Abstract
Mutations in the GJB2 gene, which encodes the gap junction protein connexin26 (Cx26), are the major cause of genetic non-syndromic hearing loss. The role of the allelic variant M34T in causing hereditary deafness remains controversial. By combining genetic, clinical, biochemical, electrophysiological and structural modeling studies, we have re-assessed the pathogenetic role of the M34T mutation. Genetic and audiological data indicate that the majority of heterozygous carriers and all five compound heterozygotes exhibited an impaired auditory function. Functional expression in transiently transfected HeLa cells showed that, although M34T was correctly synthesized and targeted to the plasma membrane, it inefficiently formed intercellular channels that displayed an abnormal electrical behavior and retained only 11% of the unitary conductance of the wild-type protein (HCx26wt). Moreover, M34T channels failed to support the intercellular diffusion of Lucifer Yellow and the spreading of mechanically induced intercellular Ca2+ waves. When co-expressed together with HCx26wt, M34T exerted dominant-negative effects on cell–cell coupling. Our findings are consistent with a structural model, predicting that the mutation leads to a constriction of the channel pore. These data support the view that M34T is a pathological variant of Cx26 associated with hearing impairment.
INTRODUCTION
Connexins comprise a multi-gene family of proteins that form the intercellular channels clustered at gap junctions, through which ions, small metabolites and second messengers are exchanged between connected cells (1). Intercellular channels are formed by two half-channels, or connexons, consisting of six connexin subunits that span the plasma membranes of communicating cells and dock in the intercellular space (2). The specific role of connexin channels in the homeostasis of different organs has been illustrated by the association of mutations in several human connexins with a variety of genetic diseases and by the specific phenotypes revealed by targeted connexin gene deletion in mice (3–5). In the inner ear, three connexin isoforms have been found to be expressed in overlapping patterns (6) and their crucial role in organ physiology has been revealed by their implication in different forms of hereditary deafness (7). Thus, mutations in human Cx26 (HCx26), HCx30 and HCx31 (GJB2, GJB6 and GJB3 genes, respectively) have been linked to both syndromic and non-syndromic forms of hearing loss (reviewed in 8).
The arrangement of gap junctions between supporting cells of the organ of Corti appears to be the structural basis for the re-circulation of cochlear K+ ions that flow from the endolymph, where their concentrations are ~150 mm, into hair cells upon sound stimulation (9). In this scheme, K+ ions flow into the hair cells through mechanically gated channels by the combined effect of the positive endolymphatic potential (~+80 mV) and the negative intracellular potential and depolarize them. K+ ions exit hair cells reaching the interstitial space of the organ of Corti, where they are partly taken up by cochlear supporting cells (10). The excess K+ concentration that results from auditory signals or sustained deleterious stimuli, such as ischemia or prolonged exposure to high sound pressure levels (reviewed in 11), is thought to be removed via an intercellular pathway delineated by intercellular channels composed of Cx26 and Cx30 that provide a spatial buffering network similar to that of astrocytic gap junctions in the brain (12). Genetic studies have demonstrated that a specific single-base deletion in Cx26, named 35delG, is the most common mutation worldwide, although its prevalence varies with ethnical groups (8). In addition, several missense mutations responsible for either recessive or dominant forms of the disease have been identified (for an updated list, see Rabionet et al. (2002) http://www.iro.es/cx26deaf.html). The first deafness-related Cx26 mutation, which was detected in a dominant pedigree of non-syndromic hearing loss, was a methionine-to-threonine substitution at position 34 (M34T) (13).
The pathogenetic role of M34T was initially supported by the results of its functional expression in Xenopus oocytes, which showed a dominant-negative effect of the M34T mutant (14). Further studies on the family first described by Kelsell et al. (13), however, uncovered the association of dermatological signs in deaf patients and identified another dominant mutation in GJB2 segregating with the disease, casting doubts on the significance of the M34T variant. Subsequently, the same M34T substitution was also reported to be responsible for a recessive form of hearing loss (15), whereas several authors described normal hearing in heterozygous carriers of M34T associated with either 35delG or other Cx26 recessive mutations (16–19) and, therefore, considered it a benign polymorphism.
More recent in vitro data have shown that in transfected HeLa cells, the M34T variant disrupts either channel formation or its oligomerization (20,21). In contrast, Thonnissen et al. (22) observed low levels of dye transfer between HeLa cells expressing M34T, providing the first evidence that this mutant could traffic to the cell membrane and form intercellular channels, although with reduced efficiency. More recently, Oshima et al. (23) reported that assembly of M34T in HeLa and Sf9 cells resembles that of wild-type human Cx26 (HCx26wt) and that dye transfer in these cells is close to normal. Finally, electrophysiological studies performed in paired Xenopus oocytes concluded that M34T was capable of forming with Cx32 functional heterotypic channels with abnormal gating properties, which allowed to suggest that the channels are not fully open at rest but are activated when positive transjunctional voltages are applied to the M34T side (24). In addition, co-injection of M34T and HCx26wt in oocytes supported a recessive role for the M34T allele, as a 1:1 ratio of mutant to wild-type RNA (as found in heterozygous individuals) did not significantly reduce wild-type coupling.
Given the apparent contradictions of these reports, we sought to re-assess the pathogenetic role of the M34T variant by combining genetic, auditory, biochemical, electrophysiological and structural modeling studies. We provide here genetic and audiological evidence that although heterozygous carriers are found among normal hearing subjects, the majority of them (62.5%) exhibited a mild to moderate hearing impairment. Furthermore, all compound heterozygotes were affected by hearing loss, supporting the view that M34T is a pathological variant. We also show that M34T is correctly synthesized and targeted to HeLa cells membrane, although it forms intercellular channels that retain an abnormal electrical activity. Interestingly, these channels fail to sustain the intercellular diffusion of Lucifer Yellow (LY) and largely inhibit the spreading of intercellular Ca2+ waves. Finally, when co-expressed with HCx26wt at a 1:1 protein ratio, M34T invariably shows dominant-negative effects on cell–cell communication. These results fit well with our structural model, predicting that the mutation induces a constriction of the channel pore.
RESULTS
Genetic and audiological features
We have studied seven families in which one or more hearing-impaired members had the M34T mutation. Besides carrying out the routine genetic tests, we examined the audiological features of all members of the families to disclose even mild auditory defects potentially associated with the mutation. Cx26 mutations in heterozygous or compound heterozygous condition were found in 21 patients, without any instance of homozygosity (Fig. 1). The M34T mutation was identified in 16 individuals, either as heterozygotes or compound heterozygotes. Moreover, in 14 of them, we found the presence of a 10 bp deletion in the 5′-untranslated region of the gene (−493del10), in cis with M34T. This deletion was absent in all other individuals enrolled in this study. Patients were divided into three groups: group I, consisting of patients negative for GJB2/GJB6 mutations; group II, which included patients carrying mutations different from the M34T and group III, with patients carrying at least the M34T mutation.
Figure 1.
Pedigrees and genotypes of the seven families studied. Individuals with hearing loss are shown in black, whereas those with normal audiological parameters are in white. Gray symbols indicate individuals in which at least one frequency threshold was impaired; asterisks denote individuals with a degree of hearing loss that matches the one expected by age and sex.
In group I, hearing loss was present only in one patient (Fig. 1; family 5, subject I.2), whose hearing threshold was higher than that of controls. The other six exhibited normal audiological features and their hearing thresholds were well in line with those of age- and sex-matched controls. A flat audiogram was recorded in four subjects, whereas high-frequency sloping and low-frequency ascending curves were observed in the other two (Fig. 2).
Figure 2.
Audiometric analysis of the seven families studied. Air conduction thresholds were measured across the indicated frequencies, and the degree of hearing impairment was classified by the pure-tone average applied to the better ear. Among M34T-carrying patients, five had flat audiograms, all of which showed the M34T/wt genotype, (families 2, 4, 6) and 10 showed high-frequency sloping curves (six gently sloping: family 1, M34T/delGJB6-D13S1830 genotype with the poorer threshold; family 2, M34T/L90P genotype; family 3, M34T/wt; families 6 and 7, with one and two members showing M34T/wt genotype, respectively; four steeply sloping: family 1, the other subject with M34T/delGJB6-D13S1830 genotype and the subject M34T/wt; families 3 and 4, males with the M34T/35delG genotype). For each individual, audiograms of the better ear are shown.
In group II, three subjects (all heterozygotes) had mild hearing loss (delGJB6-D13S1830/wt, L90P/wt, 35delG/wt). In two of them, males having 64 and 67 years (family 2, subject I.2 and family 3, subject I.2; Fig. 1), hearing impairment was similar to that of the appropriate control group, whereas in the third one (delGJB6-D13S1830/wt), hearing threshold was higher than expected across all frequencies. Finally, the two remaining subjects (35delG/wt) exhibited normal hearing. Flat audiograms were found in two patients, whereas the other three were of the high-frequency steeply sloping type. In one of the patients with a mild phenotype (delGJB6-D13S1830/wt; family 1, subject I.1), hearing loss was asymmetrical.
In group III, five M34T/wt and five compound heterozygotes were hearing impaired. Mild hearing loss was diagnosed in four patients (one M34T/wt, one M34T/35delG, one M34T/delGJB6-D13S1830 and one M34T/L90P). Moderate hearing loss was present in five patients (four M34T/wt genotypes and one M34T/35delG), whereas severe hearing loss, in the only hearing ear, was observed in one patient with an M34T/delGJB6-D13S1830 genotype (Fig. 1; family 1, subject II.1). In all M34T/wt hearing impaired patients, thresholds across all frequencies were greater than that expected for an age- and sex-matched control population (Fig. 2). Even among normal hearing [PTA <20 dB hearing level (HL)] M34T heterozygotes (six cases), thresholds were greater than that in control subjects for at least one frequency in three patients (family 3, subject I.2; family 6, subject I.2; family 7, subject I.1). Five patients had flat audiograms and 10 showed high-frequency sloping curves (six cases gently sloping and four steeply sloping). A low-frequency ascending audiogram was observed in only one patient (family 5, subject II.1). Among the 16 patients carrying the M34T mutation, six (five of which were compound heterozygotes) had asymmetrical hearing loss.
