Abstract
“T-body” or chimeric antigen receptor (CAR) technology, which combines the specificity of an antibody with the homing, tissue penetration, and target cell destruction of T cells, was first described in 1993. After many years of unmet promise, significant improvements in gene transfer, including the development of efficient retroviral vectors for transduction of human T cells, and better understanding of immunological pathways and immune cell interactions, are allowing this technology to reach a critical phase of evaluation, in which we will learn whether the approach can truly meet expectations. In this review we summarize the concept of CAR-based immunotherapy, describe the steps accomplished, and outline the future progress we need to make if this approach is truly to improve cancer immunotherapy.
Introduction
Adoptive transfer of tumor-infiltrating T lymphocytes (TILs) or antigen-specific cytotoxic T lymphocytes (CTLs) induces objective clinical responses in patients with melanoma (Rosenberg et al., 2008) and Epstein-Barr virus (EBV)-related malignancies (Rooney et al., 1998; Bollard et al., 2004; Comoli et al., 2005; Straathof et al., 2005a). Gene transfer of peptide-specific native αβT cell receptor (TCR) and genetic transfer of chimeric antigen receptors (CARs) are two means by which we can redirect the specificity of polyclonal T lymphocytes and thus more broadly extend these beneficial effects of T cell therapies to other malignancies. Both strategies are appealing because they provide a means of generating large numbers of antigen-specific CTLs directed to otherwise weakly immunogenic tumor-associated antigens. Although compelling results using gene transfer of native αβTCRs have been obtained in patients with melanoma (Morgan et al., 2006), and the approach will likely be extendable to other malignancies, in this review we focus on the use of CARs for T cell-based cancer therapeutics.
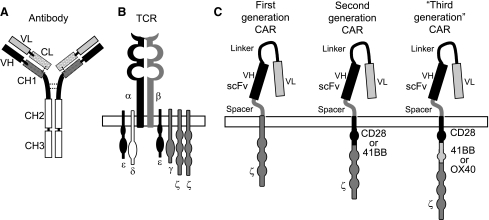
The term “T-body” was coined in 1993 to define chimeric molecules composed of a specific antigen-binding domain encoding the variable regions of a monoclonal antibody, linked together as a single chain antibody (scFv), and a signaling moiety derived from either the ζ chain of the TCR/CD3 complex or the γ chain of the FcɛRI receptor (Eshhar et al., 1993). When expressed by T cells, the chimeric molecules bind the specific antigen expressed on the cell surface of the target cells through their antibody-binding moiety, and activate the lytic pathway of the T cells on cross-linking of the chimeric ζ or γ chains that form the receptor endodomains (Fig. 1). Many researchers now term these T-bodies “chimeric antigen receptors (CARs)” or “chimeric immune receptors (CIRs),” because these hybrid receptors can also include ligands that are not antibody derived (Stastny et al., 2007). Many different CARs have now been cloned, which target antigens expressed either by hematologic malignancies (Hombach et al., 1999; Cooper et al., 2003; Jensen et al., 2003; Vera et al., 2006) or solid tumors (Kershaw et al., 2006; Lamers et al., 2006; Ahmed et al., 2007), and a comprehensive list of these is provided by Sadelain and colleagues (2009). Many of the target antigens for these CARs have been validated in preclinical and clinical studies of the native monoclonal antibody or other ligand used, which facilitates early adoption of CARs in human subjects.
FIG. 1.
Methods to Overcome Tumor Immune Evasion
Advantages of CAR-Based T Cell Immunotherapy
The advantages of CARs over the native antibodies or ligands from which they derive are a consequence of their physical association with effector T cells. Thus, CAR-modified T cells can have an active biodistribution, with migration through multiple tissue planes along chemokine gradients, and can recruit the multiple cytotoxic effector mechanisms available to a T cell, rather than the more restricted cytotoxic machinery associated with, for example, the Fc component of an antibody. CARs also offer advantages over transfer of native αβTCRs. Target cell recognition by αβTCRs is MHC restricted, precluding the design of a “universal” receptor for the treatment of patients with different HLA haplotypes. CARs, by contrast, like monoclonal antibodies, are essentially universal. Moreover, many tumors downmodulate MHC molecules and/or have dysfunctional antigen-processing machinery so that the target antigenic epitopes for αβTCR are simply not present. Because CAR-modified T cells bind directly to native proteins expressed on the surface of target cells without the need of antigen processing or MHC-restricted presentation, they are unaffected by this immune evasion strategy. Moreover, CARs can recognize nonprotein antigens, unlike conventional αβTCRs (Rossig et al., 2001). The major limitation of CARs versus αβTCRs is that they are generally unable to recognize antigens that are internal, even when these are processed to peptides presented by HLA molecules.
