Abstract
The incidence of head and neck cancer continues to increase worldwide, with tobacco exposure and human papillomavirus type 16 infections being the major etiological factors. Current therapeutic options are ineffective in approximately half of the individuals afflicted with this malignancy. Developments in the identification of molecules that sustain head and neck squamous cell carcinoma (HNSCC) growth and survival have made molecular targeting by gene therapy approaches a feasible therapeutic strategy. Although gene therapy was originally designed to correct single gene defects, it has now evolved to encompass all forms of therapeutic interventions involving engineered cells and nucleic acids that modify the overall pattern of gene expression within target tissues. Several preclinical studies and clinical trials have tested the efficacy of targeting specific molecules in patients with HNSCC, using genetic therapy approaches. This review discusses promising preclinical and clinical approaches and new directions for HNSCC gene therapy.
Introduction
Head and neck squamous cell carcinoma (HNSCC) arises in the mucosal lining of the upper aerodigestive tract. It is the tenth most common cancer worldwide, with more than 45,000 new cases reported every year in the United States alone (Jemal et al., 2007; Curado and Hashibe, 2009). Despite advances in conventional therapy including surgery, chemotherapy, and radiation, the 5-year mortality rate of patients with HNSCC has not improved. Genotoxic effects due to alcohol, tobacco, and/or oncogenic human papillomavirus type 16 (HPV16) exposure result in the development of HNSCC (Argiris et al., 2008). Dramatic changes in gene expression patterns resulting in uncontrolled growth combined with the relative accessibility of head and neck tumors to direct inoculation make HNSCC an ideal candidate for somatic gene therapy approaches.
Gene therapy may be defined as the in vivo or ex vivo introduction of nucleic acids that regulate gene expression or convert prodrugs into cytotoxic agents in target tissues, resulting in a therapeutic benefit. Gene therapy represents the use of genetic material for therapeutic purposes. One of the requirements for gene therapy is the safe and effective transfer of the therapeutic gene into the tumor cells. A major advantage of working with HNSCC tumors is that most primary and recurrent tumors are accessible for direct intratumoral injections and tissue biopsy acquisition for the evaluation of biomarkers. Thus, HNSCC is an ideal model for testing the efficacy of gene therapy strategies in a localized area with minimal systemic exposure to the agent. Several clinical trials ranging from the use of viral vectors to transduce genes, immune effectors to control tumor growth, or the direct injection of nucleic acids into the tumor have now been concluded (Karamouzis et al., 2007a). Although some of these strategies are promising, the emergence of new modalities to treat cancer using gene therapy approaches holds immense potential. The gene therapy approaches described here are not inclusive but represent those in advanced preclinical development or in clinical trial testing in HNSCC. The most critical step in gene therapy is the efficient delivery of the therapeutic gene into target tissues.
Vectors Used for Gene Transfer
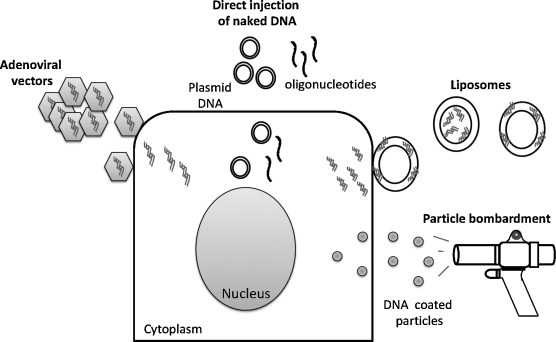
Efficient transfer and stable transgene expression in target tissues represent one of the most crucial aspects of gene therapy. Several methods of gene transfer have been developed (Xi and Grandis, 2003). These may be divided into two broad categories: (1) direct gene transfer into cells and (2) indirect methods including ex vivo modification and reimplantation of engineered cells. Implantation of engineered cells into patients is a complex process that includes risk of infection and difficulty in isolating cells, engineering them, and reimplanting them back into the patient. It is labor intensive and may require long periods of time, thus restricting use of these methods of gene transfer. A number of chemical, physical, and biological approaches have been used to introduce exogenous nucleic acids directly into mammalian cells in vivo. Ideally, vectors should protect the nucleic acids from degradation by serum enzymes, be able to easily traverse the tumor cell membrane, and transport the genetic material intact inside cells. A few selected methods employed for gene transfer in HNSCC preclinical models and clinical trials are discussed briefly below.
Direct injection of naked DNA
Although the efficiency of transfection is low, DNA can be directly injected into tumor tissue (Fig. 1). Inside cells, plasmid DNA exists mainly as extrachromosomal entities or may integrate at low levels in the genomic DNA (Wang et al., 2004). Early studies using direct injection of plasmid DNA in the muscle of mice demonstrated expression of the transgene (Wolff et al., 1990). Antisense oligonucleotides that are complementary to mRNA or genomic DNA abrogate protein synthesis by specifically inhibiting transcription or translation of the target mRNA. Preclinical studies using phosphorothioate (PTO)-modified antisense oligonucleotides or plasmid vectors engineered to express antisense DNA have demonstrated antitumor effects on direct injection of naked DNA into xenograft HNSCC tumors (Niwa et al., 2003; Thomas et al., 2008). Intraperitoneal delivery of PTO-modified antisense oligonucleotides demonstrated moderate antitumor effects in HNSCC xenograft tumors (Thomas et al., 2008). Antisense oligonucleotides have many advantages in that they are cost effective, can be synthesized rapidly, they are highly sequence specific, and can be modified to increase serum stability (Sahu et al., 2007). Modifications of oligonucleotides add to the cost of synthesis and may also affect binding to the target or activation of RNase H, an enzyme crucial for degradation of target mRNA (Baker et al., 1997). Intratumoral injections can be technically challenging and need to be a carried out by a trained individual, generally the surgeon. Intratumoral inoculations can be facilitated by ultrasound-guided injections of DNA that help identify necrotic regions and blood vessels that are best avoided while inoculating HNSCC tumors (Michaluart et al., 2008). The requirement for intratumoral injection limits the number of lesions that can be treated to those that can be easily accessed. To facilitate targeting of metastatic disease as well as less accessible lesions, DNA-based agents need to be resistant to serum nucleases and engineered to be targeted to tumor tissue on systemic administration. One method to improve serum stability is to encapsulate the DNA in a lipid bilayer.
FIG. 1.
