Abstract
Objective
During lung transplantation, cells in the pulmonary parenchyma are subjected to ischemia, hypothermic storage, and reperfusion injury. Platelets, whose granular contents include adhesion receptors, chemokines, and coactivating substances that activate inflammatory and coagulant cascades, likely play a critical role in the lung allograft response to ischemia and reperfusion. The platelet response to the pulmonary allograft, however, has never been studied. Here we report significant platelet activation immediately after lung transplantation.
Methods
We performed a prospective cohort study comparing markers of platelet activation in patients undergoing lung transplantation and patients undergoing nontransplant thoracotomy. Plasma levels of soluble P-selectin, soluble CD40 ligand, and platelet–leukocyte conjugates were measured before surgery, after skin closure, and at 6 postoperative hours.
Results
Both soluble P-selectin and soluble CD40 ligand levels increased significantly after lung transplantation but not after thoracotomy. Additionally, platelet–monocyte conjugate fluorescence was significantly higher after lung transplantation than after thoracotomy alone.
Conclusion
These findings suggest that platelet activation is significantly increased after lung transplantation beyond that expected from the postoperative state. The increase in circulating platelet–monocyte conjugates suggests an important interaction between platelets and inflammatory cells. Further research should examine whether platelet activation affects early graft function after lung transplantation.
Lung transplantation has emerged as a successful surgical therapy for end-stage pulmonary disease.1 The lung is the most richly vascular transplanted organ, and each milliliter of blood coursing through the transplanted lungs often contains more than 300,000 platelets. Platelets contain potent thrombotic and vasoactive compounds and mediate key pathways in inflammation. To our knowledge, however, the platelet response to lung transplantation has not been previously studied in human beings.
Platelets adhere to activated endothelium, crosslinking leukocytes to injured endothelial surfaces.2 Platelet-mediated leukocyte adherence, diapedesis, and infiltration of the lung allografts are integral to early graft failure after lung transplantation. Several biomarkers with potential physiologic significance reflect in vivo platelet activity. P-selectin (CD62P) is a 140-kDa transmembrane protein that is stored in the alpha and dense granules of platelets and in endothelial cells; this protein is expressed on the cell surface, and some is shed during degranulation (soluble P-selectin [sP-selectin]).3 sP-selectin binds P-selectin glycoprotein ligand-1 on leukocytes, arresting them in transit and upregulating inflammation at the blood–tissue interface.4 CD40 ligand (CD154) is a trimeric transmembrane protein that also mobilizes to the cell surface of the activated platelet before being shed (soluble CD40 ligand [sCD40L]).5,6 As the cognate interaction between CD154 and CD40 (on endothelial and other cells) can contribute not only to immune cell coactivation but also platelet clumping, this interaction is likely important in the context of lung ischemia and reperfusion.5 Platelets produce more than 95% of all sCD40L, which stimulates cyclooxygenase-2 and prostaglandin E2 synthesis, leading to inflammation.7,8 Further, circulating platelet–leukocyte conjugates, as assessed by flow cytometry, are considered to be a sensitive marker of the complex interactions that occur between platelet activation and inflammation pathways in vivo.9
Because platelets play a key role in the recruitment and binding of leukocytes to vascular sites of inflammation, platelet activation could be an integral component of early inflammatory and thrombotic processes in the reperfused lung allograft. The effects of lung transplantation on sP-selectin, sCD40L, and circulating platelet–leukocyte conjugates are, however, unknown. We therefore aimed to determine whether platelet activation is increased after lung transplantation. We hypothesized that sP-selectin, sCD40L, and circulating platelet–leukocyte conjugates would be increased in patients undergoing lung transplantation relative to patients undergoing other types of thoracic surgery.
Materials and Methods
Study Design and Subjects
We performed a prospective cohort study of patients undergoing lung transplantation and patients undergoing nontransplant thoracotomy. Patients with a known history of platelet disorders or recent antiplatelet medication use, including aspirin or ticlopidine, were excluded from study. Patients with planned cardiopulmonary bypass or a history of chemotherapy or radiation therapy to the chest were also excluded, because platelet reactivity is altered by cardiopulmonary bypass and advanced malignancy.10,11 All patients in this study underwent preoperative induction of immunosuppression with tacrolimus (0.04 mg/kg), azathioprine (2 mg/kg), methylprednisolone (1 g), and basiliximab (20 mg).
