Abstract
Perfluorooctanoic acid (PFOA), a common and persistent industrial byproduct detected in human sera, has raised health concerns. PFOA is detrimental to lactational function and postnatal mammary gland development in CD-1 mice after gestational exposure. We have examined the peripubertal period (21 through 50 days of age) as an important window of mammary gland susceptibility to environmental exposures that may affect breast cancer risk later in life. The effects of PFOA (0.1 – 10 mg/kg BW) were examined in Balb/c and C57BL/6 mice. PFOA treatment caused hepatocellular hypertrophy and delayed vaginal opening in both mouse strains. While Balb/c mice exhibited only inhibition of mammary gland and uterine development (5, 10 mg/kg), C57BL/6 mice exhibited stimulatory effects in both organs at low dose (5 mg/kg) and inhibition at higher dose (10 mg/kg). This underscores the need for caution when drawing conclusions about the effects of PFOA and possibly other environmental pollutants on the basis of studies in a single mouse strain.
Keywords: Perfluorooctanoic acid (PFOA), peripubertal exposure, mammary gland, uterus, hepatocellular hypertrophy, timing of vaginal opening, strain differences
Introduction
Perfluorooctanoic acid (PFOA) is a member of a family of synthetic perfluorinated compounds with extremely high stabilities and low surface tension. For more than 50 years, PFOA has been widely used in the production of fluoropolymers for making numerous industrial and consumer products, and is one of the most common persistent organic pollutants in the environment. Biomonitoring studies have shown that PFOA and other perfluorinated chemicals are not only found as global pollutants in air, water and soil, but also detected in blood samples from almost all organisms sampled in a global survey as well as in both general nonexposed and occupationally exposed human populations [1–3]. Although the general population exposure to PFOA and other perfluorinated compounds through air, food and drinking water seems to be limited, high blood concentration of PFOA in humans through occupational and accidental exposure has been reported [4–6]. Previous classical toxicology studies suggested that the acute toxicities of PFOA and other perfluorinated chemicals are low to moderate in mice, rats and rabbits [7–9]. However, the widespread presence of these chemicals in wild life and humans, and their accumulative and persistent properties have raised considerable health concerns over recent years. As a result, a variety of animal model studies on the toxicities of PFOA and related compounds have been recently performed.
One of the most active areas in recent PFOA toxicology studies is its developmental toxicity. Previous studies in rats and rabbits showed that maternal exposure to PFOA (0–150 mg/kg) during organogenesis (gestation day 6–17) does not result in embryo-fetal toxicity or developmental abnormalities in the offspring [8]. However, Lau et al. recently reported on the maternal and developmental toxicity of PFOA in CD-1 mice [10]. Timed-pregnant CD-1 mice were treated with 1, 3, 5, 10, 20, or 40 mg/kg PFOA by oral gavage daily from gestational day (GD) 1-17, it was found that PFOA treatment caused dose-dependent full-litter resorption and decreased weight gain in dams that carried pregnancy to term. Increased mortality and growth deficits were observed in PFOA-treated litters. In addition, significant delay in eye-opening was noted at 5 mg/kg and higher dosages, and accelerated sexual maturation was observed in male offspring. Cross-foster and restricted gestational exposure studies further indicated that these postnatal developmental effects of PFOA are due to gestational exposure, and exposure earlier in gestation generates stronger effects [11].
Since PFOA exposure causes decreased neonatal body weights and survival, its effect on maternal lactation was recently studied [12]. A significant decrease in mammary gland differentiation among dams exposed at GD 1-17 or 8-17 was observed on postnatal day (PND) 10. Delays in lactational involution were also found on PND 20. Moreover, all exposed female pups showed stunted mammary epithelial branching and growth at PND 10 and 20. These findings strongly suggest that PFOA exposure may have a significant impact on mammary gland development.
Studies have shown that many environmental pollutants can affect mammary gland development in experimental animals, and the effect can be especially significant if chemical exposure occurs during critical stages of mammary gland development such the gestational, neonatal, and peripubertal periods as well as pregnancy [13]. Since PFOA is persistent in the environment, and there is wide-spread human exposure to PFOA, it is important to examine its effect on the mammary gland during sensitive periods of mammary gland development. The peripubertal period is considered to be an important window of susceptibility of the developing breast to environmental exposures that may predispose humans to increased breast cancer risk later in life [13,14]. During the peripubertal period, the mammary gland undergoes a rapid proliferative expansion, and in rodents the peripubertal gland is known to be highly susceptible to mammary carcinogenesis [13].
Nothing is currently known about the effect of peripubertal PFOA exposure on mammary gland development. To address this issue, we investigated the effect of peripubertal exposure to PFOA on mammary gland development in C57Bl/6 and Balb/c mouse strains. We found that PFOA exposure has significant effects on mammary gland development. Importantly, the effects differ significantly between the two mouse strains.
Materials and Methods
Animals
Female, 3-week-old BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Portage, MI). Animals were weighed upon arrival and randomly distributed according to strain among four treatment groups/strain. Mice received food (8640 Harlan Teklad 22/5 Rodent Diet) and tap water ad libitum. Animal facilities were maintained on a 12:12-h light-dark cycle, at 20–24°C with 40–50% relative humidity. All animal protocols were reviewed and approved by the Michigan State University Institutional Animal Care and Use Committee.
Dosing Solution and Procedures
PFOA, as its ammonium salt (> 98% pure), was obtained from Fluka Chemical (Steinheim, Switzerland). PFOA dosing solution was prepared fresh daily in deionized water. Mice received either water vehicle (control) or PFOA at 1, 5 or 10 mg/kg body weight (BW) by oral gavage, once daily, 5 days per week for 4 weeks starting at 21 days of age.. The doses were chosen on the basis of previous work in mice that found the middle dose to affect mammary gland development in pups after gestational exposures [12].
Experimental Design
Twenty female BALB/c and 20 female C57BL/6 were divided randomly among 4 groups per strain with 5 mice treated per dose or vehicle control. Body weights (BW) and appearance of vaginal opening were monitored every day. The animals were killed, after 4 weeks of treatment, when the animals were 7 weeks of age. Two h prior to termination, mice were injected with 5-bromo-2’-deoxyuridine (BrdU) (70 mg/kg BW) for analysis of proliferation in mammary glands.