Expressing CARs in T Lymphocytes: Do We Need New Tools?
The major goal of adoptive T cell therapy in patients with cancer is to establish effective antitumor activity that can recognize and destroy malignant cells irrespective of their site in the body. Moreover, this activity should be persistent, so that there will be destruction of resurgent malignant cells, even if these arise from phenotypically distinct tumor progenitor cells that are not themselves effectively targeted. For these benefits to be produced, CAR-modified T cells need to have adequate trafficking to the tumor site, be resistant to tumor-related immunosuppressive factors, and have robust and stable expression of their transgenic CAR.
Several methods are currently used to express chimeric molecules in T lymphocytes, each with a different profile of efficiency, cost, and complexity. Because T lymphocytes are highly proliferative, most studies of CAR gene transfer have used vectors that integrate into the host cell DNA. Such vectors were typically based on gammaretroviruses or lentiviruses, but more recent advances in the development of integrating nonviral vectors are beginning to challenge this monopoly.
Viral vectors
Gammaretrovirus-mediated gene transfer to T cells has been tested in several clinical trials (Rosenberg et al., 1990; Bordignon et al., 1995; Heslop et al., 1996; Bonini et al., 1997). Such retroviruses integrate into the host genome (Miller et al., 1990; Hu and Pathak, 2000) and produce consistent gene expression in human T cells and their progeny. These vectors, however, have several limitations (Hu and Pathak, 2000). They can transduce only dividing cells (Miller et al., 1990), have limited cargo capacity (Hu and Pathak, 2000), can cause insertional mutagenesis (Hacein-Bey-Abina et al., 2008), and are expensive to produce and use clinically. Moreover, transgene expression tends to decline over time, although it can be increased after T cell activation through their CAR or native αβTCR (Pule et al., 2008). These limitations notwithstanding, retroviral gene transfer has shown an acceptable profile of efficacy and safety for expressing CAR and other transgenes in T lymphocytes (Bonini et al., 2003; Brenner and Heslop, 2003). Optimization of the vector cassette and advances in bicistronic and tricistronic vector design allow three or potentially more distinct cDNAs to be expressed by retrovirally transduced T cells (Quintarelli et al., 2007; Di Stasi et al., 2009). Most importantly perhaps, no adverse effects related to insertional mutagenesis of T lymphocytes by integrated provirus have been reported in any patient infused with retroviral gene-modified T cells during the past 20 years (Bonini et al., 2003; Brenner and Heslop, 2003). This observation markedly contrasts with experience in patients receiving hematopoietic stem cells (HSCs) that have been similarly modified with gammaretroviruses (Hacein-Bey-Abina et al., 2003, 2008). Such clinical observations parallel experimental data showing that retrovirally transduced T cells do not undergo malignant transformation in RAG1-deficient mice, unlike gene-modified HSCs (Montini et al., 2006). Overall, it seems likely that highly differentiated cells, such as mature T lymphocytes, are less likely to undergo malignant change associated with the genotoxicity of integrating gammaretroviruses.
Lentiviral vectors
Lentiviral vectors may offer certain advantages over gammaretroviral vectors. They can transduce nondividing or minimally proliferating T lymphocytes (Naldini et al., 1996; Hu and Pathak, 2000), and the reduced requirement for ex vivo activation before transduction may maximize long-term in vivo persistence of the transduced cells, by reducing activation-induced cell death or clonal exhaustion (Cavalieri et al., 2003). Compared with gammaretroviruses, lentiviruses also have enhanced cargo capacity, and reduced susceptibility to gene silencing. Although genotoxicity due to insertional mutagenesis may still occur, the frequency is apparently lower as there is a reduced probability of integration into transcriptionally sensitive sites (Montini et al., 2006). Modifications to lentiviruses to make them self-inactivating after integration may further lower this risk.
