Abstract
Aims
We investigated the prognostic performance of myeloperoxidase (MPO), and soluble CD40 ligand (sCD40L) along with B-type natriuretic peptide (BNP), high-sensitivity C-reactive protein (hsCRP), and cardiac troponin I (cTnI) for non-fatal recurrent ischaemic events in non-ST elevation acute coronary syndrome (ACS).
Methods and results
We measured plasma MPO and sCD40L in 1524 patients with ACS treated with tirofiban and randomized to early invasive vs. conservative management in the TACTICS-TIMI 18 trial who survived to 180 days. Patients with elevated baseline MPO (>884 pM) were at higher risk of non-fatal myocardial infarction or rehospitalization for ACS at 30 days (9.3 vs. 4.6%, P < 0.001). In contrast, no difference was observed with higher sCD40L (>989 pg/mL, 7.6 vs. 6.3%, P = 0.31). MPO remained associated with recurrent ischaemic events after adjustment for age, ST-deviation, diabetes, prior coronary artery disease, heart failure, cTnI, hsCRP, and sCD40L (OR 2.10; 95% CI 1.36–3.23, P = 0.001). This association was attenuated by 180 days (OR 1.26; 0.95–1.68). Stratification using baseline MPO, BNP, and cTnI identified a >3-fold gradient of risk.
Conclusion
MPO adds to BNP and cTnI for short-term risk assessment for recurrent ischaemic events in non-ST elevation ACS. sCD40L was not associated with risk in this population treated with a platelet GPIIb/IIIa receptor antagonist.
Keywords: Unstable angina, Myocardial infarction, Biomarkers, Prognosis
Introduction
Motivated by the challenge of risk assessment in a heterogeneous population and guided by advances in our understanding of the pathobiology of atherothrombosis, researchers and clinicians have maintained substantial interest in the development and application of new biomarkers for risk stratification in patients with non-ST elevation acute coronary syndrome (ACS). In particular, strategies that combine multiple biomarkers that may reflect diverse pathobiological contributors to the onset and complications of ACS have been appealing as an approach to enhance risk assessment and possibly target therapy more effectively.1 Proof-in-principle has been established with evidence for improved risk stratification using the combination of B-type natriuretic peptide (BNP), high-sensitivity C-reactive protein (hsCRP), and cardiac troponin.2–4 Nevertheless, few studies have evaluated multimarker strategies in robustly sized populations with rigorous follow-up after ACS. Moreover, comparative evaluation of newer markers is necessary to assess these candidates for integration into present strategies. In addition, because several newer markers have shown, at most, weak relationships with recurrent myocardial infarction,3,5 there is a need for assessment of their relationship with repeated ischaemic events.
Myeloperoxidase (MPO), a haemoprotein released during degranulation of neutrophils and monocytes,6 and soluble CD40 ligand (sCD40L), a shed cellular ligand released from activated platelets and stimulated lymphocytes,7 have both been investigated as prognostic biomarkers in patients with non-ST elevation ACS. The plasma concentrations of both markers have been reported to be independently associated with the risk of death or recurrent ischaemic events.8–11 However, the number of assessments of these two biomarkers in studies that have included each of the other biomarkers from an established multimarker panel2 are few.12,13 Therefore, we investigated the individual and combined relationship among MPO and sCD40L, along with BNP, hsCRP, and cardiac troponin I (cTnI) with regard to the risk of recurrent ischaemic events in patients presenting with non-ST elevation ACS and enrolled in the Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial.
Methods
Study population
Plasma samples were obtained at enrolment of patients presenting within 24 h of onset of a non-ST elevation ACS in the TACTICS-TIMI 18 trial. The design of TACTICS-TIMI 18 has been described previously.14 Patients ≥18 years old were eligible if they had symptoms consistent with unstable angina, were candidates for coronary revascularization, and had at least one of the following: ST-segment or T-wave abnormalities, elevated CK-MB or troponin, or documented coronary disease. Exclusion criteria potentially relevant to this study included recent (6 months) coronary revascularization, severe congestive heart failure, important systemic disease, or serum creatinine levels >2.5 mg/dL (221 µmmol/L). Patients were treated with aspirin, intravenous unfractionated heparin, and tirofiban (Merck and Co., West Point, PA, USA) and were randomized to an early invasive or conservative management strategy.14 Patients were followed for 180 days. The study protocol was approved by the institutional review boards of each participating hospital, and patients provided written informed consent.
