5% solution of potassium hydroxide or 16% solution of nitric acid was used as macerating agent. The final corrosion cast was blue (if using the potassium lye maceration), yellow (if using the blue Mercox and the nitric acid maceration) or red (if using the red Mercox and the nitric acid maceration).
Afterwards, 51 samples of the large intestine were studied by scanning electron microscope (SEM). They were sputtered with gold powder in the 11HD Polaron Series (sputter coater). In the SEM, voltages of 2.5 and 10 kV were applied under magnifications of 4–396 to scan the samples (Phillips ESEM XL30 with Cambridge Stereoscan 250). For details of the method, see Aharinejad et al. (1992), Kachlik (2006) and Kachlik & Hoch (2008).
The terminology used to describe the branches and plexus are based on the Terminologia Anatomica; the terms are stated in English and then in Latin (in brackets using italics) (FCAT 1998).
Ethical considerations
The study was approved by the Ethics Commission of the Third Faculty of Medicine, Charles University in Prague. All of the samples were harvested with the written informed consent of the patients.
Results
The blood vessels in the wall of the large intestine can be divided into the vasa recta, subserous plexus, submucous plexus, intermuscular plexus and mucous plexus. Each of them can be described according to its origin, course, branching and relation to the neighbouring structures.
Straight vessels of the large intestine (vasa recta intestini crassi)
The vasa recta are branches from the Drummond's marginal artery (arteria marginalis coli) or from the most peripheral (i.e. last) arcades of the colic arteries (see Fig. 1). Running straight to the intestinal wall, they abruptly change their direction when entering the serosa. Within the outermost layer of the large intestine wall their branches form a fine intermingled net called the subserous plexus, which is the only contribution of the vasa recta to the vascularization of the intestinal wall proper.
Fig. 1.
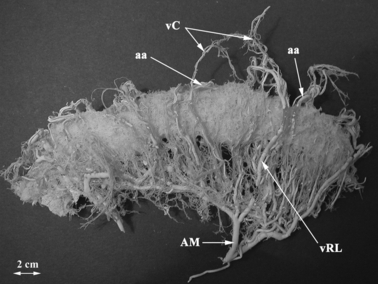
The corrosion cast of the vascular bed of the transverse colon and mesocolon with the Drummond's marginal artery (arteria marginalis coli). Bar: 2 cm. AM, arteria marginalis coli; aa, arterial arcade; vC, vasa centralia; vRL, vasa recta longa.
There are rich anastomoses between individual vessels on both surfaces of the large intestine and no regional differences are featured. They are arranged in a loose network of transverse meshes bordered by mildly tortuous arteries.
Subserous plexus (plexus subserosus)
Synonyms: subserous arteries and veins (arteriae subserosae et venae subserosae).
The vasa recta send frequent collaterals along their way, starting at their origin from the marginal artery to their termination. Before entering the external muscle layer, the short vasa recta give off branches to the mesocolic border of the large intestinal wall. These branches are scattered like the rays of a star but their short course limits the extent to which they can supply the intestinal wall. The short vasa recta only supply the area bordering the attachment of the mesocolon to the intestinal wall.
The long vasa recta have a characteristic slightly sinuous or sinusoid course in the subserous layer. On approaching the antimesocolic border, they can take an oblique or parallel turn to the intestinal wall or even change direction and turn back to the mesocolic border. There are rich anastomoses between the long vasa recta, short vasa recta and also between both types of vessels. There are also anastomoses between their collateral and terminal branches at the mesocolic and antimesocolic borders regardless of their localization. Their average diameter is approximately 80 μm.
The vessels supplying the mesocolic border and mesocolic taenia are branches of the short vasa recta. In addition, there are branches of the long vasa recta or their collaterals supplying the anterior and posterior surface without any penetration into the intestinal wall. Finally, there are branches for the distant omental taenia and free taenia, which are derived from the terminal branches of the long vasa recta before their penetrating into the intestinal wall. The length of the subserous arteries does not exceed 8 mm, usually varying between 2 and 4 mm. The subserous arteries always terminate in the same manner. As they approach any taenia or intestinal border, they bifurcate into a left and a right branch of equal calibre (30–40 μm) taking a course parallel to the longitudinal axis of the intestine.
Furthermore, in addition to the above-described branches, there are muscular and omental branches that are derived from the vasa recta, their collaterals or anastomoses. The muscular branches change their direction after a short subserous course, penetrating into the muscular layer (calibre: 30 μm). The branches for the greater omentum run perpendicularly to the longitudinal axis of the intestine and leave the intestinal wall via the omental taenia.