Protein expression and localization
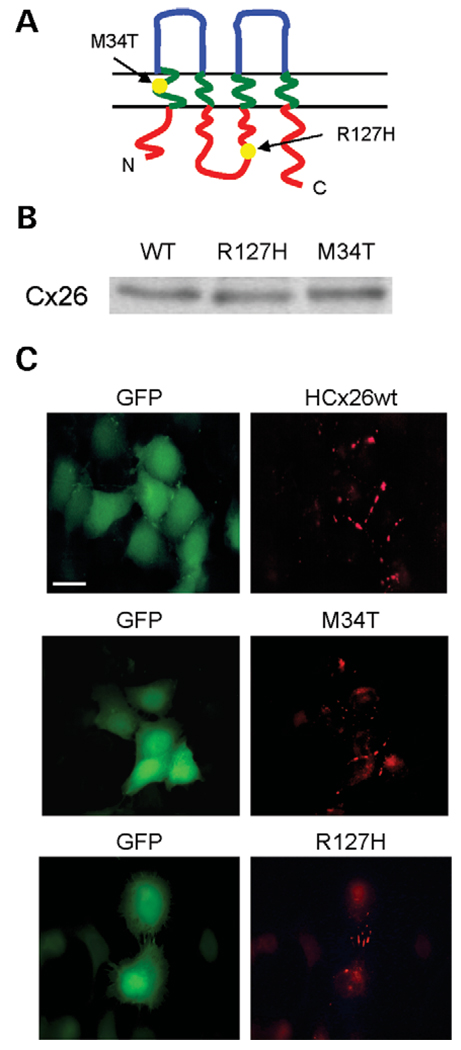
To analyze the functional impact of the M34T mutation, which is positioned in the first transmembrane domain (TM1) of Cx26, we have compared its channel-forming ability with that of the recessive R127H (25), which maps to the cytoplasmic loop (Fig. 3A). We chose these two variants because previous studies indicated that both mutated proteins form plaques in the junctional membrane (20,22,26). To examine to which extent these mutations affected the level of protein expression and their cellular localization, HCx26wt and these two variants were transiently transfected in HeLa cells, which were processed for western blot and immunofluorescence analysis 24 h post-transfection. Western blots performed on total cellular lysates demonstrated that proteins of the predicted molecular mass were detected in each experimental condition (Fig. 3B). Levels of R127H appeared comparable with those of wild-type protein, whereas M34T showed slightly increased expression, thus confirming our previous quantitative analysis (20). Transfection with HCx26wt produced, in green fluorescent protein (GFP) positive cells, a dashed, membrane-associated staining typical of gap-junctional plaques, indicating that it was correctly targeted to the plasma membrane (Fig. 3C), whereas in cells transfected with vector alone, no staining was detected (20). As previously shown (20,22,23), the M34T mutant exhibited not only a strong staining at the plasma membrane, but also the presence of cytoplasmic fluorescence, which was suggestive of partial intracellular retention (Fig. 3C). The immunolocalization of the R127H mutation gave results similar to HCx26wt, with junctional plaques present at zones of cell-to-cell apposition (Fig. 3C).
Figure 3.
Topology, expression and localization of HCx26wt and of the two variants, M34T and R127H. (A) Schematic topology of Cx26 relative to the plasma membrane, with the approximate distribution of the mutations studied. (B) Western blot analysis of the expression of wild-type and mutated Cx26 proteins in HeLa cells. Cells were transfected with the specified constructs, lysed in SDS sample buffer 24 h post-transfection and further processed for immunoblotting, as described in the Materials and Methods section. A representative blot shows that the levels of expression of the mutated proteins were comparable with those of HCx26wt. (C) Immunolocalization of HCx26wt and deafness-associated mutations. HeLa cells were transfected with the specified constructs, fixed and labeled with an anti-Cx26 antibody 24 h post-transfection, as described in the Materials and Methods section. A secondary antibody conjugated with tetramethylrhodamine isothiocyanate was used for visualization. GFP: Expression of GFP in transfected HeLa cells shows a diffuse cytoplasmic staining. Cx26: Immunolocalization of Cx26 in GFP-expressing cells shows a characteristic punctate staining, but also the presence of cytoplasmic fluorescence in the case of M34T. Bar = 10 µm.
Biophysical properties
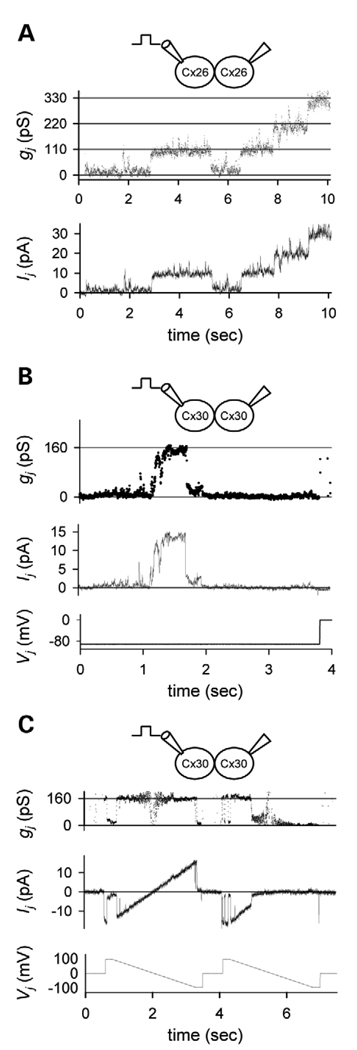
To compare the electrophysiological behavior of HCx26wt and the M34T mutant, experiments were initially carried out on cultures in which the two cDNAs were expressed through the pIRES construct (see Materials and Methods). Cell pairs expressing HCx26wt were held at a transjunctional voltage (Vj) of −95 mV, under uncoupling conditions in a medium saturated with 100% CO2 (27). Once junctional current (Ij) was reduced to below-detectable levels, the superfusion medium was switched back to normal extracellular solution (ECS) to allow Ij to recover, and a series of discrete events of similar amplitude, reflecting the opening of junctional channels, was observed (Fig. 4A). Off-line analysis yielded a unitary conductance (γ) of 114 pS for the fully open state. In contrast, when the M34T mutant was challenged under the same uncoupling conditions with a sustained Vj of −100 mV, gap junction channels tended to open more rapidly, which hampered the detection of discrete events. However, in some cases, small current transitions were sufficiently well resolved to define them as unitary gating events of ~13 pS in magnitude (Fig. 4B). Therefore, single-channel conductance of M34T is substantially (~9-fold) smaller than that of HCx26wt (Fig. 4A and B). Although such small signals were at the limit of resolution for our dual whole-cell recording conditions, this analysis indicates that the M34T variant retained partial activity and that functional homotypic/homomeric channels were assembled.
Figure 4.
Electrical properties of HCx26wt and M34T mutant. (A) Conductance changes (gj, top trace) and corresponding discrete current transitions (Ij, bottom trace) due to gating of homotypic channels formed by HCx26wt. Several opening and closing of gap junction channels were recorded with a transjunctional voltage (Vj) of −95 mV (V1 = −5 mV; V2 = −100 mV; n = 4). The calculated unitary conductance (γ) was 114 pS. (B) Conductance changes (gj, top trace) and corresponding rapid current transitions (Ij, bottom trace) due to opening of homotypic channels formed by M34T mutant recorded under a sustained Vj of −100 mV (V1 = 0 mV; V2 = −100 mV; n = 2). The estimated γ was ~13 pS.
Interactions between connexins may lead to the formation of heterotypic channels assembled by docking of two homomeric hemichannels formed from different connexins (1). To study the behavior of heterotypic channels, we used cells expressing chimeric proteins in which color variants of GFP were fused at the C-terminus of the specified constructs. When HeLa cell pairs transfected with HCx26wt-Venus/L1 recovered from CO2-induced uncoupling with a sustained Vj of −95 mV, unitary gating events indicative of the opening of single-junctional channels yielded a γ of 115 pS for the full-open state (Fig. 5A). This initial characterization indicates that the electrophysiological behavior of the tagged protein was virtually indistinguishable from that of HCx26wt.
Figure 5.
Electrical properties of tagged HCx26wt and HCx30wt. (A) Conductance changes (gj, top trace) and corresponding discrete current transitions (Ij, bottom trace) due to gating of homotypic channels formed by HCx26wt–Venus/L1 (Cx26, n = 8). Ij was recorded with a transjunctional voltage (Vj) of −95 mV (V1 = −5 mV; V2 = −100 mV). (B) Conductance changes (gj, top trace) and corresponding discrete transitions of junctional current (Ij, bottom trace) due to gating of a single homotypic channel formed by HCx30wt-CFP/L1 (Cx30, n = 4). Vj (bottom trace) was initially set at −90 mV and stepped to zero by the end of the recording period, confirming the complete closure of the channel. Conductance of the residual state, γresidual was ~27 pS (tilted arrow); conductance of the main state, γmain was 160 pS. (C) Shown are two Vj ramps from +95 to −100 mV, 2 s in duration (Vj, bottom trace), applied to a cell pair expressing homotypic HCx30wt-CFP/L1 channels (Cx30, n = 3). The Ij/Vj relationship is linear. Estimated unitary conductance (gj, upper trace) shows both main and residual (tilted arrow) states, as in (B).
Because Cx26 and Cx30 are co-localized in gap junctions of the inner ear (28,29), we next determined the biophysical properties of HCx30wt homotypic and HCx26wt/HCx30wt heterotypic channels. Pairs of HeLa cells transiently transfected with HCx30wt-CFP/L1 displayed single-channel openings when subjected to a Vj of 90 mV (Fig. 5B). Transitions occurred among the closed state, the main conductance state with γmain = 160 pS and a sub-conductance state with γresidual = 27 pS. Similar results were obtained when unitary currents (middle trace) were elicited by applying voltage ramps from +95 to −100 mV (bottom trace). Ij (top trace) was essentially linear with Vj, as expected for a normal homotypic channel, with γmain = 160 pS, as well as γresidual = 27 pS (Fig. 5C). These observations are consistent with the findings reported for mouse Cx30 by Valiunas et al. (30).