All study participants were to have blood samples obtained for biomarker testing and all available samples were tested. Owing to logistical impediments, details regarding compromise of samples during shipment, subject denial of consent for blood sampling, and depletion with prior testing, samples were not available in all subjects. This analysis was limited to patients with data for MPO and sCD40L, representing 69% of the total enrolment in TACTICS-TIMI 18. Because of depletion of samples due to prior testing of fatal cases, mortality was significantly lower in the subset of patients with the requisite biomarker data (only five of 76 patients who died by 180 days had samples available). For this reason, we limited the analyses to survivors in order to focus on the incidence of non-fatal recurrent ischaemic events. The results of the analyses presented were not different when the five subjects with MPO and sCD40L results and fatal events through 180 days were included. Patients were followed for 6 months for non-fatal recurrent ischaemic events. All potential myocardial infarctions and rehospitalizations for ACS were adjudicated according to definitions established for the TACTICS-TIMI 18 trial14 by an independent clinical endpoints committee, blinded to treatment assignment and central biomarker results.
Laboratory testing
All testings were conducted by personnel blinded to clinical outcomes and treatment allocation. The protocol specified that blood samples be obtained by trained study personnel in citrate-anticoagulated tubes, plasma isolated via centrifugation at 1500 g for >15 min, and samples frozen <60 min of acquisition. Plasma samples were storedat −22°C or colder at the enrolling site until shipped to the TIMI Biomarker Core Laboratory, where they were maintained at −80°C. MPO was measured using an enzyme-linked immunosorbent assay, the CardioMPO™ test (PrognostiX, Inc., Cleveland, OH, USA), which has been approved for clinical use by the US Food and Drug Administration. This sandwich enzyme-linked immunosorbent assay demonstrated a minimum detection limit (as calculated by interpolation of the mean plus two standard deviations) of 26 pM, with a within-run precision of 4.7%. Accuracy studies employing method of standard additions with isolated pure human MPO and multiple (n = 12) citrate plasma samples demonstrated overall recovery of 95.1 ± 4.4%. The plasma concentration of sCD40L was determined using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). The lower limit of detection is 4 pg/mL and the coefficient of variation is 6.4 and 6.0% at 437 and 1205 pg/mL concentrations, respectively. hsCRP was measured using the nephelometric method from Dade-Behring and dichotomized at 10 mg/L on the basis of prior work.15 cTnI and BNP had been measured and reported previously.16,17
Statistical methods
Plasma concentrations of each biomarker are described by the median and 25th and 75th percentiles. The baseline characteristics of patients with and without elevated levels of MPO and sCD40L were compared using the Wilcoxon rank-sum test for continuous variables and the χ2 or Fisher’s exact test for categorical variables. Correlation coefficients reported between continuous variables are based on a non-parametric method (Spearman correlation).
The unadjusted association between each marker and clinical outcome was evaluated using the Wilcoxon rank-sum test when treated continuously and the χ 2 test for dichotomously handled results. Individual biomarkers were dichotomized at established cut-points where available (hsCRP, BNP, cTnI)15 –17 and at the median for biomarkers without cutpoints established by prior studies (MPO and sCD40L). Using logistic regression, the relationship between biomarkers and outcomes was adjusted for the established major predictors of outcome in patients with non-ST elevation ACS (age, ST-deviation, diabetes mellitus, and heart failure)18 as well as currently clinically available biomarkers with established relationship with prognosis.19 Testing for heterogeneity in the effect of the invasive strategy between patients with and those without elevated levels of MPO, and sCD40L was performed using logistic regression with terms for the main effects and for the interaction of each marker with treatment allocation. All analyses were performed using Stata v9.0 (College Station, TX, USA), with two-tailed P-values <0.05 considered significant.
Results
Baseline levels of MPO and sCD40L were available for 1524 survivors of non-ST elevation ACS. Their baseline characteristics stratified by MPO and sCD40L are presented in Table 1. These characteristics are compared with those without MPO and sCD40L data in Supplementary material online, Table S1. Those with biomarker data available tended to be younger (61 vs. 65 years, P < 0.001), to have higher body weight (83 vs. 81 kg, P = 0.001), and to present less frequently with a qualifying MI (35 vs. 42%, P = 0.002).
Table 1.