The subserous plexus sends minor branches to feed the muscular layer of the intestine. They can be classified as long and short branches, and are advised to be called long and short muscular branches (rami musculares longi et breves). The short branches enter the external (longitudinal) layer, arranged in the taeniae, and supply it, reaching as deep as the intermuscular space. The long branches pass through the taeniae and send off branches to feed it. They continue and join or pass the intermuscular plexus and finally terminate in the internal (circular) muscular layer. In rare cases, they may reach the submucous plexus. This source of the arterial blood supply for the muscular layer is to be considered of minor importance (see Figs 2–6).
Fig. 2.
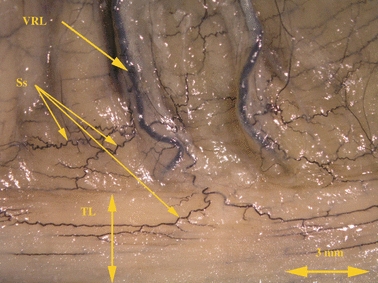
The long vasa recta longa of the transverse colon submerging under the free taenia. India ink injection. Bar: 3 mm. Ss, plexus subserosus; VRL, vasa recta longa; TL, taenia libera.
Fig. 6.
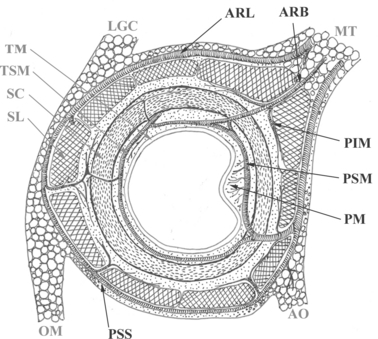
The scheme of the intramural plexuses in cross-section of the large intestine wall (freely according to Wolfram-Gabel). MT, mesocolon transversum; LGC, ligamentum gastrocolicum; TM, tunica mucosa; TSM, tunica submucosa; SC, stratum circulare; SL, stratum longitudinale; OM, omentum majus; AO, appendix omentalis, ARL, arteria recta longa; ARB, arteria recta brevis; PIM, plexus intermuscularis; PSM, plexus submucosus; PM, plexus mucosus; PSS, plexus subserosus.
Fig. 5.
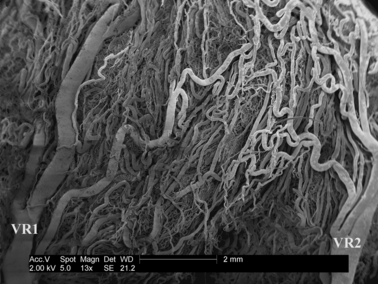
The external view of the surface of the vascular bed of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 2 mm. VR1, VR2, adjacent vasa recta.
Submucous plexus (plexus submucosus)
The submucous plexus is situated within the submucosa among the loose connective tissue, lymph vessels and nervous submucous plexus of Meissner. The vascular network is composed of vessels of a large calibre running parallel to the longitudinal axis of the intestine. This plexus sends off branches for the terminal vascularization of the inner layers of the intestinal wall. The outgoing vessels follow two opposite directions – centrifugal turn back to the muscular layer (called recurrent muscular branches; rami musculares recurrentes) and centripetal move in the direction of the intestinal lumen and give rise to the mucous plexus. In comparison with the other layers of the intestinal wall, the submucosa is rather thin (< 100 μm).
The feeding vessels are the long vasa recta. After their extramural course they turn abruptly and penetrate the whole muscular layer perpendicularly to the longitudinal axis of the intestine to reach the submucous plexus. Some branches from the subserous plexus (long muscular branches) may rarely reach the submucous plexus but their contribution to the plexus is negligible.
The centrifugal branches can be divided into several types of arteries depending on their length, branching and termination. The centripetal branches are sent off perpendicularly to the longitudinal axis of the intestine and run directly to the lumen (see Figs 3, 4, 6, 7 and 8).
Fig. 3.

The overview of the vascular bed of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 2 mm. Mc, tunica mucosa; Sm, tunica submucosa; Ms, tunica muscularis.
Fig. 4.

The cross-section of the vascular bed of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 200 μm. Mu, tunica mucosa; Sm, tunica submucosa; Ms, tunica muscularis; Ss, plexus subserosus; aR, arteria recta; vR, vena recta.
