Abstract
Vesicular stomatitis virus (VSV) has shown promise as an oncolytic agent, although unmodified VSV can be neurotoxic. To avoid toxicity, a vector was created by introducing the interferon-β (IFN-β) gene (VSV.IFN-β). We conducted this study to determine the ability of VSV.IFN-β to lyse human cancer (mesothelioma) cells and to evaluate the potential of this recombinant virus for clinical translation. Four normal human mesothelial and 12 mesothelioma cell lines were tested for their susceptibility to VSV vectors in vitro. VSV.hIFN-β did not cause cytotoxicity in any normal lines. Only 4 of 12 lines were effectively lysed by VSV.hIFN-β. In the eight resistant lines, pretreatment with IFN-β prevented lysis of cells by VSV.GFP, and VSV infection or addition of IFN-β protein resulted in the upregulation of double-stranded RNA-dependent protein kinase (PKR), myxovirus resistance A (MxA), and 2′,5′-oligo-adenylate-synthetase (2′5′-OAS) mRNA. In the susceptible lines, there was no protection by pretreatment with IFN-β protein and no IFN- or VSV-induced changes in PKR, MxA, and 2′5′-OAS mRNA. This complete lack of IFN responsiveness could be explained by marked downregulation of interferon alpha receptors (IFNARs), p48, and PKR in both the mesothelioma cell lines and primary tumor biopsies screened. Presence of p48 in three tumor samples predicted responsiveness to IFN. Our data indicate that many mesothelioma tumors have partially intact IFN pathways that may affect the efficacy of oncolytic virotherapy. However, it may be feasible to prescreen individual susceptibility to VSV.IFN-β by immunostaining for the presence of p48 protein.
Introduction
Oncolytic virotherapy is an emerging platform in cancer therapeutics (Parato et al., 2005). A group of small unmodified RNA viruses, including vesicular stomatitis virus (VSV), demonstrate inherent specificity for tumor cells which appears to be due, in part, to defective innate antiviral host defense mechanisms in tumor cells (Russell, 2002). These RNA viruses are normally relatively nonpathogenic because of their high sensitivity to the antiviral actions of type I interferons (IFNs). IFN-β, produced by normal cells as a result of viral infection, upregulates the expression of IFN-stimulated antiviral genes (ISGs), such as myxovirus resistance A (MxA), 2′,5′-oligo-adenylate-synthetase (2′5′-OAS), and double-stranded RNA-dependent protein kinase (PKR), and subsequently IFN-α in adjacent cells (Wathelet et al., 1998; Stojdl et al., 2003). These proteins potently inhibit replication of the viral genome and thus protect adjacent normal cells from the viral attack. Tumor cells of many tumor types have been reported to be relatively resistant to the antiviral protective effect of type I IFNs because of acquired mutations in the IFN pathways that presumably allow them to gain proliferative potential (Linge et al., 1995; Pfeffer et al., 1996; Wong et al., 1997; Sun et al., 1998; Matin et al., 2001; Stojdl et al., 2003; Parato et al., 2005).
VSV is a rapidly replicating, negative single-stranded RNA virus that belongs to the Rhabdoviridae family and is relatively nonpathogenic to humans (Russell, 2002; Obuchi et al., 2003; Lichty et al., 2004; Parato et al., 2005). VSV replicates more efficiently in tumor cells compared with normal “nontransformed” cells, which is attributable to flaws in cancer cells' innate immune responses involving the IFN system, tumor-related defects in translational regulation, and abnormal signaling pathways in cancer cells that support viral replication (Balachandran and Barber, 2000; Stojdl et al., 2000; Balachandran et al., 2001). VSV has shown significant promise in a variety of preclinical tumor models, such as melanoma, squamous cell cancer, ovarian cancer, colorectal carcinoma, prostate cancer, breast cancer, rhabdoid tumors, leukemia, hepatocellular carcinoma, and glioblastoma (Ebert et al., 2003, 2005; Huang et al., 2003; Stojdl et al., 2003; Ahmed et al., 2004; Shinozaki et al., 2004; Lun et al., 2006; Bergman et al., 2007; Diaz et al., 2007; Sung et al., 2008; Wu et al., 2008).
Many primary human and murine cells have been shown to exhibit significant resistance to VSV infection in vitro, yet high titers of VSV can still lead to lethal infection in vivo with death primarily due to neurologic complications (Stojdl et al., 2000; Obuchi et al., 2003). For use in human gene therapy, therefore, efforts have been made to develop approaches that will allow enhanced safety, with minimal loss of selectivity and efficacy (Stojdl et al., 2000, 2003; Obuchi et al., 2003; Lichty et al., 2004). Genetic approaches aimed at creating strains of VSV that enhance IFN production in infected cells have thus been pursued. One of strategies has been to directly introduce the IFN-β gene into the viral genome, generating very high levels of IFN-β protein as the virus replicates (Obuchi et al., 2003).
Malignant pleural mesothelioma has a dismal prognosis and is resistant to chemotherapy and radiation therapy; however, its localized nature makes it potentially amenable to gene or oncolytic viral therapy and our preclinical and clinical studies in this regard have been promising (Sterman and Albelda, 2005). Our group has been conducting preclinical and clinical trials for mesothelioma tumors with a nonreplicating adenoviral vector expressing human IFN-β (Odaka et al., 2002; Sterman et al., 2007). We have seen strong antitumor effects that are mediated primarily through virus-induced inflammation in combination with tumor cell secretion of IFN-β that leads to both innate and acquired immune-mediated antitumor responses. A replicating vector that also expresses IFN-β (i.e., VSV.hIFN-β) was thus of particular interest to us.
We postulated that a replicating vector that delivered IFN-β might provide added antitumor effects through both amplified transgene expression and direct virus-mediated tumor cell lysis. Given the promise of VSV as an oncolytic agent and the availability of a VSV–human IFN-β vector, we conducted this study to (1) study the ability of VSV to lyse human mesothelioma cells and (2) evaluate the potential of the VSV.IFN-β for clinical translation.
Materials and Methods
Cell lines
Human mesothelioma lines
The human OK1, OK2, OK5, OK6, and OK7 cell lines were provided by Dr. Claire Verschaegen (University of New Mexico). H2052, I-45, and MSTO lines were purchased from the American Tissue Type Collection (Manassas, VA). The human Pt108, REN, LRK, and M30 lines were derived from patient samples at the University of Pennsylvania. OK1, OK2, OK5, OK6, OK7, M30, REN, LRK, H2052, and MSTO were grown in Roswell Park Memorial Institute (RPMI) culture medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 2 mM penicillin/streptomycin. I-45 cells were also grown in the same supplemented RPMI medium with addition of 0.1 M nonessential amino acids (Invitrogen, Carlsbad, CA). Pt108 cells were maintained in E-medium, which consists of RPMI 1640 culture medium plus 10 μg/ml insulin, 10 μg/ml transferrin, 10 μM ethanolamine, 10 ng/ml selenium, 2 mM glutamine, 10 mM 4-(2-hydroxyethyl)-1-piperazine-ethane sulfonic acid (HEPES), 0.5 mM sodium pyruvate, 0.1 mM nonessential amino acid, penicillin/streptomycin, 1 ng/ml epidermal growth factor, 18 ng/ml hydrocortisone, and 0.1 nM T3 hormone.
Human mesothelial cell lines
LP9, HM1, and HM3 cell lines were kindly provided by Dr. James Rheinwald (Dana Farber Cancer Institute); these cell lines were grown in the special culture mediun supplemented with hydrocortisone (1000 μg per 500 ml) and epidermal growth factor as previously described (Connell and Rheinwald, 1983; Murphy and Rheinwald, 1997). PF1M cells were obtained by growing cells from nonmalignant pleural fluid in the same culture medium.
