Abstract
Background/Aims
Although increased serum uric acid (SUA) concentrations are commonly encountered in patients with risk factors for coronary artery disease (CAD), the clinical value of SUA has not been established.
Methods
The study group comprised 687 consecutive patients with suspected CAD who had undergone coronary angiography. CAD was defined as stenosis ≥ 50% of the luminal diameter. CAD severity was expressed as 1-, 2-, or 3-vessel disease. Metabolic syndrome (MS) was defined according to National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria, and aortofemoral pulse wave velocity (PWV) was obtained by arterial catheterization invasively.
Results
In total, 395 patients had CAD. SUA was higher in patients with CAD as compared to those without CAD (5.5 ± 1.0 vs. 5.2 ± 1.0 mg/dL, p = 0.004). In addition, SUA was significantly associated with the severity of CAD (p = 0.002). However, after adjusting for significant confounding factors including age, diabetes, smoking, cholesterol, MS, and PWV, SUA was not an independent risk factor for CAD (p = 0.151). Based on a subgroup analysis, SUA was more closely associated with CAD in women than in men, and in the highest quartile (≥ 6.4 mg/dL) than in the first quartile (< 4.8 mg/dL); however, these results were not significant (p = 0.062, p = 0.075, respectively). In a multivariate regression analysis, the most important determinant of SUA was MS (i.e., insulin resistance syndrome), which is strongly associated with CAD.
Conclusions
In patients with suspected CAD, SUA was not an independent risk factor for CAD and may be merely a marker of insulin resistance.
Keywords: Coronary artery disease, Insulin resistance, Uric acid
INTRODUCTION
Increased serum uric acid (SUA) levels are frequently encountered in subjects with obesity, glucose intolerance [1], renal disease [2], hyperlipidemia, atherosclerosis [3], and hypertension (HTN) [4], which all play a causal role in the pathogenesis of coronary artery disease (CAD). The clinical value of SUA for predicting CAD, however, is uncertain.
Several cohort studies [5,6] on subjects with HTN have revealed a significant association between SUA and future cardiovascular (CV) events. Additionally, based on data from the First National Health and Nutrition Examination Survey (NHANES I) [7], increased SUA levels are independently and significantly associated with ischemic heart disease and CV mortality. In contrast, results of the Framingham Heart Study [8] and Evans County Study [9], which are extensively quoted epidemiologic examinations, have shown that hyperuricemia cannot be recognized as an independent CV risk factor.
The pathophysiologic mechanisms underlying increased SUA concentrations in atherosclerotic diseases appear to be accounted for by insulin resistance [10], which is a major characteristic of metabolic syndrome (MS) and is strongly associated with CAD. Therefore, the objectives of the present study were to clarify the inde-pendent clinical value of SUA and identify determinants responsible for modulating SUA in subjects with suspected CAD.
METHODS
Study subjects
The study was retrospectively conducted with patients admitted to the cardiology department in Korea University Guro Hospital, Seoul, Korea, between March 2002 and December 2005. We included 716 consecutive patients who had undergone coronary angiography for the diagnosis or exclusion of CAD. Twenty-nine patients that met the following criteria were excluded: previous history of taking diuretics for antihypertensive medication, acute coronary syndrome, cardiomyopathy, valvular heart disease more serious than mild, postcardiac surgery, atrial fibrillation, aortic dissection, and renal insufficiency. A total of 687 subjects gave written informed consent and were enrolled in the study.
Baseline measurements and definitions
Hemodynamic measurements and blood sampling, including SUA, were performed with the patient in the supine position after 30 minutes of rest. A diagnosis of HTN was based on a systolic blood pressure of ≥ 140 mmHg or a diastolic blood pressure of ≥ 90 mmHg with repeated measurements, or antihypertensive drug therapy. Diabetes mellitus (DM) was defined as a fasting blood glucose concentration ≥ 126 mg/dL or antihyperglycemic drug treatment. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. MS was defined according to National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria (≥ 3 of the following abnormalities) [11]: waist circumference greater than 102 cm in men and 88 cm in women, serum triglyceride (TG) level of at least 150 mg/dL (1.69 mmol/L), high-density lipoprotein cholesterol (HDL-C) level of less than 40 mg/dL (1.04 mmol/L) in men and 50 mg/dL (1.29 mmol/L) in women, blood pressure of at least 130/85 mmHg, or serum glucose level of at least 110 mg/dL (6.1 mmol/L).
Coronary angiography
All patients underwent routine coronary angiography using the Judkins technique with digitized coronary angiography equipment (Philips, Eindhoven, Netherlands). All coronary angiograms were visually assessed by at least three experienced angiographers, and a consensus was reached. For this study, we defined significant CAD as minimal lumen diameter stenosis ≥ 50% on the angiogram. The severity of CAD was expressed as 1-, 2-, or 3-vessel disease.