Fig. 7.

The longitudinal section of the vascular bed of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 500 μm. Mu, tunica mucosa; SM, tunica submucosa; C, glandulae intestinales = Lieberkühn's crypts; a, artefacts (extravasates).
Fig. 8.
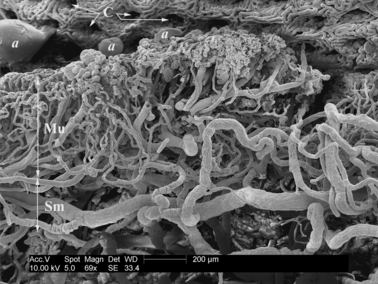
The detail of the longitudinal section of the vascular bed of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 200 μm. Mu, tunica mucosa; Sm, tunica submucosa; C, glandulae intestinales = Lieberkühn's crypts; a, artefacts (extravasate).
Intermuscular plexus (plexus intermuscularis)
Synonyms: plexus muscularis, plexus myentericus.
There is a greater number of feeding vessels that serve as a source for the muscular layer; they are issued by the submucous plexus as its centrifugal branches, by the subserous plexus as centripetal branches or emerge directly from the penetrating vasa recta.
The centrifugal branches from the submucous plexus are the most important source of arterial blood for the muscular layer of the intestinal wall. They are called the recurrent muscular branches (rami musculares recurrentes plexus submucosi). They originate in the submucous layer from trunks of the vasa recta, their collaterals or from mutual anastomoses. Their diameter ranges from 25 to 30 μm.
According to their length, they can be classified as long, middle and short. The short recurrent muscular branches (rami musculares recurrentes breves) terminate exclusively in the internal (circular) muscular layer without reaching the intermuscular plexus. They are very short, ranging from 250 to 500 μm. The middle branches (rami musculares recurrentes medii) supply the internal (circular) muscular layer and the intermuscularis plexus, which runs in a perpendicular and rectilinear direction through the circular muscle layer (750–1000 μm). They terminate in the intermuscular plexus, which is parallel to the longitudinal axis of the intestine. Finally, the long branches (rami musculares recurrentes longi) have the same orientation as the middle branches but reach as far as the muscular bundles of the taeniae (longitudinal muscular layer). Some may even join the subserous plexus. During their course, they send off branches for both muscular layers and for the intermuscular plexus, their length ranging from 1500 to 2000 μm. There are frequent anastomoses between collateral and terminal branches of all types of the recurrent muscular branches.
The second source of the arterial blood vessels, although less numerous in branches and less voluminous, is provided by arteries issued centripetally from the subserous plexus (rami musculares plexus subserosi). These can be classified as long and muscular branches. They originate from either the vasa recta or from their collaterals and anastomoses. Although not as significant as the recurrent muscular branches from the submucous plexus, these branches contribute considerably to the arterial blood supply of all three taeniae. Their diameter is approximately 25 μm.
The short muscular branches enter the taeniae (longitudinal muscular layer) running perpendicularly to the longitudinal axis of the intestine and supply them. Some terminal branches reach the intermuscularis plexus, being 200 μm long (or 400 μm via the taenia) and helping to feed it. The long muscular branches pass through the longitudinal muscular layer and send off some nourishing branches. They then join or pass the intermuscular plexus within the intermuscular space and terminate in the circular muscular layer. Their length is usually between 750 and 1500 μm. In rare cases, they may even reach the submucous plexus and contribute to its blood supply.
The branches derived from this plexus and from all vessels passing the muscular layer are called the interfascicular branches (rami interfasciculares). Their diameter ranges from 10 to 20 μm and they run rectilinearly between the muscle fascicles which they supply. There are rich mutual anastomoses. Finally, they send the fascicular branches (rami fasciculares), which are 10–15 μm in diameter, into the muscle fascicles perpendicularly to their longitudinal axis. Their terminal branches are the precapillary arterioles anastomosing with each other and forming a terminal arteriolar network giving rise to the capillary bed of the muscular fascicle (see Figs 3, 4 and 6).
Mucous plexus (plexus mucosus)
The entire thickness of the mucosa is regularly juxtaposed by intestinal glands (glandulae intestinales; Lieberkühn's crypts). The upper ends of the glands open into the intestinal lumen and the bases reach as deep as the muscularis mucosae. The mucous plexus resembles a mosaic because of its regular dimensions and shape. The diameter of its vessels ranges from 25 to 30 μm. The mucous plexus is fed by the mucosal branches from the submucous plexus. They run perpendicularly to the longitudinal axis of the intestine. Due to the presence of folds on the mucosal surface, their course is variable and they can be classified as long and short (see Figs 6–8).