Viral strains
The VSV.mIFN-β and VSV.hIFN-β vectors were originally described by Obuchi et al. (2003). VSV.GFP was generated as previously described (Diaz et al., 2007). Viral stocks were manufactured by the Core Viral Facility of Mayo Clinic (Rochester, MN). All vectors were amplified in Baby Hamster Kidney (BHK) cells and were titered by standard plaque assay of BHK cells.
MTT assay
To perform the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, cells were plated in quadruplicate on 96-well plates (5000 cells/well in RPMI or Dulbecco's modified Eagle's medium [DMEM] medium with 10% FBS) and were infected with different multiplicities of infection (MOIs) (10, 1, 0.1, 0.01, and 0) of the VSV.mIFN-β, VSV.hIFN-β, and VSV.GFP viruses. Viability was assessed at successive time points (24, 48, or 72 hr) by developing the reaction assay per manufacturer's instruction (Promega, Madison, WI). Optical density was read at 570 nm and corrected using a background control value.
Plaque assays
To evaluate the ability of VSV.hIFN-β to replicate in mesothelioma cells, LP9, REN, and LRK cells were infected at an MOI of 1. The supernatants were collected 24 hr later and titered by standard plaque assay of BHK cells. In addition, PF1M, REN, and LRK cells were pretreated with human IFN-β for 24 hr at 1 and 10,000 U/ml, followed by infecting the cells with VSV.GFP. The supernatants and cell lysates were collected at 6, 24, and 48 hr postinfection to measure the virus titers.
Preparation of IFN-β and IFN-inducible gene (MxA, 2′5′-OAS, and PKR) mRNA
To collect mRNA for reverse transcriptase–polymerase chain reaction (RT-PCR) after VSV.GFP infection or IFN-β exposure, T25 flasks of REN, LRK, and LP9 cells (1.3 × 106 cells) were left as controls, infected with VSV.GFP at an MOI of 10, or exposed to 2.5 ml of 10 U/ml hIFN-β in RPMI/10% FBS or DMEM/10% FBS. After 1 hr, the viral inoculum was aspirated in the infected flasks, cells were washed with PBS twice, and 2.5 ml of supplemented RPMI medium was applied in each of the flasks for 5 hr. After 6 hr from the time of infection or application of IFN-β, the medium was aspirated and cells were placed in TRIzol Reagent (Invitrogen) using 300 μl in each flask, which were combined to 1 ml for each condition. Samples were frozen at −80°C.
Total RNA were isolated using the protocol provided by the manufacturer, followed by removal of contaminating genomic DNA by DNase I treatment (Roche Molecular Biochemicals, Indianapolis, IN). High-quality RNA was confirmed by running an aliquot of each sample on a denaturing formaldehyde/agarose/EtBr gel.
Real-time RT-PCR
Quantitative analysis of mRNA expression was performed using real time RT-PCR. Three micrograms of total RNA were reverse transcribed to cDNA, using Oligo(dT)15 primer (Promega) and SuperScript III reverse transcriptase (Invitrogen), following the protocol provided by the manufacturer. Synthesized cDNA was normalized to β-actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), or 18S rRNA levels and quantification of tumor mRNA levels was performed as previously described (Jassar et al., 2005). Relative levels of expression of each of the selected genes (fold change in VSV.GFP-infected or IFNβ-treated vs. control) were determined. Each sample was run in quadruplicate and the experiment was repeated at least once using the Smart Cycler System (Cepheid, Sunnyvale, CA). The primers were designed based on cDNA and genomic DNA sequence available in GenBank (primer sequences available upon request).
Pretreatment assays with hIFN-β
Cells were plated on 96-well plates (5000 cells/well) and were incubated overnight. On the next day the medium was discarded and hIFN-β (PBL InterferonSource, Piscatway, NJ) was added at various dilutions made with RPMI- or DMEM-supplemented medium (1, 10, 100, 500, 1000, and 10,000 U/ml). The activity of hIFN-β was 5 pg/unit. After 24 hr of incubation with IFN-β, the medium was discarded and cells were infected with VSV.GFP at an MOI of 0.05. Four wells were used for infection with VSV.GFP alone and four wells as controls with medium only. MTT assay was performed 48 hr after infection.
IFN-β ELISA
REN, LRK, and LP9 cells (1 × 106) were plated in six-well plates in triplicate and were incubated in 1 ml of supplemented RPMI or DMEM medium overnight. On the next day, the cells were infected with VSV.GFP and VSV.hIFN-β at an MOI of 1 in a total volume of 100 μl. After 1 hr, the inoculum was aspirated, flasks were washed with PBS twice, and 400 μl of RPMI or DMEM culture medium was applied. Twenty-four hours after the time of infection, the medium was collected, spun for 10 min at 13,000 rpm to remove dead cells, and retained at −80°C to quantify human IFN-β by ELISA according to manufacturer's instructions (PBL InterferonSource).
INF-β bioassay
Human mesothelial cells and human mesothelioma cells were infected with VSV.GFP at an MOI of 10 and supernatants were harvested at 24 hr. The supernatants were inactivated for viral activity by heating at 56°C for 30 min. REN cells were plated at a density of 5000 cells/well and then incubated with the heat-inactivated supernatants for 24 hr. The supernatants were discarded and VSV.GFP at an MOI of 10 was added for 48 hr. The MTT assay was performed to assess the viability of REN cells after incubation with the supernatants. The hIFN-β levels in the supernatants were determined by comparing the survival results from the MTT assay to a standard curve performed with known concentrations of hIFN-β. For our standard curve, REN cells were plated at a density of 5000 cells/well and incubated with hIFN-β concentrations ranging from 0.01 to 10 U/ml prepared in supplemented RPMI medium. The sensitivity of the assay was 0.01 U/ml of hIFN-β.
Animal studies
SCID mice were purchased from the Wistar Institute (Philadelphia, PA). Three groups of SCID mice (n = 6–8 per group) were injected with 1 × 106 cells (REN or MSTO) on the hind flank. Once tumor size reached approximately 200 mm3, intratumoral injections were performed with control medium or 6.6 × 108 pfu of vector in 100 μL of PBS (VSV.mIFN-β and VSV.hIFN-β) once weekly for 3 consecutive weeks. Tumors were measured twice per week with calipers and mice were monitored for toxicity. Mice were euthanized if toxicity was evident or tumor burden exceeded 1500 mm3. The Animal Use Committee of the University of Pennsylvania approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
Cytology blocks of primary tumor cells in pleural effusions
To analyze actual patient samples, we obtained pleural effusion samples from mesothelioma patients who were part of a phase I trial of intrapleural administration of an adenovirus expressing IFN-β (Ad.IFN-β) (Sterman et al., 2007). Samples were collected before and 24 hr after receiving the vector, at a time when pleural fluid IFN-β levels were markedly increased. To evaluate the cells, pleural effusion fluids were spun down at 1500 rpm and the cell pellet was resuspended with molten 1% agarose in PBS. The samples were fixed with 1% formalin overnight and later embedded with paraffin for immunohistochemical staining.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded cytology blocks and a human mesothelioma tissue array (obtained from the Mesothelioma Research Bank [CDC NIOSH 1-U19-OH009077 Mesothelioma Virtual Bank for Translational Research, Pittsburgh, PA]) were sectioned at 5 μm and placed on precleaned glass microscope slides. The array contains samples for 48 mesothelioma tumors that were arrayed in triplicate.