Unadjusted association of baseline characteristics with myeloperoxidase and soluble CD40L
| MPO ≤ 884 pg/ mL (n = 762) |
MPO > 884 pg/mL (n = 762) |
P-value | sCD40L ≤ 989 pg/mL (n = 762) |
sCD40L > 989 pg/mL (n = 762) |
P-value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 61 (52, 69) | 61 (53, 70) | 0.60 | 61 (53, 70) | 60 (52, 69) | 0.09 |
| Age > 65 years | 303 (39.8) | 306 (40.2) | 0.88 | 329 (43.2) | 280 (36.8) | 0.01 |
| Female | 244 (32.0) | 259 (34.0) | 0.41 | 240 (31.5) | 263 (34.5) | 0.21 |
| Risk factors | ||||||
| Weight, kg | 83 (72, 95) | 83 (72, 96) | 0.72 | 83 (72, 95) | 83 (72, 97) | 0.38 |
| Current smoker | 218 (28.7) | 225 (29.5) | 0.72 | 215 (28.3) | 228 (30.0) | 0.46 |
| Diabetes | 191 (25.1) | 220 (28.9) | 0.09 | 191 (25.1) | 220 (28.9) | 0.09 |
| History of hypertension | 512 (67.2) | 487 (63.9) | 0.18 | 492 (64.6) | 507 (66.5) | 0.42 |
| Hypercholesterolaemia | 475 (62.3) | 485 (63.7) | 0.60 | 486 (63.8) | 474 (62.2) | 0.52 |
| Cardiovascular history | ||||||
| Prior myocardial | 303 (39.8) | 294 (38.6) | 0.64 | 287 (37.7) | 310 (40.7) | 0.23 |
| infarction | ||||||
| Prior heart failure | 50 (6.6) | 42 (5.5) | 0.39 | 48 (6.3) | 44 (5.8) | 0.67 |
| Prior PCI | 224 (29.4) | 216 (28.4) | 0.65 | 217 (28.5) | 223 (29.3) | 0.73 |
| Prior CABG | 182 (23.9) | 160 (21.0) | 0.18 | 170 (22.3) | 172 (22.6) | 0.90 |
| Presenting characteristics | ||||||
| Heart rate > 100 b.p.m. | 26 (3.4) | 55 (7.3) | 0.001 | 46 (6.1) | 35 (4.6) | 0.21 |
| Systolic | 14 (1.8) | 23 (3.0) | 0.13 | 28 (2.4) | 19 (2.5) | 0.87 |
| BP < 100 mmHg | ||||||
| ST | 224 (29.4) | 233 (30.6) | 0.62 | 235 (30.8) | 222 (29.1) | 0.47 |
| depression ≥ 1.0 mm | ||||||
| Index diagnosis MI | 231 (30.3) | 303 (39.8) | <0.001 | 274 (36.0) | 260 (34.1) | 0.45 |
Data are shown as n (%) for dichotomous variables and median (25th, 75th percentile) for continuous variables.
BP, blood pressure; MI, myocardial infarction; PCI, percutaneous interventions; CABG, coronary artery bypass grafting.
The median concentration of MPO was 884 pM with 1st, 25th, 75th, and 99th percentiles of 56, 489, 1381, and 6820 pM, respectively. The concentration of MPO was more likely to be elevated in patients presenting with a myocardial infarction compared with those with unstable angina (P < 0.001) and tended to be more frequently increased in patients with diabetes mellitus (P = 0.09, Table 1). The median concentration of sCD40L was 989 pg/mL with 1st, 25th, 75th, and 99th percentiles of 5, 311, 2798, and 9153 pg/mL, respectively. Baseline characteristics were similar between those with and without elevated levels of sCD40L, with the exception that patients with an elevated concentration of sCD40L tended to be younger (P = 0.07, Table 1). MPO was only weakly correlated with hsCRP (ρ = 0.08, P = 0.003), sCD40L (ρ = −0.12, P < 0.001), and cTnI (ρ = 0.18, P < 0.001), and not BNP (ρ = 0.03, P = 0.26), estimated GFR (ρ = −0.05, P = 0.07).