Mouse Cx26 and Cx30 have been shown to assemble heterotypic channels in functional expression systems (31). To study the behavior of HCx26wt/HCx30wt heterotypic channels, we used co-culture of cells expressing HCx26wt-Venus/L1 and HCx30wt-CFP/L1. Pairs of cells that displayed at least one bi-color fluorescent plaque at the appositional membrane were examined for functional coupling and voltage gating. Junctional currents (Ij) were recorded from the cell expressing HCx26wt-Venus/L1 while alternate negative and positive voltage steps (Vj) were applied to the cell expressing HCx30wt-CFP/L1. We assume that at both the beginning and the end of the negative Vj step, two channels were open and that, during the pulse, one channel exhibited opening and closing transitions with a calculated unitary conductance (γmain) of ~82 pS (Fig. 6A). Ij recordings during a positive Vj step showed two closing events from open to residual states of 130 pS in magnitude and one closing event of ~30 pS in magnitude (γresidual), presumably from the sub-conductance to the closed state (Fig. 6A). The different values of γmain at positive and negative Vjs, indicative of rectification, were also measured by delivering voltage ramps from +95 to −95 mV (Fig. 6B). In both experimental protocols, single-channel conductance was smaller when the HCx26wt side was made relatively positive and vice versa. The fitting curve (solid line of the gj – Vj plot in Fig. 6B) showed that the conductance of the main state at a Vj = 0 mV was ~110 pS. This value is reasonably close to the expected γmain value of ~130 pS [γeq = (1/γ26 + 1/γ30)]−1 calculated from the arrangement in series of one homotypic HCx26wt connexon (γ26 = 230 pS) with one homotypic HCx30wt connexon (γ30 = 320 pS), thus supporting the notion that these heterotypic channels retain the properties of composing hemichannels.
Figure 6.
Gating behavior of heterotypic channels. (A) A transjunctional voltage (Vj = VCx30 −VCx26, bottom trace) was imposed by stepping the HCx30wt (Cx30)-expressing cell, and junctional current (Ij, middle trace) was recorded from the HCx26wt cell (Cx26, n = 4). Top trace: junctional conductance trace (gj). (B) Junctional current (Ij, top left) was recorded from the Cx30 cell during five consecutive Vj (VCx26 − VCx30) ramps from +95 to −95 mV, 3 s in duration (bottom left, n = 4). The right-hand panel shows rectification in the junctional conductance gj = Ij/Vj, derived from the ramp responses and plotted as a function of Vj. (C) Junctional current (Ij, middle trace) was recorded from the cell expressing Cx26 in response to four consecutive Vj ramps (VM34T − VCx26, bottom trace) from +100 to −100 mV, 3 s in duration (n = 4). The calculated junctional conductance (gj, upper trace) of heterotypic M34T/HCx26wt channels shows pronounced rectification (n = 4). (D) Same protocol as in (C) shows milder rectification for heterotypic M34T/HCx30wt channels (Vj = VM34T − VCx30). Ij (middle trace) was recorded from the cell expressing Cx30 (n = 4). (E) Plot of the relationship of Vj to steady-state junctional conductance (Gjss, calculated as the ratio of steady-state to instantaneous junctional conductance measured at the offset and onset of voltage steps, respectively) obtained in cell pairs expressing homotypic M34T channels (filled circles), or heterotypic M34T/HCx26wt (open circles) and M34T/HCx30wt (filled squares) combinations (pooled data from five independent experiments). In heterotypic channels, Vj is defined as positive for depolarization and negative for hyperpolarization of the side expressing the wild-type connexins cell relative to the M34T cell. Lines through data are least square fits with quadratic functions to aid data trend visualization. Heterotypic channels display asymmetric Gj − Vj relationships because M34T is poorly voltage dependent, whereas HCx26wt and HCx30wt close at positive voltages.
To obtain heterotypic channels containing one mutated connexon, HeLa cells were transiently transfected with M34T, expressed through the pIRES vector, HCx26wt-Venus/L1 or HCx30wt-Venus/L1 (Fig. 6C and D). Separate cultures were eventually mixed and pairs for dual whole-cell patch clamp analysis were identified by the simultaneous presence of diffused GFP fluorescence in the cytosol of one cell, indicating positive M34T transfectants, and punctate Venus fluorescence on the other side of the junction, reflecting expression of tagged forms of either HCx26wt or HCx30wt. Our data show that M34T hemichannels were able to dock with either HCx26wt or HCx30wt, leading to the formation of functional heterotypic gap junction channels. In both cases Ij recordings obtained by applying voltage ramps showed a rectification that was clearly more pronounced for the M34T/HCx26wt combination (Fig. 6C) than for M34T/HCx30wt (Fig. 6D). Steady-state Gj–Vj dependence was analyzed by applying prolonged voltage steps (see Materials and Methods), and pooled data were obtained by normalizing the steady-state conductance, measured just prior to command offset, to the conductance values at command onset (Fig. 6E). These data show that M34T homotypic junctions were relatively insensitive to Vj, similarly to HCx26wt channels (32), a feature maintained in both heterotypic configurations for relative positivity of the cytoplasmic side of the M34T connexon (Fig. 6E). Thus, although M34T channels were endowed with a residual functionality, their properties were significantly altered in both homotypic and heterotypic configurations.
As a control, we examined heterotypic junctions formed by pairing HCx26wt or HCx30wt with V84L, a deafness-related recessive mutant of Cx26 that retains the ability to sustain electrical communication (32,33). Homotypic V84L channels exhibited a unitary conductance of 110 pS, very close to the value measured for HCx26wt channels (33). Heterotypic combinations (HCx26wt/V84L and HCx30wt/V84L) were obtained by mixing transiently transfected HeLa cells, as described previously. In contrast to what observed with M34T, HCx26wt/V84L channels showed a linear Ij–Vj dependence of the open state with a main conductance of ~120 pS and no residual state (Fig. 7A). Results obtained by delivering voltage commands in the range from −100 to +100 mV for 10 s (not shown) were consistent with those generated with ramp protocols. As expected on the basis of the properties of HCx26wt/HCx30wt heterotypic junctions, HCx30wt/V84L channels exhibited rectification and single-channel conductance increased from ~70 to ~150 pS when voltage ramps from −100 to +100 mV were delivered to the cell expressing V84L while recording Ij from the cell expressing HCx30wt (Fig. 7B). Thus, in contrast to what observed with M34T, the electrical characteristics of HCx30wt/V84L channels did not differ appreciably from those of HCx26wt/HCx30wt channels.
Figure 7.
Gating behavior of heterotypic channels containing the V84L mutation. (A) Conductance changes (gj, top trace) and corresponding discrete current transitions (Ij, middle trace) due to channel opening during transjunctional voltage ramps (Vj = VV84L − VCx26, bottom trace) from +100 to −100 mV (n = 3) (B) Two junctional voltage ramps (Vj = VV84L − VCx30, bottom trace) from +100 to −100 mV, 3 s in duration, are shown (n = 3). In both panels, junctional current (Ij, middle trace) shows an ohmic behavior (viz. a linear dependence) on Vj.
Intercellular dye transfer
To assess the permeability of mutated gap junction channels, we examined the cell-to-cell transfer of LY (MWionic form = 443; charge 2−) by loading one cell of a pair with a patch pipette. We did not detect LY diffusion across M34T channels despite the presence of sizeable junctional conductance (gj = 4.5 ± 1.3 nS, n = 5), measured by dual whole-cell patch-clamp (Fig. 8). It should be noted that this level of gj corresponds to approximately 450 open channels (4.5/0.013 nS) at any given time and that robust LY transfer is consistently detected with a similar number of functional HCx26wt homotypic channels (34). LY diffusion was then assayed in cultures of parental and transiently transfected HeLa cells expressing either the wild-type or the mutated connexins (Table 1). Dye coupling was scored as positive if the probe diffused to at least two neighboring cells. As expected, non-transfected HeLa cells did not display intercellular dye diffusion (not shown). Microinjection of LY into an individual cell of an HCx26wt-positive cluster resulted in intercellular coupling in all instances with 100% of GFP-positive cells filled with the probe. In contrast, no dye transfer was observed between cells expressing the M34T mutation, whereas R127H showed some incidence of dye transfer, although drastically reduced in comparison with HCx26wt, with only one or two cells of the R127H cluster receiving LY (Table 1).
Figure 8.
Permeability to LY of M34T mutant channels. Top left: bright field image of one representative pair of HeLa cells with superimposed regions of interest (ROIs) used to compute average pixel fluorescence. Top right: image of the same field illuminated at λex = 480 nm to visualize EGFP positive cells (λem = 535 nm). Bottom left: fluorescence image obtained at the end of the experiment by exciting the field at λex = 425 nm to quantify LY spread (λem = 535 nm). Cell 1 was contacted by a patch pipette containing 4% LY. Intracellular delivery of LY started 20 s after the onset of the recording. Bottom right: normalized fluorescence from the corresponding ROIs (traces numbered 1 and 2) measured concurrently with junctional conductance (gj, lowermost trace). The arrowhead marks the time at which the LY fluorescence image was acquired. Results are representative of five independent experiments. Scale bar: 10 µm.
Table 1.
Qualitative comparison of dye diffusion (LY; MW = 457 Da; charge 2−) between HeLa cells transfected with either HCx26wt or the specified mutations
| Transfection | Percentage of positive injections | n |
|---|---|---|
| HCx26wt | 100 | 135 |
| M34T | 0 | 67 |
| R127H | 14 | 95 |
| HCx26wt + M34T | 0 | 102 |
| HCx26wt + R127H | 100 | 47 |
The incidence of cell coupling (second column) was scored by recording the number of instances in which the dye diffused from the injected cell to at least two neighboring ones. In the third column, the total numbers of injections (n) is indicated.
The original report of the heterozygous M34T mutation in a family with dominant deafness (13) suggested that the mutant allele would act as a dominant-negative subunit of connexin channels, and functional expression studies performed on pairs of Xenopus oocytes (14) initially supported this view. To determine whether this mutation could interfere with the function of wild-type channels, we examined dye coupling in HeLa cells co-transfected with the same amount of HCx26wt and M34T plasmids and found that LY transfer was abolished under these experimental conditions (Table 1). In contrast, the R127H mutation, segregating with autosomal recessive deafness (25), failed to affect the ability of HCx26wt to form functional channels when the two constructs were co-transfected at an equal ratio (Table 1). These results demonstrate that the M34T mutant, but not the recessive R127H mutant, significantly inhibited the functional activity of HCx26wt when expressed in a manner mimicking the heterozygous genotype.
Intercellular calcium waves
In many cell types, connexins are involved in the radial propagation of intercellular calcium waves induced by mechanical stimulation of a single cell in a monolayer (reviewed in 35). We performed calcium-imaging experiments to determine whether the M34T variant could interfere with the intercellular diffusion of second messengers such as calcium ions and inositol 1,4,5-trisphosphate (InsP3). To minimize the contribution of ATP release to the propagation of calcium signals (36), in all experiments, the extracellular medium was supplemented with 40 U/ml apyrase grade VII.
