Abstract
Microbial organisms express pathogen-associated molecular patterns (PAMPs) that can stimulate expression of pro-inflammatory mediators following ligation of pathogen recognition receptors. However, both commensal organisms and pathogens can express PAMPs. The immune system can distinguish between commensals and pathogens in part through secretion of the key inflammatory cytokines IL-1β and IL-18. A PAMP such as lipopolysaccharide can induce production of intracellular pro-IL-1β and pro-IL-18, but not their secretion. A second “danger signal”, derived from host-cell molecules that are released from stressed or infected cells, or detected as a PAMP that is present in the cytosol, can stimulate assembly of an inflammasome that activates the protease caspase-1. Caspase-1, in turn, is responsible for processing and secretion of the mature IL-1β and IL-18. Many diverse ligands leading to inflammasome activation have been identified, but the cell signaling pathways initiated by the ligands tend to converge on a small set of common mechanisms.
Introduction
Keeping the body free from invading pathogens is an intricate task that requires cooperation between the innate and adaptive immune systems. The early response to infection is the responsibility of the innate immune system, which exhibits a broad specificity to a wide array of germline-encoded pathogen-associated molecular patterns (PAMPs) through different pattern recognition receptors (PRRs). The PRRs include Toll - like receptors (TLRs), nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), retinoid acid-inducible gene I (RIG-I)-like receptors (RLRs), and the C-type lectin receptors (CLRs) (1).
The two most-extensively studied PRRs are TLRs and NLRs, which exhibit significant interplay (1). Some NLRs can detect PAMPs and stimulate expression of pro-inflammatory genes (2–4), while other NLRs require the participation of other PRRs in order to promote processing and secretion of the key proinflammatory cytokines, IL-1β and IL-18 (5).
NLRs, a family of cytosolic receptors (also known as CATERPILLERs, NOD-LRRs or NACHT-LRRs), are comprised of three domains which include an effector N-terminus domain, a central NOD or NACHT domain, and a C-terminus LRR motif. A total of 23 NLR members have been identified in humans and are classified based on their N-terminal domain. CARD-containing NLRCs (NLRC 1-5) and PYD-containing NLRPs (NLRP 1-14) play an important role in inflammation, while the role of BIR-containing NAIPs is not well defined yet. NLRs also exist in other organisms: mice possess 34 members, and plants contain a similar subset of genes termed plant disease-resistance (R) genes (6).
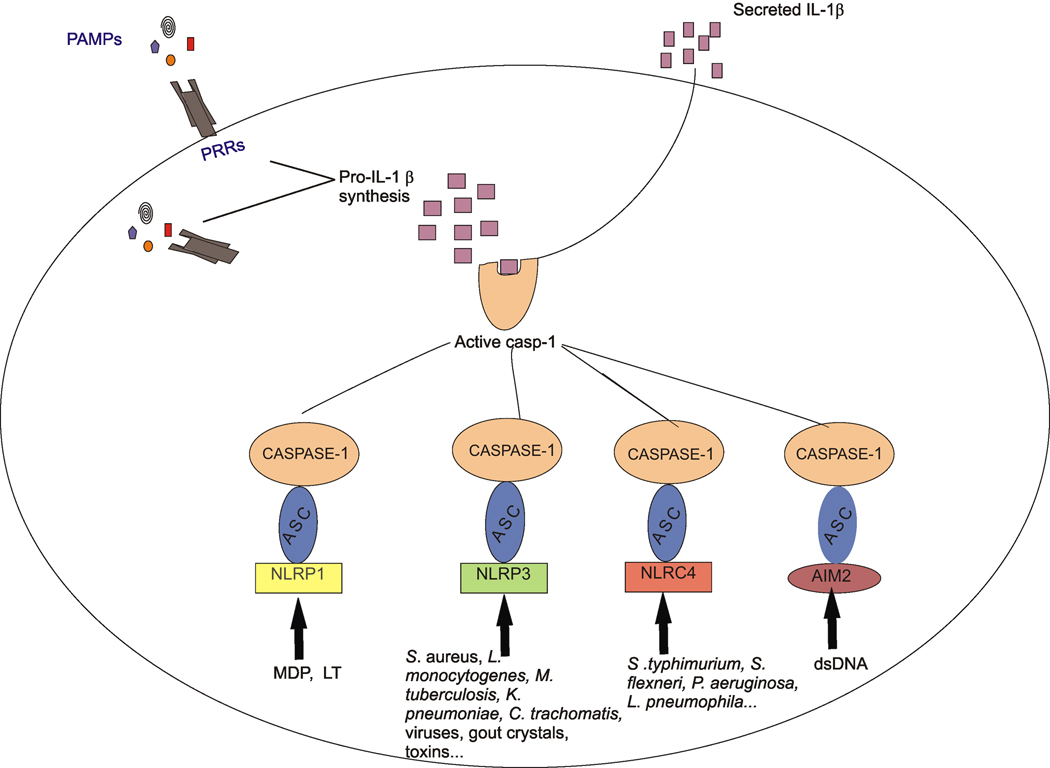