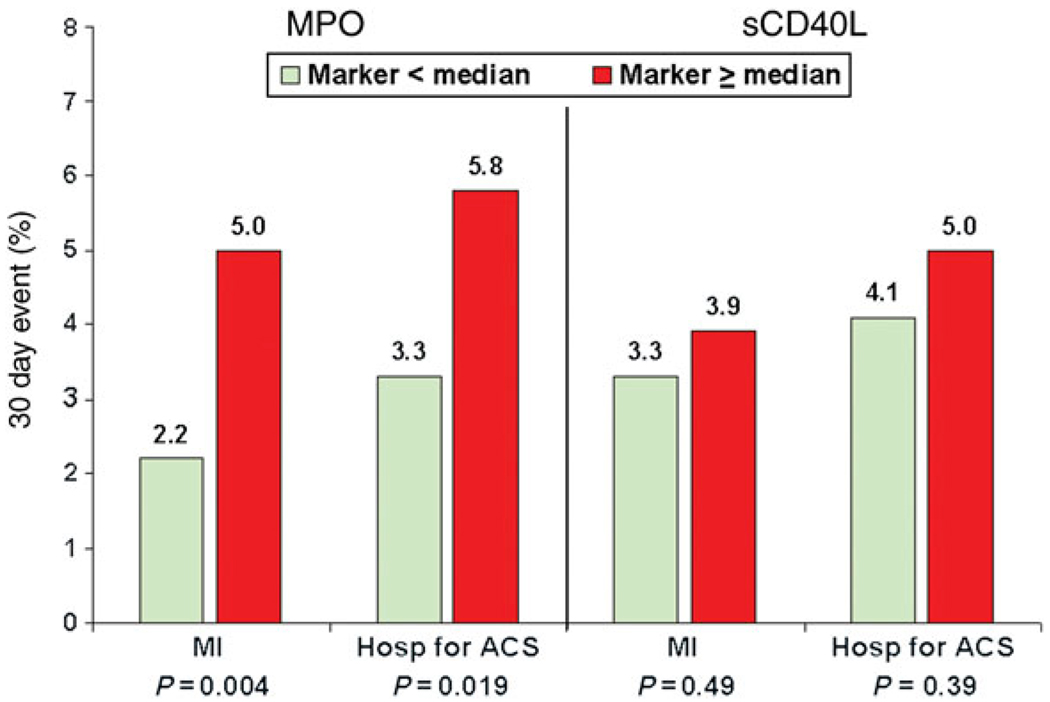
The median concentration of MPO was significantly higher in the 106 patients who subsequently suffered a non-fatal myocardial infarction or recurrent ACS event during the first 30 days (1158 pM; 25–75th percentile 729–1767 pM) compared with those without recurrent ischaemic events (866 pM; 25–75th percentile 473–1366 pM, P = 0.0003). In contrast, the median concentration of sCD40L did not differ significantly between those with recurrent ischaemia by 30 days (1091 pg/mL; 25–75th percentile 420–2871 pg/mL) and those without (971 pg/mL; 25–75th percentile 304–2795, P = 0.26). Analysis in a logistic regression model as continuous variables (log-transformed) revealed a significant relationship between MPO and the risk of recurrent ischaemic events (OR 1.42; 95% CI 1.14–1.78; P = 0.002) and a trend for sCD40L (OR 1.15; 95% CI 0.99–1.33; P = 0.07). When dichotomized at the median, an elevated baseline level of MPO (>884 pM) was associated with a two-fold higher risk of recurrent ischaemic events (MI or recurrent ACS) at 30 days (9.3 vs. 4.6%, P < 0.001), whereas patients with an elevated concentration of sCD40L (>989 pg/mL) showed only a modest trend towards higher risk (7.6 vs. 6.3%, P = 0.31). The relationship between each marker and the risk of recurrent ischaemic events is shown in Figure 1 revealing a significant association between MPO and MI (P = 0.004) and rehospitalization for ACS (P = 0.019) and no detectable difference for sCD40L (P = 0.49 and P = 0.39, respectively). The same pattern was maintained when the relationship between MPO and risk of recurrent ischaemic events was examined in those patients with a baseline cTnI below the 99th percentile (n = 629, 6.4 vs. 3.6%, P = 0.11).
Figure 1.
Relationship between myeloperoxidase (MPO) and soluble CD40L (sCD40L) and the risk of recurrent ischaemic events at 30 days. MI, myocardial infarction; Hosp for ACS, hospitalization for acute coronary syndrome.
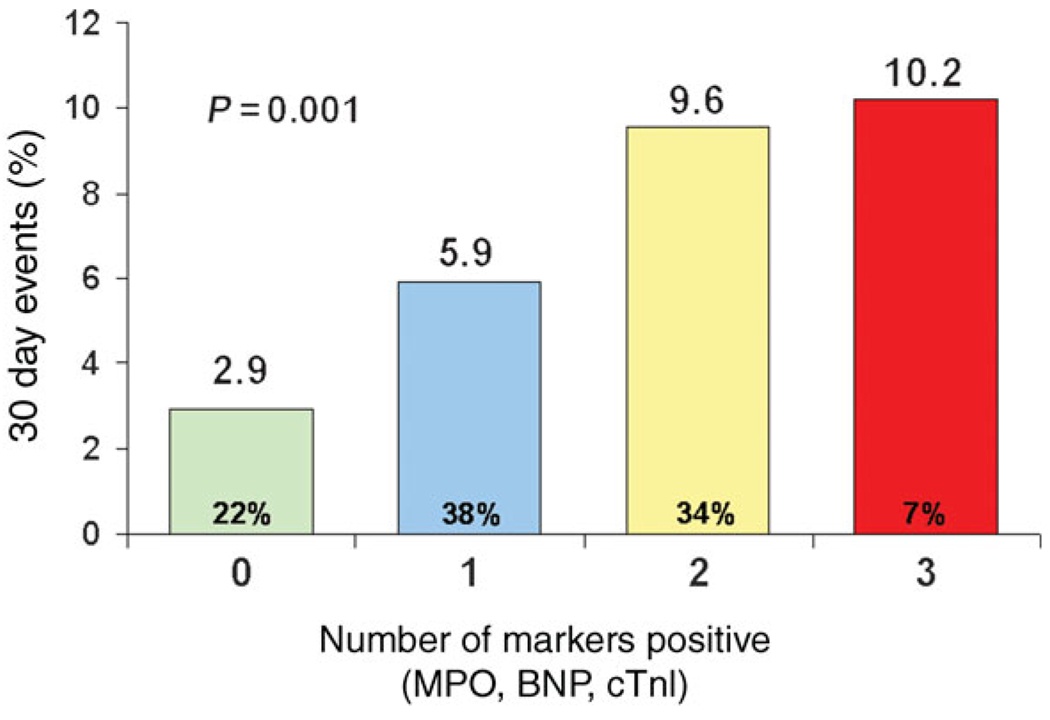
After adjustment for age, ST-deviation, diabetes mellitus, history of coronary artery disease, history of heart failure, baseline cTnI, hsCRP, and sCD40L, an elevated baseline concentration of MPO was associated with a nearly two-fold higher risk of non-fatal myocardial infarction or recurrent ACS at 30 days (adjusted OR 2.10; 95% CI 1.36–3.23, P = 0.001). An increased concentration of sCD40L was associated with a non-significant trend towards more frequent ischaemic