Selected Gene Therapy Clinical Trials in Head and Neck Squamous Cell Carcinoma
Cationic liposomes
To increase the transfection efficiency and to protect nucleic acid-based agents from serum nuclease degradation, encapsulation of DNA in liposomal formulations has been examined. Liposomes are composed of a spherical lipid bilayer surrounding an aqueous core (Fig. 1). Nucleic acids are either encapsulated or incorporated into the lipid bilayer of cationic liposomes, protecting them from degradation (Crook et al., 1996). Liposomes between 100 and 400 nm in diameter can escape the leaky vasculature present in most HNSCC tumors and fuse with the plasma membrane of tumor cells (Nagayasu et al., 1999). Blood vessels supplying normal tissue retain the liposomes, preventing gene transfer to normal tissue. The stability, persistence, and distribution of plasmid DNA complexed with 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol) liposomes was determined in athymic nude mice bearing xenograft HNSCC tumors (Thomas et al., 2003). Plasmid DNA persisted in various tissues, including the injection site, for 1 month. However, a highly sensitive polymerase chain reaction (PCR)-based method revealed the presence of plasmid DNA in other organs as well. To facilitate specific delivery to HNSCC tumors, the inclusion of tumor-targeting peptides into the lipid bilayer has been explored (Rezler et al., 2007). Because HNSCC tumors express high levels of the transferrin receptor, higher uptake of lipid complexes containing transferrin were observed in HNSCC xenograft tumors (Neves et al., 2006). Liposomes have been used clinically and are nontoxic when administered at low concentrations. Cationic liposomes have been used to deliver several genes including those encoding HLA-B7, interleukin (IL)-2, and E1A (Gleich et al., 1998; Wollenberg et al., 1999; Yoo et al., 2001). Unlike some viral vectors, liposomes do not facilitate the integration of plasmid DNA into genomic DNA, necessitating retreatment. Strategies requiring permanent integration of the gene therapy agent into the cell typically employ viral vectors for gene transfer.
Viral vectors
Viruses are designed to enter cells and express their genes (Fig. 1). Replication-deficient viruses are incapable of propagating in vivo and can be engineered to carry genes of interest into cells. Retroviruses and adenoviruses are the two main classes of viruses being tested as gene delivery vectors (Kay et al., 2001). Retroviruses typically integrate the genetic material into the host genome whereas adenoviral vectors deposit the material in an episomal manner. Another important consideration is that retroviruses infect only actively dividing cells. Compared with retroviruses, adenoviruses are relatively safe and hence have been used most often in HNSCC clinical trials (Vorburger and Hunt, 2002). Adenoviruses are DNA viruses that infect both dividing and nondividing cells. Although they do not integrate the genetic material into the host genome, avoiding potential problems inherent in random integration, adenoviruses produce transient, although high, levels of the transgene. A disadvantage of adenoviral vectors is that they trigger cellular and humoral immune responses resulting in the generation of neutralizing antibodies that reduce the effectiveness of the vector when readministered to the patient (Yang et al., 1996; Kafri et al., 1998; Ikeda et al., 1999).
Particle bombardment
Particle bombardment uses a gun with a microprojectile containing 1-μm-diameter tungsten or gold particles coated with a thin layer of DNA to force gene delivery into target tissues (Fig. 1). An explosive charge propels the microprojectile toward the tissue; as the projectile slams into a retaining plate, the tungsten or gold particles are propelled through the aperture and hit the tissue with force. Examination of the tissue reveals particles embedded in the cytoplasm (Williams et al., 1991). The gene gun approach successfully conferred systemic antitumor immunity to oral melanoma in a hamster cheek pouch model (Trimble et al., 2003). Although expression of the transgene was detected for 5 days, the number of transduced cells was rare and superficial with no significant antitumor effects (Shillitoe et al., 1998). There are currently no clinical trials in HNSCC using this method of gene transfer.
Gene Therapy Strategies
The first cancer gene therapy protocol was submitted in 1993 (Oldfield et al., 1993). To date there are more than 1000 cancer gene therapy and at least 12 HNSCC clinical protocols listed in the Clinicaltrials.gov registry. Gene therapy strategies tested for antitumor efficacy in HNSCC preclinical models and in clinical trials are discussed in the sections below.
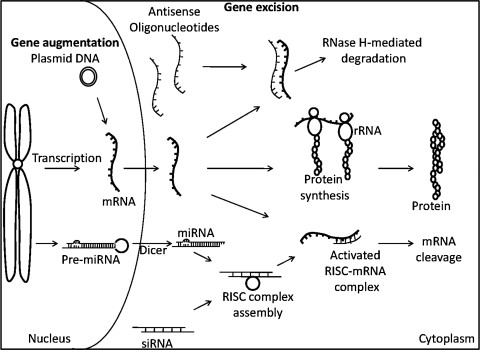
Gene augmentation therapy
Gene addition or augmentation therapy restores the normal function within cells that have low or no expression of a particular gene (Fig. 2). One of the most commonly mutated tumor suppressor genes in human cancer is p53, which plays an important role in regulation of the cell cycle (Lane, 1992). The normal function of p53 involves recognition and response to DNA damage induced by radiation and cytotoxic agents, inducing apoptotic cell death (Levine, 1997). More than 50% of tumors express mutant forms of p53 (Hollstein et al., 1991). In addition, HNSCC tumors with wild-type p53 have other mechanisms to inactivate the enzyme, resulting in resistance to apoptosis. Replacing the mutant protein with a functional wild-type p53 would increase programmed cell death in HNSCC. INGN 201 (Ad5CMV-p53, Advexin; Introgen Therapeutics/Vivante GMP Solutions, Houston, TX), a replication-impaired adenoviral vector encoding the wild-type p53 gene under the control of a cytomegalovirus promoter, has been evaluated in preclinical and clinical trials for treatment of HNSCC (Zhang et al., 1994; Gabrilovich, 2006). A phase III trial comparing survival of HNSCC patients treated with INGN 201 with those treated with methotrexate is currently underway. Preliminary results from 63 patients with recurrent HNSCC treated with INGN 201 demonstrate a significant increase in survival of patients with low levels of mutated or inactivated p53, compared with patients with high levels of mutated p53 (p < 0.0001) (Sobol et al., 2008). Patients with high levels of mutated p53 did not respond to treatment. Overall there was no significant difference in survival between methotrexate-treated patients and INGN 201-treated patients (p = 0.5492). The expression of p53-inhibitory proteins HDM2 and HDM4 was reported to be high in 93% of patients expressing wild-type p53, accounting for the response to INGN 201 in this subset of patients (Sobol et al., 2008). On the basis of the preliminary results from this study, augmenting p53 in HNSCC tumors may have antitumor effects.
FIG. 2.
Selected Gene Therapy Clinical Trials in Head and Neck Squamous Cell Carcinoma
Tumors depend on angiogenesis for growth, invasion, and metastasis. Prevention of neovascularization can negatively impact tumor progression. Endostatin is a 20-kDa protein that inhibits angiogenesis and tumor growth (O'Reilly et al., 1997). Although recombinant endostatin is well tolerated, it has a short half-life and repeated dosing is required to achieve antitumor effects (Folkman, 2006). The therapeutic efficacy of an adenoviral expression vector (E10A) engineered to express high levels of endostatin in transduced cells has been tested in HNSCC patients (Lin et al., 2007; Li et al., 2008). Fifteen patients with solid tumors, including 2 patients with HNSCC, were treated with intratumoral injections of up to 1012 viral particles on days 1 and 8 (Lin et al., 2007). There were no dose-limiting toxicities. Although indications of antitumor effects were observed, with 1 of 15 patients experiencing a minor response and 12 with stable disease, a larger phase II/III trial is necessary to determine the therapeutic efficacy of E10A adenovirus.