Nonviral vector gene transfer
Although DNA plasmid-based gene delivery has greatly reduced the cost of manufacturing and testing, the use of this approach for CAR gene transfer has been limited by the inefficiency of the process and the transience of expression due to lack of transgene integration—a lethal flaw for transduction of a rapidly dividing population such as T cells. Improvements in electroporation of T lymphocytes have significantly enhanced the efficiency of gene delivery, and reduced the toxicity of the procedure. Without a high rate of plasmid integration, however, investigators are compelled to extensively culture the transduced cells to expand the rare clones in which stable expression is achieved. Unfortunately, this often exhausts the T cells and renders them unfit to expand further and persist in vivo (Park et al., 2007; Till et al., 2008). Transposon-based gene delivery systems may overcome this limitation, by combining transposons and transposases with the gene of interest in the plasmid. Systems such as Sleeping Beauty (Huang et al., 2008; Singh et al., 2008) and PiggyBac (Wilson et al., 2007) are currently being considered to express CARs in T lymphocytes. It remains to be seen whether genomic integration mediated by transposon-based gene delivery is safe and efficient in vivo or whether insertional mutagenesis and transgene silencing will occur.
Other technologies have also been explored for gene transfer to T lymphocytes. In principle, for example, messenger RNA (Mitchell et al., 2008) or protein transfer can be used to force CAR expression in T lymphocytes. These approaches may be safe, but the transience of their effects means they are likely of limited clinical value for CAR transfer.
Initial Clinical Trials of CAR-Modified T Cells
Despite consistent and robust expression of CAR molecules in T cells, early clinical studies of the approach were disappointing. The first trials were in patients infected with HIV, who received T lymphocytes expressing a CAR (CD4ζ) binding to the HIV gp120 envelope expressed on the surface of infected cells. Infusions of syngeneic or autologous CD4ζ-modified CD4+ and CD8+ T cells were well tolerated but produced minimal antiviral activity. Although gene-modified T cells were detectable in the bloodstream for up to 1 year, and trafficked to the gut-associated HIV reservoir, the percentage of gene-modified cells was low and substantially declined with time (Mitsuyasu et al., 2000; Walker et al., 2000).
Initial studies in patients with cancer were equally discouraging. T cells with a CAR targeting the α-folate receptor (FR) were infused into patients with metastatic ovarian cancer. Fourteen patients received 3 × 109 to 1.7 × 1012 cells without toxicity. However, antitumor responses were not measurable and transgenic T cells were barely detectable in the circulation beyond 3 weeks after infusion, with only one exception of persistence 1 year after treatment, albeit at low levels (Kershaw et al., 2006). A second study was conducted in patients with neuroblastoma, using T cells electroporated with a plasmid encoding a CAR targeting the L1-cell adhesion molecule (L1-CAM) expressed by neuroblasts. A total of 6 patients with refractory neuroblastoma received up to 3 infusions of gene-modified T cells (108 and/or 109 cells). The toxicity was acceptable, although bone pain requiring narcotics was reported shortly after the infusion of T cells in a patient with extensive osseous metastases. Tumor responses were modest and incomplete, and again persistence of the infused cells was limited (Park et al., 2007). Finally, subjects with relapsed or refractory B cell lymphomas received T cells transfected by electroporation with a DNA plasmid encoding a CAR targeting the CD20 antigen. Seven patients were treated with multiple infusions and some also received subcutaneous injections of recombinant interleukin (IL)-2. No adverse events attributable to the T cell infusions were observed. T cells were documented to traffic to the bone marrow, although the number of circulating CD20+ B cells remained stable or slightly increased after treatment in all patients, with marginal antitumor effects. The administration of low-dose IL-2 prolonged the persistence of modified T cells but not beyond 9 weeks (Till et al., 2008).