events in this model when treated either dichotomized at the median (adjusted OR 1.39; 95% CI 0.93–2.09, P = 0.11) or as a continuous variable (P = 0.15). These findings were both consistent handling the biomarkers as continuous variables (log-transformed) with an adjusted OR 1.41 (95% CI 1.22–1.77; P = 0.003) for a log increase in MPO and adjusted OR 1.15 (95% CI 1.00–1.32; P = 0.056) with sCD40L. In the subset of patients with BNP also available (n = 1453), the risk related to MPO was not attenuated with the addition of BNP to the model (adjusted OR 2.36; 95% CI 1.50–3.73, P < 0.001). With MPO in the model, hsCRP was not associated with recurrent ischaemic events (P = 0.43); therefore, we updated our prior multimarker model2 for this analysis of recurrent ischaemia, with MPO replacing hsCRP. When evaluated in a multimarker approach integrating biomarkers measured and reported previously in TACTICS-TIMI 18, the combination of MPO, BNP, and cTnI identified a >3-fold gradient of risk for recurrent ischaemic events at 30 days (Figure 2).
Figure 2.
Multimarker strategy using myeloperoxidase (MPO), B-type natriuretic peptide (BNP), and cardiac troponin I (cTnI). Each elevated marker confers one point towards the multimarker score.
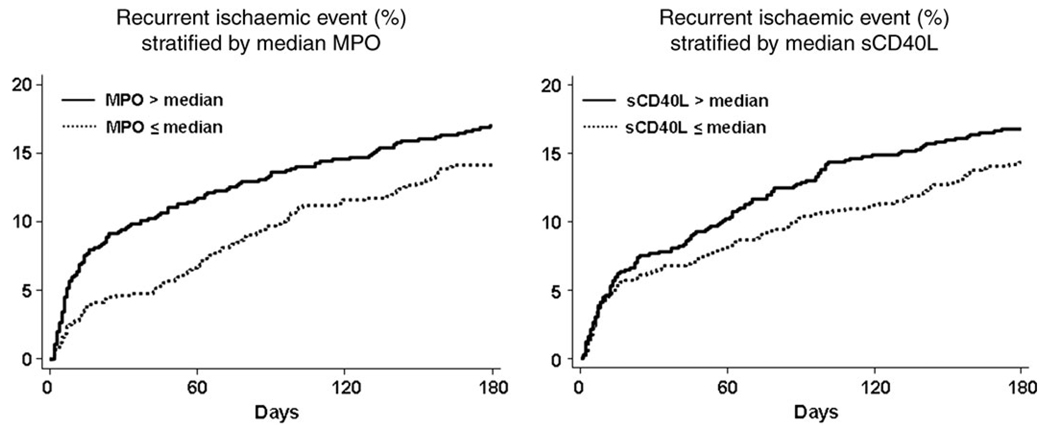
We also evaluated the relationship between MPO and sCD40L with outcomes through 6 months of follow-up. Notably, the association between MPO and the risk of myocardial infarction or recurrent ACS was attenuated by 6 months (16.7 vs. 13.9%, P = 0.14) with an adjusted OR 1.26 (95% CI 0.95–1.68). Examination of Kaplan–Meier cumulative incidence curves reveals an early separation with a gradual convergence in the risk by 6 months (Figure 3). In addition, we detected a non-significant trend between sCD40L and outcome at 6 months (16.5 vs. 14.0%, P = 0.18, Figure 3). Analyses as continuous variables with respect to outcomes at 180 days did not differ qualitatively (MPO: adjusted OR 1.15; 95% CI 0.99–1.34; P = 0.072, and sCD40L: adjusted OR 1.05; 95% CI 0.95–1.15; P = 0.33). Finally, there was no evidence for an interaction between the efficacy of the early invasive management strategy and the risk associated with MPO with respect to outcomes at 30 days (P = 0.65) or 6 months (P = 0.14). Specifically, MPO was associated with an adjusted two-fold higher risk of recurrent ischaemia in patients managed with the invasive (OR 2.17; 95% CI 1.05–4.48) or conservative strategy (OR 2.13; 95% CI 1.24–3.66).
