Abstract
Background/Aims
Acute myocardial infarction (AMI) is a leading cause of death. Inflammatory processes play an important role in atherosclerosis, which is intimately related to AMI. The aim of this study was to investigate the association between anti-inflammatory and pro-inflammatory cytokines ratios and AMI.
Methods
A total of 90 AMI patients and 90 age-and sex-matched controls were recruited in this study. Plasma cytokines and conventional risk factors were determined by standard methods.
Results
Patients with AMI showed increased interleukin (IL)-6 and tumor necrosis factor-α levels and lower anti- to pro-inflammatory cytokine ratios as compared with controls. A multivariate logistic regression analysis revealed that IL-10 to IL-6 ratio was independently associated with the occurrence of AMI (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.39 to 12.17; p < 0.0001). In contrast, IL-6 levels were no longer significant in the multivariate model (OR, 1.02; 95% CI, 0.932 to 1.12; p = 0.603). A receiver operating characteristic (ROC) curve analysis indicated that IL-6 levels and IL-10 to IL-6 ratios were a significant predictor of AMI (area under ROC curve, 0.892 and 0.851, respectively).
Conclusions
Our results suggest that the ratio of IL-10 to IL-6 is independently associated with AMI, and reduced levels of this ratio may favor the development of AMI.
Keywords: Myocardial infarction, acute; Cytokines, inflammatory; ROC curve
INTRODUCTION
Acute myocardial infarction (AMI) is a multi-factorial disease. AMI is often caused by unstable plaque in the epicardial coronary artery, in which inflammatory processes play a central role [1]. In this regard, several circulating pro-inflammatory molecules have been associated with thrombotic cardiovascular events including acute phase proteins, cellular adhesion molecules, and cytokines [2]. Moreover, few articles have explored the predictive value of inflammatory markers on cardiovascular events in cardiovascular disease-free subjects [3,4]. Far fewer studies have considered multiple markers, such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IL-10 simultaneously. In fact, most studies have focused only on C-reactive protein (CRP) [5]. A recent longitudinal study demonstrated a stronger role for IL-6 in the prediction of congestive heart failure, as compared with CRP [3].
Animal experiments suggest that IL-6 and TNF-α play important roles in the regulation of acute phase protein synthesis. Acute phase proteins, such as fibrinogen and factor VIII, are established risk factors for atherosclerosis [6]. The plasma cytokine IL-6 plays an important role in mediating inflammation and is a central stimulus for the acute phase response [7].
The effects of inflammatory stimuli are counterbalanced by anti-inflammatory mechanisms, including IL-10, IL-4, high-density lipoproteins (HDL), and vascular endothelial growth factor [8,9]. IL-10 is a centrally operating anti-inflammatory cytokine that plays a crucial role in the regulation of the innate immune system. Studies using IL-10 deficient mice have shown that IL-10 has a protective role in atherosclerosis [10]. Further, it strongly deactivates the inflammatory host response and potently inhibits the production of pro-inflammatory cytokines [11].
The present study was performed to investigate the association between anti- and pro-inflammatory cytokines ratios and AMI.
METHODS
Subjects
The study population was composed of individuals from the eastern part of India of Asian-Indian origin with a history suggestive of AMI. All cases (n = 90) were evaluated using electrocardiographic (ECG) criteria, cardiac enzyme studies, and echocardiographic evaluation (evidence of regional wall motion abnormalities and left ventricular ejection fraction > 50%). All recruited patients were admitted within 12 hours after the first episode of chest pain. Healthy age- and sex-matched controls (n = 90) included subjects without any of the following characteristics: signs of ischemic heart disease, ECG criteria suggestive of acute coronary syndrome, or a past history of cardiovascular disease and diabetes. Samples were obtained from consecutively examined patients except for those with the following exclusion criteria: patients taking known lipid altering medications; use of non-steroidal, anti-inflammatory drugs; and the presence of chronic kidney disease. All patients were admitted to the hospital within 12 hours of the onset of chest pain. Blood samples for cytokine and biochemical analysis were taken at the time of admission. Blood samples were collected by vein puncture into vacuum tubes containing EDTA. Plasma samples were processed and divided into aliquots and stored in cryovials at -700℃ until further analysis for cytokines.