Statistical analysis
Values were expressed as the mean ± SD. Categorical variables were compared using a chi-square test. Differences in the mean values between the two groups were compared using an unpaired t-test. A p value of < 0.05 was considered statistically significant. The effects of classic risk factors on SUA were analyzed using a multivariate regression analysis with stepwise selection. A multiple logistic regression analysis was used to identify independent risk factors of CAD. Classic CV risk factors included in the multivariate models were age, gender, BMI, DM, smoking, total cholesterol, pulse wave velocity (PWV), and MS. We calculated the 95% confidence interval (CI) for each odds ratio (OR) and all p values were two-tailed. Crude rates were compared using a Pearson-Mantel-Haenszel test. Statistical analyses were performed using the SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
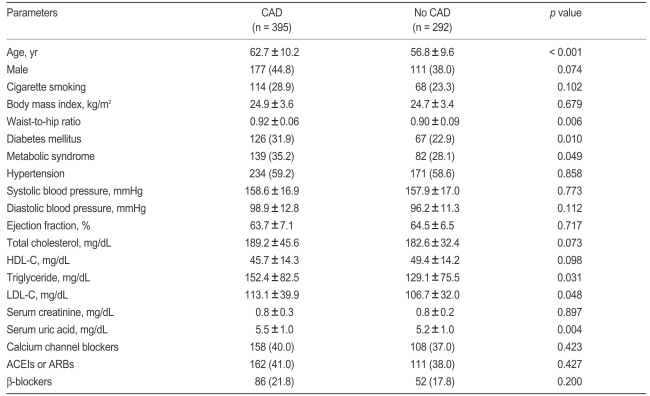
Table 1 represents clinical characteristics of the study population. The sample comprised 288 men and 399 women, and 395 patients had CAD (57.5%). The mean age of the participants was 59.3 ± 10.4 years old. Subjects who had CAD were older than those who did not (62.7 ± 10.2 vs. 56.8 ± 9.6, p < 0.001). DM (31.9 vs. 22.9%, p = 0.01) and MS (35.2 vs. 28.1%, p = 0.049) were more common among subjects with CAD. Moreover, they had a higher waist-to-hip ratio (p = 0.006), greater TG (p = 0.031), low-density lipoprotein cholesterol (LDL-C, p = 0.048), and PWV levels (p < 0.001), and increased SUA (5.5 ± 1.0 vs. 5.2 ± 1.0, p = 0.004). Gender, smoking, total cholesterol, HDL-C, and blood pressure were not significantly different between the two groups.
Table 1.
Clinical characteristics of subjects with and without coronary artery disease
Values are presented as mean ± SD or number (%) of patients.
CAD, coronary artery disease; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
Clinical value of SUA
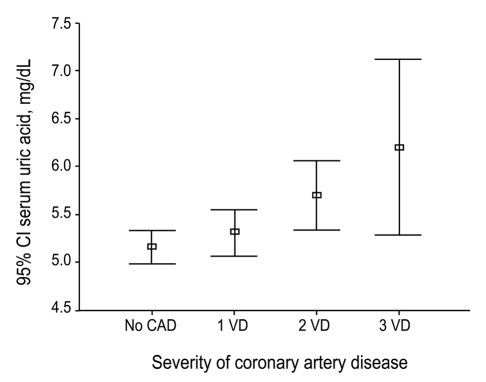
When the severity of CAD was expressed as 1-, 2-, 3-vessel disease, or no CAD, a significant association was observed between the severity of CAD and serum uric acid levels (p = 0.002, Fig. 1).
Figure 1.
Severity of coronary artery disease and serum uric acid (Kruskal-Wallis ANOVA, p = 0.002).
CAD, coronary artery disease; VD, vessel disease; CI, confidence interval.
In a multivariate logistic regression analysis, however, the significant independent risk factors of CAD were age (p = 0.001), DM (p = 0.021), and PWV (p = 0.048). In contrast, waist-to-hip ratio (p = 0.089), TG (p = 0.114), LDL-C (p = 0.167), MS (p = 0.278), and SUA (p = 0.151) were not significantly or independently related to CAD.
In a sex-specific analysis, only two factors, age (p = 0.002) and DM (p = 0.045), were significantly associated with CAD in men and women. However, a trend was detected toward MS and SUA being independent risk factors of CAD in women (p = 0.059, p = 0.062, respectively), but not men (p = 0.423, p = 0.299, respectively).