Necropsy
Mammary glands, uteri and livers were harvested at the time of termination. Uteri and livers were weighed prior to fixation. All tissues were fixed in 10% neutral formalin for 24 hrs, transferred to 70% ethanol, paraffin embedded, and then 5-µm sections were prepared and stained with hematoxylin and eosin (H & E). One pair of abdominal and inguinal mammary glands from each animal was prepared as whole mounts, following formalin fixation, by alum carmine staining, as previously described [15]; the contralateral glands were processed for histological sections. Whole mounts and histological sections were visualized by light microscopy. H&E sections of livers, uteri and mammary glands were reviewed, blind to treatment, for treatment-related differences and pathological changes.
Mammary gland whole mount analysis
Whole mount preparations of the inguinal glands were scored for growth and developmental status. Photomicrographs were made of whole mounts using a Nikon SMZ-2T microscope with QImaging MicroPublisher 5.0 RTV Camera (QImaging; Surrey B.C., Canada) at 10× magnification for quantification of 1) longitudinal growth, as determined by ductal length extending from the lymph node to the most distal terminal branches, 2) numbers of terminal end buds (TEBs), and 3) stimulated/enlarged terminal duct (TDs). TEBs were defined as enlarged (greater than 100 µm in diameter), multilayered ductal tips surrounded by adipocytes and located at the periphery of the gland. Stimulated TDs were defined as TEB-like structures (75 – 100 µm in diameter) occurring within the body of the gland and were distinguished from unstimulated TDs, which averaged 50 µm in diameter. Two independent measures were performed. Mean values for each treatment group were calculated and analyzed statistically for treatment-related differences
Analysis of mammary epithelial cell proliferation
Deparrafinized tissue sections were autoclaved at 15 psi for 20 minutes in citrate buffer (pH 6.0) for antigen retrieval. Next, sections were blocked with goat anti-mouse IgG Fab fragments (Jackson Immunoresearch Laboratories) (1:100 in 1% PBSA, 60 min), blocked with normal goat serum (Vector Laboratories) (1:1 in PBS, 30 min) and incubated for 60 min at room temperature with anti-BrdU antibody (kit from Amersham Biosciences, Piscataway, NJ). BrdU localization was detected with a biotinylated goat anti-mouse secondary antibody conjugated to Alexa 546 (Molecular Probes) (1:100 in PBS, 30 min). Nuclei were counterstained with DAPI (Molecular Probes) (1:10,000 in H2O), and sections were visualized and images captured using a Nikon inverted epifluorescence microscope (Mager Scientific, Dexter, MI) with MetaMorph software (Molecular Devices Corporation, Downington, PA). Sections treated for detection of BrdU by immunofluorescence were quantitated for the number of positive luminal epithelial cell nuclei from captured images using MetaMorph software. Positive nuclei displayed staining above luminal epithelial cytoplasmic background. Five mice per treatment group were analyzed; a minimum of 1000 total cells and three independent sections per mouse were analyzed. Mean values for percent BrdU positive cells in TEBs and stimulated TDs were not significantly different and were combined in the analyses. Mean values for mammary structures in each treatment group were calculated and analyzed statistically for treatment- and mouse strain-related differences.
Statistical Analysis
Data were evaluated for exposure effects by analysis of variance (ANOVA) using a general linear model (Statistical Analysis System [SAS] version 9.1, SAS Institute, Inc. Cary, NC). Means were evaluated and effects of the different doses within each strain and between the two strains were compared. Treatment group-specific mean BWs were calculated. Liver and uterine weights were calculated and normalized to BW. Values are presented as mean ± S.D. or mean ± S.E.M. Differences between treatment groups were determined using Student’s t-tests with SAS. Differences were considered significant at p< 0.05.
RESULTS
Body weight
All mice displayed normal behavior and looked healthy throughout the treatment period. From the beginning of the experiment until dose 18, both strains of mice showed similar BW gains for all PFOA doses. At doses 19 and 20 (of a total of 20) mice of both strains receiving the 10 mg/kg showed significant decreases in BW (Table 1).
Table 1.
Effects of PFOA treatment on body weight (BW), relative liver weight, relative uterine weight and the timing of vaginal opening (Mean ± SD, n=5)
| Treatment | BW | Relative liver weight | Relative uterine weight | Age of vaginal opening |
|---|---|---|---|---|
| (g) | (%) | (%) | (Day) | |
| Balb/c | ||||
| Vehicle | 17.3 ±0.97d | 4.9 ±0.36b,c,d | 0.52 ±0.29b,c,d | 41.8 ±2.49b |
| 1 mg/kg | 17.4 ±0.42d | 7.7 ±0.43a,c,d | 0.32 ±0.07a,c,d | 46.8 ±2.68a |
| 5 mg/kg | 16.7 ±1.04d | 10.6 ±0.38a,b | 0.11 ±0.03a,b | No Opening |
| 10 mg/kg | 15.1 ±0.55a,b,c | 10.8 ±0.50a,b | 0.08 ±0.03a,b | No Opening |
| C57BL/6 | ||||
| Vehicle | 17.4 ±0.55d | 4.5 ±0.91b,c,d | 0.33 ±0.08b,d | 40.8 ±2.77c |
| 1 mg/kg | 17.2 ±1.15d | 6.9 ±0.17a,c,d | 0.56 ±0.16a,c,d | 43.0 ±3.94c |
| 5 mg/kg | 16.8 ±1.35d | 11.3 ±0.48a,b | 0.37 ±0.12a,b,d | 47.0 ±2.24a,b |
| 10 mg/kg | 15.6 ±0.65a,b,c | 12.0 ±0.96a,b | 0.11 ±0.04a,b,c | No Opening |
p<0.05, compared to Vehicle control group;
p<0.05, compared to 1 mg/kg PFOA treatment group;
p<0.05, compared to 5 mg/kg PFOA treatment group;
p<0.05, compared to 10 mg/kg PFOA treatment group. Relative liver weight (%) = 100 ±liver weight/ BW; relative uterine weight (%) = 100 ±uterine weight/ BW.