Figure 3.
Kaplan–Meier estimated cumulative failure rates for recurrent ischaemic events (non-fatal myocardial infarction or rehospitalization for acute coronary syndrome) stratified by myeloperoxidase and soluble CD40L above and below the median concentration. Statistical comparison is provided within the text.
Discussion
With the rapid pace of emergence of new candidate biomarkers for risk assessment in patients with ACS, there is a strong need for comparative evaluation of the strongest candidates in order to guide further development as well as potential clinical application. MPO and sCD40L have appeared among the strongest candidate biomarkers of inflammation based upon prognostic performance in initial studies of patients with ACS.8 – 10,20 In our concurrent analysis of these biomarkers alone, and in combination with established prognostic markers (cTnI, BNP, hsCRP), we found that the baseline concentration of MPO was associated with an ~2-fold higher adjusted short-term risk of non-fatal recurrent ischaemic events. In contrast, the concentration of sCD40L was associated with only a weak trend towards increased risk in our study in which all patients were treated with a glycoprotein IIb/IIIa receptor antagonist. Neither marker remained significantly associated with recurrent cardiac events by 6 months.
Evaluation of myeloperoxidase
Experimental and epidemiological studies have supported a potential role for MPO in the assessment of risk among patients presenting with ACS.21 In addition to its microbiocidal properties, MPO appears to have both pro-atherogenic and destabilizing effects on atheromatous plaque through its oxidation of LDL,22 activation of metalloproteinases,23 consumption of nitric oxide as substrate,24 and the modification of apoA1 in HDL.25,26 The plasma concentration of MPO is elevated among patients presenting with ACS, including those without myonecrosis evident at initial presentation.8 In addition, at least three studies have shown that an elevated concentration of MPO measured early after presentation with ACS is associated with a higher risk of death and/or recurrent ischaemic events.8,9,11 Our analysis not only provides an important additional validation of the relationship between MPO and the short-term risk of recurrent ischaemic events, but also demonstrates that this relationship is independent of other novel markers including sCD40L, hsCRP, and BNP, as well as cardiac troponin. Although serial samples for the evaluation of troponin were not available, the application of a contemporary low cut-point for cTnI at presentation is also a strength of this analysis. In addition, the strong association of MPO with recurrent ischaemic events distinguishes this biomarker from others, such as BNP, and hsCRP, which are more closely related to the risk of death and heart failure, but have shown a more modest relationship with recurrent myocardial infarction.3,5 However, the absence of an ability to predict a greater benefit of early invasive management with MPO differs from troponin and highlights the need for additional investigation to define specific therapeutic actions in response to an increased concentration of MPO. In addition, the attenuation of the risk related to MPO by 6 months of follow-up contrasts with results from prior studies and points to a need for additional evaluation of the prognostic performance of MPO with respect to long-term clinical outcomes. However, our results lend support to consideration of MPO as a biomarker for risk assessment early after presentation.8
Evaluation of soluble CD40 ligand
sCD40L is released from stimulated lymphocytes and activated platelets27 and thus has attracted substantial interest as a marker of the combined activation of inflammatory and procoagulant pathways identified as central to the pathogenesis of ACS.1 Prior work by our group and others had indicated an association between the plasma level of sCD40L early after presentation with ACS and the risk of death and recurrent ischaemic events.10,20 However, since the time of these studies, preanalytic influences, such as the anticoagulant used, speed of centrifugation, temperature, and time to analysis, have achieved recognition as bearing importantly on measurement of sCD40L and presented as barriers to potential practical clinical application.28,29 Although we followed the same pre-analytic methods as used in our prior work20 using citrated plasma as supported by comparative studies,30 we could not detect a significant relationship between sCD40L and outcome in our present study. It is possible that the uniform treatment of patients in this study with a platelet glycoprotein IIb/IIIa receptor antagonist, which has been shown to attenuate the risk associated with sCD40L,10 impacted on the strength of the relationship. Nevertheless, these results, at a minimum, contribute to a mixed evidence-base and raise some caution with regard to the strengths and weaknesses of sCD40L as a biomarker for clinical application, particularly when viewed relative to other candidate markers of inflammation such as MPO. This note of caution is corroborated by a smaller study (n = 427) with infrequent use of glycoprotein IIb/IIIa receptor antagonists in which any association between sCD40L and outcome was attenuated compared with prior studies.12 Together, these findings underscore the need for both a thorough characterization of the preanalytic and analytic performance of newer markers and establishment of a strong base of evidence with repeated validation before novel biomarkers are considered for introduction into clinical application.