The short mucous branches (rami mucosi breves) run to the segments of mucosa located between two semilunar folds. They originate from the adjacent submucous plexus and send off two to four collateral branches. Their length ranges from 500 to 1000 μm. The long mucous branches (rami mucosi longi) run in the mucosa of the semilunar folds. Their course is more rectilinear and sometimes slightly flexed. The long mucous branches range in length from 2 to 5 mm. Each branch bifurcates at a variable distance from the lumen into two branches of the first order (15 μm in diameter). These branches then send off two or three more branches of second order, respectively. All of these collaterals successively send the precapillary arterioles (10 μm in diamater). The arterioles coil round the bases and bodies of the intestinal glands and descend as far as the furthermost luminal surface. Some of the arterioles run a straight course but the majority form waves and intermingle with each other, sending off capillaries. A dense network of capillaries covers the glands, which are embedded in a vascular cushion (see Fig. 8).
The luminal surface is covered by capillaries. They change their direction from perpendicular to horizontal and then they turn back and run deep to join a venule. They may also curve along the circular opening brim of the gland orifice, encircling it like rods of a wreath (see Fig. 9).
Fig. 9.

The overview of the vascular bed luminal surface of the large intestine in the scanning electron microscope. Corrosion cast. Capillaries encircling the luminal orifice of the glandulae intestinales. Bar: 100 μm. Feeding capillaries enwrap the walls and encircle the luminal gland orifice.
The wreath-like structures are formed by one to five capillaries and have various widths. The capillaries anastomose with each other to encircle the circumference of the gland orifice. The length of each individual capillary is different, ranging from a narrow spot, enabling the capillary to change the ascending course into the descending course, to one-half of the gland orifice circumference (see Fig. 10). Some capillaries connect adjacent wreathes of vessels, usually in more anastomosing sites. This mosaic (carpet) is identical to the SEM picture of the normally-shaped intestinal mucosa. The outline of the glands is maintained mainly by the dense network of the superficially laid capillaries forming a system of vascular wreaths mutually connected and merging to form a carpet. The diameter of the gland orifice varies from 60 to 90 μm and the depth of the gland ranges from 250 to 300 μm.
Fig. 10.

The overview of the vascular bed luminal surface of the large intestine in the scanning electron microscope. Corrosion cast. Bar: 200 μm. Hexagons of the bee honeycomb arrangement of glandulae intestinales.
The total view of the gland carpet may resemble a honeycomb (see Fig. 8). The gland orifices are arranged in a hexagonal web; the centre of one gland orifice is situated in the middle of a hexagon and the corners are located in the orifices of six adjacent glands (see Fig. 11). This strictly regular disposition organization of the intestinal gland reflects the arrangement of the large intestine mucosa. The total width of the mucous plexus varies from 200 to 300 μm.
Fig. 11.
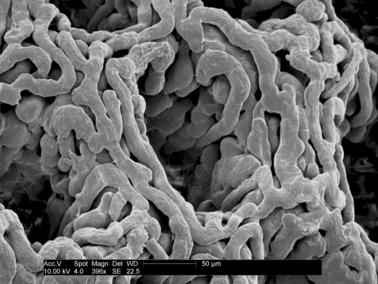
The luminal orifices of the glandulae intestinales (Lieberkühn's crypts) of the vascular bed of the large intestine. Corrosion cast. Bar: 50 μm.