Effect of PFOA on mammary gland development
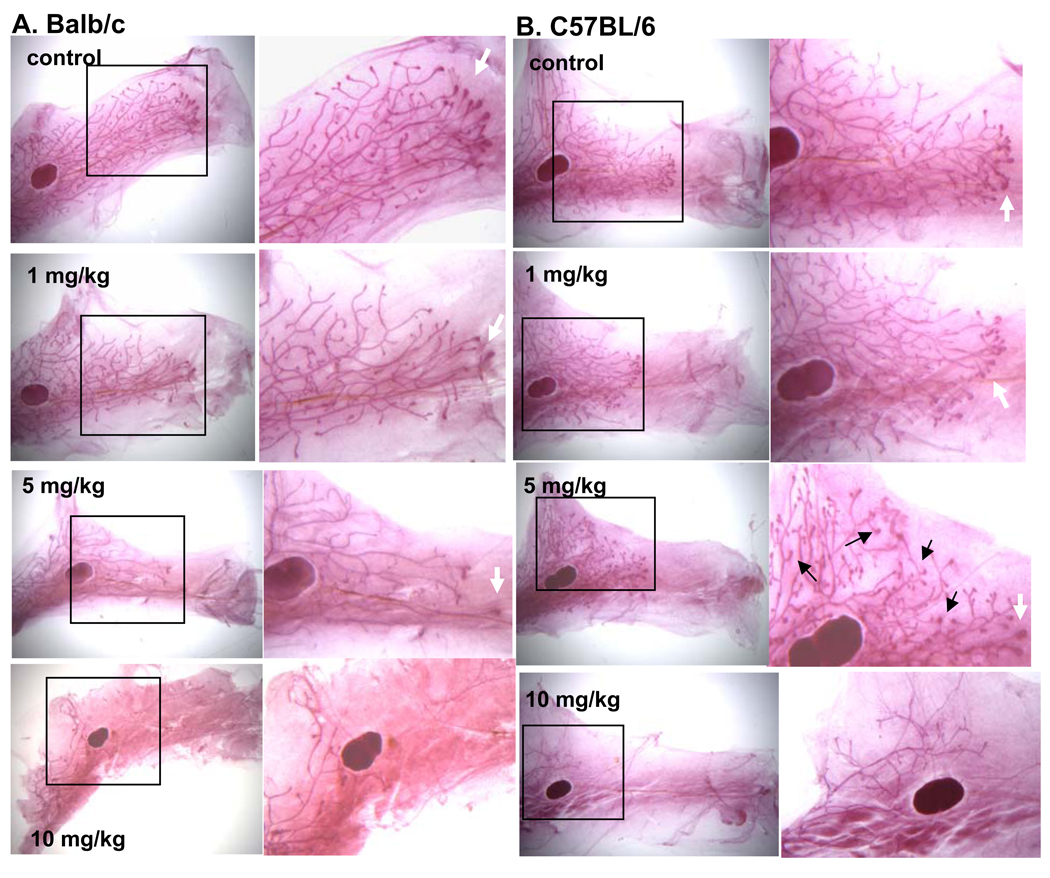
Analysis of mammary gland whole mounts revealed that PFOA treatment caused mammary gland growth inhibition in the Balb/c strain, as evidenced by reduced ductal length, as well as decreased numbers of terminal end buds (TEB) and stimulated TDs; significant inhibition was detected at the 5 and 10 mg/kg doses (p<0.05) (Table 2, Fig. 1A).
Table 2.
Effects of PFOA treatment on mammary gland ductal length, the number of terminal end buds (TEBs) and the number of stimulated terminal ducts (TDs) (Mean ± SD, n=5)
| Treatment | Ductal Length (relative units) | Number of TEBs | Number of stimulated TDs |
|---|---|---|---|
| Balb/c | |||
| Vehicle | 7.60 ± 0.94c,d | 8.20 ± 1.92c,d | 6.20 ± 2.39c,d |
| 1 mg/kg | 7.28 ± 0.73c,d | 6.00 ± 3.74d | 3.80 ± 1.64d |
| 5 mg/kg | 3.99 ± 0.36a,b | 3.99 ± 0.36a,d | 1.20 ± 1.30a,d |
| 10 mg/kg | 3.40 ± 4.77a,b | 0.00 ± 0.00a,b,c | .00 ± 0.00a,b,c |
| C57BL/6 | |||
| Vehicle | 4.76 ± 1.19d | 7.80 ± 2.39c,d | 6.60 ± 2.88c,d |
| 1 mg/kg | 4.06 ± 0.85d | 10.40 ± 3.36d | 9.80 ± 2.77c,d |
| 5 mg/kg | 4.01 ± 0.71d | 11.20 ± 1.30a,d | 16.60 ± 5.90a,b,d |
| 10 mg/kg | 0.30 ± 0.67a,b,c | 0.00 ± 0.00a,b,c | 0.00 ± 0.00a,b,c |
p<0.05, compared to Vehicle control group;
p<0.05, compared to 1 mg/kg PFOA treatment group;
p<0.05, compared to 5 mg/kg PFOA treatment group;
p<0.05, compared to 10 mg/kg PFOA treatment group.
Fig. 1. Effect of PFOA treatment on mammary gland development.
Mice were treated with vehicle control or indicated doses of PFOA as described in Materials and Methods. Twenty-four h after the last vehicle or PFOA treatment, mice were killed and mammary gland whole mounts were prepared as described in Materials and Methods. Representative mammary gland whole mount photomicrographs from control- or PFOA-treated (A) Balb/c or (B) C57BL/6 mice. Black arrows indicate stimulated TDs and white arrows indicate TEBs. (Mag. X 2.5 ;Mag of insets X 4.5).
In striking contrast, PFOA treatment in the C57Bl/6 strain resulted in increased numbers of TEBs and stimulated TDs at the 5 mg/kg dose (p<0.05) (Table 2, Fig. 1B). There were no differences in ductal length among the control, 1 and 5 mg/kg doses. An inhibitory effect on all measures of mammary gland development was seen at the 10 mg/kg dose; significant decreases in ductal length, numbers of TEBs and stimulated TDs were observed (p<0.05).