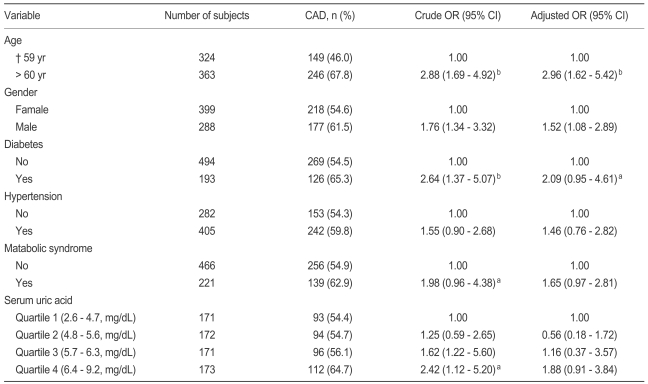
Results of the adjusted ORs are reported in Table 2. After adjusting for age, gender, DM, HTN, and MS, SUA in the highest quartile (≥ 6.4 mg/dL) showed an increased risk for CAD (OR, 1.88; 95% CI, 0.91 to 3.84) as compared with SUA in the first quartile (< 4.8 mg/dL); however, this result was not significant (p = 0.075).
Table 2.
Odds ratio of coronary artery disease according to prognostic variables
Adjusted for age, gender, diabetes, hypertension, and metabolic syndrome.
CAD, coronary artery disease; OR, odds ratio; CI, confidence interval.
ap < 0.05.
bp < 0.01.
In the stepwise multivariate regression analysis, significant determinants responsible for modulating SUA levels included MS (p < 0.001), DM (p = 0.018), and gender (p = 0.039). In a sex-specific analysis, MS was the only independent determinant of SUA levels in men (p = 0.001) and women (p = 0.039).
DISCUSSION
In the present study, SUA was not an independent risk factor of CAD after adjusting for potential confounding variables. Subgroup analyses, however, showed that SUA was more strongly associated with an increased risk of CAD in women (p = 0.062) than in men (p = 0.299). These findings are consistent with results of previous studies [8,12,13]. In contrast, the Framingham Heart Study [8] detected no association between SUA and CV events after adjusting for age, office blood pressure, total cholesterol, smoking, DM, and diuretics therapy. In addition, sex-specific analyses in the Framingham Heart Study [8] revealed a strong and graded association between baseline uric acid levels and an increased risk for CAD, death from CV disease, and death from all causes among women. This risk, however, was substantially reduced after adjusting for age, and was eliminated completely in the multivariate model. The NHANES I epidemiologic study [12] also showed no association between SUA and CV events in men; however, among women, SUA was predictive of all-cause mortality and ischemic heart disease. These associations persisted even after excluding the first 10 years of follow-up and were independent of antihypertensive agent and diuretics use, diastolic blood pressure, obesity, and other characteristics. The underlying mechanisms governing the higher association of SUA with adverse events in women as compared with men remain uncertain.
Nevertheless, several recent reports [5,6] have identified epidemiologic evidence to support the contention that SUA is an independent risk factor for hypertension-associated morbidity and mortality. The third NHANES [14] revealed that hypertensive people with SUA levels in the second (5.0 to 5.9 mg/dL) and third quartiles (6.0 to 6.9 mg/dL) have a significantly higher relative risk (RR) for both heart attack (RR = 1.32) and stroke (RR = 1.15). Additionally, those in the fourth quartile (SUA > 7.0 mg/dL) were at substantially higher RR for heart attack and stroke (2.2, 1.5, respectively). Based on our results, SUA levels in the highest quartile (≥ 6.4 mg/dL) are associated with an increased risk of CAD (OR, 1.88; 95% CI, 0.91 to 3.84) as compared with the first quartile (< 4.8 mg/dL); however, this result was not significant (p = 0.075).
The mechanisms underlying SUA increases in patients with atherosclerotic disease remain unknown. Laboratory and clinical evidence suggests that SUA plays a role in platelet adhesiveness [15], the formation of free radicals [16], and oxidative stress [17]. Recently, several reports [18,19] have linked SUA with MS, which is a cluster of metabolic abnormalities, with insulin resistance as the major characteristic. Since selective insulin resistance and hyperinsulinism facilitates the active reabsorption of uric acid [10], this concept may provide a physiological clue as to why MS is closely linked to SUA. In the present study, the only significant determinant of SUA levels in both men and women was MS (p = 0.001, p = 0.039, respectively). This result suggests that insulin resistance may be a pathophysiologic mechanism of hyperuricemia in patients with CAD. A trend was observed toward SUA being an independent risk factor for CAD in women (p = 0.059, p = 0.062, respectively), but not in men (p = 0.423, p = 0.299, respectively).
The fact that SUA in the highest quartile showed an increased risk of CAD, but the significance disappeared after the multivariate regression analysis, suggests that the influence of SUA on CHD is explained by the secondary association of SUA with other established etiological risk factors such HTN, dyslipidemia, hyperinsulinemia, obesity, and MS. Regardless, SUA can used to predict CAD as a high risk of vascular disease.