Discussion
Resin corrosion injections are the most suitable technique to depict and study the arrangement of blood vessels in the intestinal wall at both the macroscopic and microscopic level (Shikata & Shida, 1985; Christofferson & Nilsson, 1988; Krstić, 1991; Aharinejad et al. 1992; Hossler, 1998). Our studies confirm this. As for the microcirculatory bed of the wall of the large intestine, there are several studies reporting the successful application of this technique in animals (Shikata & Shida, 1985; Browning & Gannon, 1986; Christofferson & Nilsson, 1988; Krstić, 1991; Aharinejad et al. 1992; Skinner et al. 1995; Hossler, 1998; Sangiorgi et al. 2007) but only few articles concerning the application in humans (Gannon et al. 1984; Sun et al. 1992; Skinner et al. 1995; Araki et al. 1996; Fait et al. 1998). In animals, the principal study was published by Shikata & Shida (1985). They applied this technique in the experimental study of colon anastomosis in healing after the stapler operation using instruments of various origins in dogs. Gannon et al. (1984) carried out an experiment involving vascular injections followed by in-vivo microscopy of the laboratory rat large intestine. Details of the morphological relationship of individual plexuses to the corresponding intestinal wall layers and the level of their location were reported by Bernardes & Patrício (2004), Boulter & Parks (1960), Goerttler (1932), Horstmann (1943), Ohtsuka et al. (1988), Wolfram-Gabel & Maillot (1981) and Wolfram-Gabel et al. (1982, 1983, 1984, 1986). To compare the data of various segments of the gastrointestinal tube, see studies by Wolfram-Gabel et al. (1984, 1986); the duodenum was scrutinized by Szyika (1976) and Wolfram-Gabel et al. (1982), and the small intestine and stomach by Barclay & Bentley (1949).
The vascular bed of the large intestine, as described above, resembles a similar arrangement of the intramural arteries in the stomach (Barclay & Bentley, 1949; Gannon et al. 1984) and small intestine (Szyika, 1976). It also reflects the vascular arrangement in the striated muscle, which was described by Saunders et al. (1957) as ‘macromesh’ with a finer plexus within it called the ‘micromesh’. In the large intestine, the term ‘micromesh’ can be applied to the mucous plexus between the epithelium of the mucosa and the submucosa. The rich mucous vascular bed was termed the ‘carpet’ by Brockis & Moffat (1958) who discovered the similarity of the loops around the intestinal glands with the carpet pattern.
As for the terminology, we followed the terms proposed by Wolfram-Gabel & Maillot (1981) and Wolfram-Gabel et al. (1982, 1983, 1984, 1986) and added the Latin terms to all of the branches and plexus described (in brackets using italics) because the Terminologia Anatomica (1998) and Terminologia Histologica (2007) do not include any terms concerning the microcirculatory bed of the intestine (FCAT 1998; FICAT, 2008).
As early as 1736, Albinus described the branching of the intramural vessels in the intestinal wall but his study was limited to the small intestine. This was proven by the translation of his thesis (Albinus, 1736; Kachlik, 2006; Kachlik & Hoch, 2008). Bourgery (1839) described the subserous plexus as the principal plexus issuing arteries for the deeper layers of the intestinal wall. Mellière (1927) assumed the vasa recta to be branches from the marginal artery, opening out in the subserous connective tissue into a plexus nourishing all deeper layers.
There is extensive branching of the subserous arteries, especially in anastomotic branches that are rich in the subserous layer and can be divided into anastomoses of the first and second order, respectively. The first-order anastomoses are situated either between two collateral branches, between two terminal branches or between the collateral and terminal branch of the adjacent vasa recta. Their diameter is approximately 50 μm and they are arranged in a regular network with distances between adjacent vessels from 1.4 to 2.0 mm. This creates an impression of the continuous and regular striation. The second-order anastomoses (being of the same dimensions) are branches of the previous level of anastomoses, situated mostly transversely and perpendicularly to the longitudinal axis of the intestine, thus forming the second striation. Such an arrangement, first described in detail by Wolfram-Gabel et al. (1984), resembles that reported by Szyika (1976) in the jejunum and ileum. Apart from nourishing the subserous layer, the main purpose of the vasa recta is to penetrate the muscular layer. There are several terminal branches penetrating deep into the wall to the submucous layer (Griffiths, 1961 reported four main sites without any detailed data).
Ernst (1851) observed that the long vasa recta penetrating the muscular layer send off branches supplying it. Later, Heller (1872) added the description of the blood supply of the muscular layer directly from the subserous plexus (for more details, see Hou-Jensen, 1931 and Wolf-Heidegger, 1942). The intermuscular plexus itself is interposed between the internal (circular) and external (longitudinal) muscular layers (arranged in the taeniae in the majority of the large intestine) in the intermuscular space. It is rather thin but it supplies the thickest layer of the large intestinal wall. This is the reason why there are accessory vessels directly derived from the adjacent plexuses (subserous and submucous). These vessels contribute to the supply of the muscle bundles and fibres without interpolating in any further plexus. The plexus helps to hold both muscular layers in place. The long recurrent muscular branches have the same course as the middle branches but reach as far as the longitudinal muscular layer (taeniae). Some even join the subserous plexus, as stated by Wolfram-Gabel et al. (1984). The second source of the arterial blood vessels, although less frequent in branches and less voluminous, is provided by arteries issued by the subserous plexus centripetally. Wolfram-Gabel et al. (1984) called them the ‘arteriae musculares’ but we recommend the term muscular branches from subserous plexus (rami musculares plexus subserosi). As for the mucous plexus, our results of the diameter of the intestinal gland orifices (varying from 60 to 90 μm) are similar to those reported by Wolfram-Gabel et al. (1983) (80–100 μm).