Since we observed an inhibitory effect at a lower dose (5mg/kg) in Balb/c mice compared to C57BL/6 mice (10mg/kg), we considered the possibility that these two strains might have a different dose range for stimulatory effects of PFOA. To see if this was the case, we also tested a 0.1 mg/kg dose in Balb/c mice. No differences were observed in mammary gland parameters between the control and 0.1mg/kg groups (data not shown).
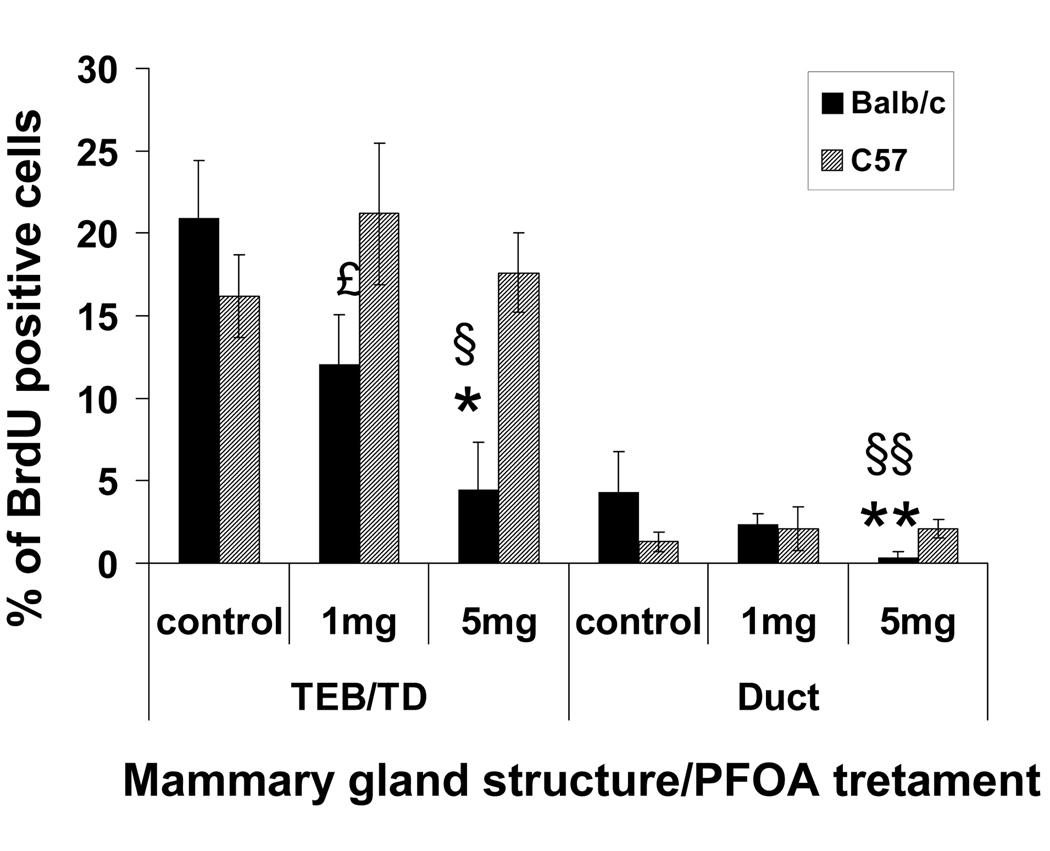
We also examined the effect of PFOA on the proliferation index (percent of BrdU positive cells) of mammary epithelial cells in ducts, TEBs and stimulated TDs in the control, 1 and 5 mg/kg treatment groups. To accomplish this, mice were injected with BrdU 2 hrs prior to killing and BrdU incorporation was detected in histological sections by immunoflourescence staining.
In the Balb/c strain, there were significant decreases in BrdU positive cells in TEBs, stimulated TDs and ducts at the 5 mg/kg dose (Fig. 2.) While control-treated Balb/c and C57BL/6 mice showed similar percentages of proliferating cells, Balb/c glands had significantly fewer proliferating cells in TEBs and stimulated TDs at the 1 and 5 mg/kg doses and in ducts at the 5 mg/kg dose than C57BL/6 glands (Fig. 2).
Fig. 2. Effect of PFOA treatment on mouse mammary gland epithelial cell proliferation.
Mouse mammary epithelial cell proliferation was determined by BrdU incorporation assay. Two h before killing, mice were injected with BrdU (70 mg/kg of body weight). BrdU incorporation was detected in histological sections by immunoflourescence staining as described in Materials and Methods. Cell proliferation was quantitated in TEBs, stimulated TDs and ducts by counting the number of BrdU positive luminal epithelial cell nuclei from captured images using MetaMorph software as described in Materials and Methods. Five mice per treatment group were analyzed, and a minimum of 1000 total cells and three independent sections per mouse were analyzed. The values for TEBs and stimulated TDs were combined. Data are presented as Mean ± S.E.M. (n=5). * p<0.05 compared with vehicle-treated Balb/c TEB/TD; ** p<0.05 compared with vehicle-treated Balb/c duct; £ p<0.05 PFOA (1mg/kg)-treated C57BL/6 TEB/TD compared with PFOA (1 mg/kg)-treated Balb/c TEB/TD; § p<0.05 PFOA (5mg/kg)-treated C57BL/6 TEB/TD compared with PFOA (5 mg/kg)-treated Balb/c TEB/TD; §§ p< 0.05 PFOA (5mg/kg)-treated C57BL/6 duct compared with PFOA (5 mg/kg)-treated Balb/c duct.
In C57BL/6 mice, TEBs, stimulated TDs and ducts exhibited similar percentages of BrdU positive, proliferating cells in the control, 1 and 5 mg/kg treatment groups (Fig 2). Since there were significantly more TEBs and stimulated TDs in the 5 mg/kg treatment group (Table 2), this indicated that overall mammary epithelial cell proliferation was increased in this treatment group.