The cytology and tissue sections were deparaffinized in xylene and rehydrated through graded alcohol solutions. The slides were placed in a coplin jar filled with 10 mM sodium citrate (pH 6), as the antigen retrieval agent, and boiled in a microwave for 5 min. The slides were blocked with appropriate serum for 30 min before addition of the primary antibodies. The primary anti-human antibodies used were anti-p48 (sc-496; Santa Cruz Biotechnology, Santa Cruz, CA), anti-STAT1 (sc-592; Santa Cruz Biotechnology), anti-IFNAR1 (ab45172; Abcam, Cambridge, MA), anti-PKR (sc-100378; Santa Cruz Biotechnology), and anti-OAS1 (sc-100639; Santa Cruz Biotechnology). After overnight incubation with the primary antibodies at 4°C, the slides were washed with PBS twice, followed by incubating in a 3% hydrogen peroxide solution for 30 min to quench any endogenous peroxidase activity. The appropriate biotinylated secondary antibodies (1:200 dilution) was added to the sections and incubated for 30 min at room temperature. The slides were washed again and incubated with ABC-peroxidase for 30 min (Vector Elite, Burlingame, CA). After a final rinse in PBS, the cytology and tissue sections were incubated with 3,3′ diaminobenzidine (DAB) substrate (0.02% DAB, 0.005% hydrogen peroxide) for 1–5 min to develop the colorimetric reaction, counterstained with hematoxylin, and then mounted with glass coverslips with Aqua-poly. The immunoreactive score for each tissue section was calculated using our modified Remmele's scoring system (see below).
Modified Remmele's scoring system
The 20 × objective was used to evaluate the whole section by two observers who were blinded with respect to the sample identities and treatment assignment. The immunoreactive score for each sample was calculated by multiplying the percent score with the intensity score (Remmele and Stegner, 1987). The percent score indicates the percentage of cells positive: 76–100% tumor cells, score 4; 51–75% tumor cells, score 3; 26–50%, score 2; 1–25%, score 1. An example of the intensity score is shown in Fig. 4A, with a scale of 0 (absent) to 3+ (strongly positive). An immunoreactive score of 0–2 indicates absence or extremely low expression of that particular protein.
FIG. 4.
Immunostaining of mesothelioma tumors from patients. (A) Mesothelioma tumor samples from 48 patients were stained with an antibody against p48. Sections from four patients showing examples of our grading system (Grade 0, no staining; Grade 3, strongly positive) are shown at 20 × magnification. (B) Pleural effusion cells of three patients from our Ad.IFN-β Phase I trial were collected prior to and 24 hr after receiving the vector. Cells were immunostained for p48 (upper panels) and the IFN-response protein oligo-adenylate-synthetase 1 (OAS1) before vector (middle panels) and 24 hr after vector (lower panels) to evaluate their IFN responsiveness as assessed by upregulation of OAS1. Cells are shown at a 100 × magnification (see Supplemental Fig. S3 for a low-power, wide-field view). The large cells are mesothelioma cells, and the smaller cells are macrophages. All three patients showed baseline p48 staining and an clear upregulation of OAS1 after exposure to IFN.
Statistical analyses
For the RT-PCR and flank tumor studies comparing differences between two groups, we used unpaired Student t-tests. For flank tumor studies comparing more than two groups, we used analysis of variance with appropriate post hoc testing. Differences were considered significant when p < 0.05. Data are presented as mean ± standard error of the mean.
Results
Oncolytic activities of VSV vectors in vitro in mesothelioma cells
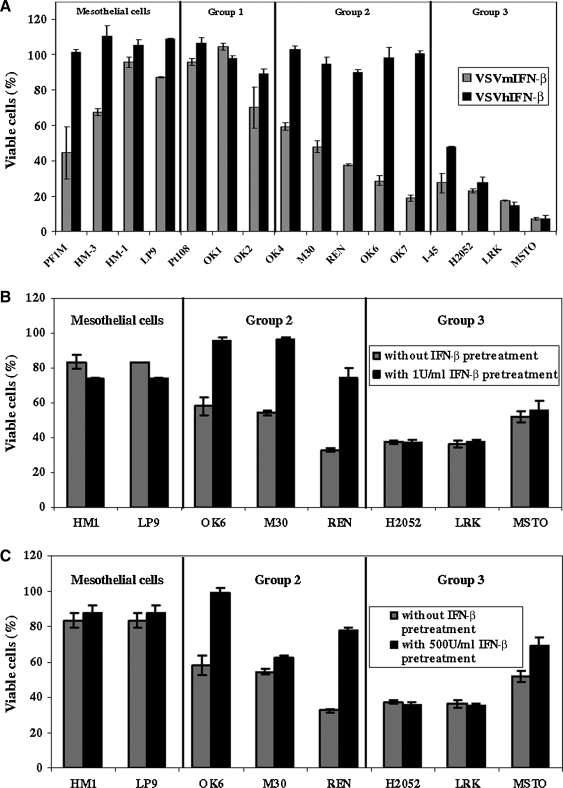
Nontransformed human mesothelial lines and human mesothelioma lines were tested for their in vitro susceptibility to the VSV vectors. As control vectors, we used a VSV expressing either green fluorescent protein (VSV.GFP) or murine IFN-β (VSV.mIFN-β). VSV.mIFN-β serves as an ideal control for the VSV.hIFN-β, because IFN proteins are strictly species specific with no cross reactivity. Our test vector was a VSV expressing human IFN-β (VSV.hIFN-β).
Four nontransformed human mesothelial lines, PF1M, HM3, HM1, and LP9, were studied (Fig. 1A). PF1M was a primary cell line derived in our laboratory. Forty-eight hours after infection (at an MOI of 0.1), VSV.mIFN-β and VSV.GFP (data not shown) induced about 50% cell death in the normal primary human mesothelial cells (PF1M), but only minimal cell death in the three immortalized human mesothelial cell lines. All four of the nontransformed human mesothelial lines were completely resistant to VSV.hIFN-β.
FIG. 1.
Virus Titers in the Culture of PF1M Primary Mesothelial Cells Receiving Interferon-β Pretreatment Prior to Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein
Twelve human mesothelioma lines were tested. Lines Pt 108, M30, REN, and LRK were primary cell lines derived in our laboratory, used in early passage. Three types of responses were seen. Three of the mesothelioma lines (Group 1: Pt108, OK1, and OK2) seemed quite resistant to cell death induced by either VSV.mIFN-β or VSV.hIFN-β and thus resembled the normal mesothelial lines. Five mesothelioma lines (Group 2: OK6, M30, REN, OK4, and OK7) were moderately sensitive to killing (50–75% cell death) by VSV.mIFN-β, but were completely resistant to killing by VSV.hIFN-β. Four mesothelioma lines (Group 3: H2052, LRK, I45, and MSTO) were efficiently killed by both VSV.mIFN-β and VSV.hIFN-β. Additional data for a range of MOIs at different time points are shown in Supplemental Fig. S1 (available online at www.liebertonline.com/hum).
These data demonstrate that IFN-β transgene protects normal mesothelial cells from VSV-mediated cell lysis, but also protects more than half of mesothelioma lines tested.
Ability of VSV to replicate in human mesothelioma cells
Because of different susceptibilities of mesothelioma lines to VSV.IFN-β strains, the ability of VSV.hIFN-β to replicate in a nontransformed mesothelial cell line (LP9), a mesothelioma line resistant to VSV.hIFN-β (REN), and a mesothelioma line sensitive to VSV.hIFN-β (LRK) was investigated in more detail. These cell lines were infected with VSV.hIFN-β at an MOI of 1, and the culture supernatants were collected at 24 hr later to measure the virus titer using plaque assay. The supernatant of LP9 culture contained 1 × 104 pfu/ml/1 million cells, and the supernatant of REN culture contained 1.35 × 106 pfu/ml/1 million cells, showing only minimal replication. In contrast, VSV.hIFN-β was able to replicate substantially better in LRK cells (1.55 × 108 pfu/ml/1 million cells), an amount that was 2 logs higher than in REN cells and 4 logs higher than in LP9 cells.