CAR-Modified T Cells and Lack of Costimulation
Although the results of these studies were clinically disappointing, they proved instrumental in showing that a major problem of CAR-modified T cells is their lack of expansion and persistence in vivo, which is in sharp contrast with the behavior of adoptively transferred antigen-specific CTLs (Heslop et al., 1996). A number of factors likely contribute to these differences. Elimination of the cells by an immune response directed to the CAR can certainly occur (Lamers et al., 2006), and it is also true that the culture conditions used to generate T cells were suboptimal. Thus activation and expansion of naive T cells before gene transfer require optimal costimulation provided by CD28 and tumor necrosis factor (TNF) family members (Paulos et al., 2008; Boesteanu and Katsikis, 2009). Because the clinical-grade reagents for this costimulation are not readily available, investigators have cross-linked CD3 with monoclonal antibodies, with suboptimal consequences for T cell survival. More recently, activation with beads coexpressing OKT3 and CD28 has been used and may produce a more active and persistent product (Paulos et al., 2008). The substitution of cytokine cocktails such as IL-7, IL-15, and IL-21 for IL-2 alone also serves to better preserve the subset of T cells with a central-memory phenotype (TCM), and thereby favor long-term persistence (Ma et al., 2006; Andorsky and Timmerman, 2008).
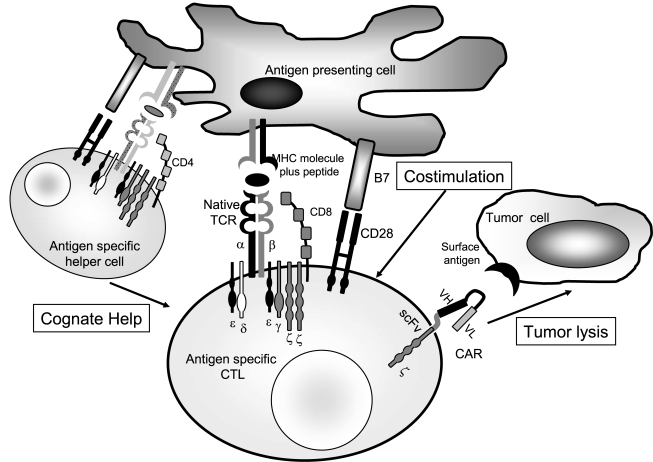
While the previously described mechanisms may help to explain the poor in vivo persistence and activity of CAR-modified T cells, it is increasingly apparent that a major factor is the inability of CAR engagement alone to recapitulate the costimulatory events that follow the physiologic engagement of the native αβTCR. Full activation and proliferation of T cells require not only TCR engagement (first signal) but also costimulation provided by antigen-presenting cells (APCs, second signal) and cytokines (third signal). A multiplicity of these costimulatory receptor–ligand and cytokine signals is required, in an optimal temporal and spatial sequence. CAR-redirected T cells lack any such costimulation when they engage tumor cells, because these target cells are deficient in costimulatory molecule expression (e.g., CD80 and CD86) and do not release helper cytokines. Moreover, CAR-modified T cells cannot receive activation through stimulation provided by professional APCs in secondary lymphoid organs because the native receptors on CAR-modified T cells are not specifically directed toward antigens on the hosts' APCs.
Two approaches have been adopted to compensate for the lack of costimulation after CAR engagement
Costimulatory endodomains
The first approach is to incorporate costimulatory signaling domains as part of the CAR itself. Thus, domains derived from CD28 (Finney et al., 1998; Maher et al., 2002; Kowolik et al., 2006; Vera et al., 2006), 4-1BB (Imai et al., 2004), or OX40 (Pule et al., 2005) molecules have been incorporated as single or multiple (Pule et al., 2005; Milone et al., 2009) endodomains in tandem in the CAR molecule to generate “second- and third-generation” CARs (Fig. 1). Antigen engagement of these CARs is followed by T cell activation, proliferation, and IL-2 secretion, even without cross-presentation by APCs (Maher et al., 2002; Vera et al., 2006). Experiments in severe combined immunodeficient (SCID) mice are being conducted to define the best combinations of costimulation required for CAR-modified T cells. So far the data seem to suggest that the optimal combination may depend on the affinity of the single-chain antibody, the level of CAR expression and its intrinsic structure (hinge region, spacer region, and transmembrane domain), as well as on the density of antigen expression by tumor cells and the tumor environment. It is difficult for a murine SCID system to accurately model all these variables, particularly because CAR-modified T cells also interact with other immune system cells in an immunocompetent individual. Because of these limitations, it is essential to validate any conclusions from animal models in small early-phase clinical trials. Such studies using polyclonal activated T lymphocytes expressing these novel “second-generation” CARs are currently open at several institutions. It will likely remain difficult to predict which costimulatory combination will be superior in any given setting, and a side-by-side comparison of CARs containing different costimulatory endodomains infused simultaneously in the same patient may be the most efficient way of optimizing the approach.