Effect of PFOA on the uterus and on the timing of vaginal opening
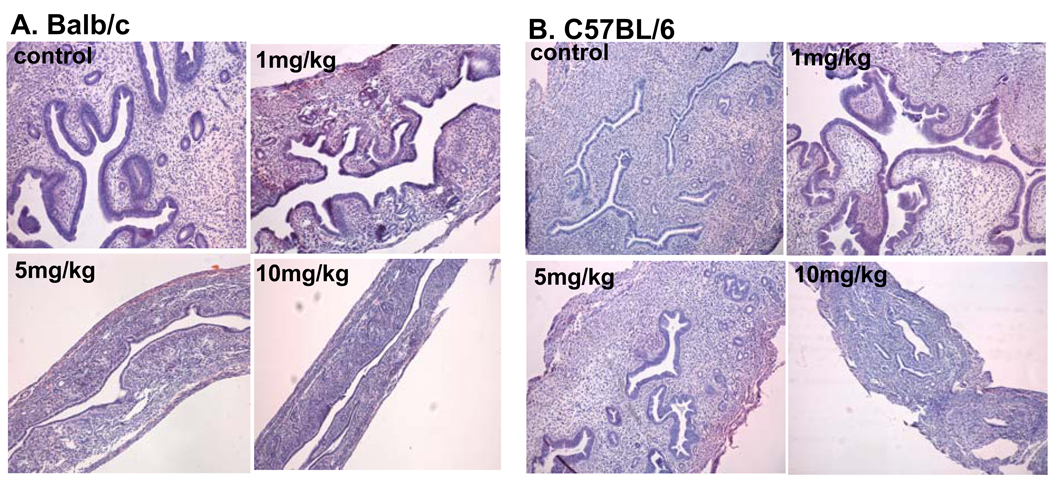
A dose-dependent inhibitory effect on the uterus was observed in the BALB/c mouse strain, as evidenced by a significant decrease of relative uterine weight (Table 1). Histological evaluation showed decreased uterine size and glandular development (Fig.3A). In contrast, the C57BL/6 mouse strain showed a significant increase in relative uterine weight at the 1 mg/kg PFOA dose (Table1; p< 0.05). A significant decrease in relative uterine weight was seen only at the 10 mg/kg dose (p< 0.05). The stimulatory effect of PFOA treatment on the relative uterine weight in C57Bl/6 mice corresponded to the histological findings (Fig 3B). The stimulatory effect at the 1 mg/kg dose was reflected in an overall increased size of the uterus, along with well-developed endometrial lining, glands and stoma. Decreased uterine size and development were found in mice treated with the10 mg/kg PFOA dose. We considered the possibility that the stage of estrus cycle when the mice were sacrificed may have confounded the interpretation of the effect of PFOA on uterine weight and histology. Therefore, we examined the stage of estrous cycle in relation to uterine weight and morphology for both the Balb/c and C57BL/6 mice treated with PFOA. In the case of the Balb/c strain, the decrease in uterine weight at the 1 mg/kg dose was not due to a difference in the distribution of animals in the various stages of the cycle ( proestrus, estrus or diestrus) since the distribution was very similar between the PFOA and control-treated mice, but rather was due to a lower uterine weight at each stage of the cycle in the PFOA-treated mice (data not shown). In the case of the C57BL/6 mice, again there was no difference in the distribution of mice in the various stages of the estrus cycle between the control and 1mg/kg PFOA- treated animals but rather an increase in uterine weight and morphology at each stage of the cycle in the PFOA-treated mice (data not shown). Therefore, the changes in uterine weight and morphology appear to be related to the effect of PFOA treatment and not due to altered distribution of mice in various stages of the estrus cycle in the various treatment groups.
Fig. 3. Effect of PFOA treatment on the uterus.
Mice were treated with vehicle control or indicated doses of PFOA as described in Materials and Methods. At the time of termination, uteri were harvested and fixed in 10% neutral formalin. H & E staining of uterus was performed as described in Materials and Methods. Representative photomicrographs of uterine sections from vehicle control- or PFOA-treated (A) Balb/c or (B) C57BL/6 mice. (Mag. X 40).
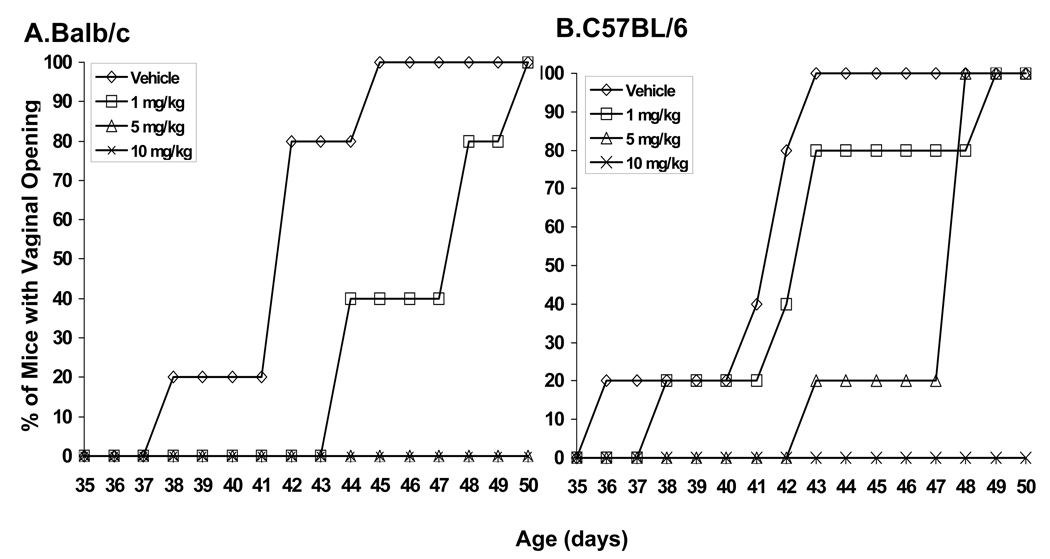
The effects of PFOA treatment on the timing of vaginal opening were comparable in both mouse strains. The mean age at vaginal opening was similar in control-treated Balb/c and C57BL/6 mice (Table 1). In the Balb/c strain, mean age at vaginal opening was significantly greater in the 1 mg/kg treatment group (p< 0.05) and vaginal opening was completely absent at the 5 and 10 mg/kg doses (Fig. 4A). In the C57Bl/6 mice, mean age at vaginal opening was significantly greater at the 5 mg/kg dose (p< 0.05), and completely absent at the 10 mg/kg dose (Fig. 4B).