Several members of the NLR gene family are involved in the assembly of a macromolecular protein complex termed the ‘inflammasome” that leads to the activation of the cysteine protease, caspase-1 (also known as interleukin-1 converting enzyme or ICE), which in turn cleaves pro-IL-1β or pro-IL-18, resulting in secretion of the mature cytokines (7–9). NLRP3, NLRP1 and NLRC4 are among the NLR members demonstrated to activate the inflammasome in cells, while other NLR members have been shown to activate the inflammasome in a cell-free system (Fig. 1). In this review, we will discuss the different types of inflammasomes and focus on recent findings regarding their activation by pathogens and other triggers.
Figure 1.
Both a pathogen recognition receptor (PRR) and Nod-like receptor (NLR) family member are usually required for secretion of IL-1β. Binding of a pathogen-associated molecular patterns (PAMP) to its PRR results in pro-IL-1β synthesis, but not always secretion of IL-1β. A second signal, derived from an extracellular “danger signal” such as ATP or gout crystals, or an intracellular PAMP such as muramyl-dipeptide (MDP) from peptidoglycan activate an inflammasome consisting of caspase-1, the adaptor protein ASC, and a NLR family member or AIM2. LT, lethal toxin from Bacillus anthracis; dsDNA, double-stranded DNA. Inflammasome-dependent caspase-1 activation results in processing and secretion of the mature IL-1β.
The NLRP3 Inflammasome
The NLRP3 inflammasome (also known as Nalp3, cryopyrin, PYPAF1, CIAS1, and CLR1.1), is the most extensively studied inflammasome to date and is responsible for caspase-1 activation by a vast number of pathogens and other stimuli. NLRP3 inflammasome activation usually requires two signals: the first signal, from PAMPs such as lipolysaccharide (LPS), stimulates a TLR expressed on the cell surface or in an endosome and promotes the production and intracellular accumulation of immature cytokines (10, 11). The second signal, usually derived from danger signals (DSs), also known as damage-associated molecular patterns (DAMPs), such as host-derived ATP or uric acid crystals, ligates the danger signal receptor (DSR) and results in processing and secretion of mature cytokines. Initially, DAMPs were thought to be restricted to molecules of microbial origin, but recent work has expanded the concept to include host-cell material released from dying, infected or other stressed cells, including ATP, adenosine, uric acid, and the chromosomal protein, high mobility group box 1 (HMGB1) (12–17). Stimulation with any of these second signals leads to formation of a large complex containing NLRP3, caspase-1, and the adaptor protein, ASC.