The main limitation of the present study is that subjects who were at higher risk of CAD as compared to a population-based sample were assessed. As a consequence, a higher percentage of DM and MS was observed (28.1%, 32.2%, respectively) as compared to previous studies [6,20]. Therefore, caution is necessary when applying the results of this study to different clinical settings.
Because we did not measure insulin resistance indices, such as homeostasis model assessment (HOMA) insulin resistance, we do not know if elevated SUA is related to insulin resistance in this population. However, we may assume the possibility of elevated SUA and increased insulin resistance due to the strong relationship between MS and insulin resistance. Nevertheless, the present study has several potential strengths as compared with other studies. We only enrolled subjects without a previous history of taking diuretics or antihypertensive drugs, which confounds the link between SUA and the risk of CAD. Also, we identified the significant determinants responsible for modulating SUA levels, thereby allowing a basic understanding of why SUA is frequently elevated in subjects at risk for CV.
The present study demonstrates a strong association between the severity of CAD and SUA levels in subjects with suspected CAD. Nonetheless, SUA was not an independent risk factor after adjusting for concomitant CAD risk factors. The significant determinant of SUA levels was MS, which is inextricably linked to insulin resistance and is commonly associated with CAD. Therefore, one can safely conclude that SUA may be merely a marker of insulin resistance, which plays a causal role in the pathogenesis of CAD.
Acknowledgements
Si Heung Park is a student at Woodberry Forest School, Virginia, USA. We are grateful to Mr. SH Park for data collection and analysis. This study was supported by grants from KIOM and the Seoul R&BD Program, Republic of Korea (10526).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee J, Sparrow D, Vokonas PS, Landsberg L, Weiss ST. Uric acid and coronary heart disease risk: evidence for a role of uric acid in the obesity-insulin resistance syndrome: the Normative Aging Study. Am J Epidemiol. 1995;142:288–294. doi: 10.1093/oxfordjournals.aje.a117634. [DOI] [PubMed] [Google Scholar]
- 2.Frohlich ED. Uric acid: a risk factor for coronary heart disease. JAMA. 1993;270:378–379. doi: 10.1001/jama.270.3.378. [DOI] [PubMed] [Google Scholar]
- 3.Puig JG, Michan AD, Jimenez ML, et al. Female gout: clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151:726–732. doi: 10.1001/archinte.151.4.726. [DOI] [PubMed] [Google Scholar]
- 4.Puig JG, Ruilope LM. Uric acid as a cardiovascular risk factor in arterial hypertension. J Hypertens. 1999;17:869–872. doi: 10.1097/00004872-199917070-00001. [DOI] [PubMed] [Google Scholar]
- 5.Franse LV, Pahor M, Di Bari M, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP) J Hypertens. 2000;18:1149–1154. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Schillaci G, Reboldi GP, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension: the PIUMA Study. Hypertension. 2000;36:1072–1078. doi: 10.1161/01.hyp.36.6.1072. [DOI] [PubMed] [Google Scholar]
- 7.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992: National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 8.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 9.Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J. Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago Heart Association Detection Project in Industry. J Clin Epidemiol. 1989;42:257–267. doi: 10.1016/0895-4356(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M. Uric acid metabolism and tubular sodium handling: results from a population-based study. JAMA. 1993;270:354–359. [PubMed] [Google Scholar]
- 11.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of serum uric acid to mortality and ischemic heart disease: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 13.Alderman MH, Kivlighn S, Beauchard L, Cohen H, Madhaven S. Increased serum uric acid associated with increased cardiovascular disease in treated hypertensive patients. J Hypertens. 1998;16(Suppl 2):S5. [Google Scholar]
- 14.Ward HJ. Uric acid as an independent risk factor in the treatment of hypertension. Lancet. 1998;352:670–671. doi: 10.1016/S0140-6736(05)60816-1. [DOI] [PubMed] [Google Scholar]
- 15.Emmerson BT. Atherosclerosis and urate metabolism. Aust N Z J Med. 1979;9:451–454. doi: 10.1111/j.1445-5994.1979.tb04180.x. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez-Vivar J, Santos AM, Junqueira VB, Augusto O. Peroxynitrite-mediated formation of free radicals in human plasma: EPR detection of ascorbyl, albumin-thiyl and uric acid-derived free radicals. Biochem J. 1996;314:869–876. doi: 10.1042/bj3140869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anker SD, Leyva F, Poole-Wilson PA, Kox WJ, Stevenson JC, Coats AJ. Relation between serum uric acid and lower limb blood flow in patients with chronic heart failure. Heart. 1997;78:39–43. doi: 10.1136/hrt.78.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM. Syndrome X: 6 years later. J Intern Med Suppl. 1994;736:13–22. [PubMed] [Google Scholar]
- 20.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]