Fig. 4. Effect of PFOA treatment on the timing of vaginal opening.
Mice were treated with vehicle control or indicated doses of PFOA as described in Materials and Methods. Appearance of vaginal opening was monitored and recorded daily. Time-course of the timing of vaginal opening in vehicle- or PFOA-treated (A) Balb/ c or (B) C57BL/6 mice. Data are presented as the percent (%) of mice with vaginal opening at indicated day of age.
Effects of PFOA treatment on the liver
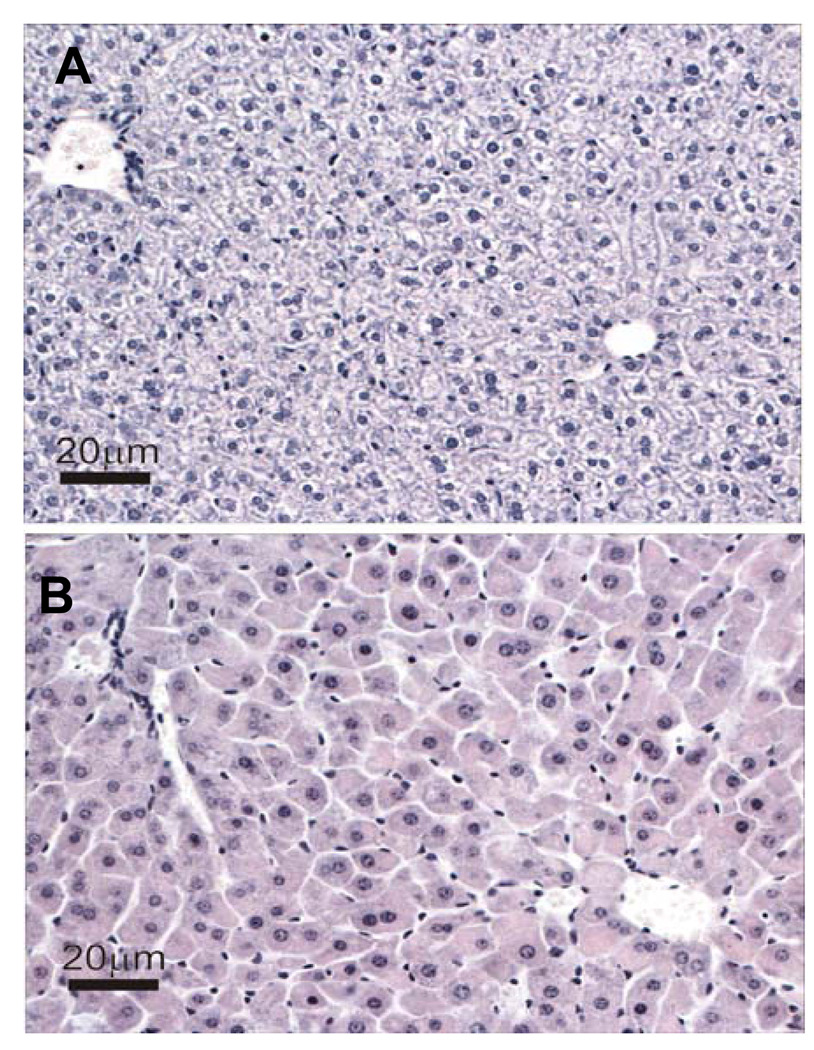
Dose-dependent increases in relative liver weights were detected in both strains (Table 1). Analysis of histological sections revealed that the principal morphologic alteration in the livers of both strains of mice was a dose-dependent increase in hepatocellular hypertrophy. However, at each dose the extent and severity of the hypertrophy was greater in C57Bl/6 mice (data not shown). Fig. 5 shows representative images of liver histology from control- and PFOA-treated (5 mg/kg) C57Bl/6 mice.
Fig. 5. Effect of PFOA treatment on liver.
Mice were treated with vehicle control or indicated doses of PFOA as described in Materials and Methods. At the time of termination, livers were harvested and fixed in 10% neutral formalin. H & E staining of livers was performed as described in Materials and Methods. Representative photomicographs of liver sections from (A) vehicle- or (B) PFOA-treated (5 mg/kg) C57BL/6 mice. (Scale bar = 20 µm).
Discussion
Because of its widespread presence in the environment, in wildlife and humans, and its persistence and accumulation properties, PFOA has raised significant health concerns. The peripubertal period is considered to be an important window of susceptibility for the developing breast to environmental exposures that may predispose humans to increased breast cancer risk later in life [13,14]. During the peripubertal period, the mammary gland undergoes exponential growth in rodents and humans. During this period, highly proliferative TEBs are present at the growing edge of the gland [16,17]. Once the gland has grown to the limits of the mammary fat pad, the TEBs disappear from the mature gland. Several studies have determined that TEB structures are sensitive to chemical carcinogens in rodent models, and their presence at the time of carcinogen exposure is positively associated with tumor multiplicity [18,19]. Environmental exposures that increase the numbers of TEBs or prolong their presence in the developing gland could enhance the sensitivity of the gland to the action of chemical carcinogen. Alternatively, environmental exposures that decrease the abundance of TEBS and/or inhibit mammary growth might confer protection from breast cancer risk [13].
Recent studies have shown that gestational plus lactational PFOA exposure in CD-1 mice leads to general developmental toxicity in offspring, such as decreased neonatal body weight and survival, and stunted mammary gland development. These effects may be caused by abnormal lactational performance due to a significant reduction in mammary gland differentiation of exposed dams [10,12]. More recent studies (Fenton et al Reproductive Toxicology, this issue) have observed that lactation-only exposure in CD-1 mice also delays mammary gland development.