A rapidly increasing number of studies have demonstrated the activation of the NLRP3 inflammasome in response to microbial infections. Bacteria including S. aureus and L. monocytogenes can activate the NLRP3 inflammasome by triggering K+ efflux due to their toxins listeriolysin O, and α-toxin, β -toxin or γ-toxin (18). M. tuberculosis and M. marinum use their ESX-1 secretion system to regulate secretion of IL-1β and IL-18 in a process that requires the NLRP3 inflammasome (19). Interestingly a recent study showed that K. pneumonia requires the NLRP3 inflammasome not only for caspase-1 dependent IL-1β secretion, but also for inducing macrophage necrosis and release of HMGB1, independently of caspase-1 (20). Extracellular HMGB1 binds to its specific receptor, RAGE, inducing secretion of TNFα from macrophages (21), which would thus allow the NLPR3 inflammasome to affect secretion of inflammatory cytokines other than IL-1β and IL-18. Similarly, N. gonorrhoeae infection of monocytes causes IL-1β production, pyronecrosis, and HMGB1 release in an NLRP3 dependent manner that requires the activity of the cysteine protease cathepsin B (22). C. trachomatis, utilizing its type III secretion system, can stimulate NLRP3 inflammasome dependent caspase-1 activation by causing K+ efflux-dependent and ROS production in epithelial cells – moreover, caspase-1 was deemed crucial for chlamydial growth and survival (23). As chlamydial infection also results in release of HMGB1 from infected epithelial cells (24), it is tempting to speculate that the NLRP3 inflammasome may amplify the inflammatory response during chlamydial infection through HMGB1. In contrast, P. gingivalis infection on its own can not activate an inflammasome: instead it induces synthesis of pro-IL-1β, which can be secreted following NLRP3-dependent activation in infected cells that are treated with the danger signal ATP (25). Conversely, Mycobacterium tuberculosis subverts the innate immune response by inhibiting NLPR3 inflammasome activation (26).
The NLRP3 inflammasome can also be activated by viruses such as the Sendai virus and influenza A virus, whose infections can cause caspase-1 dependent IL-1β secretion (27, 28). Conversely, NLPR3 inflammasome activation by the influenza virus is also required for lung tissue repair (29). Recently, Modified Vaccinia Virus Ankara (MVA), an attenuated poxvirus used as a vector for AIDS vaccines, was shown to activate the NLRP3 inflammasome, leading to secretion of IL-1β (30).
Furthermore, the fungus C. albicans, signaling through the tyrosine kinase Syk, causes NLRP3 inflammasome mediated caspase-1 activation and IL-1β secretion in a process triggered by K+ efflux and ROS production (31, 32). The transition of Candida from the yeast to the filamentous form (hyphae formation) is important for activation of the NLRP3 inflammasome (33). Heat killed S. cerevisiae can also activate NLRP3 inflammasome (34).
Both abiotic and biological crystals can also activate the NLRP3 inflammasome. Thus, asbestos, silica and amyloid-β fibrils induce NLRP3-dependent caspase-1 activation following “frustrated phagocytosis” of the large crystals or lysosomal leakage (35–38). In this context, NLRP3 could play both beneficial and deleterious roles, by inducing inflammation in response to environmental particles or during Alzheimer’s disease. The ability of host cells to respond to DAMPs through inflammasomes also explains the ability of alum, chitosan and QuilA/saponin to behave as potent adjuvants (39, 40).
More recently, hemozoin, a heme crystal produced by malaria causing parasite Plasmodium, was shown to similarly activate NLRP3 inflammasome by acting as a danger signal through the Lyn/Syk kinase pathway (41, 42).
The NLRP3 inflammasome initially gave the impression of being rather promiscuous, as it could be activated by a wide variety of stimuli, ranging from toxins, ATP and uric acid crystals. However, these disaparate stimuli stimulate NLRP3 through a small number of shared mechanisms (Fig. 2). Thus, the K+ efflux is induced by microbial toxins (18, 43), imidazoquinoline derivatives (44), and infection by C. albicans or C. trachomatis (23, 31–33), and extracellular ATP (45, 46). ATP can stimulate K+ efflux due to the association of the purinergic receptor, P2X7, with the recently-discovered hemichannel, pannexin-1 (47, 48), which allows formation of large non-selective pores in the membrane. Pannexin-1 in fact has been shown to be necessary for ATP-induced IL-1β secretion (47, 49).
Figure 2.
Diverse danger signals lead to NLRP3 inflammasome activation through common mechanisms. Extracellular damage-associated molecular patterns (DAMPs) can lead to K+ efflux and then ROS production, or directly to ROS production. Large particles can also cause lysosomal destabilization and release of enzymes such as cathepsin B. Either high ROS levels or cytosolic cathepsin B can stimulate the NLRP3 inflammasome, leading to caspse-1 activation.