In control cells transfected with the empty vector, mechanical stimulation induced a [Ca2+]i increase that remained largely confined to the stimulated cell, and in the few instances in which the signal propagated to neighboring cells, it reached only one cell of the first order (not shown). As expected, in monolayers expressing HCx26wt, the [Ca2+]i response of a mechanically stimulated cell was consistently followed by the intercellular spreading of a calcium wave (Fig. 9A; n = 33) that involved all first-order cells (91% of the observations), and in 21% of the cases, cells of the second order. The propagation of calcium waves was completely inhibited by the gap junction blocker carbenoxolone (CBX, 100 µm) in all experiments (Fig. 9B; n = 13). In M34T-expressing cells, calcium spreading was largely blocked, being observed only in 27% of the cases (Fig. 9C; n = 37), with only one cell of the first order being recruited. Similar results (blockade in 70% of the experiments) were also obtained when cells were co-transfected with a 1:1 ratio of HCx26wt and M34T plasmids (Fig. 9D; n = 12).
Figure 9.
Mechanically-induced intercellular calcium waves in transfected HeLa cells. HeLa cells expressing the specified constructs were analyzed by calcium imaging 24 h post-transfection. Monolayers loaded with fura-2/AM were subjected to mechanical stimulation of single cells (cell 1 in all panels) by briefly deforming the cell surface with a micropipette, as described in the Materials and Methods section. Apyrase (40 U/ml) was added in all experiments. For all conditions, intercellular calcium waves shown are representative of at least 12 experiments from four independent transfections. (A) HCx26wt transfected cells (n = 32). (B) HCx26wt transfectants incubated in the presence of the gap junction blocker CBX (100 µm, n = 18). (C) M34T transfected cells (n = 45). (D) HCx26 wt + M34T co-transfected cells (n = 12). In all panels, insets show the clusters of EGFP-positive cells included in the experiment with the cell numbers corresponding to the individual fluorescence traces (see color-coding below the abscissa). Scale bar: 10 µm.
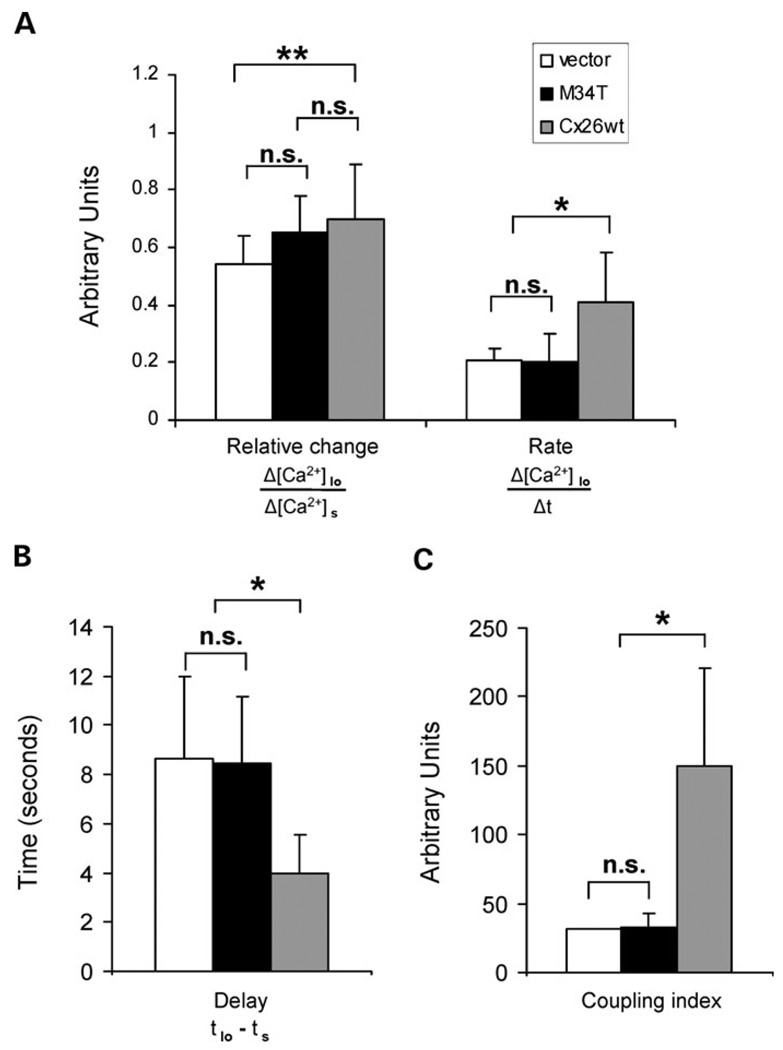
The evidence that in a minority of cases a calcium wave was observed in non-transfected and in M34T-expressing cells was not entirely surprising, given the complexity of the molecular mechanisms governing this phenomenon. On the one hand, the contribution of paracrine signals other than ATP could not be entirely excluded, and on the other hand, the recruitment of stretch-activated channels and downstream pathways could also account for the occasional calcium response of the cell(s) tightly connected to the stimulated one. To exclude an involvement of connexin-unrelated mechanisms in the propagation of calcium waves, we refined our investigation by restricting the analysis to neighboring cells involved in the wave. In M34T transfectants, the [Ca2+]i elevation in the cell(s) of the first order relative to the stimulated one (Δ[Ca2+]Io/Δ[Ca2+]s) decreased slightly with respect to that of HCx26wt (Fig. 10A). In addition, the rate of the rising phase in the first-order cells (Δ[Ca2+]Io/Δt) was reduced to less than 50% compared with that of HCx26wt pairs and similar results were obtained in control cells transfected with the empty vector (Fig. 10A). The delay in the calcium response of the first-order cell(s), measured as the time required to reach the half maximal change (EC50) in fluorescence ratio with respect to that of the stimulated cell (tIo − ts) (37), was also altered, being significantly reduced in M34T transfectants with respect to that observed in HCx26wt-expressing cells (Fig. 10B). Finally, to complete this quantitative analysis, we derived a coupling index among different transfectants by multiplying the percentage of successful stimulations (those inducing an intercellular Ca2+ wave) by the number of cells displaying an increase of cytosolic Ca2+. The value calculated for M34T-expressing cells was similar to that of controls and five times lower than that of HCx26wt transfected cells (Fig. 10C).
Figure 10.
Quantitative analysis of critical parameters of calcium wave propagation. HeLa cells expressing the specified constructs were analyzed by calcium imaging in response to mechanical stimulation, as described in the Materials and Methods section. Apyrase (40 U/ml) was added in all experiments. Changes in intracellular Ca2+ concentration were estimated by calculating the excitation ratio of fura-2 fluorescence at 340 and 380 nm (see Materials and Methods). (A) Δ[Ca2+]Io/Δ[Ca2+]s describes the calcium concentration change in cells of the first order relative to the stimulated cell. Δ[Ca2+]Io/Δt calculates the rise rate of the calcium signal in cells of the first order relative to the stimulated cell. (B) tIo −ts indicates the delay in the calcium response of first order cells, measured as the time required to reach the half maximal (EC50) fluorescence ratio change with respect to that of the stimulated cell. (C) The coupling index of different transfectants is derived by multiplying the percentage of positive experiments (i.e. the experiments in which intercellular calcium spreading was detected) by the number of coupled cells. Results are shown as means ± SD of the number of experiments indicated in the legend of Figure 9. Statistical significance was assessed by the unpaired Student’s t-test. *P < 0.05; **P = 0.05; ns, not significant.
Structural model of the M34T mutant
These genetic and functional data indicate that the M34 residue in Cx26 is critical for channel function. Fleishman et al. (38) have recently published a structural model of the transmembrane (TM) region of Cx32 (PDB code 1TXH) on the basis of a combination of theoretical methods and low-resolution electron cryo-microscopy map of Cx43 (39). Given the high conservation of the TM helices among the connexin family, these data are expected to serve as a general template for the TM regions of other connexins. In this model, M34 mediates putative contacts between two consecutive connexins (38). However, because only the positions of the Cα atoms were determined, the evaluation of the possible consequences of point mutations is not immediate. To gain further structural insights into the consequence of the M34T mutation on channel structure, we undertook the theoretical construction of all-atoms models of HCx26wt and M34T using the Cα structure of Cx32 as a reference template. In this atomic model, the side chains of Tyr152 within TM3, which is considered to be the main pore-lining helix (2), determine the maximum constriction region of the pore of HCx26wt channels near the extracellular side (Fig. 11A). M34 does not face the pore lumen, but is partially exposed to the lipid face, where it also establishes hydrophobic contacts with residues of the adjacent connexin chain (Fig. 11B, inset).
Figure 11.
Structural models of HCx26 wt and M34T channels. (A) Atomic model of HCx26wt as seen from the intracellular side after full EM. The different colors of the ribbons depict the six subunits forming the connexon. The Y152 residue corresponding to the minimum diameter of the pore lumen is represented in space-filling spheres. Hydrogen atoms are not shown for visual clarity. (B) Lateral view of the transmembrane part of the HCx26wt connexon obtained rotating the structure in (A) by 90 degrees. The position of M34 is indicated with its corresponding space-filling representation. Inset: a representative close-up of the M34 neighborhood. Residues within 0.5 nm are shown in sticks. M34 may establish hydrophobic interactions with the lipid phase and also with residues belonging to the second transmembrane helix (TM2) from the nearby connexin. (C) Root mean square deviation (RMSD) calculated over the Cα atoms plotted versus simulated time for Cx26 wild-type (blue) and M34T (red) connexons. (D) Top panel: averaged distance between the center of the mass of the last four Cα atoms of couples of opposite TM3 helices (see Materials and Methods). Middle panel: averaged distance between the center of the mass of the first four Cα atoms of couples of opposite TM3 helices. Bottom panel: average distance between OH atoms belonging to opposite Y152 for HCx26wt (dark blue) and M34T (red). (E) Trajectory of the inter-helical angle between opposite TM3 helices within the connexon. (F) The central scheme illustrates the conformational change produced by the M34T substitution (red cylinders) with respect to wild-type (blue cylinders).