Assessment of functional antiviral IFN-β responses
The data above suggest the hypothesis that in some of the tumor cells, IFN (of the appropriate species specificity) produced by the transgene in the VSV vector inhibited replication and subsequent oncolysis, implying some partial sensitivity to the antiviral effects of IFN. We therefore directly evaluated the antiviral activity of IFN-β on representative cell lines by pretreating them with IFN-β for 24 hr and then testing the ability of VSV.GFP to replicate and lyse these pretreated cells.
The nontransformed human mesothelial cell lines (HM1 and LP9) were resistant to VSV.GFP-mediated oncolysis with or without human IFN-β pretreatment (Fig. 1B). On the other hand, pretreatment with 1 and 500 U/ml of human IFN-β for 24 hr almost completely protected the Group 2 OK6 and REN mesothelioma lines from VSV-mediated oncolysis (Fig. 1B and C). The M30 mesothelioma line was also protected by IFN-β; however, the cell viability decreased when M30 cells were pretreated with 500 U/ml of IFN-β. In contrast, the Group 3 H2052 and LRK cell lines were not protected from VSV-induced oncolysis after pretreatment (Fig. 1B and C). In fact, these cells were killed by VSV.GFP when pretreated with hIFN-β at doses as high as 10,000 U/ml (Supplemental Fig. S2, available online at www.liebertonline.com/hum). MSTO cells were only protected at doses greater than 1000 U/ml of hIFN-β (Supplemental Fig. S2).
To confirm that the IFN-β-mediated viral protection is due to a decrease in virus replication in cells, the viral titers in the cultures of mesothelioma cell lines (REN and LRK) and primary mesothelial cells (PF1M) after infecting with VSV.GFP were investigated (Table 1). In the PF1M primary mesothelial cell line (Table 1A), the initial viral titer at 1 hr postinfection was 2.8 × 103 pfu/ml/106 cells. Viral replication peaked at 48 hr, when the titer reached 2.5 × 105 pfu/ml/106 cells (a 2-log increase). Viral replication was markedly inhibited by either 1 unit (150-fold) or 10,000 units (340-fold) of IFN-β. In the partially sensitive REN line (Table 1B), the initial viral titer at 1 hr postinfection was 1.3 × 104 pfu/ml/106 cells. Viral replication peaked earlier at 24 hr, when the titer reached 1.25 × 106 pfu/ml/106 cells (a 2-log increase). In this case, 1 unit of IFN-β had virtually no effect on viral replication; however, 10,000 units of IFN-β markedly inhibited viral production (1000-fold). In the highly IFN-resistant LRK line (Table 1C), the initial viral titer at 1 hr postinfection was lower at 9 × 102 pfu/ml/106 cell. However, viral replication was very rapid, peaking at 6 hr, when the titer reached 8.5 × 104 pfu/ml/106 cells (again, a 2-log increase). At this time point, pretreatment with IFN-β had no effect; titers were actually higher at 1 × 105 pfu/ml/106 cells. Even at the extremely high dose of 10,000 units of IFN-β, replication was only inhbited by less than threefold.
Table 1A.
Virus Titers in the Culture of PF1M Primary Mesothelial Cells Receiving Interferon-β Pretreatment Prior to Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein
Table 1B.
Virus Titers in the Culture of REN Mesothelioma Cell-Line Receiving Interferon-β Pretreatment Prior to Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein
Table 1C.
Virus Titers in the Culture of LRK Mesothelioma Cell-Line Receiving Interferon-β Pretreatment Prior to Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein
| |
Virus titer |
|||
|---|---|---|---|---|
| Interferon-β (U/ml) | 1 hra | 6 hra | 24 hra | 48 hra |
| 0 | 900 | 85,000 | 80,000 | 3200 |
| 1 | NM | 107,500 | 59,500 | 1900 |
| 10,000 | NM | 29,000 | 11,000 | 2050 |
The unit for the virus titers is pfu/ml/million cells.
Abbreviation: NM, not measured.
Time after infection (hr).
Table 1.
These data demonstrate that some mesothelioma cell lines have a partial or fully intact IFN response that protects them from VSV-mediated cell lysis by inhibiting virus replication.
Oncolytic activities of VSV in vivo
We next examined the effect of VSV treatment in vivo using the two human cell lines that were able to grow as xenografts in SCID mice. Both lines were killed in vitro by VSV.mIFN-β, although MSTO was more sensitive. However, only one line (MSTO) was killed by VSV.hIFN-β in vitro, whereas the other line (REN) was resistant to VSV.hIFN-β in vitro. When flank tumors reached approximately 200 mm3 in size, they were injected three times with saline or 6.6 × 108 pfu of vector intratumorally at weekly intervals. MSTO flank tumors treated with either VSV.hIFN-β or VSV.mIFN-β showed statistically significant (p < 0.05) tumor growth inhibition (approximately 75%) compared with the control tumors (Fig. 2A). The growth inhibition induced by VSV.hIFN-β was equivalent to that of VSV.mIFN-β. REN flank tumors treated with VSV.mIFN-β showed statistically significant (p < 0.01) tumor growth inhibition (approximately 80%) compared to control (Fig. 2B). However, tumors treated with VSV.hIFN-β were not statistically significantly smaller (p = 0.2) than control tumors and were statistically larger (p < 0.05) than the tumors treated with VSV.mIFN-β.
FIG. 2.
Upregulation of Interferon-β mRNA and Protein in Human Mesothelioma Cells After Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein at an MOI of 10 for 6 hr, Measured Using Real-Time Reverse Transcriptase–Polymerase Chain Reaction, or 24 hr, Using Interferon-β Bioassay
Mechanistic studies
To understand the basis for this variable resistance of mesothelioma cells to infection with VSV.IFN-β, we focused additional studies on three human cell lines that appeared to be representative of each type of response to VSV.mIFN-β and VSV.hIFN-β: (1) LP9, a nontransformed mesothelial cell line that was resistant to both vectors; (2) REN, a mesothelioma cell line that was sensitive to VSV.mIFN-β, but resistant to VSV.hIFN-β; and (3) LRK, a mesothelioma cell line that was sensitive to both vectors.
Ability of human mesothelioma cells to produce IFN-β message and protein in response to VSV
We infected the aforementioned cells and evaluated the ability of VSV to stimulate the production of IFN-β message. This response is a key initial antiviral cellular defense mechanism mediated through the IRF3 pathway (Decker et al., 2002; Lichty et al., 2004). At baseline, there was no detectable mRNA for IFN-β in any cell line. As shown in Table 2, VSV infection upregulated the expression levels of IFN-β message in the LP9 and REN cell lines to a similar degree. In contrast, the LRK cells showed no induction of IFN-β.
Table 2.
Upregulation of Interferon-β mRNA and Protein in Human Mesothelioma Cells After Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein at an MOI of 10 for 6 hr, Measured Using Real-Time Reverse Transcriptase–Polymerase Chain Reaction, or 24 hr, Using Interferon-β Bioassay
| |
mRNA levels (fold change) |
Interferon-β protein levels (U/ml/million cells) |
||
|---|---|---|---|---|
| Cell lines | Control | VSV.GFP infecteda | Control | VSV.GFP infected |
| LP9 | u/d | 7325% ± 6%b | u/d | 0.225 |
| REN | u/d | 11,428% ± 12.5% | u/d | 0.007 ± 0.003 |
| LRK | u/d | u/dc | u/d | u/d |
Abbreviations: LP9, normal mesothelial cells; u/d, undetectable; VSV.GFP, vesicular stomatitis virus expressing green fluorescent protein.
Levels of mRNA are expressed in arbitrary units.
Expression levels are presented as means of triplicates with ± the coefficient of variation (standard error of the mean/mean).
p-Value is < 0.05 compared with LP9.