ATP stimulation of macrophages can also induce formation of reactive oxygen species, which lead to caspase-1 activation (50). The antioxydant N-acetylcysteine can inhibit both ATPmediated caspase-1 activation and, interestingly, NLRP3 inflammation activation due to treatment with ionophores such as nigericin (43, 50).
Finally, destabilization of lysosomes by silica crystals, amyloid-β fibrils and alum salts has been shown to activate the NLPR3 inflammasome (36, 37). Rupture of lysosomes causes release of the lysosomal protease, cathepsin B, which is somehow sensed by the NLRP3 inflammasome.
The NLRP1 Inflammasome
NLRP1 (also known as Nalp1, CARD7, NAC, DEFCAP, and CLR17.1) forms an inflammasome containing caspase-1 and ASC. It is stimulated by the presence of cytosolic muramyl-dipeptide (MDP), which results in activation of caspase-1 (51). Studies with a reconstituted NLRP1 inflammasome in a cell-free system demonstrated that caspase-1 activation occurs in a two-step mechanism whereby MDP triggers a conformational change in NLRP1, allowing it to oligomerize into the inflammasome after binding nucleotides (52).
Separate studies on the NLRP1 inflammasome were carried out in mice, which possess three NLRP1 paralogues (NLRP1a, NLRP1b and NLRP1c), compared to the sole NLRP1 gene in humans. Susceptibility of macrophages to lethal toxin (LT), a metalloproteinase responsible for B. anthracis pathogenesis, is mediated by an NLRP1b inflammasome through an unknown mechanism (53). Transfection of fibroblasts with NLRP1b and caspase-1 conferred susceptibility of these cells to anthrax LT (54).
The NLRC4 Inflammasome
The NLRC4 inflammasome (also known as IPAF, CARD12, CLAN, and CLR2.1) is activated by some Gram-negative bacteria possessing type III or type IV secretion systems. These bacteria include S. typhimurium, S. flexneri, P. aeruginosa and L. pneumophila (55–60). An initial study showed that caspase-1 activation and subsequent IL-1β and IL-18 maturation in response to S. typhimurium infection requires the assembly of the NLRC4 inflammasome (56), and later work demonstrated that NLRC4 senses the presence of flagellin in the cytosol aided by the type III secretion apparatus (61, 62). Similarly, L. pneumophila utilizes its Dot-Icm type IV secretion system to activate the NLRC4 inflammasome and caspase-1, which then restricts growth of Legionella in macrophages (60). Flagellin from Legionella was shown to be the trigger for assembly of the NLRC4 inflammasome (63). A recent study showed that L. pneumophila can also induce caspase-1 activation independently of NLRC4, through a pathway involving ASC and triggered by loss of intracellular K+ (64).
Unlike Salmonella and Legionella, S. flexneri does not possess flagellin, yet is still capable of activating the NLRC4 inflammasome through a process that requires an intact type III secretion system (59). Although initial studies showed that NLRC4 inflammasome activation following P. aeruginosa infection requires flagellin and an intact type III secretion system (55, 57), it was also found that either the non-flagellated strain PA103ΔU or the flagellin-deficient mutant strain PAKΔfliC can also activate the NLRC4 inflammasome, leading to IL-1β secretion (58). These results suggest the existence of a flagellin-independent mechanism for NLRC4 inflammasome assembly.
Inflammasome Activation by AIM2
Four recent studies have reported that cytoplasmic dsDNA is sensed by a PYHIN (pyrin and HIN domain-containing protein) family member: AIM2 (absent in melanoma 2) (65–68). Although it is not an NLR family member, AIM2, upon binding cytoplasmic dsDNA through its carboxy terminal domain, oligomerizes and interacts with ASC through its amino terminal pyrin domain. This interaction causes the formation of an ASC pyroptosome or AIM2 inflammasome which ultimately leads to activation of caspase-1 (66, 67) and NF-κB (67).
Concluding Remarks
All the cases studied until now point to the importance of inflammasomes in the innate immune response against infection or nonliving particles. There is a clear interplay between TLRs and NLRs for optimal production of IL-1β and IL-18. Even though the flurry of recent reports have clarified our comprehension of the mechanisms underlying inflammasome activation, many unanswered questions remain.