Blood Sampling
After informed consent was obtained, blood sampling was performed (1) before induction of anesthesia and immunosuppression, (2) immediately postoperatively, after skin closure, and (3) 6 hours later. Clinical data, including platelet counts, were recorded. Phlebotomy was performed through a fresh needle stick at each point with a 21-gauge butterfly into Vacutainer tubes (BD, Franklin Lakes, NJ) containing EDTA. Whole blood was spun at 7000g for 15 minutes, and plasma was frozen at −80°C until batch analysis. All blood samples were processed within 15 minutes of phlebotomy.
Plasma sP-selectin and sCD40L levels were measured in triplicate with commercially available assays (R&D Systems, Minneapolis, Minn) according to the manufacturer protocol.
Platelet–Leukocyte Conjugates
Circulating platelet–leukocyte conjugates were quantified by flow cytometry, as previously described.2,12,13 Briefly, 200 μL whole blood was used from each sample for analysis. Three samples were run in parallel: a negative control, an unstimulated sample, and 10-mmol/L adenosine diphosphate–stimulated sample. The samples were labeled sequentially with phycoerythrin-labeled antihuman platelet CD42 antibody (BD) and then fluorescein isothiocyanate–labeled antihuman leukocyte CD14 antibody (BD). After labeling, all samples were fixed and then lysed with Optilyse solution (Beckman Coulter, Inc, Fullerton, Calif) according to manufacturer protocol. All tubes were then immediately analyzed on a FACSCalibur Flow Cytometer (BD) according to the method of Michelson with Cell Quest Pro software.14 Isotype-matched fluorescein isothiocyanate–labeled IgG (BD) served as negative control preparations. Particles expressing both CD42 and CD14 were identified as platelet–leukocyte conjugates and then further subdivided into either platelet–monocyte or platelet–neutrophil conjugates. Differences in granularity and the intensity of CD14 expression allowed differentiation between monocyte and neutrophil populations.2,12,13 Measures of conjugates were expressed as the mean intensity of phycoerythrin-labeled CD42 per particle, reflecting the number of platelets bound per monocyte-neutrophil conjugate, and as the percentage of the particles positive for phycoerythrin-labeled CD42 and fluorescein isothiocyanate–labeled CD14. Flow cytometric platelet–leukocyte conjugate data are presented as fractional change from baseline.
Statistical Analysis
Continuous variables are summarized with mean ± SE or median with interquartile range, as appropriate. Comparison of preoperative with postoperative plasma biomarkers and flow cytometric results was performed with signed rank tests. We compared patients undergoing lung transplantation with those undergoing thoracotomy alone at each postoperative time point with Mann–Whitney U tests. Multivariate linear regression was also performed for postoperative plasma biomarker levels with adjustment for baseline levels.
Before the study, we performed power analyses that indicated that a sample size of 7 patients undergoing transplantation and 10 control patients would provide 80% power (α = .05) to detect a difference between the groups of 1.5 SD, the effect size seen in previous studies of platelet activation.15-17 Statistical analyses were performed using Analyze-it! (Analyse-it Software, Ltd, Leeds, UK) and STATA (Stata Corporation, College Station, Tex) software packages.
Ethical Guidelines
This study was approved by the Western Institutional Review Board (Nov 16, 2004, WIRB Protocol No. 2198). All patients voluntarily participated after signing a consent form before the scheduling of their surgery. There were no patient exposures to any dangerous chemical or toxic substance. Funding agencies did not participate in data analysis or manuscript preparation.
Results
Study Population
Twenty-six patients were enrolled between March and August 2005. Ten patients were enrolled in the lung transplant study group; 3 were subsequently excluded because of unplanned cardiopulmonary bypass. Four patients underwent bilateral transplantation. The indications for transplantation were pulmonary fibrosis (n = 5) and cystic fibrosis (n = 2). Sixteen patients were recruited into the reference thoracotomy group; 6 were excluded intraoperatively after the procedure was aborted because of advanced malignancy. The indications for thoracotomy in the final reference group were cancer (n = 9) and thymic cyst (n = 1). Lobectomy was performed in 7 cases, thymectomy in 1, and pleurodesis in 2, after formal resection was abandoned because of unexpected advanced malignancy. The final study population comprised 7 patients who underwent lung transplantation and 10 patients who underwent nontransplant thoracotomy (Table 1).
TABLE 1.