After VSV infection, the mRNA of IFN-β must be effectively transported to the cytoplasm, translated into protein, and secreted to exert its effects on ISGs (Decker et al., 2002; Lichty et al., 2004). VSV has evolved mechanisms (such as the M protein) to block this translation, although it appears that normal cells can at least partially overcome this block. Given that both LP9 and REN cells produced similar (and high) levels of IFN-β mRNA after VSV infection and that both cell lines were able to respond to exogenous IFN-β, we need to find out whether the protein levels of IFN-β would be upregulated after VSV infection. We thus evaluated the supernatant fluid from LP9, REN, and LRK cells that had been infected with VSV.GFP and measured IFN-β levels by a standard ELISA. The IFN-β levels from all three cell lines were below the level of detection (threshold of 25 pg/ml or 2.5 U/ml). We therefore used a bioassay comparing the ability of supernatants (after inactivation of virus) to protect REN cells from VSV.GFP compared with very low amounts of IFN-β. We found that this assay was sensitive to levels of IFN-β as low as 0.01 U/ml. As shown in Table 2, LP9 cells infected with VSV.GFP at an MOI of 10 for 24 hr produced 0.22 U/ml of IFN-β per 1 million cells infected, whereas REN cells produced only 0.01 U/ml per 1 million cells. In contrast, LRK cells produced no detectable amounts of IFN-β.
Finally, we compared the low levels of IFN-β produced after VSV.GFP infection with the levels of IFN-β after infection with VSV.hIFN-β. IFN-β production after 24 hr of infection was easily detected in the supernatants of cells infected with VSV.hIFN-β; however, levels were markedly affected by the ability of the vector to replicate. The susceptible LRK cells produced more than 353 U/ml/106 cells at 24 hr after infection with VSV.hIFN-β at an MOI of 1. LP9 and REN cells made clearly detectable but much lower amounts, 5 and 7.2 U/ml/106 cells, respectively.
Ability of human mesothelioma cells to upregulate the mRNA expressions of ISGs after external hIFN-β exposure
We next sought to determine how well each cell line could respond to external IFN-β stimulation by measuring the ability of these cells to upregulate and synthesize the mRNAs for three well-described ISGs, MxA, 2′5′-OAS, and PKR, which are known to inhibit the replication of VSV in normal host cells (Balachandran and Barber, 2000; Lichty et al., 2004; Brin et al., 2006; Sterman et al., 2006). We therefore incubated each cell line with 10 U/ml hIFN-β (approximately 50 pg/ml) for 6 hr and then harvested the cells for quantification of the mRNA levels of each gene using real-time RT-PCR (Table 3A).
Table 3A.
Incubation with 10 U/ml Human Interferon-β for 6 hr
Table 3.
Upregulation of Interferon-β-Inducible Antiviral Genes MxA, 2′5′-OAS and PKR Using Real-Time Reverse Transcriptase–Polymerase Chain Reaction in Human Mesothelial and Mesothelioma Cells After Incubation with 10 U/ml Human Interferon-β or Infection with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein at an MOI of 10.
At baseline, all three cell lines had very low or undetectable levels of message for MxA or 2′5′-OAS. There was variable baseline expression of PKR (Table 3A). However, after exposure to IFN-β, the LP9 and REN cells showed marked upregulation of the mRNA levels of all three IFN-inducible proteins. The response was most robust in the LP9 line, with a statistically (p < 0.05) lesser degree of induction in the REN cell line. There was no stimulation of any of these mRNAs in the LRK cell line.
Ability of human mesothelioma cells to upregulate the mRNA expressions of ISGs after VSV.GFP infection
We next examined the ability of VSV to upregulate the expression of ISGs (Table 3B) by infecting LP9, REN, and LRK cell lines with VSV.GFP at an MOI of 10. Six hours postinfection, VSV.GFP was able to upregulate the expression all three ISG mRNAs in the LP9 and REN cell lines; however, the level of upregulation was reduced by 30–50% in the REN cell line compared with the LP9 cell line (p < 0.05). Consistent with the lack of the LRK cell line to respond to IFN-β, we saw no upregulation of ISGs after VSV infection. It should be noted that in comparison to Table 3A, consistent with the low levels of IFN-β made after VSV infection, the levels of upregulation of the ISGs mRNAs after viral infection are much lower than those seen after exposure to 10 U of IFN-β.
Table 3B.
Incubation with Vesicular Stomatitis Virus Expressing Green Fluorescent Protein at an MOI of 10 for 6 hr
| |
MxA mRNAa |
2′5′-OAS mRNA |
PKR mRNA |
|||
|---|---|---|---|---|---|---|
| Cell lines | Control | VSV.GFP infected | Control | VSV.GFP infected | Control | VSV.GFP infected |
| LP9 | u/d | 82% ± 2.2%b | u/d | 105% ± 1.7% | 615% ± 9.1% | 958% ± 1.7% |
| REN | 13% ± 12.2% | 30% ± 5.9%c | u/d | 23% ± 6.3%c | 45% ± 9.7% | u/d |
| LRK | u/d | u/d | u/d | u/d | u/d | u/d |
Abbreviations: MxA, myxovirus resistance A; 2′5′-OAS, 2′,5′-oligo-adenylate-synthetase; PKR, double-stranded RNA-dependent protein kinase; VSV.GFP, vesicular stomatitis virus expressing green fluorescent protein.
Levels of mRNA are expressed in arbitrary units based on a standard curve.
Expression levels are presented as means of triplicates with ± the coefficient of variation (standard error of the mean/mean).
p-Value is < 0.05 compared with LP9.
Baseline mRNA expression levels of various components on IFN signaling pathway within different human mesothelioma lines
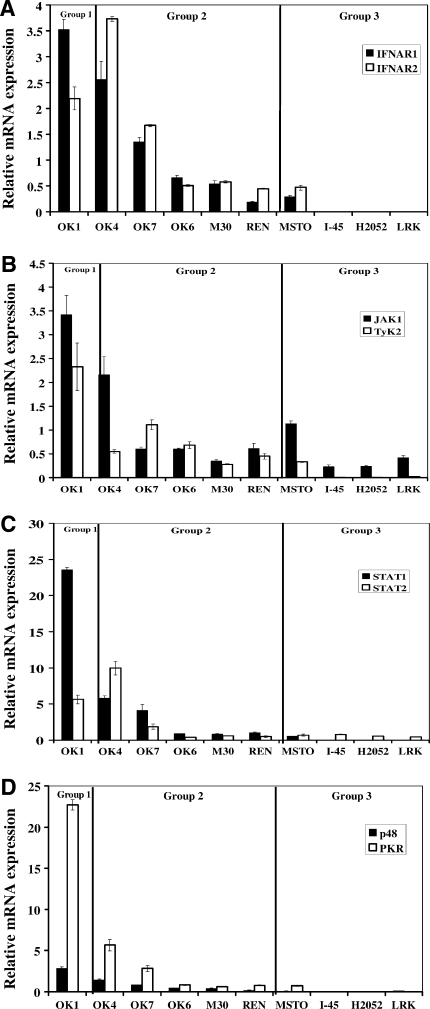
The heterogeneity in IFN responsiveness among mesothelioma lines could potentially be explained by losses of key components on the IFN signaling pathway as a result of oncogenesis (Linge et al., 1995; Pfeffer et al., 1996; Wong et al., 1997; Sun et al., 1998; Matin et al., 2001; Parato et al., 2005) or by functional defects in their IFN responses after stimulation. With a goal toward finding baseline deficits that could be used clinically (i.e., by PCR or immunohistochemistry) to predict responses to oncolytic viruses, we extracted total RNA from 10 human mesothelioma lines and performed quantitative real-time PCR to compare the baseline expression levels of various components on the IFN signaling pathway between the primary mesothelial cells (PF1M) and the established and primary mesothelioma cell lines (Fig. 3). Expression of these mRNAs was very high in the Group 1 cell line and similar to the levels seen in the normal mesothelial cell line in the Group 2 cells. Interestingly, IFNAR1, IFNAR2, TyK2, STAT1, p48, and PKR mRNA levels were highly downregulated or undetectable in three IFN-nonresponsive Group 3 lines (H2052, I45, and LRK) compared with the primary mesothelial cells. MSTO, a Group 3 cell line not responsive to IFN, had detectable levels of IFNAR1 and 2, JAK1, STAT1, STAT2, and PKR, but virtually undetectable levels of p48. Together, these results indicate that baseline deficiencies in key molecules of the type I IFN signaling pathway within a subset of mesothelioma lines likely explains why these cells fail to respond to IFN and are thus killed by VSV.hIFN-β.