Al-Fallouji (1984) was the first to emphasize that there are no regional differences in the arrangement of the intramural plexuses of the large intestine. Any segment of the large intestine can be used as the terminal end for a postresection anastomosis. There is no morphological background for the development of ischaemic consequences after the anastomosis of two segments of the large intestine following resection. Rather, the factors of primary importance include: the calibre of the colon lumen, width of the colon wall and the mobility of the colon segment. The danger of ischaemia is due to the extensive traction of the mesocolon or the newly anastomosed large intestine.
The existence of the continuous intramural plexuses within the whole length of the small and large intestine allows us to confirm the theory of a compact intestinal intramural vascular canal. Such a connection is like one huge anastomosis that can replace the blood supply of one source artery if some disruption in flow occurs. A blockage due to either iatrogenic (ligation) or pathological (embolization, thrombosis, obturation or arteriosclerosis) causes can be compensated in varying degrees by the anastomoses, which allow a change in the direction of blood flow. Especially in the rectum, the theory of the inverted blood flow from the middle and inferior rectal artery to the network of the superior rectal artery, and even more orally, was suggested by McGregor (1957) and Sunderland (1942). This theory is pivotal for the denial of the physiological function of the critical points. These points exist as places of the interrupted blood supply. In certain cases, the macroscopically visible anastomoses may be absent. In the otherwise healthy and unaffected patient, they have no physiological function. However, in cases of any pathological affliction or embryological discrepancy [e.g. in infant necrotizing colitis, arteriosclerosis (abdominal angina), venous thrombosis and states with disrupted haemocoagulation], these points indicate predisposed sites of vulnerability.
The architecture of the capillary network in the large intestine segments was qualitatively described by Aharinejad & Lametschwandtner (1992) in rats and guinea pigs, by Browning & Gannon (1986), Sangiorgi et al. (2007) and Skinner et al. (1995) in rats, and in humans only by Sun et al. (1992) in normal tissue, transitional mucosa and adenocarcinoma, by Skinner et al. (1995) comparing rat and human colon and by Araki et al. (1996) and Fait et al. (1998). Interestingly, comparisons to the microvascular architecture of the small intestine revealed some differences. Skinner et al. (1995) presented different types of microvascular architecture within the individual villi. He discerned a fountain-like arrangement, in which the arterioles reach the top of the villus without branching (‘fountain-type’), a deeply interconnected type (‘step ladder’) and a ‘tuft-type’. Unfortunately, this classification cannot be applied to the large intestine. Browning & Gannon (1986) described a gradual reduction of both the mucous capillary density and of the average network width towards the aboral end of the large intestine. Aharinejad & Lametschwandtner (1992) perceived it as a reflection of successive decrease of the water reabsorption towards the aboral end of the colon. These findings are in stark contrast to the results stated by Fait et al. (1998) and those obtained by us. He suggested that the discrepancy between species is based on different intestinal functions and nutrition. The rodent colon is comparatively much longer than that of the human. Fait et al. (1998) was the first to give a quantitative description of a microvascular unit based on three parameters: intercapillary distances (107.2 ± 27.6 μm), interbranching distances (51.3 ± 28.5 μm) and branching angles (87.4 ± 29.2°) in various segments of the large intestine. He found no regional differences. However, Araki et al. (1996) reported several differences in microvascular architecture between the ascending and sigmoid colon. The subsurface capillary network of the ascending colon is honeycomb-shaped and multi-layered, whereas that of the sigmoid colon is almost single-layered. He speculated that the capillary network of the ascending colon is closely related to the greater degree of water absorption and electrolyte transport activities compared with those of the sigmoid colon.
Concluding remarks
This article brings together thorough and comprehensive information on the arrangement of the blood supply to the large intestine wall. All vascular plexuses are connected in a long channel running through the whole length of the intestine. If necessary, it can serve as a huge collateral. This collateral is sufficient only in an otherwise non-afflicted intestine (without developmental defect, immune, vascular or connective tissue diseases, etc.). It presents a model that can serve as a background for other studies concerning the changes of the large intestine wall blood supply in various pathological states, such as colorectal cancer, inflammatory bowel disease, especially Crohn's disease, etc.