Patient characteristics of the lung transplant and thoracotomy groups
| Transplant (n = 7) | Thoracotomy (n = 10) | |
|---|---|---|
| Age (y, mean ± SD) | 51 ± 16 | 59 ± 13 |
| Sex (% male) | 57% | 70% |
| Transplant type (% bilateral) | 57% | — |
| Ischemia time (min) | ||
| First lung | 180 | — |
| Second lung (n = 4) | 247 | — |
| Lung resection | — | 70% |
| Platelets (103 cells/cm3, mean ± SE) | ||
| Preoperative | 365 ± 174 | 221 ± 48 |
| Day 1 | 205 ± 114 | 194 ± 69 |
| Day 2 | 159 ± 83 | 183 ± 61 |
| Day 3 | 160 ± 83 | 184 ± 64 |
sP-Selectin Levels
Baseline pre-operative sP-selectin levels in the lung transplant group were similar to those in the thoracotomy group (Figure 1). Significant increases in sP-selectin levels were seen in the lung transplant group at both the postoperative and 6-hour times relative to baseline (P < .05), however, no change in sP-selectin was observed in the thoracotomy group. Mean sP-selectin levels were significantly higher in the lung transplant group than in the thoracotomy group during the immediate postoperative period (59 vs 33 pg/mL, P = .03) and at 6 hours (104 vs 40 pg/mL, P = .001).
Figure 1.
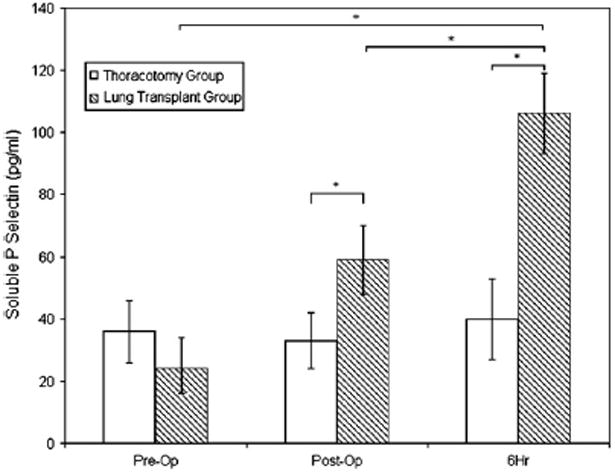
sP-selectin levels before surgery (Pre-Op), immediately after surgery (Post-Op), and 6 hours later (6Hr). Asterisk indicates P <.05.
Higher sP-selectin levels immediately after lung transplantation were associated with a greater decrease in platelet count on routine phlebotomy from baseline to postoperative day 1 (P = .006) and day 2 (P =0.002). Similar associations between the 6-hour postoperative sP-selectin level and changes in platelet counts at days 1 and 2 were shown, although these were not statistically significant (P = .10 and P = .06, respectively).
sCD40L Levels
Mean sCD40L levels were similar between the lung transplant and thoracotomy groups at baseline (481 vs 524 ng/mL, P = .53; Figure 2). The lung transplant group demonstrated a significant increase in sCD40L at the 6-hour point relative to the baseline value (P < .05), whereas no difference was observed in the control group. sCD40L levels were higher in the transplant group than in the thoracotomy group after skin closure (577 vs 524 ng/mL, p = 0.08), but this difference did not reach statistical significance. sCD40L levels at the 6-hour time point were significantly higher in the lung transplant recipients than in the control group (815 vs 547 ng/mL, P = .022).
Figure 2.
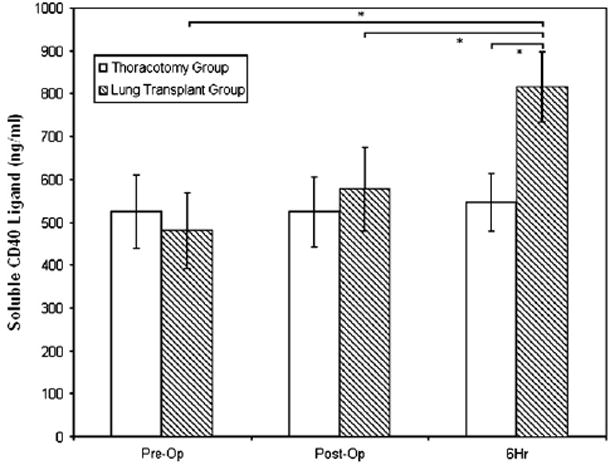
SCD40L levels before surgery (Pre-Op), immediately after surgery (Post-Op), and 6 hours later (6Hr). Asterisk indicates P <.05.