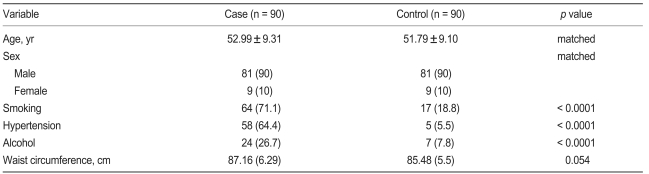
The demographic characteristics of patients and control subjects are shown in Table 1. The subjects of the present study were part of a health examination between the Immunotechnology Section (presently Division of Molecular Medicine), Bose Institute Kolkata, and the Department of Cardiology, NRS Medical College and Hospital, Kolkata. Participants between 45 and 65 years of age were recruited between November 2006 and September 2007. The institutional ethics committee approved the study protocol.
Table 1.
Characteristic of Asian Indian population in Eastern part of India in the case and control group
Values are presented as mean ± SD or number (%).
Blood biochemistry
Detailed self-reported questionnaires containing information on the history of hypertension, smoking status, and alcohol consumption were collected at baseline and used for analysis. Waist circumferences were also measured according to standard methods. The following parameters were measured using standard methods in our laboratory: glucose, total cholesterol, and HDL cholesterol (HDL-C). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald equation [12] and non-HDL-C levels were calculated as follows: Non-HDL-C = Total cholesterol - HDL-C.
IL-6, TNF-α, and IL-10 levels were measured from frozen stored plasma. Cytokines were measured in duplicate using an enzyme-linked immunosorbance assay kit from Diaclone Research, Besancon, France. These assay kits had a lower limit of detection of 8 pg/mL for TNF-α, 2 pg/mL for IL-6, and 5 pg/mL for IL-10.
Statistical analysis
Results are presented as the means ± SD and the percentage of the population studied. Student's t tests and Mann-Whitney U tests were used to examine differences between the two groups. For determination of optimal cut-off values and diagnostic performance of these continuous variables, receiver operating characteristic curves analysis was performed. Optimal cut-off points for these risk factors were determined based on the convergence of sensitivity and specificity. Stepwise logistic regression analysis was used to assess the independent adjusted relationship between different variables and AMI with independent variables being those with p ≤ 0.05 in univariate analysis. All statistical tests were two-sided. A p value < 0.05 was considered to be statistically significant. SPSS (SPSS Inc., Chicago, IL, USA) was used for all calculations and MedCalc® version 9.2.0.1 statistical software (MedCalc Software, Mariakerke, Belgium) was used only for comparison between areas under receiver operating characteristic curves of different variables.
RESULTS
General characteristics of the study subjects
Baseline characteristics of study participants are shown in Table 1. Among these study participants, 90 AMI patients (81 males [90%] and 9 females [10%]) and 90 age-and sex-matched healthy controls (81 males [90%] and 9 females [10%]) were recruited. Individuals who suffered from AMI were more likely to smoke, drink alcohol, suffer hypertension, and have a higher mean waist circumference as compared with healthy controls.
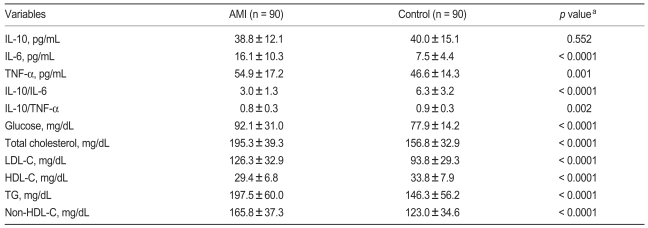
Study population characteristics were compared with controls (Table 2). AMI patients had lower levels of HDL-C and IL-10, and higher fasting glucose, triglyceride, LDL-C, non-HDL-C, total cholesterol, and plasma levels of IL-6 and TNF-α as compared with control. Furthermore, the ratio of IL-10 to IL-6 and the ratio of IL-10 to TNF-α were both lower in AMI patients as compared with the control group.
Table 2.
The demographic information in AMI group and control groups
Values are presented as mean ± SD.
aNon-paired student's t-test for comparisons between case and control group.
AMI, acute myocardial infarction; IL, interleukin; TNF-α, tumor necrosis factor-alpha; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride.