Gene excision therapy
Another approach to inhibit tumor growth is to inhibit oncogenes or molecules that tumor cells depend on for survival. Transcription of mRNA can be hindered by the binding of antisense DNA, resulting in steric hindrance and degradation of the target mRNA by RNase H (Fig. 2). The epidermal growth factor receptor (EGFR) is highly upregulated in more than 90% of HNSCC patients (Rubin Grandis et al., 1998). Ligand binding to the receptor triggers downstream signaling pathways that mediate HNSCC growth and survival (Kalyankrishna and Grandis, 2006). In 2006, an EGFR-specific antibody, cetuximab, was reported to improve HNSCC patient survival when combined with radiation compared with radiation treatment alone (Bonner et al., 2006). Although cetuximab is now approved by the U.S. Food and Drug Administration (FDA) for the treatment of HNSCC patients, as a monotherapy the response rate is less than 10% (Karamouzis et al., 2007b). Antisense approaches demonstrate better antitumor effects compared with inhibitors that prevent EGFR phosphorylation (Grandis et al., 1997). Plasmid DNA encoding an EGFR antisense fragment was injected intratumorally into HNSCC xenografts, resulting in antitumor effects (He et al., 1998). On the basis of these studies a dose escalation phase I clinical trial was carried out with intratumoral administration of the EGFR antisense plasmid DNA (Lai et al., 2009). No dose-limiting toxicity was observed at any of the doses. Results from the phase I trial demonstrated an ∼29% response in HNSCC patients. Further, response to treatment correlated with EGFR levels. EGFR protein levels can also be altered by small interfering RNA (siRNA) and by regulating microRNA (Fig. 2).
Preclinical studies using siRNA expression plasmid vectors targeting human S-phase kinase-associated protein (Skp) demonstrated antitumor effects in HNSCC xenografts (Fang et al., 2008). Antitumor effects were reported in xenograft HNSCC tumors transduced with retroviral vectors expressing urokinase plasminogen activator receptor (uPAR) siRNA (Zhou et al., 2009). More recent developments have identified small noncoding RNAs, called microRNAs (miRNAs), involved in posttranscriptional regulation (Bushati and Cohen, 2007). Each miRNA regulates hundreds of mRNA targets (Lewis et al., 2005). Tumor-suppressive miRNAs are reported to be downregulated by DNA hypermethylation in HNSCC (Kozaki et al., 2008). Treatment of cells with 5-aza-2-deoxycytidine restored expression of miR-137 and miR-193a. Further, transfection of HNSCC with miR-137 and miR-193a reduced growth in all HNSCC cell lines. Suppression of HNSCC cell invasion and induction of apoptosis were reported on transfection with miR-138 (Liu et al., 2009). Targeting HNSCC by siRNA and miRNA approaches is still at its infancy and has not yet been tested in the clinical setting. Another genetic approach to targeting tumor cells is to introduce a gene-directed enzyme that would convert a prodrug into a cytotoxic agent within the tumor cells.
Prodrug activation gene therapy
Although more than 20 strategies for suicide gene therapy have been developed, few strategies have undergone preclinical evaluation in HNSCC (Coukos and Rubin, 2001; Portsmouth et al., 2007). Thus far there have been no clinical trials conducted with suicide gene therapy in HNSCC. The most widely tested strategy in HNSCC involves the herpes simplex virus thymidine kinase (HSV-TK) gene expressing an enzyme that transforms the prodrug ganciclovir into a cytotoxic substance triggering cell death of host cells by interfering with DNA synthesis. In addition, neighboring cells acquire the cytotoxin via gap junctions or by phagocytosis of vesicles generated from the dying cells, resulting in cell death due to a bystander effect (Freeman et al., 1993). A drawback of this strategy is that it does not affect tumor cells that are quiescent. Transfer of the HSV-TK gene into HNSCC tumors has been demonstrated with replication-deficient adenoviral vectors (O'Malley et al., 1996; Kothari et al., 2009). Antitumor effects in HNSCC xenograft tumors induced by the bystander effect have been reported on intratumoral inoculation of cells engineered ex vivo to express HSV-TK (Thomas et al., 1998). More recently, nanoparticle-mediated HSV-TK gene therapy has demonstrated antitumor effects in HNSCC (Yu et al., 2008). Lipid-mediated transduction in HNSCC xenografts has also been used for HSV-TK gene transfer (Neves et al., 2009). Strategies combining IL-2 or the histone deacetylase (HDAC) inhibitor valproic acid with HSV-TK/ganciclovir gene therapy have been reported to be effective against HNSCC xenograft tumor growth (O'Malley et al., 1996; Kothari et al., 2009). Regression of contralateral tumors has been reported in animals being treated intratumorally by HSV-TK gene therapy, suggesting the recruitment of immune cells that are then activated by the dying tumor cells (Bi et al., 1997). Thus expression of a strong exogenous antigen such as HSV-TK in the tumor can recruit immune cells to the region. Unmasking of weak tumor antigens as a result of dying tumor cells sensitizes immune cells to the tumor, producing a systemic antitumor response (Plautz et al., 1993; Vile et al., 1994; Gagandeep et al., 1996).
Immune-modulatory gene therapy
Cancer immunotherapy sensitizes the patient's immune cells to control tumor growth. It is well established that HNSCC patients are deficient in several immune cell types including natural killer (NK) and lymphokine-activated killer (LAK) cells (Whiteside, 2005). Reduced dendritic cell function has also been reported in HNSCC patients (Tas et al., 1993). In addition, HNSCC tumors express immune inhibitory cytokines including transforming growth factor (TGF)-β and IL-10 (Wanebo et al., 1993). Thus, HNSCC cells are capable of evading immune recognition and suppress immune cell function. Boosting immune recognition and function in HNSCC patients may result in tumor ablation (Whiteside, 2007). Antitumor activity has been reported in preclinical models of HNSCC treated by local expression of IL-2 (Sacchi et al., 1990; Rabinowich et al., 1992). Because HNSCC tumors have lower levels of class I major histocompatibility complex (MHC), the cells are less immunogenic because of poor antigen presentation to cytotoxic T lymphocytes (CTLs) (Ferris, 2004). Expression of MHC-I molecules has been reported to reverse the oncogenesis of transformed cells (Tanaka et al., 1985). Further, rejection of leukemic cells has been reported after gene transfer of MHC (Hui et al., 1984). On the basis of these studies an MHC alloantigen gene therapy strategy was developed and tested in preclinical models and in patients with HNSCC (Gleich et al., 1998, 2001, 2003). Allovectin-7 (Vical, San Diego, CA) is plasmid DNA formulation that expresses the allo-MHC class I molecule HLA-B7 heavy chain and β2-microglobulin required for stabilization of HLA-B7. Patients with recurrent HNSCC were treated with direct intratumoral injections of the agent in order to elicit an inflammatory antitumor response. In an initial phase I trial, nine patients with advanced HNSCC were administered four doses of Allovectin-7 (10 μg/dose). No significant toxicities were reported and four patients experienced a partial response, with a gradual reduction in tumor size. Two multicenter phase II studies involving a low dose (10 μg) and a high dose (100 μg) of Allovectin-7 were carried out in patients with recurrent HNSCC (Table 1) (Gleich et al., 2001; Galanis, 2002). Of 60 patients treated, one cycle of treatment resulted in a partial response rate of 10% and a stable disease rate of 23% in patients with advanced, refractory HNSCC. Twenty responders underwent a second treatment cycle. Of the 20 patients, 1 had a complete response, 4 had partial responses, and 6 had stable disease.