Immediately after lung transplantation, higher sCD40L levels were associated with a decrease in platelet count on postoperative day 1 or 2 (P = .058 and P = .05, respectively). There were no associations between sCD40L levels at 6 hours and the change in platelet count on days 1 or 2 (P > .50 for both).
Circulating Platelet–Leukocyte Conjugates
Figure 3 shows platelet–monocyte conjugates, expressed as the median (with interquartile range) fluorescent intensity of platelets per conjugate. No differences in the fluorescent intensity of platelets per platelet–monocyte conjugate were observed at the preoperative point, nor in the immediate postoperative period (P = .55). A significant increase in the fluorescent intensity of platelets per conjugate from baseline, however, was seen at 6 postoperative hours in transplant recipients relative to thoracotomy patients (P =.02). No differences in the percentage of monocytes engaged in circulating platelet–monocyte conjugates were observed between lung transplant and thoracotomy groups (Figure 4). Also, no differences were seen between patient groups in the fluorescent intensity of platelets per platelet–neutrophil conjugate or in the percentage of neutrophils with bound platelets (data not shown).
Figure 3.
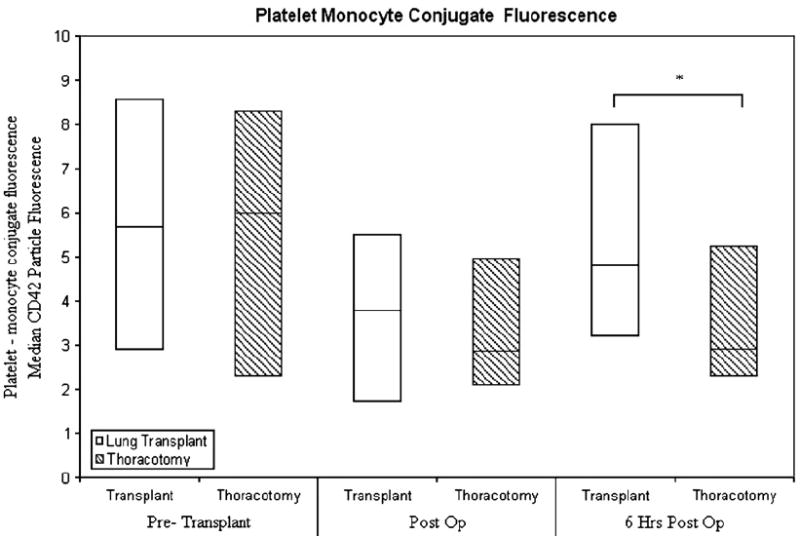
Platelet-monocyte conjugate fluorescence before surgery (Pre-Transplant), immediately after surgery (Post Op), and 6 hours later (6 Hrs Post Op). Asterisk indicates P <.05.
Figure 4.
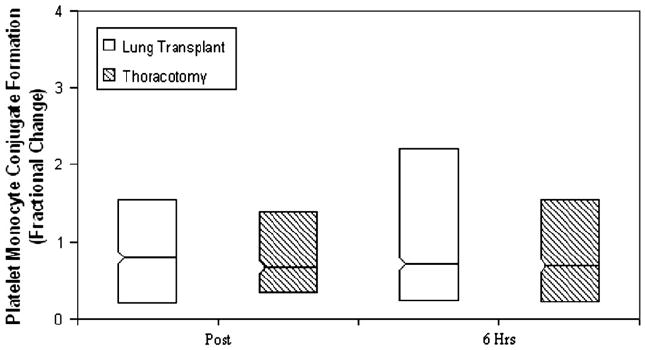
Fractional change from baseline in platelet–monocyte conjugate formation immediately after surgery (Post) and 6 hours later (6 Hrs). Asterisk indicates P <.05.
Discussion
To our knowledge, this is the first study to measure platelet activation before and after human lung transplantation. Although any surgical procedure might be expected to result in systemic platelet activation, we have shown that sP-selectin, sCD40L, and platelet–monocyte conjugates increase after lung transplantation to a greater degree than after other types of thoracic surgery. This implies that platelet activation is particularly pronounced after lung transplantation, which could modulate graft function or failure after transplantation.