Cytokines
Mean concentrations of anti-inflammatory cytokine IL-10 were lower in the AMI group as compared with the control group; however, this difference was not significant (38.81 vs. 40.03 pg/mL, p = 0.552, Table 2). AMI patients had significantly higher plasma concentrations of the pro-inflammatory cytokines IL-6 and TNF-α as compared with controls (IL-6 concentration, 16.10 ± 10.32 vs. 7.52 ± 4.36 pg/mL; p < 0.0001), (TNF-α concentration, 54.94 ± 17.19 vs. 46.61 ± 14.33 pg/mL; p = 0.001). No significant differences in anti-inflammatory cytokine values were observed; however, the anti- to pro-inflammatory cytokine ratio was significantly lower in AMI patients as compared with controls ([IL-10 to IL-6 ratio, 2.96 ± 1.31 vs. 6.31 ± 3; p < 0.0001], [IL-10 to TNF-α ratio, 0.76 ± 0.29 vs. 0.91 ± 0.34; p = 0.002]).
ROC analysis
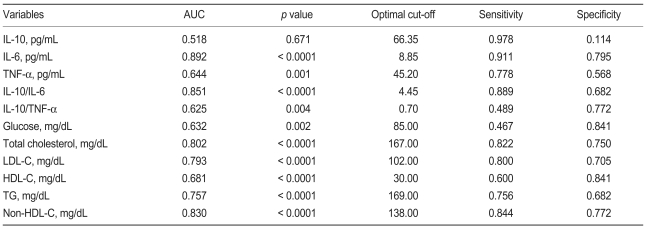
For discrimination between patients and controls, we found that the area under the receiver operating characteristic (ROC) curve ranged from 0.518 to 0.892 (Table 3). As shown in Table 3, IL-6 (area under the curve [AUC], 0.892; p < 0.0001), IL-10 to IL-6 ratios (AUC, 0.851; p < 0.0001), and non-HDL-C (AUC, 0.830; p < 0.0001) showed superior performance against other parameters. IL-6 had the highest area under the curve; however, this value was not significantly higher than the area under the curve for the IL-10 to IL-6 ratio (difference, 0.041; p = 0.069) or non-HDL-C (difference, 0.062; p = 0.111). The area for the IL-10 to IL-6 ratio, which is a balance between anti- and pro-inflammatory cytokine, was slightly larger than the area for non-HDL-C (difference, 0.021; p = 0.615). This analysis demonstrates that IL-6 shows superior performance against the other parameters in our study population. IL-6 concentrations at an optimal cut-off level of 8.85 pg/mL showed a sensitivity and specificity of 91% and 79%, respectively (Table 3). Diagnostic performance and sensitivity and specificity values for other parameters are also shown in Table 3.
Table 3.
Area under the receiver operating characteristic curve and optimal cut off values of different variables
AUC, area under the curve; IL, interleukin; TNF-α, tumor necrosis factor-alpha; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride.
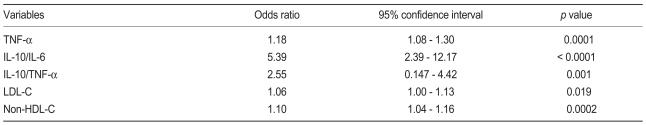
Logistic regression analysis
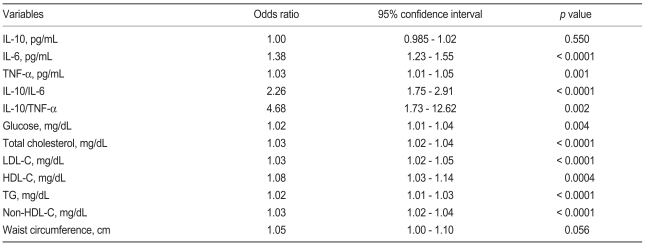
In the univariate logistic regression analysis we found that the ratios of IL-10 to TNF-α and IL-10 to IL-6 showed greater odds ratios than the other variables (Table 4). To further delineate a potential independent association between IL-6 and TNF-α, a multivariate analysis was performed, which included the ratio of anti- to pro-inflammatory cytokines markers; baseline characteristics, which showed significant discriminating abilities in the ROC curve analysis; and independent variables found in the univariate analysis. Anti- to pro-inflammatory cytokine ratios, TNF-α, LDL-C, and non-HDL-C remained significant. IL-6 was no longer significant (Table 5) after the IL-10 to IL-6 ratio was introduced into the model (odds ratio, 1.38; p < 0.0001 without IL-10 to IL-6 ratio).