Table 1.
Selected Gene Therapy Clinical Trials in Head and Neck Squamous Cell Carcinoma
| Vector | Agent | Phase | HNSCC | No. of HNSCC patients | Response | Toxicities | Ref. |
|---|---|---|---|---|---|---|---|
| Liposome | E1A DC-Chol | II | Recurrent | 24 | 1-CR, 2-PR, 7-SD | No significant toxicities | Villaret et al. (2002) |
| Allovectin-7: HLA-B7 DNA and DMRIE/DOPE | II | Recurrent | 60 | 6-PR, 14-SD | No toxic effects | Gleich et al. (2001) | |
| Viral vectors | ONYX-015 | II | Recurrent | 30 (standard dose) 10 (hyperfractionated dose) |
2-CR, 2-PR, 10-SD 1-CR, 3-SD |
Fever and pain at injection site | Nemunaitis et al. (2001b) |
| H101 plus 5-FU and cisplatin | II | End stage | 18 | 4-CR + PR | Fever, pain at injected site, hepatic dysfunction, and hematological toxicities | Lu et al. (2004) | |
| ONYX-015 plus cisplatin and 5-FU | II | Recurrent | 14 | 3-CR, 3-PR, 2-SD | Nausea, vomiting, mucositis, pain at injection site, constipation, and fatigue | Lamont et al. (2000) | |
| II | Recurrent | 37 | In 30 evaluable patients: 8-CR, 11-PR | Pain at injection site, flulike symptoms, kidney failure, and anorexia | Khuri et al. (2000) | ||
| Naked DNA | EGFR antisense plasmid DNA | I | Advanced | 17 | 2-CR, 3-PR, 2-SD | No dose-limiting toxicities | Lai et al. (2009) |
| DNA-hsp65 | I | III/IV | 21 | 4-PR | Moderate pain, edema, and infections | Michaluart et al. (2008) |
Abbreviations: CR, complete response; DC-Chol, 3β-[N-(N′, N′-dimethylaminoethane)-carbomoyl] cholesterol; DMRIE/DOPE, 1,2-dimyristyloxypropyl-3-dimethylhydroxyethyl ammonium bromide/dioleoyl-phosphatidylethanolamine; EGFR, epidermal growth factor receptor; 5-FU, 5-fluorouracil; HNSCC, head and neck squamous cell carcinoma; PR, partial response; SD, stable disease.
Oncolytic viruses
Although oncolytic viruses do not modulate gene expression in cancer cells, these viruses are being developed as gene delivery vectors (Nemunaitis et al., 2001a). There are two key issues that need to be addressed in order to maximize the antitumor effects of oncolytic virus, namely, oncolytic efficiency and tumor specificity. Overexpression of the adenovirus death protein (ADP) has been reported to enhance viral spread and the efficacy of tumor cell lysis (Doronin et al., 2000). ADP is a membrane glycoprotein required at late stages of infection for efficient cell lysis and release of viral particles from cells. Several strategies have been tested to enhance tumor-specific targeting with oncolytic viruses (Dobbelstein, 2004). Promoters that are specifically active in tumor cells can be introduced into the viral genome to regulate the expression of essential viral genes necessary for viral replication. These engineered viral particles will preferentially replicate in tumor and not in normal tissues. Cyclooxygenase-2 (COX-2), primarily responsible for prostaglandin production, is virtually undetectable in most tissues under physiological conditions but is overexpressed in several cancers including HNSCC (Chan et al., 1999; Komhoff et al., 2000; Half et al., 2002). A COX-2 promoter-driven replication-selective adenoviral vector, Ad-COX2-E1a, demonstrated antitumor effects against COX-2-expressing HNSCC in vitro and in vivo (Tanaka et al., 2005; Nakagawa et al., 2009).
Adenoviral particles that selectively replicate within tumor cells, resulting in cell lysis, have been tested in clinical trials for HNSCC and premalignant lesions (Alemany, 2007). The most widely tested oncolytic virus in HNSCC is ONYX-015, which is deficient in E1B (55-kDa) protein. The E1B protein was originally believed to be required for inactivation of p53 in normal cells, allowing the virus to selectively replicate in tumor cells while normal cells expressing wild-type p53 remained unaffected. However, it was demonstrated that normal cells have a defective late viral RNA export mechanism that restricts the replication of ONYX-015 within the cells (O'Shea et al., 2004). Tumor cells are permissive to the transport of the late RNA viral proteins, allowing viral replication and consequent cell death. Initial phase I trials included dose escalation studies in HNSCC patients with poor prognosis and easily accessible tumors (Ganly et al., 2000). Replication of the virus was demonstrated on day 8 postinjection with evidence of clinical response in 5 of 22 patients. The treatment was well tolerated with only mild flulike symptoms. In a phase II trial ONYX-015 was administered at two doses with a standard dose of 2 × 1011 particles for five consecutive days or twice daily for 2 weeks (hyperfractionated) in patients with recurrent HNSCC (Nemunaitis et al., 2001b) (Table 1). Tumor regression was observed in 14 and 10% of patients treated with standard and hyperfractionated doses, respectively. In addition, 40–60% of the patients demonstrated stable disease. Adenoviral injections induced mild to moderate fever in 67% of cases. Further, 47% of patients experienced pain at the injection site. Transient viremia was reported in 41% of patients 5–6 days after treatment initiation. Although the treatment was well tolerated, only modest clinical responses were achieved. ONYX-015 mouthwash was examined in the treatment of premalignant dysplasia (Rudin et al., 2003). Histologic resolution of dysplasia was reported in 37% of the (7 of 19) patients. However, the responses were transient and a larger sample size is needed to determine the effectiveness of ONYX-015 mouthwash in the treatment of premalignant oral dysplasia. Higher response rates were seen in patients treated with a combination of ONYX-015 and chemotherapy (Khuri et al., 2000) (Table 1). Limited response to ONYX-015 may be due to several reasons including poor distribution of the injected virus and the high level of fibrotic tissue, particularly in patients treated with radiation (McCormick, 2001). Attempts to improve the antitumor efficacy by combining ONYX-015 with chemotherapy have yielded favorable results (Khuri et al., 2000; Lamont et al., 2000).