Previous studies have demonstrated pulmonary platelet deposition 24 hours after lung transplantation in dogs.18 Platelet deposition has also been reported during reperfusion injury of rat and human lungs.19-21 In a rat model of lung transplantation, platelet accumulation was shown to contribute to postischemic graft dysfunction.22 Okada and coworkers23 reported that treatment with a prostacyclin analog with antiplatelet activity attenuated reperfusion injury by abrogating platelet sequestration in rat lung grafts.23 In addition, antiplatelet agents attenuate hyperacute xenograft failure.24
We presume that the site of platelet release of sP-selectin and sCD40L and monocyte interaction is the newly reperfused pulmonary vascular bed. Evidence suggests that leukocyte activation, adhesion, and infiltration are integral to the development of ischemia–reperfusion injury after lung transplantation.25,26 P-selectin binding is an important element of this process, as evidenced by the reduction of lung injury with P-selectin blockade at the time of reperfusion.27 Further, there is strong link between CD154/CD40 interaction and interleukin 8 upregulation, which also happens to be associated with poorer graft function.28,29 The results of these studies, in combination with our findings, suggest an important role of platelets in mediating ischemia–reperfusion injury after lung transplantation. Future studies should focus on the relationships among platelet activation, the risk of ischemia–reperfusion injury, and clinical outcomes after lung transplantation. Platelets may offer a promising target in preventing or treating ischemia–reperfusion injury and primary graft dysfunction, which currently have no specific medical therapy, in lung transplant recipients.
Although the number of platelets bound per monocyte conjugate increased after lung transplantation, we were unable to detect an increased percentage of monocytes with bound platelets. A possible explanation for this finding is that there may be a rapid sequestration of activated platelets in the lung allograft, as previously described,20,21,23 limiting the number of platelets that would interact with circulating monocytes. We suggest that this could occur to a greater extent in lung than in other solid organ transplantation because of the large surface area of dysfunctional endothelium. In addition, the low-pressure pulmonary circulation may facilitate platelet endothelial binding, as previous histologic studies of fresh lung allografts have shown the deposition of platelets in the lungs early after reperfusion.20
There are several possible limitations of this study. First, endothelial release of sP-selectin or sCD40L could account for the findings in our study. The vast majority of sCD40L is platelet-derived, however, and it is, therefore, a highly specific marker of platelet activation.7 Second, biomarker analysis was performed at two time points after transplantation; we cannot exclude the possibility that assessment after reperfusion might show even more substantial differences. Third, the sample size was small; however, we detected differences consistent with our hypothesis, likely attributable to the prospective planning and sample size calculations based on previous work. It should be noted that this is the first direct study of platelet activation after human lung transplantation, as well as the first study to use the sensitive techniques afforded by flow cytometry to examine platelet–leukocyte conjugates directly. Fourth, we did not investigate the association between platelet biomarkers and indicators of graft function (eg, ratio of PaO2 to inspired oxygen fraction). Larger, multicenter studies will be necessary to study whether indicators of platelet activation correspond to such clinical indices. Fifth, all lung transplant recipients received preoperative induction of immunosuppression before transplantation, and the possibility of interactions of these pharmacologic agents with platelets has not been studied.
In summary, this study shows that sP-selectin, sCD40L, and platelet–leukocyte interactions are significantly increased after human lung transplantation but not after thoracotomy alone. Future studies should attempt to replicate our findings and focus on the relationship to clinical markers of reperfusion injury with sP-selectin, sCD40L, and platelet–leukocyte conjugate formation. Abrogation of the platelet activation and adhesion cascades in lung transplantation may offer a future clinical avenue to decrease the impact of reperfusion injury.
Acknowledgments
Supported by grants HL007854, HL072866, HL67771, HL085149, HL55397, and HL076857 from the National Institutes of Health and the Department of Surgery at Columbia University.