FIG. 3.
Baseline mRNA expression of IFN pathway-associated genes among human mesothelioma lines. Total RNA was extracted from the primary mesothelial cell line, PF1M, and 10 human mesothelioma lines and used to synthesize cDNA. Specific primer sets were made to amplify eight IFN pathway-associated genes: (A) IFNAR1, IFNAR2, (B) JAK1, TyK2, (C) STAT1, STAT2, (D) p48, and double-stranded RNA-dependent protein kinase (PKR). Each sample was run in quadruplicate and the sample loading was adjusted in relation to their β-actin or GAPDH expression. The expression of each gene is normalized to the expression in the PF1M line and the fold change ± standard deviation is shown.
Distribution of IFN-response pathway proteins at baseline in mesothelioma tumors
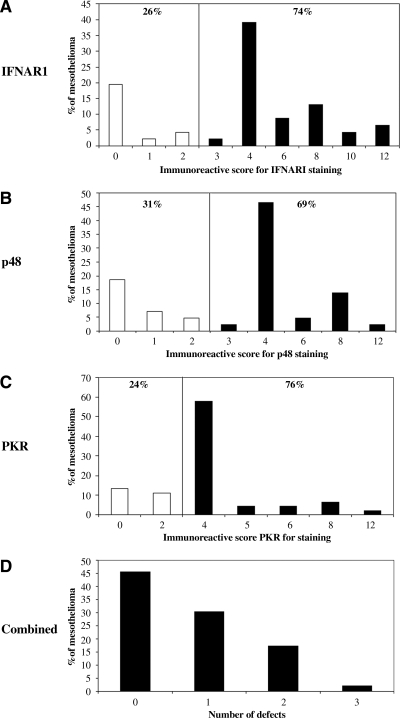
To see if these changes in cell lines were reflected in actual clinical samples, we performed immunostaining with antibodies to IFNAR1, p48, and PKR on a mesothelioma tissue array consisting of 48 tumor biopsies. As detailed in the Materials and Methods, for each biopsy, we graded the intensity of staining (Fig. 4A for examples using the p48 antibody) and the percentage of cells positive and combined these factors into a immunoreactive score. The score ranged from 0 to 12, with an immunoreactive score of 0–2 indicating absence or extremely low expression of a particular protein. Figure 5 shows the distribution of scores for each antibody. There were 26%, 31%, and 24% of mesothelioma tumors that had absence or low expression of IFNAR1, p48, and PKR, respectively (Fig. 5A–C). Approximately 50% of mesothelioma tumors had at least one component of the IFN pathway missing at baseline (Fig. 5D).
FIG. 5.
A mesothelioma tissue array, which consists of 48 primary tumor biopsies, was stained with antibodies against IFNAR1 (A), p48 (B), and PKR (C) and an immunoreactive staining score (see Materials and Methods section) was determined for each tumor. (A–C) The distribution of the immunoreactive score for each antibody. An immunoreactive score of 2 or less indicates an absence or low expression of that particular protein. (D) The number of defects per tumor is plotted. Forty-five percent of tumors had no defects, approximately 30% of tumors had lost staining in one protein, and 25% had lost staining in two or three of the proteins.
Response of mesothelioma tumor cells to IFN-β treatment in vivo
To determine if the baseline expression of IFN response proteins might correlate with the ability of the tumor cell to respond to IFN-β in vivo, we were able to take advantage of unique specimens stored from a previous gene therapy clinical trial conducted at our institution in which an adenovirus expressing hIFN-β was instilled intrapleurally (Sterman et al., 2007). We were able to find three patients in whom pleural fluid samples containing clearly identifiable mesothelioma cells were available before treatment and 1 day after vector instillation (a time when pleural IFN-β levels were very high).
We stained each sample for p48, which gave the most clear staining and seemed to be our best predictor of response in the cell lines, and the IFN-response protein OAS1 as a marker of IFN responsiveness. The staining revealed that before treatment, the tumor cells from all three of these patients had clearly detectable basal p48 expression (Fig. 4B and Supplemental Fig. S3, available online at www.liebertonline.com/hum) and very low levels of OAS1 expression. Twenty-four hours after gene transfer, OAS1 expression clearly increased (Fig. 4B and Supplemental Fig. S3). Unfortunately, we did not have a patient with p48 absent among these three examples; however, these results suggest that it would be feasibile to prescreen patients for their responsiveness to IFNs by looking at baseline p48 levels.
Discussion
Rare therapeutic success with current therapies for malignant pleural mesothelioma indicates a need for new therapeutic modalities. Our group and others have shown in preclinical studies that delivery of type I IFNs using an adenoviral vector induces strong antitumor immune responses and has been highly effective in eliminating tumors in mice (Ahmed et al., 2001; Odaka et al., 2002; Brin et al., 2006). This antitumor activity appears to be present in patients also. Intrapleural administration of a replication-incompetent adenoviral vector expressing IFN-β to patients with mesothelioma and malignant pleural effusions resulted in humoral and cellular antitumor immune responses and some significant clinical responses (Sterman et al., 2006, 2007). Given the potential challenges of giving intravenous injections of oncolytic vectors such as VSV (Lun et al., 2006) and the potential induction of neutralizing antibodies (Power et al., 2007), the ability to safely and effectively deliver local gene therapy vectors to patients with tumors of the pleural space make mesothelioma an excellent disease target (Sterman et al., 2007). In recently completed work (Willmon et al., 2009), we have shown the efficacy of a VSV vector expressing murine IFN in a mouse model of mesothelioma with increased safety features. Importantly, this cell line was resistant to effects of murine IFN-β, thus supporting replication. Given this potential advantage of a replicating vector expressing mouse IFN-β in our preclinical models, the purpose of this study was to evaluate the potential antitumor efficacy of a replicating oncolytic virus expressing human IFN-β, VSV.hIFN-β, in a large panel of human mesothelioma cell lines.
Taking the advantages of species-specific binding for IFN-β and its receptor, a panel of human mesothelioma cells were screened for their susceptibility to VSV vectors expressing either human IFN-β (which is species specific and only interacts with the human IFN type I receptor) or mouse IFN-β (which only interacts with the mouse IFN type I receptor). Nine of 12 human mesothelioma lines (including a number of primarily isolated lines) were rapidly lysed by our control vector, VSV.mIFN-β (or by another control vector, VSV.GFP), whereas the four nontransformed mesothelial lines showed more limited toxicity with this vector (Fig. 1A). These data are consistent with previous findings showing that tumor cells, compared with their normal counterparts, are more susceptible to the lytic effects of VSV (Balachandran and Barber, 2000; Stojdl et al., 2000), a result that suggests that majority of mesothelioma lines have some defects in the IFN pathway. In addition, the VSV-mediated cell lysis was completely abolished in our nontransformed mesothelial lines when the vector expressed human IFN-β, a finding that supports the initiative for inserting this antiviral cytokine gene to improve safety (Obuchi et al., 2003).