A modified version of ONYX-015, called H101 and developed by Sunway Biotech (Shanghai, China), was tested in patients with advanced cancers including HNSCC (Lu et al., 2004). Results from a phase II trial demonstrated a 30.4% response rate in 46 patients administered intratumoral H101 in combination with chemotherapy. Of 15 patients with HNSCC evaluated, 4 demonstrated a response to the combination (Table 1). The treatment was well tolerated with some side effects including fever and pain at the injection site. In November 2005, H101 was approved by the Chinese State Food and Drug Administration for cancer treatment (Jia and Kling, 2006). Although these are encouraging reports, further studies are required to enhance the potency of oncolytic viruses. In addition, techniques that facilitate systemic administration of viral particles in order to target metastatic tumors and tumors that are inaccessible to intratumoral injections are necessary.
Transcription factor decoys
Activated transcription factors regulate gene expression by binding to specific sequences in the promoter region. DNA mimetics with sequences homologous to specific transcription factor binding sites can bind to activated transcription factors, sequestering them and preventing the transcription of molecules that are required for tumor cell growth and survival. Transcription factor decoys have been used in preclinical models and in clinical trials for several tumor types. In HNSCC, preclinical studies have demonstrated that a transcription factor decoy that binds to the signal transducer and activator of transcription-3 (STAT3) results in cell death in vitro and reduced xenograft tumor growth compared with control tumors (Leong et al., 2003). Transfer of the double-stranded, short DNA-based STAT3 decoy can be achieved by intratumoral administration in HNSCC. Modifications of the decoy will be required to enhance resistance to serum nucleases and proteases and enable systemic delivery.
Combining gene therapy with conventional therapies
Combining gene therapy strategies with chemotherapy or radiation treatment has been reported to confer enhanced antitumor effects in preclinical studies. Addition of docetaxel to intratumorally or systemically delivered EGFR antisense gene therapy resulted in significant reduction in HNSCC xenograft growth in vivo (Niwa et al., 2003; Thomas et al., 2008). Partial surgical resection of HNSCC xenograft tumors followed by administration of a human IL-2 plasmid formulation resulted in significant antitumor efficacy (Li et al., 1999). A significant problem in the radiotherapy of HNSCC tumors involves local failure and toxicity to adjacent critical structures. To increase the radiosensitivity of HNSCC tumors, a gene therapy approach was adopted (Rhee et al., 2007). Adenoviral particles encoding the Nijmegen breakage syndrome (NBS1) protein were used to transduce HNSCC cells. Expression of the full-length NBS1 protein resulted in enhanced sensitivity of HNSCC xenograft tumors to ionizing radiation. Combining chemoradiation and gene therapy has also been tested in preclinical models. When transduction of HNSCC xenografts with a replication-competent adenoviral vector encoding tissue inhibitor of matrixmetalloprotease-2 (TIMP-2) was combined with cisplatin treatment and radiotherapy, a significant additive antitumor effect was obtained compared with treatment with any single agent alone (McNally et al., 2009). These studies demonstrate the benefit of combining gene therapy with conventional therapies for the management of HNSCC.
Future Considerations
Since the first approved clinical gene therapy trial, when a 4-year-old girl was administered autologous T cells engineered to express the adenosine deaminase gene as a therapeutic agent for severe combined immunodeficiency, a wide array of gene therapy strategies has been developed (Blaese et al., 1995). In this review several gene therapy approaches that modify gene expression impacting survival and immune surveillance of HNSCC tumors have been outlined. Although targeting single genes has demonstrated antitumor effects in HNSCC, approaches combining gene therapy with chemotherapy, radiotherapy, or surgery seem to have enhanced tumor-ablative effects (O'Malley et al., 1996; Li et al., 1999; Rhee et al., 2007). Results from clinical trials have demonstrated that cancer gene therapy is well tolerated and effective in subsets of HNSCC patients. Despite our increased understanding of molecules that play important roles in cancer biology, moderate responses have been observed in cancer gene therapy trials (Gottesman, 2003). Further improvements in the safety and efficacy of gene delivery systems will improve clinical responses to gene therapy interventions. Increased understanding of the potential molecular targets in HNSCC will facilitate a more rational selection of gene therapy strategies that may improve clinical outcome.
Acknowledgments
This work was supported by a developmental research grant from P50 CA097190 (to S.M.T.), a pilot project grant from 5P50 CA090440-08S1 (to S.M.T.), grants RO1 CA77308, RO1 CA101840, P50 CA097190 and an American Cancer Society Clinical Research professorship CRP-08-229-01 (to J.R.G.).
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Alemany R. Cancer selective adenoviruses. Mol. Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Argiris A. Karamouzis M.V. Raben D. Ferris R.L. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B.F. Lot S.S. Condon T.P. Cheng-Flournoy S. Lesnik E.A. Sasmor H.M. Bennett C.F. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- Bi W. Kim Y.G. Feliciano E.S. Pavelic L. Wilson K.M. Pavelic Z.P. Stambrook P.J. An HSVtk-mediated local and distant antitumor bystander effect in tumors of head and neck origin in athymic mice. Cancer Gene Ther. 1997;4:246–252. [PubMed] [Google Scholar]
- Blaese R.M. Culver K.W. Miller A.D. Carter C.S. Fleisher T. Clerici M. Shearer G. Chang L. Chiang Y. Tolstoshev P. Greenblatt J.J. Rosenberg S.A. Klein H. Berger M. Mullen C.A. Ramsey W.J. Muul L. Morgan R.A. Anderson W.F. T lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Bonner J.A. Harari P.M. Giralt J. Azarnia N. Shin D.M. Cohen R.B. Jones C.U. Sur R. Raben D. Jassem J. Ove R. Kies M.S. Baselga J. Youssoufian H. Amellal N. Rowinsky E.K. Ang K.K. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Bushati N. Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Chan G. Boyle J.O. Yang E.K. Zhang F. Sacks P.G. Shah J.P. Edelstein D. Soslow R.A. Koki A.T. Woerner B.M. Masferrer J.L. Dannenberg A.J. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- Coukos G. Rubin S.C. Gene therapy for ovarian cancer. Oncology. 2001;15:1197–1204. 1207. discussion 1207–1198. [PubMed] [Google Scholar]