Limitations
This study has limitations that must be recognized in its interpretation. First, because of limited availability of samples for testing of MPO and sCD40L, we could only evaluate the associations with non-fatal outcomes, and thus the generalizability of our findings with respect to MPO and multimarker testing may be impacted. Although there was clearly a strong selection pressure on the availability of this cohort and a strong possibility of selection bias, we believe that the qualitative findings of our results are not likely to be altered by this limitation. Most importantly, previous data indicate that a significant, directionally similar association has been demonstrated between MPO and mortality.11 Therefore, it is unlikely that the directionality of our results would have been qualitatively different had patients with fatal complications been included. Moreover, in a sensitivity analysis in which we randomly imputed an MPO value above or below the median for the 71 subjects without MPO data who died and included them in the analysis, the relationship between MPO and the risk of death or recurrent ischaemic events at 30 days remained significant (OR 1.7; 95% CI 1.2–2.3; P = 0.003). In addition, our findings with respect to MPO and recurrent ischaemic events are consistent with work from two prior studies. Nevertheless, additional studies of the relationship of MPO alone and in combination with other biomarkers with short and long-term mortality will be important. For example, on the basis of prior results, we expect that hsCRP is likely to remain in the multimarker model when deaths are included in the outcome.3,5 Secondly, serial sampling of biomarkers was not available in TACTICS-TIMI 18. Therefore, our analyses pertain to ascertainment at presentation and we cannot exclude the possibility that the performance of MPO might be different at later points in time when serial data for troponin become available to the clinician. In addition, the prognostic association of BNP appears stronger when assessed later after presentation (e.g. prior to discharge) and thus the relative contribution of BNP may also have been greater if assessed serially.31 Finally, because all patients in this study were treated with tirofiban, our design does not permit us to eliminate an influence on the association between sCD40L and outcome.
Conclusion
The concentration of MPO at presentation with non-ST elevation ACS is associated with the short-term risk of recurrent ischaemic events independently of clinical risk factors, cardiac troponin, and other novel biomarkers, including BNP, sCD40L, and hsCRP. This finding supports continued investigation to define a possible role of MPOin the clinical evaluation and management of patients with ACS.
Supplementary Material
Acknowledgments
Funding
TACTICS-TIMI 18 was supported by Merck and Co. Support for reagents and testing of sCD40L was provided by Beckman-Coulter (Chaska, MN, USA). Testing of MPO was supported by National Institutes of Health grant P01 HL076491. D.A.M. and M.S.S. are supported in part by NIH grant U01 HL083-1341.
Footnotes
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: Disclosures of Relationships with Industry. The TIMI Study Group has received significant research grant support from Accumetrics, Amgen, Astra-Zeneca, Bayer Healthcare, Beckman Coulter, Biosite, Bristol-Myers Squibb, CV Therapeutics, Eli Lilly and Co, GlaxoSmithKline, Inotek Pharmaceuticals, Integrated Therapeutics, Merck and Company, Merck-Schering Plough Joint Venture, Millennium Pharmaceuticals, Novartis Pharmaceuticals, Nuvelo, Ortho-Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi-Aventis, Sanofi-Synthelabo, and Schering-Plough. Dr. Morrow has received honoraria for educational presentations from Bayer Diagnostics, Beckman-Coulter, Dade-Behring, Sanofi-Aventis, and Roche Diagnostics. He has served as a consultant for GlaxoSmithKline and Sanofi-Aventis and on advisory boards for Critical Diagnostics, Genentech, OrthoClinical Diagnostics and Beckman-Coulter. Dr. de Lemos has received grant support from Merck, Biosite and Roche Diagnostics, has served as a consultant to Roche Diagnostics, Biosite, Inverness Medical, and Bayer Diagnostics, and has received speaker honoraria from Merck and Biosite. Dr. Cannon serves on advisory boards for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck and Company, Pfizer, Sanofi-Aventis and Schering-Plough, and has received lecture fees or honoraria for educational materials from Accumetrics, AstraZeneca, Bristol-Myers Squibb, Merck and Company, Pfizer, Sanofi-Aventis, Schering-Plough, BGB New York, DIME, and NCME. Dr. Hazen is named as co-inventor on pending patents filed by the Cleveland Clinic Foundation that relate the use of biomarkers, including myeloperoxidase, for inflammatory and cardiovascular diseases. Dr. Hazen is the scientific founder of PrognostiX Inc. Dr. Hazen has received honoraria for educational presentations from Merck, BioSite, Abbott, Pfizer, Lilly, Wyeth, Esperion, GlaxoSmithKline and Atherogenics. He has served as a consultant for Merck, Pfizer, PrognostiX, Wyeth, Biophysical, and is on the advisory board of PrognostiX.