In the present study, we have examined the effects of peripubertal PFOA exposure (from 21 to 50 days of age) in Balb/c and C57BL/6 mice. Peripubertal PFOA treatment caused similar hepatocellular hypertrophy and delayed vaginal opening in both mouse strains. However, the effects of PFOA treatment on the uterus and mammary gland were strikingly different between the two mouse strains. In Balb/c mice there were dose-dependent inhibitory effects on uterus and mammary gland, while in the C57Bl/6 strain, there were stimulatory effects on the mammary gland and uterus at low doses and inhibitory effects at higher doses (10mg/kg). Specifically, in the C67BL/6 mammary gland, the 5mg/kg PFOA dose caused increased numbers of TEBs and an overall, generalized stimulation of gland seen as an increased abundance and enlargement of numerous TDs. The stimulatory effect in C57BL/6 mammary glands at the 5 mg/kg dose was confirmed by an analysis of mammary cell proliferation. While the percentage of proliferating cells in TEBs, stimulated TDs and ducts was similar in the control and 5 mg/kg treatment groups, the increased numbers of TEBs and stimulated TDs indicate an overall increase in the abundance of proliferating mammary epithelial cells. Over a dose range of 0.1, 1, 5 and 10 mg/kg, Balb/c mice display only an inhibitory effect (at 5 and 10 mg/kg doses). Similar patterns of PFOA-induced stimulation in the C57BL/6 strain and PFOA-induced inhibition in the Balb/c strain were observed in the uterus. However, the maximal stimulatory effect in the uterus in C57BL/6 mice was seen at the 1 mg/kg dose, whereas the maximal stimulatory effect in the mammary gland was seen at the 5 mg/kg dose. Taken together, these results indicate that the two strains exhibit inherent, genetically determined differences in mammary gland and uterine responses to PFOA. The genetic and mechanistic basis for the differences between the two strains is not currently known.
Peroxisome proliferator-activated receptor α (PPARa) plays a critical role in various toxicities of PFOA in rodents [20–22]. Toxicological studies have shown that PFOA is an agonist for PPARα and induces hepatocellular tumors, testicular Leydig cell adenomas, and pancreatic acinar cell tumors in rats [7,8]. The general developmental toxicity of PFOA resulting from gestational/prenatal exposure in CD-1 mice depends on the expression of PPARa [21]. Since PFOA is a known PPARα agonist, effects on the mammary gland may involve this pathway. However, it is known that PPARα is not a critical element of mammary gland development in the neonate, since PPARα null mice exhibit normal mammary gland development and function [23]. Therefore, the effects of gestational PFOA exposure on neonatal mammary tissue are not thought to be mediated through this pathway [12]. It is not known if PFOA’s activity as a PPARa agonist that we observed in this study plays a major role in the differential effects of peripubertal PFOA exposure on mammary gland and uterine development observed in this study on Balb/c and C57Bl/6 mice. Studies of peripubertal PFOA treatment of PPARa knockout mice (C57BL/6 genetic background) are planned to clarify this issue.
The most well-known environmental pollutants that are capable of causing abnormal mammary gland development are estrogenic endocrine disrupting chemicals (EDCs) (those found in the external environment that can mimic or inhibit the effect of endogenous estrogen ) [13,24,25]. Exposure to EDCs during critical periods leads to defective mammary gland development. For example, gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the aryl-hydrocarbon receptor and a persistent EDC, inhibits mammary gland development in the female offspring of Long-Evans rats [26]. The mammary gland and uterus are estrogen target tissues. The stimulatory effects observed in the C57BL/6 mammary gland and uterus are similar to estrogen-induced effects in these tissues. Estrogen-like properties of PFOA and other perfluorinated chemicals have recently been evaluated [27]. Using an estrogen receptor a (ERa) - positive human breast cancer cell line (MCF-7), Maras et al. reported that PFOA does not possess estrogen-dependent proliferation capacity [28]. Similarly, using a yeast two-hybrid assay that employs the interaction between the human ERa or ERb ligand binding domain and the coactivator TIF2 (transcription intermediary factor 2), Ishibashiet et al. observed an estrogenic effect for 6:2 FTOH (1H,1H,2H,2H-perfluorooctan-1-ol) and 8:2 FTOH (1H,1H,2H,2H-perfluoro-decan-1-ol), but not PFOA [29]. Together, these findings suggest that PFOA may not possess direct estrogenic activity. However, a recent study performed in rare minnows (Gobiocypris rarus) revealed estrogen-like properties of PFOA. It was found that PFOA can disturb the activity of estrogen in mature male rare minnows by inducing the expression of the hepatic estrogen-responsive genes, vitellogenin and ERb, and inhibiting female reproduction [30]. It is not clear if these findings are due to a direct or indirect effect of PFOA, or are relevant to the effects of PFOA in mammals.
The most widely reported effect of PFOA is its liver toxicity [7,8]. In this regard, we observed PFOA-induced hepatocytomegaly in both strains of mice, and this was more severe in the C57BL/6 strain. Another mechanism through which PFOA might indirectly affect mammary gland development is by alteration of gene expression in the liver. PFOA has been shown to alter gene regulation in the liver through a PPARa-independent mechanism(s) [31,32]. Recent studies found that about 15% of genes altered by PFOA exposure in the liver are independent of PPARa, and the PPARa-independent genes regulated by PFOA include those involved in lipid homeostasis, amino acid metabolism, and xenobiotic metabolism [31,32]. Moreover, expression of the majority of the genes controlling xenobiotic metabolism are up-regulated in PFOA-treated, PPARa knockout mice [32]. It is possible that up-regulation of these xenobiotic metabolism enzymes might affect the levels of serum estradiol and/or other steroid hormones, which in turn could affect uterine and mammary gland development and these events may be differentially regulated in various mouse strains. We are currently analyzing serum estradiol levels in control- and PFOA-treated mice.