Both HCx26wt and M34T models showed alike root mean square deviation (RMSD) behaviors, reaching a stabilization plateau after nearly 250 ps, thereafter oscillating around a value of ~0.3 nm (Fig. 11C). The average RMSD of each connexin was ~0.15 nm, suggesting that, although the helical conformation was fairly well maintained, the relative orientation of the chains suffered significant changes upon relaxation. Comparison of the average distances between the backbone extremities of two opposite TM3 helices at the intracellular sides of both variants indicated that HCx26wt suffered a decrease of ~0.2 nm, whereas M34T showed an opposite behavior increasing the internal diameter by 0.1 nm (Fig. 11D). A similarly opposite behavior was also retrieved at the extracellular side, where the backbone extremities of the TM3 helices remained 0.2 nm closer in M34T (Fig. 11D). Thus, the inter-helical angle between opposite TM3 helices of HCx26wt decreased to almost 50 degrees, whereas in the M34T mutant, it increased up to ~65 degrees (Fig. 11E). This implies a sort of angular bending between the principal angles of the helices (Fig. 11F) that leads, as a consequence of this movement, to a higher constriction of the pore in the M34T mutant. Hence, the average distance between couples of OH atoms in opposite Tyr152 residues remained nearly within its initial value of 2 nm for HCx26wt, whereas a more pronounced reduction to ~1.7 nm was observed for the M34T mutant (Fig. 11D).
DISCUSSION
Our data demonstrate that the presence of the M34T variant of HCx26 is indeed associated with mild-to-severe forms of deafness. This conclusion is supported by comprehensive biophysical and functional studies showing that, although M34T retains partial functionality, the mutant channels are defective with respect to wild-type in both electrical and metabolic couplings.
Genotype–phenotype correlation
Although M34T has been described both as an autosomal dominant (13) and recessive mutation (15,40–42), this interpretation has remained controversial due to the frequent findings of normal hearing in individuals heterozygous for the M34T variant (16–19) and even in compound heterozygotes with 35delG (15). Thus, it has been suggested that the pathological effect of the M34T allele might depend on the mutations segregating in the opposing allele, such as 167delT (43,44) or 35delG (45). However, in a recent multicenter study, all patients with a 35delG/M34T genotype were found to have mild-to-moderate hearing impairment (46). These discrepancies may reflect a reduced penetrance of M34T or be the consequence of a diagnostic bias toward more severe forms of hearing impairment, as persons with mild deficits are less likely to undergo audiological or genetic testing (46).
The audiometric features of the seven families reported here revealed that hearing loss was present in 14 subjects. Considering heterozygous subjects carrying the M34T mutation, 10/16 (62.5%) were mildly to severely impaired with respect to the audiometric parameters of age- and sex-matched controls. Of note, none of the five M34T compound heterozygotes displayed normal hearing, being classified as mildly to severely impaired. The most compromised frequencies were in the range of 2000–8000 Hz, which resulted in an audiogram configuration of the sloping type. This observation may account for the discrepancy with many previous studies in which the degree of hearing impairment was assessed by pure tone average of only three frequencies (500, 1000, 2000 Hz). A clinical re-assessment of those subjects with a wider range of frequencies may help uncover mild cases of hearing loss associated with M34T mutation. In fact, the only other report that examined air conduction thresholds across the same range of frequencies, as in this study, found that the two subjects with M34T/wt genotype displayed mild-to-moderate hearing loss (47). A moderate hearing loss affected the heterozygous M34T child (48); in this study, the authors included only deaf children with hearing thresholds > 56 dB HL. Together, these observations indicate a pathogenetic role of the M34T mutation and are consistent with most recent surveys associating M34T compound heterozygosis to mild-to-moderate hearing loss (15,46,47,49,50).
The finding that 60% of patients carrying only the M34T mutation were also hearing impaired, thus suggesting the involvement of this variant even in dominant forms of deafness, is not easily explained. It has been noted, however, that M34T segregates with a 10 bp deletion in the 5′-unranslated regions of the Cx26 gene (15). It is not known whether this deletion is of any significance, but its association with M34T may account for the variability in the phenotype of M34T heterozygous, as it could alter the expression level of the M34T allele in vivo. Of note, this deletion was present, in cis, in 14 out of the 16 M34T subjects, whereas it was absent in all other individuals enrolled in the study. Hence, this study and functional studies (14) suggest that M34T could be regarded as a dominant allele, but its dominant-negative activity may be affected by modifying factors such as the level of RNA expression, as recently suggested (51), thus explaining the observed phenotypic variability. Although a population-based study may also give information on the penetrance of the M34T allele, the pedigrees of our families suggest that environmental factors alone cannot explain the hearing deficits of M34T heterozygotes (see, for example, family 7 in Fig. 1).
Impact of M34T on channel function
By combining electrophysiology, dye transfer and dynamic calcium imaging, our data demonstrate that M34T mutant channels, although correctly synthesized and targeted to the plasma membrane, are functionally defective. This conclusion is based on several lines of evidence. First, unitary conductance of M34T was ~10-fold lower than that of HCx26wt gap junction channels. Secondly, heterotypic HCx26wt/M34T channels exhibited a strong Ij–Vj rectification that can have functional implications for communicating cells residing at different transmembrane potentials. Thirdly, intercellular transfer of LY was prevented, and finally, M34T channels failed to sustain the propagation of intercellular Ca2+ waves. These results demonstrate that the mutation alters significantly the molecular cut-off size of the pore and therefore should be considered as a pathological variant of Cx26.
In addition, we found that M34T exerted a dominant-negative inhibition of metabolic coupling when expressed in a manner mimicking a heterozygous genotype, an effect not displayed by the recessive R127H mutation. Our data support the conclusion reached by previous functional studies performed in paired Xenopus oocytes expressing M34T (14). Although this dominant-negative effect was later attributed to an artifact because of the lack of control of the expression levels of mutant and wild-type RNA in the exogenous system (40), a dominant inhibition of channel function has been confirmed in a recent study conducted on Cx26 hemichannels in single oocytes (52).
These observations are difficult to reconcile with a recent study demonstrating that M34T, although not capable of forming functional gap junction channels on its own in Xenopus oocytes, did not exhibit a dominant-negative effect on cell–cell coupling when co-expressed at a 1:1 ratio with HCx26wt (24). Such a discrepancy cannot be explained easily. It should be noted, however, that White et al. (14) and we have tested the dominant-negative action of M34T in cells that were all programmed to express this variant together with HCx26wt, whereas Skerrett et al. (24) have paired oocytes co-injected with HCx26wt and M34T in heterotypic fashion, with oocytes expressing only the wild-type protein. Thus, these studies are not directly comparable. An additional difference in the observed functional properties of M34T may stem from the relative expression levels of the wild-type and mutant proteins, a parameter that cannot be fully controlled in cells co-expressing the two HCx26 sequences, as there are no antibodies that can distinguish between them. As found from this study and from the work of D’Andrea et al. (20), although the amount of M34T expression was found to be comparable with that of HCx26wt in either single transfectants or in oocytes injected with the cognate RNA (14), it is possible that changes in the protein ratios may also account for the differences between these three studies. Indeed, increasing the ratio of mutated to wild-type RNA injection (M34T:HCx26wt = 2:1) led to a considerable decrease of intercellular coupling (24). Differences in protein levels are an inherent drawback of in vitro functional expression systems. Thus, the residual permeability of the M34T variant to fluorescent tracers, as previously reported (22,23), is likely to depend on the over-expression of the low conductance channel, as suggested by the same authors.
A structural model of M34T channels
The stringency of the requirements of distinct connexin channels in different tissues strongly suggests that altering such composition in vivo would result in the development of functional abnormalities (53). Indeed, targeted ablation of Cx26 in the epithelial network of the organ of Corti has elegantly illustrated its essential role for cochlear function and cell survival (54). Our functional analysis demonstrates that a methionine residue at position 34 in Cx26 substantially influences homotypic, as well as heterotypic and heteromeric channels formed by association with both HCx26wt and HCx30wt and, together with the genetic and clinical data, further suggests that the consequences of this single-amino acid substitution cannot be compensated by other connexin co-expressed in the cochlea.
M34 is located in the TM1 domain of connexins, which is likely to be one of the pore-lining helices (38,55,56) and interacts with other transmembrane helices, stabilizing the TM1 structure and ensuring the open-channel state (23). Our molecular dynamic simulations demonstrate that the M34T substitution would cause a reduction of the side chain size at this position, leading to a partially closed channel, consistent with decreased channel permeability and unitary conductance. Caution should be exerted, however, in the interpretation of these models, which are affected by several approximations whose consequences are difficult to estimate because of the lack of input data at atomic resolution (38). Nonetheless, the results of these simulations are in good agreement with our own, as well as previously published experimental data, suggesting that this model captures at least the gross determinants of the M34T mutation.
Calcium wave propagation and M34T pathogenicity
Intercellular calcium waves propagating in the network of supporting cells have been observed in the organ of Corti in response to mechanical stimulation of single-hair cells (57). This phenomenon depends on two underlying mechanisms: (i) the release of an extracellular messenger, believed to be ATP because purinergic receptor antagonists and apyrase, which hydrolyzes ATP, block the propagation of Ca2+ waves in cochlear organotypic cultures (57); and (ii) the diffusion of inositol trisphosphate (InsP3) through gap junctions (35,58), because agents that interfere with InsP3 signaling or gap junction communication effectively inhibit wave propagation between many cell types (59–61), including supporting cells of the organ of Corti (33). We speculate, therefore, that the disruption of this intercellular signaling pathway by the M34T mutation may interfere with re-cycling of K+ ions back into the endolymph, leading to accumulation of K+ ions and excitotoxic death of hair and supporting cells in vivo, which would eventually result in hearing impairment (62). Our data are consistent with a mechanistic explanation of the pathogenesis of deafness which postulates that InsP3 permeability between the epithelial cell network of the cochlea is crucial for normal hearing (33).
MATERIALS AND METHODS
Reagents
LY, apyrase grade VII, aprotinin, chymostatin and leupeptin were obtained from Sigma (St Louis, MO). The polyclonal anti-rat Cx26 antibody employed was from Zymed Laboratories (San Francisco, CA). For immunoblot detection, the secondary antibody was a goat anti-rabbit IgG conjugated with alkaline phosphatase from Calbiochem (San Diego, CA). For immunofluorescence, the secondary antibody was a tetramethylrhodamine isothiocyanate (TRITC)-conjugated affinity purified goat anti-rabbit IgG from Jackson ImmunoResearch Laboratories (West Grove, PA). The chemiluminescence substrate (CSPD) and enhancer (Nitro-Block) for alkaline phosphatase were from Tropix (Bedford, MA). Restriction enzymes were from New England Biolabs (Beverly, MA). Nitrocellulose was from Schleicher & Schuell (Dassel, Germany). All other reagents were from Sigma, unless otherwise specified.