Antigen-specific CTLs
The second approach for providing costimulation is to express the CAR in antigen-specific CTLs, whose native αβTCR is targeting for an antigen known to be present on host professional APCs. In this way, native receptor engagement ensures all costimulation is received physiologically, and chimeric receptor engagement serves exclusively as a means of retargeting an already costimulated cell. This approach has been validated preclinically in vitro (Rossig et al., 2002; Landmeier et al., 2007) and in vivo (Duraiswamy et al., 2003; Savoldo et al., 2007), and we extended it to EBV antigen-specific CTLs redirected to the GD2 antigen present on many human tumors including neuroblastoma (Pule et al., 2008) (Fig. 2). Adoptive transfer of EBV-specific CTLs effectively treats EBV-driven lymphomas after allogeneic hematopoietic stem cell (HSC) transplantation (Rooney et al., 1995, 1998). Studies using a “gene-marking” approach demonstrated the persistence in vivo of EBV-specific CTLs for more than 10 years after their adoptive transfer (Heslop et al., 1996). These clinical observations indicate that the infused CTLs, although phenotypically predominantly effector T cells (TE) and effector-memory T cells (TEM) (Pule et al., 2008), contain a fraction of cells that have functionally long-term memory properties and persist when receiving an efficient costimulation from latently EBV-infected APCs (Heslop et al., 1996). We therefore engrafted EBV-specific CTLs and primary T cells with distinguishable GD2-specific CARs and infused both sets of autologous cells into patients with neuroblastoma. We found that CAR-modified EBV-specific CTLs had enhanced and prolonged survival compared with polyclonal activated T cells expressing the same CAR, and produced objective tumor responses including complete remission (Pule et al., 2008). We are now using this approach for patients with lymphoma, comparing the expansion and persistence of EBV-specific CTLs expressing a “first-generation” CAR targeting the CD19 molecule with that of polyclonal T lymphocytes expressing a “second-generation” CAR targeting the same CD19 molecule but also encoding the CD28 endodomain. This study will allow us to formally evaluate whether physiologic or synthetic costimulation is superior for CAR-modified human T cells in vivo. Finally, in addition to appropriate in vivo costimulation, expressing CARs on antigen-specific CTLs of defined specificity may allow the use of vaccination to boost CAR cellular immune responses.
FIG. 2.
Methods to Overcome Tumor Immune Evasion
Does CAR Expression in Specific T Cell Subsets Matter?
In the previous section we discussed the potential advantages of expressing a CAR in a defined CTL population. There are, of course many other subsets of T cells in which CAR can be expressed, and some of these may have superior overall functionality to others. In addition to polyclonal activated T lymphocytes, other T cell subsets including γδT cells and natural killer (NK) cells have been used as platforms for CAR modification.
γδT lymphocytes
γδT lymphocytes represent less then 5% of human T cells, and are preferentially localized in organs containing epithelial cells (skin, lung, intestine, and genitourinary tract) (Nanno et al., 2007). They have MHC-unrestricted cytotoxic activity. Ex vivo-expanded human Vγ9Vδ2+ T cells have antitumor activity against several human malignancies and, when engineered to express CAR molecules, they acquire specific cytotoxic activity against other tumor cells (Rischer et al., 2004). Despite the scarcity of γδT cells in the peripheral blood, these cells can be expanded ex vivo and in vivo using a combination of IL-2 and zoledronate, a pharmacologic agent that promotes the accumulation of metabolites, such as isopentenyl pyrophosphate, which act as endogenous ligands to stimulate Vγ9Vδ2+ T cells (Dieli et al., 2007). Clinical trials using CAR-modified γδT cells are planned, although we know little about the likely persistence of these cells in vivo after adoptive transfer. However, their high tropism for epithelia may be useful for treating several types of carcinomas.