The inflammasome complexes differ from each other with respect to their activating stimuli. NLRC4 is activated during infection with mostly flagellated bacteria (55–60), but the mechanisms through which non-flagellated bacteria activate NLRC4 remain unknown (58). NLRC4 is hypothesized to directly or indirectly sense membrane damage caused by these pathogens via their type III secretion system and not through any pathogen-derived molecules. This might also be the case for F. tularensis, which requires ASC but not NLRC4 in order to induce caspase-1-mediated cell death in macrophages (69).
NLRP1 in humans senses the presence of MDP, a subcomponent of peptidoglycan (51, 52), but its putative paralogue in mice, NLRP1b, exhibits a different ligand-specificity as it is activated by LT during B. anthracis infection (53). Functional homologs of these NLR members may be present in humans and mice, as the equivalent inflammasomes in the two species have not been identified through sequence homology.
Until far, NLRP3 has been associated with the widest variety of stimuli, which makes it unlikely that all these activators trigger inflammasome assembly by direct interaction with NLRP3. Instead they appear to stimulate common signaling pathways that converge on NLRP3 activation. Some of the events leading to NLRP3 activation are K+ efflux (18, 43), ROS production (43, 50), and cathepsin B release from lysosomes (36, 37).
The link between K+ efflux and ROS production in NLRP3 activation also deserves more attention. Results from at least two studies suggest that K+ efflux precede ROS production: the use of anti-oxydants prevents ionophore-induced NLRP3 activation (43), and blocking K+ efflux inhibits ROS production (23). Plasma membrane depolarization has been previously shown to activate NADPH oxidase (70, 71), suggesting a mechanism for ROS production in cells subjected to danger signals that stimulate NLRP3 assembly.
The inflammasome field is expanding quickly, attracting some of the most talented researchers in immunology and cell biology. Future studies will undoubtedly elucidate the functions of remaining NLRs as well as the ultimate triggers responsible for their activation.
Acknowledgements
Research in the authors’ laboratories relating to inflammasomes was supported by NIH grants R01DE016593 and R01DE019444.
References
- 1.Baccala R, Gonzalez-Quintial R, Lawson BR, Stern ME, Kono DH, Beutler B, Theofilopoulos AN. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 2.Girardin SE, Boneca IG, Carneiro LAM, Antignac A, Jéhanno M, Viala J, Tedin K, Taha M-K, Labigne A, Zähringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 3.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 4.Welter-Stahl L, Ojcius DM, Viala J, Girardin S, Liu W, Delarbre C, Philpott D, Kelly KA, Darville T. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell. Microbiol. 2006;8:1047–1057. doi: 10.1111/j.1462-5822.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 6.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 8.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariathasan S. ASC, Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect. 2007;9:664–671. doi: 10.1016/j.micinf.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 12.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 13.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 14.Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. The double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;16:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 16.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 17.Sitkovsky MV, Ohta A. The "danger" sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 22.Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungas T, Verbeke P, Darville T, Ojcius DM. Cell death, BAX activation, and HMGB1 release during infection with Chlamydia. Microbes Infect. 2004;6:1145–1155. doi: 10.1016/j.micinf.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Ö, Abdul-Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell. Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 29.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 33.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting Edge: Candida albicans Hyphae Formation Triggers Activation of the Nlrp3 Inflammasome. J Immunol. 2009 doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willingham SB, Ting JP. NLRs and the dangers of pollution and aging. Nat Immunol. 2008;9:831–833. doi: 10.1038/ni0808-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiemi Shio M, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 44.Kanneganti T-D, Ozoren N, Body-Malapel M, Amer A, Park J-H, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 45.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 46.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 47.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 49.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 52.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 54.Liao KC, Mogridge J. Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin. Infect Immun. 2009 doi: 10.1128/IAI.00276-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 56.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 57.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 61.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nature Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 62.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 63.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 66.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 69.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol. 2005;289:L954–L961. doi: 10.1152/ajplung.00210.2005. [DOI] [PubMed] [Google Scholar]
- 71.Cameron AR, Nelson J, Forman HJ. Depolarization and increased conductance precede superoxide release by concanavalin A-stimulated rat alveolar macrophages. Proc Natl Acad Sci U S A. 1983;80:3726–3728. doi: 10.1073/pnas.80.12.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]