DNA analysis
DNA was extracted from peripheral blood according to standard protocols after obtaining informed consent. The whole Cx26 coding region was amplified by polymerase chain reaction (PCR) using the following forward (CX26ORF-F: TGCTTACCCAGACTCAGAGAA) and reverse (CX26ORF-R: GACTGAGCCTTGACAGCTGAG) primers. PCR reaction was performed in a total volume of 50 µl containing 15 pmol of each primer, 100 µm dNTP, 5 µl 10× reaction buffer (100 mm Tris pH 8.3, 500 mm KCl, 15 mm MgCl2, 0.01% gelatin), 2.5 U Ampli Taq Gold Polymerase (Perkin Elmer, Foster City, CA) and 100 ng of DNA template, using an automated Thermal Cycler 9700 (Perkin Elmer). The cycling profile consisted of an initial denaturation at 94°C for 12 min, which was followed by 35 cycles of 94°C × 45 s, 60°C × 45 s, 72°C × 80 s and a final extension step at 72°C for 10 min. Exon I and flanking splice sites were amplified using oligonucleotides EX1CX26F (tcaaaggaactaggagatcgg) and EX1CX26R (aaggacgtgtgttggtccag) as forward and reverse primers, respectively.
When genetic analysis was either negative for GJB2 mutations or a heterozygous condition was found, samples were further tested for GJB6 mutations. The Δ(GJB6-D13S1830) deletion of the GJB6 gene was detected by PCR amplification as suggested by del Castillo et al. (63). Amplicons were directly sequenced with the same PCR primers, using an ABI-PRISM big-dye terminator cycle reaction kit (Perkin Elmer), and samples were then analyzed on an automated sequencer (ABI 3100, Perkin Elmer). Patients were eventually divided into three groups: group I, negative for GJB2/GJB6 mutations; group II, carrying mutations different from M34T and group III, carrying at least the M34T mutation.
Patients
We have studied eight probands with mild-to-severe sensorineural hearing loss, in which genetic analysis had identified the presence of the M34T mutation as a heterozygous or a compound heterozygous condition and their respective families (seven in total). A total of 28 subjects, aged between 5 and 69 years (median = 37 years and 5 months) were studied. The follow-up period ranged from a minimum of 18 months to a maximum of 12 years, and in all patients, serial audiograms were available. None reported a relevant history of otological disease, noise exposure or assumption of ototoxic drugs.
A detailed history was collected from all patients, followed by otoscopic and audiological examination comprehensive of pure tone audiometry (or conditioned infantile audiometry in one patient), tympanometry, stapedial reflex threshold and auditory brainstem evoked potentials (ABR). Otoacoustic emissions were not available in all patients. In all cases, the audiological study revealed cochlear site of lesion, and there were no clinical features suggestive of a syndromic deafness. None of them accused vertigo or dizziness, so vestibular function was not tested. Audiological features of hearing loss were then analyzed according to the GenDeaf study group recommendations (64). Air conduction thresholds were measured across the following frequencies: 125, 250, 500, 1000, 2000, 4000 and 8000 Hz, whereas for bone conduction, the range was between 250 and 4000 Hz. The dB values relate to dB HL. The degree of hearing impairment was classified by the pure-tone average (PTA) applied to the better ear at 500, 1000, 2000 and 4000 Hz. In the case of normal hearing, PTA is ≤20 dB; in mild hearing loss, PTA is >20 ≤ 40 dB; in moderate hearing loss, PTA is >40 ≤ 70 dB; severe hearing loss is characterized by a PTA >70 ≤ 95 dB, and hearing loss is defined as profound when PTA >95 dB.
Hearing impairment is progressive when a deterioration of ≥15 dB in the PTA occurs within 10 years. Depending on its configuration, the audiometric curve is classified as flat, if the difference between 125 and 8000 Hz is <15 dB; mid-frequency U-shaped, when difference is ≥15 dB between the poorest thresholds in the mid-frequencies (>500 ≤ 2000 Hz) and those at higher (>2000 ≤ 8000 Hz) and lower (≤500 Hz) frequencies; low frequency-ascending, when there are ≥15 dB between the poorer low frequency thresholds and the higher frequencies; high frequency-gently sloping, if the difference between the mean of 500 and 1000 Hz and the mean of 4000 and 8000 Hz is within 15–29 dB; high frequency-steeply sloping, with ≥30 dB difference between the latter frequency pairs. Hearing impairment is classified as (i) asymmetrical, if >10 dB difference occurs between the ears in at least two frequencies, with the PTA in the better ear exceeding 20 dB HL and (ii) unilateral, when one ear has either a PTA >20 dB or one frequency threshold exceeding 50 dB while the other ear has a PTA ≤20 dB. Hearing thresholds were compared with those of age- and sex-matched controls (ISO 7029:2000). Differences in audiological parameters between groups were determined by Fisher’s exact test and P-values <0.05 were considered to be significant.
Molecular cloning
The coding region of HCx30 (PubMed-NCBI accession number HSA005585), HCx26wt (PubMed-NCBI accession number AF281280) or HCx26 mutations was amplified by PCR from genomic DNA of normal hearing subjects and patients with different forms of sensorineural deafness. Amplicons were subcloned into the bicistronic vector pIRES-EGFP (Clontech, Palo Alto, CA), which permits an efficient selection of transfected cells for functional studies by monitoring the expression of the enhanced form of Aequorea victoria GFP, as previously described (32). For electrophysiological recordings, we employed HCx26 proteins fused to either EYFP (HCx26wt–EYFP) or the circularly permuted yellow mutant of the GFP, called Venus (65) (HCx26wt–Venus), by two linkers of different lengths. For the generation of fusion proteins, the open reading frame (ORF) of the specified constructs was amplified by PCR using oligonucleotide primers that introduced the desired restriction enzyme sites at both ends of the coding sequence and deleted the stop codon. The following primers, which introduced EcoRI–BamHI sites, were used for Linker L1 (six amino acids: DPPVAT):
26forward: 5′-CGGAATTCAGATGGATTGGGGCACG-3′; 26reverse: 5′-CGGGATCCACTGGCTTTTTTGACTT-3′; 30-forward: 5′-CGGAATTCCCAGCGCAATGGATTGG-3′; 30-reverse: 5′-CGGGATCCCTTGGGAAACCTGTGAT-3′. For linker L2, which introduced EcoRI–XhoI sites and comprised 17 residues (RILQSTVPRARDPPVAT), the primers used were 26forward: 5′-GGTCCTCGAGATGGATTGGGGCACGCTGC-3′; 26reverse: 5′-CCCGAATTCGAACTGGCTTTTTTGACTTCCC-3′.
The resulting PCR products were digested with the two enzymes and ligated into the respective sites of pEGFP-N1 (Clontech). All constructs were sequenced using the Dye terminator (Perkin-Elmer), as recommended by the manufacturer, to verify that PCR amplification did not introduce unwanted mutations. The length of the linker did not interfere with the electrophysiological profile of the recombinant gap junction channels, as assessed by measuring their unitary conductance with a dual whole-cell patch clamp technique.
Cell culture and transfection
A clone of HeLa cells essentially devoid of connexins (66) was kindly provided by Professor Klaus Willecke (University of Bonn, Germany) and cultured according to standard procedures. Twenty-four hours after plating, cells were transfected with the expression vectors described previously, using lipofectamine (Gibco/Invitrogen, Leek, The Netherlands). Experiments were performed the following day.
Protein immunoblot analysis
Proteins were dissolved in lysis buffer (50 mm Tris pH 6.8, 2% SDS, 10 µg/ml each of aprotinin, chymostatin and leupeptin) from cells grown to confluence. Prior to electrophoresis, protein concentration was measured by the bicinchoninic acid method (Pierce, Rockford, IL) with bovine serum albumin as the standard. For each sample, 20 µg of total proteins were separated on 13% SDS gel and then transferred to nitrocellulose. Coomassie blue staining of identical gels run in parallel confirmed the equal loading of the samples. Nitrocellulose filters were blocked with 1% bovine serum albumin in Tween-added PBS (TPBS: 137 mm NaCl, 2.7 mm KCl, 8.1 mm Na2PO4, 1.5 mm KH2PO4, pH 7.4, supplemented with 0.1% Tween 20) and incubated overnight with the primary anti-Cx26 antibody under continuous shaking. Blots were further incubated for 1–2 h with the alkaline phosphatase (AP)-conjugated secondary antibody, processed for protein visualization with ECL and exposed to CL-Xposure Films (Pierce) for 10 or 20 min.
Immunofluorescence
Cells grown onto glass coverslips were fixed with 2% paraformaldehyde and incubated with the primary anti-Cx26 antibody for 1 h at 4°C. Following a 30 min incubation at 4°C in the presence of a secondary antibody, coverslips were mounted onto glass slides and visualized under a Leica DMLS fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Dual-patch clamp recordings
For electrophysiological recordings, cells were grown on thin (no. 0) coverslips and transferred to an experimental chamber mounted on the stage of an upright microscope equipped with an infinity-corrected water-immersion objectives 60×, 0.90 numeric aperture (NA); LUMPlanFl, Olympus, Tokyo, Japan. Cells were constantly superfused with ECS containing (in mm) 150 NaCl, 4 KCl, 1 MgCl2, 5 Hepes, 2 CaCl2, 2 Pyruvate, 5 Glucose, 2 CsCl and 1 BaCl2 (pH 7.4, 330 mOsm). Glass capillaries for patch clamp recordings were formed on a vertical puller (PP-83, Narishige, Japan) from 1.5 mm outer diameter soda glass (Harvard Apparatus, Edenbridge, UK) and filled with an intracellular solution (ICS-1) containing (in mm) 130 KCl, 10 Na–Aspartate, 0.26 CaCl2, 5 TEA–Cl, 1 MgCl2, 5 Hepes, 2 EGTA and 3 ATP–Mg (pH 7.2). Current and voltage were sampled at rates between 1 and 20 kHz. In the whole-cell configuration, the patch electrodes acquired an access resistance of 10–12 MOhm, on average, whereas the input resistance of untransfected HeLa cells was >0.5 GOhm. To measure junctional conductance, each cell of an isolated pair was voltage-clamped independently with one of two List EPC-7 amplifiers and kept at the same holding potential (Vh). By stepping the voltage in one cell (cell 1) while keeping the potential of cell 2 at Vh, thus establishing a transient transjunctional voltage Vj ≡ V1 −V2 = ΔV1, junctional current (Ij) was measured directly as the current change in the unstepped cell (i.e. Ij = −ΔI2). Values of junctional conductance (gj) were calculated by dividing Ij/Vj, corrected for the error due to the access resistance of both pipettes. In the study of the voltage dependence of normalized conductance (Gjss = gjss/gj(0), where gjss is the steady-state conductance measured at the end of a prolonged voltage step and gj(0) is the instantaneous conductance measured at the earliest time resolution of Ij after the imposition of a voltage step), pairs coupled by conductances larger than 10 nS were discarded, thereby limiting the effect of series resistance on these measurements. To block gap junction channels, cells were transiently superfused in ECS saturated with 100% CO2 to produce carbonic acid (H2CO3), which, in its non-dissociated form, is membrane permeable and causes a rapid closure of the gap junction channels. The current flowing through single-gap junction channels was detected as discrete step-like events while the cells recovered from cytoplasm acidification during washout of the CO2.