Acknowledgments
Grant support: GAUK 108/1999/C, GAUK 25/2001/C and GAUK 103/2004/C. Special thanks to Prof. Alois Lametschwandtner (Department of Organismic Biology, Vascular and Muscle Research Unit, University of Salzburg, Austria) for the use of the SEM laboratory.
Author contributions
D.K. – substantial contributions to the conception and design, performance of the injections, corrosion casting, acquisition of data or analysis and interpretation of data. V.B. – substantial contributions to the conception and design, performance of the injections, corrosion casting, acquisition of data or analysis and interpretation of data. J.S. – senior author, final approval of the article.
References
- Aharinejad SH, Lametschwandtner A. Microvascular Corrosion Casting. New York: Springer Verlag; 1992. [Google Scholar]
- Aharinejad S, Gangler P, Hagen D, et al. Studies on the microvascularization of the digestive tract by scanning electron microscopy of vascular corrosion casts. 1. Large intestine in rats and guinea pigs. Acta Anat. 1992;141:278–283. doi: 10.1159/000147315. [DOI] [PubMed] [Google Scholar]
- Albinus BS. Dissertatio De Arteriis Et Venis Intestinorum Hominis. Leidae Batavorum: Theodorum Haak; 1736. [Google Scholar]
- Al-Fallouji MAR. The surgical anatomy of the colorectal intramural blood supply. Vasc Surg. 1984;18:364–371. [Google Scholar]
- Araki K, Furuya Y, Kobayashi M, et al. Comparison of the mucosal microvasculature between the proximal and distal human colon. J Electron Microsc. 1996;45:202–206. doi: 10.1093/oxfordjournals.jmicro.a023433. [DOI] [PubMed] [Google Scholar]
- Barclay AE, Bentley FH. The vascularisation of the human stomach. Br J Radiol. 1949;22:62–67. doi: 10.1259/0007-1285-22-254-62. [DOI] [PubMed] [Google Scholar]
- Bernardes A, Patrício J. Adaptation de la microvasculari-sation du côlon à différents degrés d'ischémie: etude anatomo-chirurgicale. E-mémoir Acad Nat Chir. 2004;3:26–33. [Google Scholar]
- Boulter PS, Parks AG. Submucosal vascular patterns of the alimentary tract and their significance. Br J Surg. 1960;47:546–550. doi: 10.1002/bjs.18004720518. [DOI] [PubMed] [Google Scholar]
- Bourgery JM. Traité complet de l’Anatomie de l’Homme. Paris: Delaunay; 1839. Vol. 5 Splanchnology; pp. 169–179. [Google Scholar]
- Brockis JG, Moffat DB. The intrinsic blood vessels of the pelvic colon. J Anat (London) 1958;92:52–57. [PMC free article] [PubMed] [Google Scholar]
- Browning J, Gannon B. Mucosal microvascular organization of the rat colon. Acta Anat. 1986;126:73–77. doi: 10.1159/000146191. [DOI] [PubMed] [Google Scholar]
- Christofferson RH, Nilsson BO. Microvascular corrosion casting with analysis in the scanning electron microscope. Scanning Microsc. 1988;10:43–63. [Google Scholar]
- Ernst F. Über die Anordnung der Blutgefässe in den Darmhäuten. Zürich: Universität Zürich; 1851. [Google Scholar]
- Fait E, Malkusch W, Gnoth AH, et al. Microvascular patterns of the human large intestine: morphometric studies of vascular parameters in corrosion casts. Scanning Microsc. 1998;12:641–651. [Google Scholar]
- FCAT. Terminologia Anatomica. Stuttgart: Thieme Verlag; 1998. [Google Scholar]
- FICAT. Terminologia Histologica: International Terms for Human Cytology and Histology. Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Gannon B, Browning J, O’Brien P, et al. Mucosal microvascular architecture of the fundus and body of human stomach. Gastroenterology. 1984;96:866–875. [PubMed] [Google Scholar]
- Goerttler K. Der konstruktive Bau der menschlischen Darmwand. Morph Jb. 1932;69:329–380. [Google Scholar]
- Griffiths JD. Extramural and intramural blood supply of the colon. Br Med J. 1961;4:323–326. doi: 10.1136/bmj.1.5222.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A. Über die Blutgeffässe des Dünndarmes. Ber Verh Kgl Sächs Ges Wiss. 1872;24:165–171. [Google Scholar]