However, we observed that more than half of the mesothelioma cell lines were protected from VSV-induced lysis in the presence of the hIFN-β transgene, revealing substantial tumor heterogeneity in their IFN responsiveness. We noted three classes of tumor cells with respect to their responses to IFN. Group 1 cells (3 of 12 lines) appeared to be resistant to both control VSV vectors and to VSV.hIFN-β and thus resembled the nontransformed mesothelial lines. Group 2 cells (5 of 12 lines) were lysed by control VSV vectors, but were resistant to lysis by the VSV.hIFN-β vector. In contrast, Group 3 cells (4 of 12 lines) appeared to be completely resistant to the antiviral effect of IFN-β and were thus lysed by both control and VSV.hIFN-β. These in vitro findings were consistent with animal studies conducted with flank mesothelioma tumors in SCID mice. Using the IFN nonresponsive MSTO line, flank tumor growth was significantly inhibited and showed similar sensitivity to VSV.hIFN-β and VSV.mIFN-β (Fig. 2A). In the IFN-responsive REN flank model in SCID mice, significant tumor inhibition was seen with the VSV.mIFN-β, whereas the VSV.hIFN-β vector did not induce a statistically significant difference in tumor size compared with the control group (Fig. 2B).
The heterogeneity in response to type I IFNs seen in our mesothelioma cell lines has been reported in other tumor cell lines in the literature, including cells derived from melanomas (Linge et al., 1995; Wong et al., 1997), lymphomas (Sun et al., 1998), bladder cancers (Matin et al., 2001), renal cancers (Pfeffer et al., 1996), and prostate cancers (Ahmed et al., 2004). This issue was addressed comprehensively by Stojdl et al. (2000) using the NCI 60 tumor cell panel. They found that 81% of lines tested were “nonresponsive” to either IFN-α or IFN-β pretreatment, defined as 5 units of IFN being unable to significantly affect (<10-fold) the effective concentration required to induce 50% effect (EC50) of cells infected with wild-type VSV for 48 hr. The degree of IFN responsiveness (partial vs. total) was not explored in detail, however. Our data in mesothelioma lines, however, show a higher percentage of IFN-sensitive tumor cells.
The mechanism of this heterogeneity was explored in more detail by comparing the LP9 nontransformed line, the partially sensitive REN tumor line (Group 2), and the completely resistant LRK tumor line (Group 3). The resistant line (LRK) had multiple defects. Unlike the normal LP9 cells, no IFN-β mRNA and protein were upregulated in LRK cells following VSV infection (Table 2), and ISG mRNA levels were also unchanged in this cell line after treatment with IFN-β (Table 3A) or after infection with VSV (Table 3B). In contrast, the partially sensitive REN line appeared to be intermediate in these responses. Although the REN cells produced high amounts of IFN-β message after VSV infection (Table 2), their ability to upregulate the message levels of IFN-dependent antiviral proteins after treatment with IFN-β (Table 3A) and after VSV infection (Table 3B) was significantly less than that seen with the nontransformed LP9 cell line. These findings are similar to those described in a panel of glioblastoma cells (Wollmann et al., 2007). In that study, normal human astrocytes were resistant to VSV infection, whereas four of five lines tested were highly susceptible to VSV infection, even in the presence of IFN-α. All of the susceptible cell lines showed defects in their ability to upregulate IFN-regulated proteins, such as MxA, and half had defects in their ability to produce IFN-β message after VSV infection.
To further investigate the mechanisms of heterogeneity in IFN response, quantitative real-time PCR analyses were performed to define possible defects in the type-I IFN signaling pathway among the mesothelioma lines (Fig. 3). Group 1 tumor cells (resistant to all types of VSV) appeared to have highly elevated levels of mRNA for Type I IFN activation pathway genes compared with primary mesothelial cells (Fig. 3). Group 2 tumor cells (sensitive to control VSV but resistant to lysis by VSVh.IFN-β) tended to have similar levels of mRNA for Type I IFN activation pathway genes as the primary mesothelial cells (Fig. 3). Group 3 tumor cells (sensitive to control and VSV.hIFN-β) had markedly reduced or undetectable levels of mRNA of most of the Type I IFN activation pathway genes. The results (with the exception of the MSTO line) indicate that there are losses of multiple components of the IFN pathway which explains why these cells do not respond to IFN.
We also extended these observations to mesothelioma tumors from patients. When we used immunohistochemistry to screen a tissue array of 48 primary tumors for their basal levels of three key components of IFN pathway that were downregulated in cell lines (IFNAR1, p48, and PKR), we found that about half of the primary tumors tested had no defects in any of these components (Fig. 5D), suggesting they would be responsive to IFN. It is interesting to note that p48 (which was uniformly markedly downregulated in our IFN-nonresponsive cell lines) is encoded by a gene on chromosome 14q. A recent “loss of heterozygosity” analysis done by De Rienzo et al. (2000) showed that approximately 43% of mesotheliomas displayed allelic losses from chromosome 14q, perhaps explaining these findings.
Using specimens from a recently conducted clinical trial (Sterman et al., 2007) of intrapleural delivery of Ad.IFN-β in mesothelioma patients, we were also able to directly determine if p48 status might predict response to IFN. In the three cases with sufficient cells, we observed clear p48 expression at baseline and this was associated with strong upregulation of the IFN-dependent protein OAS after Ad.IFN-β gene transfer (Fig. 4 and Supplemental Fig. S3). Although more studies are needed (e.g., in specimens with p48 absent), these data suggest that screening tissue samples using immunohistochemistry for basal expression of proteins such as p48 might be useful for predicting whether viral replication of an INF-containing vector will be likely.
A clinical trial proposing the use of VSV.hIFN-β in patients with liver tumors has already been submitted to the DNA Recombinant Advisory Council and others will likely soon follow. Our data support studies to examine the use of VSV mutant vectors in patients with mesothelioma; however, it is likely that there would be heterogeneity in susceptibility to tumor lysis by this vector and suggests that tests to evaluate the sensitivity of a given tumor to a specific VSV vector might be useful (“personalized medicine”). This could include testing tumor samples for their ability to support VSV replication (Wollmann et al., 2007; Nguyen et al., 2008); however, it would be much more practical to use immunohistochemistry or PCR analysis on tumor biopsies to search for loss of key components of the IFN pathway, such as IFNARs, TyK2, or p48 (Wong et al., 1997). Our data have demonstrated that it is feasible to prescreen patients' responsiveness to IFN prior to treatment. This sort of susceptibility analysis may also be important in predicting the efficacy of other oncolytic viruses (i.e., measles, Newcastle Disease Virus, or Reovirus) that depend on defects in tumor IFN responses for efficacy. In addition to predicting patients who might have optimal response, knowing the IFN status of a tumor might also have practical therapeutic applications. The recent report that histone deacetylase inhibitors blunt the IFN pathway in tumor cells (Nguyen et al., 2008) suggests that these agents might be useful adjuncts in patients who have tumors with some residual IFN sensitivity.
Supplementary Material
Acknowledgments
The authors thank Kirsten Langfield (Mayo Clinic) and Tim Kottke (Mayo Clinic) for their assistance in the preparation of vectors. The mesothelioma tissue array was obtained from the Mesothelioma Research Bank (CDC NIOSH 1-U19-OH009077-01 Mesothelioma Virtual Bank for Translational Research). This work was supported by the Schulze Family Foundation (to R.G.V., M.J.F., and S.J.R.), an NCI (National Cancer Institute) grant P01 CA66726 (to S.M.A.), and an NRSA (National Research Service Award) training grant 5-T32 HL07748 (to L.-C.S.W.).