Intracellular delivery of LY and fluorescence imaging
For dye transfer assays, cell 1 (donor) was patch-clamped using patch pipettes filled with a 4% LY solution in ICS-2, containing (in mm) 60 LiCl and 10 HEPES (adjusted to pH 7.2 with KOH; 320 mOsm). Illumination wavelength was set at 425 nm by a fast switching monochromator (Polychrome IV, TILL Photonics, Martinsried, Germany). At image recording onset, cell 1 was maintained in the cell-attached configuration for a few seconds to establish a baseline. Subsequently, the patch of membrane under the pipette was ruptured, allowing LY to fill the cell while leaving the seal intact (whole-cell recording conditions), and fluorescence emission (F) was selected around 535 nm using a D535/30 m filter (Chroma, Rockingham, VT). Images were formed on a scientific grade CCD camera (SensiCam, PCO Computer Optics, Kelheim, Germany) using either 40× or 60× water-immersion objectives (LumPlan FL, 0.8 and 0.9 NA, respectively; Olympus) on a motorized upright microscope (BX61, Olympus) and displayed as (F − Fbck)/(Fmax −Fbck), where Fmax is the asymptotic maximal value reached in the injected cell and Fbck is background fluorescence. At the end of image recording (3–6 min), cell 2 (acceptor) was contacted by a second pipette containing an identical intracellular solution. The whole-cell configuration was achieved in cell 2 also, and the responses to a sequence of 13 mV voltage pulses, fed to the patch clamp amplifier connected to cell 1, were used to measure junctional conductance. Data were analyzed offline using the Matlab 7.0 software package (The MathWorks, Natick, MA).
Intercellular dye transfer
Glass capillaries were prepared with a dual-step puller (Narishige, Tokyo, Japan) and filled with a 5% solution of LY (dissolved in 0.33 m lithium chloride). Individual cells in GFP-expressing clusters were injected with a pneumatic PLI-100 pico-injector (Medical Systems Corp., Greenvale, NY) and mounted onto a Zeiss-inverted Axiovert 35 TV microscope (Carl Zeiss, Jena, Germany). For both GFP and LY, the filter set was Zeiss 09 (BP450-490/LP520). Fluorescence images were collected through an oil immersion objective (40×, 1.8 NA), captured by a low light level CCD camera (Hamamatsu Photonics, Tokyo, Japan) and fed into a digital image processor developed in the laboratory. Intercellular LY diffusion was revealed by capturing three different images for each experiment: image 1 (GFP), showing GFP-expressing cells before injection; image 2 (GFP + LY), capturing the same field of cells 3–5 min after LY injection and containing the fluorescence of both GFP and LY; image 3 (LY), resulting from the subtraction of image 1 from image 2, thus displaying LY fluorescence alone. Subsequent processing for false color display was performed with Corel Photo-Paint software (Corel Corporation, Ottawa, Canada).
Ca2+ imaging
Transfected HeLa cells grown onto coverslips were incubated in modified Krebs solution (KRH), containing (in mM) 25 Hepes/NaOH buffer, pH 7.4, 125 NaCl, 5 KCl, 1 MgSO4, 1.2 KH2PO4, 2 CaCl2, 10 glucose, supplemented with 0.5% BSA and loaded at room temperature with a mixture (1:2, vol:vol in DMSO) of fura-2/AM (final concentration in KRH = 1 µm) and 20% Pluronic gel (Molecular Probes, Eugene, OR; final concentration in KRH = 0.04%). After 45 min, the loading solution was removed and the cells washed and kept in KRH for 45 min to allow complete de-esterification of the dye. Videomicroscopy and Ca2+ measurements were carried out at room temperature. The fura-2 loaded cultures were observed on an inverted microscope (Zeiss Axiovert 35 TV) with an oil immersion 40× objective (1.8 NA; Carl Zeiss). Cells were excited at wavelengths between 340 and 380 nm with a monochromator device equipped with integrated light source (Polychrome IV, Till Photonics). Excitation light was separated from the light emitted from the sample using a 395 nm dichroic mirror. Images of emitted fluorescence >510 nm were recorded at a frequency of one image per second by a cooled slow-scan interline transfer camera (IMAGO CCD camera, Till Photonics) and simultaneously displayed on a 19 inch Vivitron color monitor. The imaging system was controlled by an integrating imaging software package (TILLvisION, Till Photonics) using a personal computer. Video frames were then digitized, integrated and processed offline to convert fluorescence data into Ca2+ maps by computing a ratio of 340/380 nm excitation wavelength values. Mechanical stimulation of single cells in confluent monolayer was performed by briefly deforming the cell surface with a micropipette. A fire-polished glass micropipette with a tip diameter ~1 µm was positioned over a single cell by a micromanipulator (Narishige). The pipette was briefly deflected downward manually to transiently distort the membrane. Experiments were either discontinued or discarded when there was evidence of damage to the plasma membrane, as revealed by leak of the fluorescent probe from the cell. Results are shown as mean ± SD. Comparisons between two populations of data were made using the Student’s unpaired t-test and P-values of 0.05 or less were considered to be significant.
Generation of the all-atoms model
Our analysis is based on the Cα model of mouse Cx32 reported by Fleishman et al. (38; PDB entry code 1TXH). This model includes the transmembrane (TM) α-helices corresponding to the segments Ala19–Ala40 (TM1), Leu76–Ala96 (TM2), Leu131–Val152 (TM3) and Val189–Val209 (TM4). After sequence alignment using T-COFFEE (67), these fragments corresponded in HCx26 to: Ser19–Glu42 (TM1), Arg75–Ala96 (TM2), Leu132–Val153 (TM3) and Glu187–Leu209 (TM4). Charged residues found immediately next to those segments were also added in order to consider eventual electrostatic interactions. Mapping of the Cα coordinates from the original mouse Cx32 model generated a rough Cα model of HCx26. Subsequently, all the remaining atoms of the amino acids were added using the canonical conformation with the XLEAP module of AMBER8.0 software suite (68). Additionally, all helices were capped with acetyl and N-methyl groups in order to avoid the spurious effects of terminal zwitterionic charges.
This procedure generated an all-atoms model with a large number of steric clashes and internal tensions that were relaxed using energy minimization (EM) and molecular dynamics (MD). Simulations were performed in the presence of implicit solvent with the generalized Born approach (69) as implemented in the SANDER module of AMBER8.0 with a 2 ps time-step. After EM, 50 ps of constrained MD were performed, in which the initial harmonic constraints on the Cα carbons were linearly reduced from 1 to 0 kcal/mol-Å2, followed by 450 ps of unconstrained MD. Simulations were stopped when the systems reached stabilization of the RMSD. A dielectric constant of 78, a cutoff distance of 1.8 nm and a salt concentration of 0.15 m were used. The simulations used the Amber94 force field (70) keeping the temperature at 100 K using Langevin dynamics with a collision frequency of 2 ps−1. The shake algorithm was used to constraint all chemical bonds. This procedure is expected to allow the entire complex to explore the energy landscape more efficiently than a simple EM, obtaining a better relaxation of the protein complex. A similar strategy has already been proven to produce reliable results in other protein–RNA and protein–protein complexes (71,72).
RMSDs were calculated from the last minimization step over the Cα- atoms. The distances between the extremes of the pore-lining TM3 helices were calculated between the centers of mass of the first (last) four Cα atoms of two opposite TM3 helices. The inter-helix angles were calculated between the lines defined by centers of mass of the first and last four Cα carbons of each helix TM3. The distances between OH atoms of Tyr152, corresponding to the maximum constriction of the pore, were calculated between couples of residues in opposite TM3 helices in the connexon. All the distances and angles were averaged over the three possible pairs of opposite helices in the hexamer.
ACKNOWLEDGEMENTS
This work has been funded by grants from Regione Friuli Venezia-Giulia (to P.D’A.), Telethon Italy (GP0043Y02 to F.M. and P.D’A. and GGP05131 to F.M.), the Sixth Research Frame Program of the European Union (FP6 Integrated Project EuroHear, LSHG-CT-20054-512063 to F.M.), Fondazione CARIPARO (to S.P.) and National Institutes of Health (RO1-NS036706 to F.F.B.).
Footnotes
Conflict of Interest statement. None declared.