- Horstmann W. Űber die Mesenterialgefässe und ihren Einbau in die Darmwand. Morph Jb. 1943;89:249–279. [Google Scholar]
- Hossler FE. Vascular corrosion casting can provide quantitative as well as morphological information on the microvasculature of organs and tissues. Micros Today. 1998;6:14–15. [Google Scholar]
- Hou-Jensen HM. Über die Anordung der Blutgefässe im Intestinum ileum. Z Anat Entw-Gesch. 1931;94:68–93. [Google Scholar]
- Kachlik D. Blood Supply of the Large Intestine – Thesis. Praha: Author-published; 2006. in Czech. [Google Scholar]
- Kachlik D, Hoch J. The Blood Supply of the Large Intestine. Praha: Karolinum Publishing; 2008. [Google Scholar]
- Krstić RV. Human Microscopic Anatomy. Berlin: Springer-Verlag; 1991. [Google Scholar]
- McGregor AL. A Synopsis of Surgical Anatomy. 8th edn. Baltimore: Williams & Wilkins Company; 1957. [Google Scholar]
- Mellière J. Étude de la vascularisation des tuniques du segment gauche du colon. Ann Anat Pathol Méd-Chir. 1927;4:867–888. [Google Scholar]
- Ohtsuka A, Ohtani O, Murakami T. Microvascularization of the alimentary canal as studied by scanning electron microscopy of corrosion casts. In: Motta PM, Fujita H, editors. Ultrastructure of the Digestive Tract. Boston: Martinus Nijhoff Publishers; 1988. pp. 202–212. [Google Scholar]
- Sangiorgi S, Boni L, Dionigi G, et al. Patterns microvascular distribution of the colonic wall studied by electron microscopy: experimental study in rats. J Med Sci Res. 2007;15:13–18. [Google Scholar]
- Saunders RLCH, Lawrence J, Maciever DA, et al. The anatomic basis of the peripheral circulation in man or the concept of the macromesh and micromesh as illustrated by the blood supply of muscle in man. In: Redish W, Tango F, editors. Peripheral Circulation in Health and Disease. New York: Grune & Stratton; 1957. p. 144. [Google Scholar]
- Shikata J, Shida T. Experimental studies by the resin-casting method on the vascular structure of the colon following stapler anastomoses. Dis Colon Rectum. 1985;28:341–346. doi: 10.1007/BF02560437. [DOI] [PubMed] [Google Scholar]
- Skinner SA, Frydman GM, O’Brien PE. Microvascular structure of benign and malignant tumors of the colon in humans. Dig Dis Sci. 1995;40:373–384. doi: 10.1007/BF02065424. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Edinburgh: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- Sun XF, Zhang H, Wu XC, et al. Microvascular corrosion casting of normal tissue, transitional mucosa and adenocarcinoma in the human colon. Acta Oncol. 1992;31:37–40. doi: 10.3109/02841869209088262. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Blood supply of the distal colon. Austr NZJSurg. 1942;11:253–263. [Google Scholar]
- Szyika AS. Systématisation de l'angioarchitectonie du jéjunum et de l'iléon chez l’Homme adulte. Arch Anat Hist Embr Norm et Exp. 1976;59:79–142. [PubMed] [Google Scholar]
- Wolf-Heidegger G. Der intramurale Verlauf der Dünndarmgefässe. Gastroenterologie. 1942;66:56. [Google Scholar]
- Wolfram-Gabel R, Maillot C. Vascularisation des tuniques sous-muqueuse et muqueuse des première et deuxième portions du duodénum chez l’Homme adulte. Arch Anat Hist Embr Norm et Exp. 1981;64:129–171. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritké JG. Les réseaux vasculaires de la couche sous-séreuse du côlon chez l’Homme. Archs Anat Histol Embryol. 1982;65:77–98. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritké JG. La vascularisation des tunique sous-muquese et muquese du chez l’Homme. Archs Anat Histol Embryol. 1983;66:67–98. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritké JG. Les réseaux vasculaires de la tunique musculeuse du côlon chez l’Homme. Archs Anat Histol Embryol. 1984;67:57–76. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritké JG. Systématisation de l'angioarchitectonie du côlon chez l'homme adulte. Acta Anat. 1986;125:65–72. [PubMed] [Google Scholar]