Abbreviations and Acronyms
- sCD40L
soluble CD40 ligand
- sP-selectin
soluble P-selectin
References
- 1.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944–55. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–6. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 3.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–79. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, et al. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270:11662–70. doi: 10.1074/jbc.270.19.11662. [DOI] [PubMed] [Google Scholar]
- 5.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 6.Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–54. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 7.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–21. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Goodall AH, Hjemdahl P. A sensitive flow cytometric assay for circulating platelet-leucocyte aggregates. Br J Haematol. 1997;99:808–16. doi: 10.1046/j.1365-2141.1997.4993305.x. [DOI] [PubMed] [Google Scholar]
- 10.Weerasinghe A, Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg. 1998;66:2145–52. doi: 10.1016/s0003-4975(98)00749-8. [DOI] [PubMed] [Google Scholar]
- 11.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell–induced platelet aggregation. Br J Pharmacol. 2004;143:819–26. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Z, Théroux P. Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol. 2004;43:1982–8. doi: 10.1016/j.jacc.2003.10.071. [DOI] [PubMed] [Google Scholar]
- 13.Scholz T, Zhao L, Temmler U, Bath P, Heptinstall S, Lösche W. The GPIIb/IIIa antagonist eptifibatide markedly potentiates platelet-leukocyte interaction and tissue factor expression following platelet activation in whole blood in vitro. Platelets. 2002;13(7):401–6. doi: 10.1080/0953710021000024367. [DOI] [PubMed] [Google Scholar]
- 14.Linden MD, Frelinger AL, 3rd, Barnard MR, Przyklenk K, Furman MI, Michelson AD. Application of flow cytometry to platelet disorders. Semin Thromb Hemost. 2004;5:501–11. doi: 10.1055/s-2004-835671. [DOI] [PubMed] [Google Scholar]
- 15.Vanacore R, Guida C, Urciuoli P, Mazzoni A, Bianco I, Urbani L, et al. High levels of circulating monocyte-platelet aggregates can predict rejection episodes after orthotopic liver transplantation. Transplant Proc. 2003;35:1019. doi: 10.1016/s0041-1345(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 16.Rinder CS, Gaal D, Student LA, Smith BR. Platelet-leukocyte activation and modulation of adhesion receptors in pediatric patients with congenital heart disease undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;107:280–8. [PubMed] [Google Scholar]
- 17.Rinder CS, Bonan JL, Rinder HM, Mathew J, Hines R, Smith BR. Cardiopulmonary bypass induces leukocyte-platelet adhesion. Blood. 1992;79:1201–5. [PubMed] [Google Scholar]
- 18.Hoyer J, Lebreuil G, Sicardi F, Noirclerc M. Effects of experimental lung transplantation on blood coagulation and platelets. Eur Surg Res. 1974;6:110–6. doi: 10.1159/000127710. [DOI] [PubMed] [Google Scholar]
- 19.Okada Y, Marchevsky AM, Zuo XJ, Pass JA, Kass RM, Matloff JM, et al. Accumulation of platelets in rat syngeneic lung transplants: a potential factor responsible for preservation-reperfusion injury. Transplantation. 1997;64:801–6. doi: 10.1097/00007890-199709270-00002. [DOI] [PubMed] [Google Scholar]
- 20.Colombat M, Castier Y, Lesèche G, Rufat P, Mal H, Thabut G, et al. Early expression of adhesion molecules after lung transplantation: evidence for a role of aggregated P-selectin-positive platelets in human primary graft failure. J Heart Lung Transplant. 2004;23:1087–92. doi: 10.1016/j.healun.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Zenati MYS, Dowling RD, Stein KL, Griffith BP, Zenati M, Yousem SA, Dowling RD, Stein KL, Griffith BP. Transplantation. 1990;50:165–7. doi: 10.1097/00007890-199007000-00034. [DOI] [PubMed] [Google Scholar]
- 22.Naka Y, Chowdhury NC, Liao H, Roy DK, Oz MC, Michler RE, et al. Enhanced preservation of orthotopically transplanted rat lungs by nitro-glycerin but not hydralazine. Requirement for graft vascular homeostasis beyond harvest vasodilation. Circ Res. 1995;76:900–6. doi: 10.1161/01.res.76.5.900. [DOI] [PubMed] [Google Scholar]
- 23.Okada Y, Marchevsky AM, Kass RM, Matloff JM, Jordan SC. A stable prostacyclin analog, beraprost sodium, attenuates platelet accumulation and preservation-reperfusion injury of isografts in a rat model of lung transplantation. Transplantation. 1998;66:1132–6. doi: 10.1097/00007890-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer S, Zorn GL, 3rd, Zhang JP, Giorgio TD, Robson SC, Azimzadeh AM, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75:953–9. doi: 10.1097/01.TP.0000058517.07194.90. [DOI] [PubMed] [Google Scholar]
- 25.de Perrot M, Young K, Imai Y, Liu M, Waddell TK, Fischer S, et al. Recipient T cells mediate reperfusion injury after lung transplantation in the rat. J Immunol. 2003;171:4995–5002. doi: 10.4049/jimmunol.171.10.4995. [DOI] [PubMed] [Google Scholar]
- 26.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-nduced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 27.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci U S A. 1997;94:757–61. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–5. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 29.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365:654–7. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]