- Crook K. Mclachlan G. Stevenson B.J. Porteous D.J. Plasmid DNA molecules complexed with cationic liposomes are protected from degradation by nucleases and shearing by aerosolisation. Gene Ther. 1996;3:834–839. [PubMed] [Google Scholar]
- Curado M.P. Hashibe M. Recent changes in the epidemiology of head and neck cancer. Curr. Opin. Oncol. 2009;21:194–200. doi: 10.1097/CCO.0b013e32832a68ca. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M. Replicating adenoviruses in cancer therapy. Curr. Top. Microbiol. Immunol. 2004;273:291–334. doi: 10.1007/978-3-662-05599-1_9. [DOI] [PubMed] [Google Scholar]
- Doronin K. Toth K. Kuppuswamy M. Ward P. Tollefson A.E. Wold W.S. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol. 2000;74:6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L. Hu Q. Hua Z. Li S. Dong W. Growth inhibition of a tongue squamous cell carcinoma cell line (Tca8113) in vitro and in vivo via siRNA-mediated down-regulation of skp2. Int. J. Oral Maxillofac. Surg. 2008;37:847–852. doi: 10.1016/j.ijom.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Ferris R.L. Progress in head and neck cancer immunotherapy: Can tolerance and immune suppression be reversed? ORL J. Otorhinolaryngol. Relat. Spec. 2004;66:332–340. doi: 10.1159/000081891. [DOI] [PubMed] [Google Scholar]
- Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp. Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Freeman S.M. Abboud C.N. Whartenby K.A. Packman C.H. Koeplin D.S. Moolten F.L. Abraham G.N. The “bystander effect”: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- Gabrilovich D.I. INGN 201 (Advexin): Adenoviral p53 gene therapy for cancer. Expert Opin. Biol. Ther. 2006;6:823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- Gagandeep S. Brew R. Green B. Christmas S.E. Klatzmann D. Poston G.J. Kinsella A.R. Prodrug-activated gene therapy: Involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- Galanis E. Technology evaluation: Allovectin-7, Vical. Curr. Opin. Mol. Ther. 2002;4:80–87. [PubMed] [Google Scholar]
- Ganly I. Kirn D. Eckhardt G. Rodriguez G.I. Soutar D.S. Otto R. Robertson A.G. Park O. Gulley M.L. Heise C. Von Hoff D.D. Kaye S.B. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- Gleich L.L. Gluckman J.L. Armstrong S. Biddinger P.W. Miller M.A. Balakrishnan K. Wilson K.M. Saavedra H.I. Stambrook P.J. Alloantigen gene therapy for squamous cell carcinoma of the head and neck: Results of a phase-1 trial. Arch. Otolaryngol. Head Neck Surg. 1998;124:1097–1104. doi: 10.1001/archotol.124.10.1097. [DOI] [PubMed] [Google Scholar]
- Gleich L.L. Gluckman J.L. Nemunaitis J. Suen J.Y. Hanna E. Wolf G.T. Coltrera M.D. Villaret D.B. Wagman L. Castro D. Gapany M. Carroll W. Gillespie D. Selk L.M. Clinical experience with HLA-B7 plasmid DNA/lipid complex in advanced squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 2001;127:775–779. [PubMed] [Google Scholar]
- Gleich L.L. Li Y.Q. Li S. Gluckman J.L. Stambrook P.J. Alloantigen gene therapy for head and neck cancer: Evaluation of animal models. Head Neck. 2003;25:274–279. doi: 10.1002/hed.10258. [DOI] [PubMed] [Google Scholar]
- Gottesman M.M. Cancer gene therapy: An awkward adolescence. Cancer Gene Ther. 2003;10:501–508. doi: 10.1038/sj.cgt.7700602. [DOI] [PubMed] [Google Scholar]
- Grandis J.R. Chakraborty A. Melhem M.F. Zeng Q. Tweardy D.J. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene. 1997;15:409–416. doi: 10.1038/sj.onc.1201188. [DOI] [PubMed] [Google Scholar]
- Half E. Tang X.M. Gwyn K. Sahin A. Wathen K. Sinicrope F.A. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- He Y. Zeng Q. Drenning S.D. Melhern M.F. Tweardy D.J. Huang L. Grandis J. EGFR antisense RNA transcribed from the U6 promoter inhibits human squamous cell carcinoma growth in vivo. J. Natl. Cancer Inst. 1998;90:1080–1087. doi: 10.1093/jnci/90.14.1080. [DOI] [PubMed] [Google Scholar]
- Hollstein M. Sidransky D. Vogelstein B. Harris C.C. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hui K. Grosveld F. Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984;311:750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Ichikawa T. Wakimoto H. Silver J.S. Deisboeck T.S. Finkelstein D. Harsh G.R.T. Louis D.N. Bartus R.T. Hochberg F.H. Chiocca E.A. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Ward E. Murray T. Xu J. Thun M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jia H. Kling J. China offers alternative gateway for experimental drugs. Nat. Biotechnol. 2006;24:117–118. doi: 10.1038/nbt0206-117. [DOI] [PubMed] [Google Scholar]
- Kafri T. Morgan D. Krahl T. Sarvetnick N. Sherman L. Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: Implications for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyankrishna S. Grandis J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- Karamouzis M.V. Argiris A. Grandis J.R. Clinical applications of gene therapy in head and neck cancer. Curr. Gene Ther. 2007a;7:446–457. doi: 10.2174/156652307782793487. [DOI] [PubMed] [Google Scholar]
- Karamouzis M.V. Grandis J.R. Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007b;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- Kay M.A. Glorioso J.C. Naldini L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Khuri F.R. Nemunaitis J. Ganly I. Arseneau J. Tannock I.F. Romel L. Gore M. Ironside J. Macdougall R.H. Heise C. Randlev B. Gillenwater A.M. Bruso P. Kaye S.B. Hong W.K. Kirn D.H. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Komhoff M. Guan Y. Shappell H.W. Davis L. Jack G. Shyr Y. Koch M.O. Shappell S.B. Breyer M.D. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am. J. Pathol. 2000;157:29–35. doi: 10.1016/S0002-9440(10)64513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari V. Joshi G. Nama S. Somasundaram K. Mulherkar R. HDAC inhibitor valproic acid enhances tumour cell kill in adenovirus-HSVtk mediated suicide gene therapy in HNSCC xenograft mouse model. Int. J. Cancer. 2009 doi: 10.1002/ijc.24700. (in press). [DOI] [PubMed] [Google Scholar]