The basis for the strain-specific differences in the uterine and mammary gland responses to PFOA treatment is not known. It is possible that some aspects of the strain differences could be due to differences in pharmacokinetics, tissue distribution and/or metabolic handling of PFOA. Unrelated studies in our laboratory have shown that ovariectomized adult mice in the two strains, treated with exogenous estradiol, show differential sensitivity to estrogenic stimulation of mammary gland proliferation with the C57BL/6 strain being highly sensitive and the Balb/c strain being less sensitive ( Aupperlee & Haslam, unpublished observations). This suggests that if PFOA-treatment increases endogenous estradiol levels, it is more likely to be detected as a stimulatory effect in the C57BL/6 mammary gland than in the Balb/c gland. Clearly, high doses PFOA have negative effects on the liver, uterus, age at vaginal opening, and mammary gland development in both strains of mice. It is also possible that the two strains differ in their sensitivity to inhibitory effects of PFOA with the Balb/c strain being more sensitive.
Although the mechanism(s) for how peripubertal PFOA exposure causes abnormal mammary gland development have yet to be determined, the findings from the present study are significant in three aspects. First, our findings on the peripubertal effects of PFOA extend the previous observations of the detrimental effects of gestational PFOA exposure on mammary gland development and function in CD-1 mice [12]. Second, the demonstration that peripubertal PFOA exposure affects mouse mammary gland development provides a strong rationale for further study to understand the underlying mechanisms of PFOA-induced effects and to determine if peripubertal PFOA exposure increases susceptibility to mammary tumorignenesis. Obtaining clear answers about these issues is of importance. Accidental release of large quantities of PFOA into soil and surface water has occurred in several places, and high concentrations of PFOA in humans have been detected in the general population in the affected communities [4–6]. Therefore, the possibility exists that mammary gland development may be affected in pubertal girls in the contaminated areas. If further studies indicate that peripubertal PFOA exposure increases or decreases susceptibility to mammary cancer in experimental animals, this may have implications about breast cancer risk in PFOA-exposed girls. Third, the finding of striking differences in the effects of peripubertal PFOA exposure (both stimulatory and inhibitory) in two genetic backgrounds in the same species is critically important. Most rodent studies on the environmental pollutants generally use one mouse or rat strain. Our findings suggest that caution should be used when drawing conclusions about the effects of PFOA or other environmental pollutants on the basis of studies in a single strain. These studies also have potential implications for identifying the effects of PFOA and other environmental exposures in genetically heterogeneous human populations.
Acknowledgements
The authors thank Dr Jianwei Xie for the statistical analyses. This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science (NIEHS) and the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH.
Abbreviations
- BrdU
5-bromo-2’-deoxyuridine
- BW
body weight
- DAPI
4',6'-diamidino-2-phenylindole
- EDC
endocrine disrupting chemical
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- GD
gestational day
- H & E
hematoxylin and eosin
- PFOA
Perfluorooctanoic acid
- PND
postnatal day
- PPARα
peroxisome proliferator-activated receptor α
- TDs
terminal ducts
- TEBs
terminal end buds
Footnotes
Conflict of interest: the authors declare that there are no conflicts of interest.
References
- 1.Hekster FM, Laane RW, de Voogt P. Environmental and toxicity effects of perfluoroalkylated substances. Rev Environ Contam Toxicol. 2003;179:99–121. doi: 10.1007/0-387-21731-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Betts KS. Perfluoroalkyl acids: what is the evidence telling us? Environ Health Perspect. 2007;115:A250–A256. doi: 10.1289/ehp.115-a250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds - Exposure assessment for the general population in western countries. Int J Hyg Environ Health. 2008 Jun 17; doi: 10.1016/j.ijheh.2008.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N, Wilhelm M. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect. 2008;116:651–657. doi: 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy GL, Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 9.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90:510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 11.Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, Thibodeaux J, Das KP, White SS, Lau CS, Abbott BD. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95:462–473. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 12.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96:133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 13.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 14.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000 108; suppl 3:451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee MR, Wood BG, Lin FK, Crump LR. Organ culture of whole mammary gland of the mouse. Tissue Culture Association Manual. 1976;2:457–462. [Google Scholar]
- 16.Daniel CW, Silberstein GB. Postnatal development of the rodent mammary gland. In: Neville MC, Daniel CW, editors. The mammary gland: development, regulation, and function. New York: Plenum Press; 1987. pp. 3–36. [Google Scholar]
- 17.Russo J, Russo IH. Development of the human mammary gland. In: Neville MC, Daniel CW, editors. The mammary gland: development, regulation, and function. New York: Plenum Press; 1987. pp. 67–93. [Google Scholar]
- 18.Russo IH, Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenzanthracene. J Natl Cancer Inst. 1978;61:1439–1449. [PubMed] [Google Scholar]
- 19.Russo IH, Russo J. Experimentally induced mammary tumors in rats. Br Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Xie Y, Alexson SE, Nelson BD, DePierre JW. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol. 2002;63:1893–1900. doi: 10.1016/s0006-2952(02)00923-1. [DOI] [PubMed] [Google Scholar]
- 21.Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, Lau C. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci. 2007;98:571–581. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- 22.Asakawa A, Toyoshima M, Harada KH, Fujimiya M, Inoue K, Koizumi A. The ubiquitous environmental pollutant perfluorooctanoicacid inhibits feeding behavior via peroxisome proliferator-activated receptor-alpha. Int J Mol Med. 2008;21:439–445. [PubMed] [Google Scholar]
- 23.Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisomeproliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20:63–75. doi: 10.1016/j.beem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Hotckiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after "Wingspread"- Environmental Endocrine Disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol Sci. 2008 Feb 16; doi: 10.1093/toxsci/kfn030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 28.Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ Health Perspect. 2006;114:100–105. doi: 10.1289/ehp.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishibashi H, Ishida H, Matsuoka M, Tominaga N, Arizono K. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro. Biol Pharm Bull. 2007;30:1358–1359. doi: 10.1248/bpb.30.1358. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, Wang Z. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus) Environ Toxicol Chem. 2007;26:2440–2447. doi: 10.1897/07-008R1.1. [DOI] [PubMed] [Google Scholar]
- 31.Rosen MB, Abbott BD, Wolf DC, Corton JC, Wood CR, Schmid JE, Das KP, Zehr RD, Blair ET, Lau C. Gene Profiling in the Livers of Wild-Type and PPAR{alpha}-Null Mice Exposed to Perfluorooctanoic Acid (PFOA) Toxicol Pathol. 2008 May 8; doi: 10.1177/0192623308318208. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci. 2008;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]