Table 4.
Univariate logistic regression analysis
IL, interleukin; TNF-α, tumor necrosis factor-alpha; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride.
Table 5.
Multivariate logistic regression analysis
TNF-α, tumor necrosis factor-alpha; IL, interleukin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol.
DISCUSSION
The assessment of inflammatory markers as additional risk factors in the evaluation of cardiovascular events should be encouraged. Further studies are necessary to determine whether inflammatory markers may represent valid targets for new medications that can be used to slow the atherosclerosis process. Plasma concentrations of cytokines in virtually all of the subjects tested was determined, and it was found that a variety of phenomena were associated with changes in plasma cytokines. In our study, IL-10 to IL-6 cytokines ratios showed more consistent results than any other cytokines.
IL-6 and TNF-α are known to influence lipid metabolism in animals via two separate mechanisms [13,14]. First, these cytokines stimulate fatty acid synthesis by the liver and secondly, TNF-α stimulates lipolysis by adipocytes. These changes may explain the association between cytokines and plasma triglyceride. The observation that alcohol consumption is associated with reduced TNF-α is consistent with studies that have shown suppression of TNF-α production by macrophages with alcohol consumption [6]. The association of alcohol with reduced plasma TNF-α may explain the relationship between alcohol and increased HDL-C concentrations.
Circulating IL-6 concentrations may be the result of a variety of stimuli, including various clinical risk factors that lead to the release of IL-6 from numerous cell types, including smooth muscle cells and macrophage/foam cells found in atheromatous plaques [15]. IL-6 can be released from adipocytes and may stimulate a response from the hypothalamic pituitary-adrenal axis, resulting in hypertension and obesity [16]. In this study we also found that increased IL-6 levels are associated with hypertension. Consistent with previous studies [17], we found that circulating levels of IL-6 were increased in obese patients as compared with lean patients. Prior studies of AMI suggest that cytokines are preferentially produced by inflammatory cells in the pre-infarct zone, and persistent elevation of cytokines results from an increased infiltration of inflammatory cells [18]. In our study, individuals with elevated levels of TNF-α were at an increased risk for AMI. Thus, these data are consistent with the hypothesis that inflammation plays a major role in atherosclerosis [19].
Less information is available regarding the role of anti-inflammatory cytokines in AMI. It recently has been demonstrated that the anti-inflammatory cytokine IL-10 may act as a protective factor in atherosclerosis. IL-10 is expressed in both early and advanced human atherosclerotic plaques [20] and inhibits many cellular processes including metalloproteinase production and tissue factor expression, which may play a role in the clinical expression of atherosclerotic plaque rupture or erosion. IL-10 concentrations were particularly low in the AMI study group. IL-10 is a powerful suppressor of the immune response, and is produced by T-cells, B cells, monocytes, and macrophages [11,21]. IL-10 inhibits pro-inflammatory cytokines such as TNF-α and IL-6 [11] and has multifaceted anti-inflammatory properties, including inhibition of prototypic pro-inflammatory transcription factors, i.e., nuclear factor κB, which leads to the suppression of cytokine production [22]. IL-10 is expressed in advanced human atherosclerosis and is associated with low inducible nitric oxide synthase expression and low levels of apoptosis [23]. Our study found that plasma IL-10 con-centrations are lower in AMI patients; however, this difference is not statistically significant as compared with the control group. This result suggests that decreased plasma IL-10 concentrations are associated with clinical instability. Pro-inflammatory cytokine (IL-6 and TNF-α) plasma concentrations were significantly higher in AMI patients as compared with controls. In contrast, lower plasma concentrations of the anti-inflammatory cytokine IL-10 were found in the AMI group as compared with controls; however, this difference was not statistically significant. The beneficial effects of elevated IL-10 plasma levels were restricted to patients with elevated IL-6 and TNF-α plasma levels, which is an indication of an enhanced systemic inflammatory response. These data support the hypothesis that the balance between anti-inflammatory and pro-inflammatory cytokines may reflect the intensity of occult plaque inflammation and vulnerability to rupture [24,25]. Therefore, we examined anti- and pro-inflammatory cytokine ratios and found that IL-10 to IL-6 ratios were strongly associated with AMI. A low IL-10 to TNF-α ratio is associated with severe malarial anemia, and an insufficient IL-10 response to high TNF-α concentrations may have had a central role in the progression of this disease [26].