- Kozaki K. Imoto I. Mogi S. Omura K. Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- Lai S.Y. Koppikar P. Thomas S.M. Childs E.E. Egloff A.M. Seethala R.R. Branstetter B.F. Gooding W.E. Muthukrishnan A. Mountz J.M. Lui V.W. Shin D.M. Agarwala S.S. Johnson R. Couture L.A. Myers E.N. Johnson J.T. Mills G. Argiris A. Grandis J.R. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: First human application and potential antitumor mechanisms. J. Clin. Oncol. 2009;27:1235–1242. doi: 10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont J.P. Nemunaitis J. Kuhn J.A. Landers S.A. McCarty T.M. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann. Surg. Oncol. 2000;7:588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- Lane D.P. Cancer: p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Leong P.L. Andrews G.A. Johnson D.E. Dyer K.F. Xi S. Mai J.C. Robbins P.D. Gadiparthi S. Burke N.A. Watkins S.F. Grandis J.R. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lewis B.P. Burge C.B. Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li D. Jiang W. Bishop J.S. Ralston R. O'Malley B.W., Jr. Combination surgery and nonviral interleukin 2 gene therapy for head and neck cancer. Clin. Cancer Res. 1999;5:1551–1556. [PubMed] [Google Scholar]
- Li H.L. Li S. Shao J.Y. Lin X.B. Cao Y. Jiang W.Q. Liu R.Y. Zhao P. Zhu X.F. Zeng M.S. Guan Z.Z. Huang W. Pharmacokinetic and pharmacodynamic study of intratumoral injection of an adenovirus encoding endostatin in patients with advanced tumors. Gene Ther. 2008;15:247–256. doi: 10.1038/sj.gt.3303038. [DOI] [PubMed] [Google Scholar]
- Lin X. Huang H. Li S. Li H. Li Y. Cao Y. Zhang D. Xia Y. Guo Y. Huang W. Jiang W. A phase I clinical trial of an adenovirus-mediated endostatin gene (E10A) in patients with solid tumors. Cancer Biol. Ther. 2007;6:648–653. doi: 10.4161/cbt.6.5.4004. [DOI] [PubMed] [Google Scholar]
- Liu X. Jiang L. Wang A. Yu J. Shi F. Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W. Zheng S. Li X.F. Huang J.J. Zheng X. Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J. Gastroenterol. 2004;10:3634–3638. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Cancer gene therapy: Fringe or cutting edge? Nat. Rev. Cancer. 2001;1:130–141. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- McNally L.R. Rosenthal E.L. Zhang W. Buchsbaum D.J. Therapy of head and neck squamous cell carcinoma with replicative adenovirus expressing tissue inhibitor of metalloproteinase-2 and chemoradiation. Cancer Gene Ther. 2009;16:246–255. doi: 10.1038/cgt.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluart P. Abdallah K.A. Lima F.D. Smith R. Moyses R.A. Coelho V. Victora G.D. Socorro-Silva A. Volsi E.C. Zarate-Blades C.R. Ferraz A.R. Barreto A.K. Chammas M.C. Gomes R. Gebrim E. Arakawa-Sugueno L. Fernandes K.P. Lotufo P.A. Cardoso M.R. Kalil J. Silva C.L. Phase I trial of DNA-hsp65 immunotherapy for advanced squamous cell carcinoma of the head and neck. Cancer Gene Ther. 2008;15:676–684. doi: 10.1038/cgt.2008.35. [DOI] [PubMed] [Google Scholar]
- Nagayasu A. Uchiyama K. Kiwada H. The size of liposomes: A factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv. Drug Deliv. Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Tanaka H. Shirakawa T. Gotoh A. Hayashi Y. Hamada K. Tsukuda M. Nibu K. Cyclooxygenase 2 promoter-based replication-selective adenoviral vector for hypopharyngeal cancer. Arch. Otolaryngol. Head Neck Surg. 2009;135:282–286. doi: 10.1001/archoto.2008.549. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J. Cunningham C. Buchanan A. Blackburn A. Edelman G. Maples P. Netto G. Tong A. Randlev B. Olson S. Kirn D. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: Safety, feasibility and biological activity. Gene Ther. 2001a;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J. Khuri F. Ganly I. Arseneau J. Posner M. Vokes E. Kuhn J. McCarty T. Landers S. Blackburn A. Romel L. Randlev B. Kaye S. Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J. Clin. Oncol. 2001b;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- Neves S. Faneca H. Bertin S. Konopka K. Düzgüneş N. Pierrefite-Carle V. Simões S. Pedroso de Lima M.C. Transferrin lipoplex-mediated suicide gene therapy of oral squamous cell carcinoma in an immunocompetent murine model and mechanisms involved in the antitumoral response. Cancer Gene Ther. 2009;16:91–101. doi: 10.1038/cgt.2008.60. [DOI] [PubMed] [Google Scholar]
- Neves S.S. Sarmento-Ribeiro A.B. Simões S.P. Pedroso de Lima M.C. Transfection of oral cancer cells mediated by transferrin-associated lipoplexes: Mechanisms of cell death induced by herpes simplex virus thymidine kinase/ganciclovir therapy. Biochim. Biophys. Acta. 2006;1758:1703–1712. doi: 10.1016/j.bbamem.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Niwa H. Wentzel A.L. Li M. Gooding W.E. Lui V.W. Grandis J.R. Antitumor effects of epidermal growth factor receptor antisense oligonucleotides in combination with docetaxel in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2003;9:5028–5035. [PubMed] [Google Scholar]
- Oldfield E.H. Ram Z. Culver K.W. Blaese R.M. DeVroom H.L. Anderson W.F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum. Gene Ther. 1993;4:39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- O'Malley B.W. Cope K.A. Chen S.H. Li D. Schwartz M.R. Woo S.L. Combination gene therapy for oral cancer in a murine model. Cancer Res. 1996;56:1737–1741. [PubMed] [Google Scholar]
- O'Reilly M.S. Boehm T. Shing Y. Fukai N. Vasios G. Lane W.S. Flynn E. Birkhead J.R. Olsen B.R. Folkman J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- O'Shea C.C. Johnson L. Bagus B. Choi S. Nicholas C. Shen A. Boyle L. Pandey K. Soria C. Kunich J. Shen Y. Habets G. Ginzinger D. McCormick F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Plautz G.E. Yang Z.Y. Wu B.Y. Gao X. Huang L. Nabel G.J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portsmouth D. Hlavaty J. Renner M. Suicide genes for cancer therapy. Mol. Aspects Med. 2007;28:4–41. doi: 10.1016/j.mam.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Rabinowich H. Vitolo D. Altarac S. Herberman R.B. Whiteside T.L. Role of cytokines in the adoptive immunotherapy of an experimental model of human head and neck cancer by human IL-2-activated natural killer cells. J. Immunol. 1992;149:340–349. [PubMed] [Google Scholar]
- Rezler E.M. Khan D.R. Tu R. Tirrell M. Fields G.B. Peptide-mediated targeting of liposomes to tumor cells. Methods Mol. Biol. 2007;386:269–298. doi: 10.1007/978-1-59745-430-8_10. [DOI] [PubMed] [Google Scholar]
- Rhee J.G. Li D. Suntharalingam M. Guo C. O'Malley B.W., Jr. Carney J.P. Radiosensitization of head/neck squamous cell carcinoma by adenovirus-mediated expression of the Nbs1 protein. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:273–278. doi: 10.1016/j.ijrobp.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rubin Grandis J. Melhem M.F. Gooding W.E. Day R. Holst V.A. Wagener M.M. Drenning S.D. Tweardy D.J. Levels of TGF-α and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- Rudin C.M. Cohen E.E. Papadimitrakopoulou V.A. Silverman S., Jr. Recant W. El-Naggar A.K. Stenson K. Lippman S.M. Hong W.K. Vokes E.E. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J. Clin. Oncol. 2003;21:4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi M. Snyderman C.H. Heo D.S. Johnson J.T. D'Amico F. Herberman R.B. Whiteside T.L. Local adoptive immunotherapy of human head and neck cancer xenografts in nude mice with lymphokine-activated killer cells and interleukin 2. Cancer Res. 1990;50:3113–3118. [PubMed] [Google Scholar]