Author Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- Ahmed C.M.I. Johnson D.E. Demers G.W. Engler H. Howe J.A. Wills K.N. Wen S.F. Shinoda J. Beltran J. Nodelman M. Machemer T. Maneval D.C. Nagabhushan T.L. Sugarman B.J. Interferon alpha2b gene delivery using adenoviral vector causes inhibition of tumor growth in xenograft models from a variety of cancers. Cancer Gene Ther. 2001;8:788–795. doi: 10.1038/sj.cgt.7700364. [DOI] [PubMed] [Google Scholar]
- Ahmed M. Cramer S.D. Lyles D.S. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. doi: 10.1016/j.virol.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Balachandran S. Barber G.N. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- Balachandran S. Porosnicu M. Barber G.N. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman I. Griffin J.A. Gao Y. Whitaker-Dowling P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int. J. Cancer. 2007;121:425–430. doi: 10.1002/ijc.22680. [DOI] [PubMed] [Google Scholar]
- Brin E. Atencio I. Helmich B.K. Maneval D. Laface D. Adenovirus delivery provides extended interferon-alpha exposure and augments treatment of metastatic carcinoma. Cancer Gene Ther. 2006;13:664–75. doi: 10.1038/sj.cgt.7700942. [DOI] [PubMed] [Google Scholar]
- Connell N.D. Rheinwald J.G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983;34:245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- De Rienzo A. Jhanwar S.C. Testa J.R. Loss of heterozygosity analysis of 13q and 14q in human malignant mesothelioma. Genes Chromosomes Cancer. 2000;28:337–341. [PubMed] [Google Scholar]
- Decker T. Stockinger S. Karaghiosoff M. Muller M. Kovarik P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 2002;109:1271–1277. doi: 10.1172/JCI15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R.M. Galivo F. Kottke T. Wongthida P. Qiao J. Thompson J. Valdes M. Barber G. Vile R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Ebert O. Harbaran S. Shinozaki K. Woo S.L. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- Ebert O. Shinozaki K. Huang T.G. Savontaus M.J. Garcia-Sastre A. Woo S.L. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- Huang T.G. Ebert O. Shinozaki K. Garcia-Sastre A. Woo S.L. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 2003;8:434–440. doi: 10.1016/s1525-0016(03)00204-1. [DOI] [PubMed] [Google Scholar]
- Jassar A. Suzuki E. Kapoor V. Sun J. Silverberg M. Cheung L. Burdick M. Strieter R. Ching L.-M. Kaiser L.R. Albelda S.M. Activated tumor-associated macrophages and CD8+ T-cells are the key mediators of anti-tumor effects of the vascular disrupting agent 5,6 di-methylxanthenone-4-acetic acid (DMXAA) in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- Lichty B.D. Power A.T. Stojdl D.F. Bell J.C. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Linge C. Gewert D. Rossmann C. Bishop J.A. Crowe J.S. Interferon system defects in human malignant melanoma. Cancer Res. 1995;55:4099–4104. [PubMed] [Google Scholar]
- Lun X. Senger D.L. Alain T. Oprea A. Parato K. Stojdl D. Lichty B. Power A. Johnston R.N. Hamilton M. Parney I. Bell J.C. Forsyth P.A. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J. Natl. Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- Matin S.F. Rackley R.R. Sadhukhan P.C. Kim M.S. Novick A.C. Bandyopadhyay S.K. Impaired alpha-interferon signaling in transitional cell carcinoma: lack of p48 expression in 5637 cells. Cancer Res. 2001;61:2261–2266. [PubMed] [Google Scholar]
- Murphy J.E. Rheinwald J.G. Intraperitoneal injection of genetically modified, human mesothelial cells for systemic gene therapy. Hum. Gene Ther. 1997;8:1867–1879. doi: 10.1089/hum.1997.8.16-1867. [DOI] [PubMed] [Google Scholar]
- Nguyen T. Abdelbary H. Arguello M. Breitbach C. Leveille S. Diallo J.-S. Yasmeen A. Bismar T.A. Kirn D. Falls T. Snoulten V.E. Vanderhyden B.C. Werier J. Atkins H. Vaha-Koskela M.J.V. Stojdl D.F. Bell J.C. Hiscott J. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuchi M. Fernandez M. Barber G.N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka M. Wiewrodt R. DeLong P. Tanaka T. Zhang Y. Kaiser L. Albelda S. Analysis of the immunologic response generated by Ad.IFN-beta during successful intraperitoneal tumor gene therapy. Mol. Ther. 2002;6:210–218. doi: 10.1006/mthe.2002.0656. [DOI] [PubMed] [Google Scholar]
- Parato K.A. Senger D. Forsyth P.A. Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Canc. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Pfeffer L.M. Wang C. Constantinescu S.N. Croze E. Blatt L.M. Albino A.P. Nanus D.M. Human renal cancers resistant to IFN's antiproliferative action exhibit sensitivity to IFN's gene-inducing and antiviral actions. J. Urol. 1996;156:1867–1871. [PubMed] [Google Scholar]
- Power A.T. Wang J. Falls T.J. Paterson J.M. Parato K.A. Lichty B.D. Stojdl D.F. Forsyth P.A. Atkins H. Bell J.C. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- Remmele W. Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathology. 1987;8:138–140. [PubMed] [Google Scholar]
- Russell S.J. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9:961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- Shinozaki K. Ebert O. Kournioti C. Tai Y.S. Woo S.L. Oncolysis of multifocal hepatocellular canrcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol Ther. 2004;9:368–376. doi: 10.1016/j.ymthe.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Sterman D.H. Albelda S.M. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–283. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- Sterman D.H. Gillespie C.T. Carroll R.G. Coughlin C.M. Lord E.M. Sun J. Haas A. Recio A. Kaiser L.R. Coukos G. June C.H. Albelda S.M. Vonderheide R.H. Interferon beta adenoviral gene therapy in a patient with ovarian cancer. Nat. Clin. Pract. Oncol. 2006;3:633–639. doi: 10.1038/ncponc0658. [DOI] [PubMed] [Google Scholar]
- Sterman D.H. Recio A. Carroll R.G. Gillespie C.T. Haas A. Vachani A. Kapoor V. Sun J. Hodinka R. Brown J.L. Corbley M.J. Parr M. Ho M. Pastan I. Machuzak M. Benedict W. Zhang X.Q. Lord E.M. Litzky L.A. Heitjan D.F. June C.H. Kaiser L.R. Vonderheide R.H. Albelda S.M. Kanther M. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin. Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B. Knowles S. Marius R. Atkins H. Sonenberg N. Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B.D. TenOever B.R. Paterson J.M. Power A.T. Knowles S. Marius R. Reynard J. Poliquin L. Atkins H. Brown E.G. Durbin R.K. Durbin J.E. Hiscott J. Bell J.C. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Sun W.H. Pabon C. Alsayed Y. Huang P.P. Jandeska S. Uddin S. Platanias L.C. Rosen S.T. Interfeon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood. 1998;91:570–576. [PubMed] [Google Scholar]
- Sung C.K. Choi B. Wanna G. Genden E.M. Woo S.L. Shin E.J. Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma. Laryngoscope. 2008;118:237–242. doi: 10.1097/MLG.0b013e3181581977. [DOI] [PubMed] [Google Scholar]
- Wathelet M.G. Lin C.H. Parekh B.S. Ronco L.V. Howley P.M. Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- Willmon C.L. Saloura V. Fridlender Z.G. Wongthida P. Diaz R.M. Thompson J. Kottke T. Federspiel M. Barber G. Albelda S.M. Vile R.G. Expression of IFN-β enhances both efficacy and safety of oncolytic vesicular stomatitis virus (VSV) for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G. Robek M.D. van den Pol A.N. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J. Virol. 2007;81:1479–1491. doi: 10.1128/JVI.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L.H. Krauer K.G. Hatzinisiriou I. Estcourt M.J. Hersey P. Tam N.D. Edmondson S. Devenish R.J. Ralph S.J. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- Wu Y. Lun X. Zhou H. Wang L. Sun B. Bell J.C. Barrett J.W. McFadden G. Biegel J.A. Senger D.L. Forsyth P.A. Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clin. Cancer Res. 2008;14:1218–1227. doi: 10.1158/1078-0432.CCR-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.