REFERENCES
- 1.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 2.Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim. Biophys. Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 4.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res. Brain Res. Rev. 2000;32:163–166. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 7.Petit C, Levilliers J, Hardelin JP. Molecular genetics of hearing loss. Annu. Rev. Genet. 2001;35:589–646. doi: 10.1146/annurev.genet.35.102401.091224. [DOI] [PubMed] [Google Scholar]
- 8.Sabag AD, Dagan O, Avraham KB. Connexins in hearing loss: a comprehensive overview. J. Basic Clin. Physiol. Pharmacol. 2005;16:101–116. doi: 10.1515/jbcpp.2005.16.2-3.101. [DOI] [PubMed] [Google Scholar]
- 9.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 10.Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- 11.Nuttall AL. Sound-induced cochlear ischemia/hypoxia as a mechanism of hearing loss. Noise Health. 1999;2:17–32. [PubMed] [Google Scholar]
- 12.Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J. Comp. Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- 13.Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 14.White TW, Deans MR, Kelsell DP, Paul DL. Connexin mutations in deafness. Nature. 1998;394:630–631. doi: 10.1038/29202. [DOI] [PubMed] [Google Scholar]
- 15.Houseman MJ, Ellis LA, Pagnamenta A, Di WL, Rickard S, Osborn AH, Dahl HH, Taylor GR, Bitner-Glindzicz M, Reardon W, et al. Genetic analysis of the connexin-26 M34T variant: identification of genotype M34T/M34T segregating with mild-moderate non-syndromic sensorineural hearing loss. J. Med. Genet. 2001;38:20–25. doi: 10.1136/jmg.38.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, et al. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum. Mol. Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- 17.Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, Smith SD, Kimberling WJ. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am. J. Hum. Genet. 1998;62:792–799. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott DA, Kraft ML, Carmi R, Ramesh A, Elbedour K, Yairi Y, Srisailapathy CR, Rosengren SS, Markham AF, Mueller RF, et al. Identification of mutations in the connexin 26 gene that cause autosomal recessive nonsyndromic hearing loss. Hum. Mutat. 1998;11:387–394. doi: 10.1002/(SICI)1098-1004(1998)11:5<387::AID-HUMU6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Feldmann D, Denoyelle F, Loundon N, Weil D, Garabedian EN, Couderc R, Joannard A, Schmerber S, Delobel B, Leman J, et al. Clinical evidence of the nonpathogenic nature of the M34T variant in the connexin 26 gene. Eur. J. Hum. Genet. 2004;12:279–284. doi: 10.1038/sj.ejhg.5201147. [DOI] [PubMed] [Google Scholar]
- 20.D’Andrea P, Veronesi V, Bicego M, Melchionda S, Zelante L, Di Iorio E, Bruzzone R, Gasparini P. Hearing loss: frequency and functional studies of the most common connexin26 alleles. Biochem. Biophys. Res. Commun. 2002;296:685–691. doi: 10.1016/s0006-291x(02)00891-4. [DOI] [PubMed] [Google Scholar]
- 21.Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum. Mol. Genet. 1999;8:2369–2376. doi: 10.1093/hmg/8.13.2369. [DOI] [PubMed] [Google Scholar]
- 22.Thonnissen E, Rabionet R, Arbones ML, Estivill X, Willecke K, Ott T. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum. Genet. 2002;111:190–197. doi: 10.1007/s00439-002-0750-2. [DOI] [PubMed] [Google Scholar]
- 23.Oshima A, Doi T, Mitsuoka K, Maeda S, Fujiyoshi Y. Roles of Met-34, Cys-64 and Arg-75 in the assembly of human connexin 26. Implication for key amino acid residues for channel formation and function. J. Biol. Chem. 2003;278:1807–1816. doi: 10.1074/jbc.M207713200. [DOI] [PubMed] [Google Scholar]
- 24.Skerrett IM, Di WL, Kasperek EM, Kelsell DP, Nicholson BJ. Aberrant gating, but a normal expression pattern, underlies the recessive phenotype of the deafness mutant Connexin26M34T. FASEB J. 2004;18:860–862. doi: 10.1096/fj.03-0763fje. [DOI] [PubMed] [Google Scholar]
- 25.Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, D’Agruma L, Mansfield E, Rappaport E, Govea N, Mila M, et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–398. doi: 10.1016/S0140-6736(97)11124-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang HL, Chang WT, Li AH, Yeh TH, Wu CY, Chen MS, Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J. Neurochem. 2003;84:735–742. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- 27.Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- 29.Forge A, Marziano NK, Casalotti SO, Becker DL, Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun. Adhes. 2003;10:341–246. doi: 10.1080/cac.10.4-6.341.346. [DOI] [PubMed] [Google Scholar]
- 30.Valiunas V, Manthey D, Vogel R, Willecke K, Weingart R. Biophysical properties of mouse connexin30 gap junction channels studied in transfected human HeLa cells. J. Physiol. 1999;519:631–644. doi: 10.1111/j.1469-7793.1999.0631n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahl E, Manthey D, Chen Y, Schwarz HJ, Chang YS, Lalley PA, Nicholson BJ, Willecke K. Molecular cloning and functional expression of mouse connexin-30, a gap junction gene highly expressed in adult brain and skin. J. Biol. Chem. 1996;271:17903–17910. doi: 10.1074/jbc.271.30.17903. [DOI] [PubMed] [Google Scholar]
- 32.Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D’Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- 33.Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 34.Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D’Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem. Biophys. Res. Commun. 2003;305:1024–1033. doi: 10.1016/s0006-291x(03)00868-4. [DOI] [PubMed] [Google Scholar]
- 35.Allbritton NL, Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993;14:691–697. doi: 10.1016/0143-4160(93)90095-n. [DOI] [PubMed] [Google Scholar]
- 36.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 37.Scemes E, Dermietzel R, Spray DC. Calcium waves between astrocytes from Cx43 knockout mice. Glia. 1998;24:65–73. doi: 10.1002/(sici)1098-1136(199809)24:1<65::aid-glia7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleishman SJ, Unger VM, Yeager M, Ben-Tal N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol. Cell. 2004;15:879–888. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, Kelly T, Collins V, Wilcox LJ, McKinlay Gardner RJ, Kamarinos M, et al. High frequency hearing loss correlated with mutations in the GJB2 gene. Hum. Genet. 2000;106:399–405. doi: 10.1007/s004390000273. [DOI] [PubMed] [Google Scholar]
- 41.Kenneson A, Van Naarden Braun K, Boyle C. GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet. Med. 2002;4:258–274. doi: 10.1097/00125817-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Wu BL, Lindeman N, Lip V, Adams A, Amato RS, Cox G, Irons M, Kenna M, Korf B, Raisen J, et al. Effectiveness of sequencing connexin 26 (GJB2) in cases of familial or sporadic childhood deafness referred for molecular diagnostic testing. Genet. Med. 2002;4:279–288. doi: 10.1097/00125817-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Cucci RA, Prasad S, Kelley PM, Green GE, Storm K, Willcox S, Cohn ES, Van Camp G, Smith RJ. The M34T allele variant of connexin 26. Genet. Test. 2000;4:335–344. doi: 10.1089/109065700750065063. [DOI] [PubMed] [Google Scholar]
- 44.Kenna MA, Wu BL, Cotanche DA, Korf BR, Rehm HL. Connexin 26 studies in patients with sensorineural hearing loss. Arch. Otolaryngol. Head Neck Surg. 2001;127:1037–1042. doi: 10.1001/archotol.127.9.1037. [DOI] [PubMed] [Google Scholar]
- 45.Marlin S, Garabedian EN, Roger G, Moatti L, Matha N, Lewin P, Petit C, Denoyelle F. Connexin 26 gene mutations in congenitally deaf children: pitfalls for genetic counseling. Arch. Otolaryngol. Head Neck Surg. 2001;127:927–933. doi: 10.1001/archotol.127.8.927. [DOI] [PubMed] [Google Scholar]
- 46.Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am. J. Hum. Genet. 2005;77:945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux AF, Pallares-Ruiz N, Vielle A, Faugere V, Templin C, Leprevost D, Artieres F, Lina G, Molinari N, Blanchet P, et al. Molecular epidemiology of DFNB1 deafness in France. BMC Med. Genet. 2004;5:5. doi: 10.1186/1471-2350-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levi H, Tell L, Cohen T. Sensorineural hearing loss in Jewish children born in Jerusalem. Int. J. Pediatr. Otorhinolaryngol. 2004;68:1245–1250. doi: 10.1016/j.ijporl.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Liu XZ, Pandya A, Angeli S, Telischi FF, Arnos KS, Nance WE, Balkany T. Audiological features of GJB2 (connexin 26) deafness. Ear Hear. 2005;26:361–369. doi: 10.1097/00003446-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Cryns K, Orzan E, Murgia A, Huygen PL, Moreno F, del Castillo I, Chamberlin GP, Azaiez H, Prasad S, Cucci RA, et al. A genotype-phenotype correlation for GJB2 (connexin 26) deafness. J. Med. Genet. 2004;41:147–154. doi: 10.1136/jmg.2003.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilch E, Zhu M, Burkhart KB, Regier M, Elfenbein JL, Fisher RA, Friderici KH. Expression of GJB2 and GJB6 is reduced in a novel DFNB1 allele. Am. J. Hum. Genet. 2006;79:174–179. doi: 10.1086/505333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmada M, Schmalisch K, Bohmer C, Schug N, Pfister M, Lang F, Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol. Dis. 2006;22:112–118. doi: 10.1016/j.nbd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 53.White TW, Bruzzone R. Gap junctions: fates worse than death? Curr. Biol. 2000;10:R685–R688. doi: 10.1016/s0960-9822(00)00689-8. [DOI] [PubMed] [Google Scholar]
- 54.Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou XW, Pfahnl A, Werner R, Hudder A, Llanes A, Luebke A, Dahl G. Identification of a pore lining segment in gap junction hemichannels. Biophys. J. 1997;72:1946–1953. doi: 10.1016/S0006-3495(97)78840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J. Gen. Physiol. 2003;122:389–405. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr. Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol. Cell. Endocrinol. 1994;98:173–187. doi: 10.1016/0303-7207(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 59.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 60.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 62.Bruzzone R, Cohen-Salmon M. Hearing the messenger: Ins(1,4,5)P3 and deafness. Nat. Cell Biol. 2005;7:14–16. doi: 10.1038/ncb0105-14. [DOI] [PubMed] [Google Scholar]
- 63.del Castillo I, Villamar M, Moreno-Pelayo MA, del Castillo FJ, Alvarez A, Telleria D, Menendez I, Moreno F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N. Engl. J. Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- 64.GenDeaf Study Group. Mazzoli M, Van Camp G, Newton V, Giarbini M, Declau F, Parving A. Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiological Medicine. 2003;1:148–150. [Google Scholar]
- 65.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 66.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 68.Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Lou R, Merz KM, Wang B, Pearlman DA, et al. AMBER 8.0. Amber 8.0. 2004 [Google Scholar]
- 69.Tsui V, Case DA. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers. 2000;56:275–291. doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 70.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmayer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 1995;117:5179–5197. [Google Scholar]
- 71.Ayala YM, Pantano S, D’Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C. elegans TDP43: nucleic acid binding properties and splicing regulatory function. J. Mol. Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 72.Pantano S, Marcello A, Ferrari A, Gaudiosi D, Sabo A, Pellegrini V, Beltram F, Giacca M, Carloni P. Insights on HIV-1 Tat: P/CAF bromodomain molecular recognition from in vivo experiments and molecular dynamics simulations. Proteins. 2005;62:1062–1073. doi: 10.1002/prot.20805. [DOI] [PubMed] [Google Scholar]