- Sahu N.K. Shilakari G. Nayak A. Kohli D.V. Antisense technology: A selective tool for gene expression regulation and gene targeting. Curr. Pharm. Biotechnol. 2007;8:291–304. doi: 10.2174/138920107782109985. [DOI] [PubMed] [Google Scholar]
- Shillitoe E.J. Noonan S. Hinkle C.C. Marini F.C., III Kellman R.M. Transduction of normal and malignant oral epithelium by particle bombardment. Cancer Gene Ther. 1998;5:176–182. [PubMed] [Google Scholar]
- Sobol R. Nemunaitis J. Clayman G. Hamm J. Licato L. Chada S. Menander K. Roth J. Goodwin J. Tumor p53 biomarkers personalize selection of adenoviral p53 gene therapy or methotrexate as potentially efficacious treatments in different and complementary groups of recurrent squamous cell carcinoma of the head and neck (SCCHN) [abstract]. Poster presented at the 2nd AACR Centennial Conference on Translational Cancer Medicine; Jul 20–23;2008 ; Monterey, CA. 2008. Abstract no. B36. [Google Scholar]
- Tanaka H. Shirakawa T. Zhang Z. Hamada K. Gotoh A. Nibu K. A replication-selective adenoviral vector for head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 2005;131:630–634. doi: 10.1001/archotol.131.7.630. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Isselbacher K.J. Khoury G. Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985;228:26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Tas M.P. Simons P.J. Balm F.J. Drexhage H.A. Depressed monocyte polarization and clustering of dendritic cells in patients with head and neck cancer: In vitro restoration of this immunosuppression by thymic hormones. Cancer Immunol. Immunother. 1993;36:108–114. doi: 10.1007/BF01754410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.M. Naresh K.N. Wagle A.S. Mulherkar R. Preclinical studies on suicide gene therapy for head/neck cancer: A novel method for evaluation of treatment efficacy. Anticancer Res. 1998;18:4393–4398. [PubMed] [Google Scholar]
- Thomas S.M. Zeng Q. Dyer K.F. Suscovich T.J. Kanter P.M. Whalen J.D. Watkins S.F. Grandis J.R. Tissue distribution of liposome-mediated epidermal growth factor receptor antisense gene therapy. Cancer Gene Ther. 2003;10:518–528. doi: 10.1038/sj.cgt.7700567. [DOI] [PubMed] [Google Scholar]
- Thomas S.M. Ogagan M.J. Freilino M.L. Strychor S. Walsh D.R. Gooding W.E. Grandis J.R. Zamboni W.C. Antitumor mechanisms of systemically administered epidermal growth factor receptor antisense oligonucleotides in combination with docetaxel in squamous cell carcinoma of the head and neck. Mol. Pharmacol. 2008;73:627–638. doi: 10.1124/mol.107.041160. [DOI] [PubMed] [Google Scholar]
- Trimble C. Lin C.T. Hung C.F. Pai S. Juang J. He L. Gillison M. Pardoll D. Wu L. Wu T.C. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- Vile R.G. Nelson J.A. Castleden S. Chong H. Hart I.R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- Villaret D. Glisson B. Kenady D. Hanna E. Carey M. Gleich L. Yoo G.H. Futran N. Hung M.C. Anklesaria P. Heald A.E. A multicenter phase II study of tgDCC-E1A for the intratumoral treatment of patients with recurrent head and neck squamous cell carcinoma. Head Neck. 2002;24:661–669. doi: 10.1002/hed.10107. [DOI] [PubMed] [Google Scholar]
- Vorburger S.A. Hunt K.K. Adenoviral gene therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- Wanebo H.J. Blackinton D. Kouttab N. Mehta S. Contribution of serum inhibitory factors and immune cellular defects to the depressed cell-mediated immunity in patients with head and neck cancer. Am. J. Surg. 1993;166:389–394. doi: 10.1016/s0002-9610(05)80339-3. [DOI] [PubMed] [Google Scholar]
- Wang Z. Troilo P.J. Wang X. Griffiths T.G. Pacchione S.J. Barnum A.B. Harper L.B. Pauley C.J. Niu Z. Denisova L. Follmer T.T. Rizzuto G. Ciliberto G. Fattori E. Monica N.L. Manam S. Ledwith B.J. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Whiteside T.L. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- Whiteside T.L. Anti-tumor vaccines in head and neck cancer: Targeting immune responses to the tumor. Curr. Cancer Drug Targets. 2007;7:633–642. doi: 10.2174/156800907782418310. [DOI] [PubMed] [Google Scholar]
- Williams R.S. Johnston S.A. Riedy M. Devit M.J. McElligott S.G. Sanford J.C. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2726–2730. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.A. Malone R.W. Williams P. Chong W. Acsadi G. Jani A. Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wollenberg B. Kastenbauer E. Mundl H. Schaumberg J. Mayer A. Andratschke M. Lang S. Pauli C. Zeidler R. Ihrler S. Lohrs Naujoks K. Rollston R. Gene therapy—phase I trial for primary untreated head and neck squamous cell cancer (HNSCC) UICC stage II–IV with a single intratumoral injection of hIL-2 plasmids formulated in DOTMA/Chol. Hum. Gene Ther. 1999;10:141–147. doi: 10.1089/10430349950019273. [DOI] [PubMed] [Google Scholar]
- Xi S. Grandis J.R. Gene therapy for the treatment of oral squamous cell carcinoma. J. Dent. Res. 2003;82:11–16. doi: 10.1177/154405910308200104. [DOI] [PubMed] [Google Scholar]
- Yang L. Hwang R. Pandit L. Gordon E.M. Anderson W.F. Parekh D. Gene therapy of metastatic pancreas cancer with intraperitoneal injections of concentrated retroviral herpes simplex thymidine kinase vector supernatant and ganciclovir. Ann. Surg. 1996;224:405–414. doi: 10.1097/00000658-199609000-00017. discussion 414–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo G.H. Hung M.C. Lopez-Berestein G. Lafollette S. Ensley J.F. Carey M. Batson E. Reynolds T.C. Murray J.L. Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin. Cancer Res. 2001;7:1237–1245. [PubMed] [Google Scholar]
- Yu D. Wang A. Huang H. Chen Y. PEG-PBLG nanoparticle-mediated HSV-TK/GCV gene therapy for oral squamous cell carcinoma. Nanomedicine. 2008;3:813–821. doi: 10.2217/17435889.3.6.813. [DOI] [PubMed] [Google Scholar]
- Zhang W.W. Fang X. Mazur W. French B.A. Georges R.N. Roth J.A. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994;1:5–13. [PubMed] [Google Scholar]
- Zhou H. Tang Y. Liang X. Yang X. Yang J. Zhu G. Zheng M. Zhang C. RNAi targeting urokinase-type plasminogen activator receptor inhibits metastasis and progression of oral squamous cell carcinoma in vivo. Int. J. Cancer. 2009;125:453–462. doi: 10.1002/ijc.24360. [DOI] [PubMed] [Google Scholar]