References
- 1.Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250–252. doi: 10.1161/01.CIR.0000078080.37974.D2. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 3.James SK, Armstrong P, Barnathan E, Califf R, Lindahl B, Siegbahn A, Simoons ML, Topol EJ, Venge P, Wallentin L. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–924. doi: 10.1016/s0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- 4.James SK, Lindback J, Tilly J, Siegbahn A, Venge P, Armstrong P, Califf R, Simoons ML, Wallentin L, Lindahl B. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: a GUSTO-IV substudy. J Am Coll Cardiol. 2006;48:1146–1154. doi: 10.1016/j.jacc.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, White HD, Fox KA, Califf RM, Braunwald E. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 8.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 9.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 11.Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. doi:10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Apple FS, Pearce LA, Chung A, Ler R, Murakami MM. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2007;53:874–881. doi: 10.1373/clinchem.2006.080192. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA. Appraisal of myeloperoxidase for evaluation of patients with suspected acute coronary syndromes. J Am Coll Cardiol. 2007;49:2001–2002. doi: 10.1016/j.jacc.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann F, Robertson DH, De Lucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban (TACTICS-TIMI 18) N Engl J Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 15.Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in myocardial infarction. J Am Coll Cardiol. 1998;31:1460–1465. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, Cannon CP, Rifai N, Frey MJ, Vicari R, Lakkis N, Robertson DH, Hille DA, DeLucca PT, DiBattiste PM, Demopoulos LA, Weintraub WS, Braunwald E for the TACTICS-TIMI 18 Investigators. Ability of minor elevations of troponin I and T to identify patients with unstable angina and non-ST elevation myocardial infarction who benefit from an early invasive strategy: results from a prospective, randomized trial. JAMA. 2001;286:2405–2412. doi: 10.1001/jama.286.19.2405. [DOI] [PubMed] [Google Scholar]
- 17.Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos L, DiBattiste P, McCabe C, Gibson CM, Cannon CP, Braunwald E. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST elevation MI: BNP and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41:1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 18.Cannon CP. Evidence-based risk stratification to target therapies in acute coronary syndromes. Circulation. 2002;106:1588–1591. doi: 10.1161/01.cir.0000030416.80014.f4. [DOI] [PubMed] [Google Scholar]
- 19.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 20.Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, Gibson CM, Cannon CP, Braunwald E, Schonbeck U. Soluble CD40L: risk prediction after acute coronary syndromes. Circulation. 2003;108:1049–1052. doi: 10.1161/01.CIR.0000088521.04017.13. [DOI] [PubMed] [Google Scholar]
- 21.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacology & Therapeutics. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 23.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 25.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 28.Halldorsdottir AM, Stoker J, Porche-Sorbet R, Eby CS. Soluble CD40 ligand measurement inaccuracies attributable to specimen type, processing time, and ELISA method. Clin Chem. 2005;51:1054–1057. doi: 10.1373/clinchem.2005.048199. [DOI] [PubMed] [Google Scholar]
- 29.Weber M, Rabenau B, Stanisch M, Elsaesser A, Mitrovic V, Heeschen C, Hamm C. Influence of sample type and storage conditions on soluble CD40 ligand assessment. Clin Chem. 2006;52:888–891. doi: 10.1373/clinchem.2005.062083. [DOI] [PubMed] [Google Scholar]
- 30.Thom J, Gilmore G, Yi Q, Hankey GJ, Eikelboom JW. Measurement of soluble P-selectin and soluble CD40 ligand in serum and plasma. J Thromb Haemost. 2004;2:2067–2069. doi: 10.1111/j.1538-7836.2004.00962.x. [DOI] [PubMed] [Google Scholar]
- 31.Lindahl B, Lindback J, Jernberg T, Johnston N, Stridsberg M, Venge P, Wallentin L. Serial analyses of N-terminal pro-B-type natriuretic peptide in patients with non-ST-segment elevation acute coronary syndromes: a Fragmin and fast Revascularisation during In Stability in Coronary artery disease (FRISC)-II substudy. J Am Coll Cardiol. 2005;45:533–541. doi: 10.1016/j.jacc.2004.10.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.