Limitations of previous studies on cytokine markers in AMI include the inclusion of only one or two cytokines that are pro-inflammatory or anti-inflammatory. Multiple cytokines are involved in the inflammatory process, and have overlapping, antagonistic, and synergetic effects on many cell types. In addition, they up-and down-regulate the production of other cytokines and inflammatory markers. In our study, we included two pro-inflammatory cytokines and one anti-inflammatory cytokine. The limitation of the present study is the lack of follow-up data, mostly due to the lack of patient compliance. This additional data may have provided important information about anti- to pro-inflammatory cytokine ratios as markers for risk stratification and prognosis.
In conclusion, inflammation and thrombosis are interconnected pathologies in the natural history of atherosclerosis and measurements of inflammatory markers may aid in predicting the prognosis of patients with AMI. IL-10 to IL-6 ratios and IL-6 measurements were equivalent in their ability to discriminate between persons with and without AMI. Data from the multivariate model, however, suggest that the IL-10 to IL-6 ratio is associated with AMI, whereas IL-6 levels are not. Thus, our data suggest that the IL-10 to IL-6 ratio, and not IL-6 levels, may be a valuable indicator of AMI. Our results also suggest that reduced anti- to pro-inflammatory cytokine ratios may favor atheromatous plaque instability and may be implicated in the development of AMI.
Acknowledgements
The authors are grateful to the staff of the Deptartment of Cardiology, N.R.S. Medical College & Hospital, Kolkata, India for their cooperation during sample collection. We acknowledge Mr. Ranjit Kumar Das for his kind help in this work.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardio-vascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infraction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 4.Piche ME, Lemieux S, Weisnagel SJ, Cornean L, Nadean A, Bengeron J. Relation of high sensitivity C-reactive protein, interleukin-6, tumor factor-alpha and fibrinogen to abdominal, adipose tissue, blood pressure and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 6.Baumann H, Gaudie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 7.Papanicolaou DA, Wilder RL, Manologa SC, Chrousos GP. The pathophysiologic roles of interleukine-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Blake GJ, Ridker PM. Novel Clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 10.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukine-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 11.Moore KW, de Waal-Malefyt R, Coffiman RL, O'Gara A. Interleukine-10 and interleukine-10 receptor. Annu Rev Immol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levey RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Feingold K, Grunfeld C. Role of cytokines in inducing hyperlipidaemia. Diabetes. 1992;41(Suppl 2):97–101. doi: 10.2337/diab.41.2.s97. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisgli G, Shargill N, Spiegelman B. Adipose expression of tumor necrosis factor alpha: direct role in obesity linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotension II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 16.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukine-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 17.Mendall MA, Patel P, Asante M, et al. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart. 1997;78:273–277. doi: 10.1136/hrt.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx N, Neumann FJ, Ott I, et al. Induction of cytokine expression in leukocytes in acute myocardial infarction. J Am Coll Cardiol. 1997;30:165–170. doi: 10.1016/s0735-1097(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. Atherosclerosis: as inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20.Serneri GG, Abbate R, Gori AM, et al. Transient intermittent lymphocyte activation is responsible for the instability of angina. Circulation. 1992;86:790–797. doi: 10.1161/01.cir.86.3.790. [DOI] [PubMed] [Google Scholar]
- 21.Williams K, Dooley N, Ulvestad E, Becher B, Antel JP. IL-10 production by adult human derived microglial cells. Neurochem Int. 1996;29:55–64. doi: 10.1016/0197-0186(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukine (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 23.Mallat Z, Heymes C, Ohan J, Faggin E, Leseche G, Tedgui A. Expression of interleukine-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19:611–616. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Allergy. 2003;2:257–265. doi: 10.2174/1568010033484106. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka M, Yamaguchi S, Okuyama M, Tomoike H. Anti-inflammatory cytokine profile in human heart failure: behavior of interleukin-10 in association with tumor necrosis factor-alpha. Jpn Circ J. 1999;63:951–956. doi: 10.1253/jcj.63.951. [DOI] [PubMed] [Google Scholar]
- 26.Kurtzhals J, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]