Natural killer cells
NK cells represent less than 15% of lymphocytes and are characterized by the expression of CD56 (bright or dim) and of Fcγ receptor III (FcγRIII, CD16) and by their lack of CD3 (Cooper et al., 2001). Although the mechanisms by which NK cells recognize target cells are complex and incompletely understood, NK receptors for MHC class I molecules are crucial for discriminating normal from pathogenic cells (Cooper et al., 2001). NK cells have long been considered as important for tumor immune surveillance (Cooper et al., 2001) and after HLA-haploidentical HSC transplantation for patients with myeloid malignancies recognition of killer-immunoglobulin receptors (KIRs) and MHC disparities may contribute greatly to the graft-versus-leukemia effect (Ruggeri et al., 2008). NK cells have been used as an alternative lymphocyte platform for the expression of CARs, and in preclinical studies they have potent antitumor activity (Imai et al., 2005). As for γδT cells, little is known of the survival and persistence of NK cells after adoptive transfer.
Central memory cells
Studies in nonhuman primates have shown that virus-specific CTLs reexpanded from the CD62L+ fraction have the highest capacity to persist in vivo after adoptive transfer (Berger et al., 2008). Preclinical evaluations of CAR-modified T cells obtained from the CD62L+ subpopulation will determined whether they are superior to unfractionated T lymphocytes in establishing a long-term memory pool.
Sustaining in Vivo Expansion of Adoptively Transferred CAR-Modified T Cells
Several studies of adoptive T cell therapy suggest that robust and sustained clinical responses are best obtained by ensuring in vivo expansion of adoptively transferred antigen-specific CTLs (Dudley and Rosenberg, 2003; Robbins et al., 2004). Although costimulation is undoubtedly one component of successful longevity, the contribution of exogenous cytokines is likely also to be considerable. These cytokines, including IL-2, IL-7 and IL-15, promote both T cell expansion and survival in vivo (Ma et al., 2006).
At the simplest level, systemic administration of recombinant IL-2 has been used to support adoptive T cell therapies, including gene-modified T cells by CAR or transgenic αβTCR expression (Yee et al., 2002; Dudley and Rosenberg, 2003; Rosenberg et al., 2008; Till et al., 2008). Unfortunately, prolonged administration is associated with significant toxicity (Dudley et al., 2005; Rosenberg et al., 1994, 2008) and the concomitant expansion of IL-2 receptor-positive regulatory T cells (Treg) may progressively impair the function of effector T cells (Ahmadzadeh and Rosenberg, 2006). One means of capturing the benefits of the other cytokines important in T lymphocyte expansion and survival is to produce lymphodepletion before T cell infusion (Gattinoni et al., 2005). This manipulation induces release of homeostatic cytokines such as IL-7 and IL-15, promoting expansion of adoptively transferred lymphocytes including CAR-modified T cells (Dudley et al., 2005). However, the toxicities of agents required to produce such profound lymphodepletion may be unacceptable in patients who have already endured multiple rounds of intensive chemotherapy, and may promote adverse effects mediated by abrupt cytokine release by infused T cells (Brentjens et al., 2009).
Administration of the relevant cytokines directly may obviate the requirement for lymphodepletion. Studies in nonhuman primates have shown the safety and clinical benefits of systemic administration of IL-15, and this cytokine will soon be tested in clinical trials (Berger et al., 2009). Transgenic production of IL-15 by CAR-modified T cells may also be an option (Quintarelli et al., 2007), with the advantage of providing the cytokine only where needed. Similarly, IL-7 is essential for homeostatic expansion of naive T cells (Schluns et al., 2000; Ma et al., 2006) and for maintaining the memory T cell population (Kaech et al., 2003; Nanjappa et al., 2008). Administration of IL-7 accelerates immune reconstitution in murine models (Alpdogan et al., 2003) and the cytokine has been well tolerated in early-phase clinical trials, in which it produced polyclonal expansion of naive CD4+ and CD8+ T lymphocytes and no evident Treg expansion (Rosenberg et al., 2006; Sportes et al., 2008). IL-7 administration could enhance the expansion of adoptively transferred T cells if they physiologically reexpressed the IL-7 receptor α chain (Powell et al., 2005) or if such expression were forced by genetic manipulation (Vera et al., 2009).
Rendering CAR-Modified T Cells Resistant to Tumor Immune Evasion
Although CAR-modified T cells need to be present in sufficient numbers and for long enough to eradicate resurgent tumor cells, achieving these end points will likely not, per se, be sufficient to eradicate tumors. CAR-modified T cells must also efficiently traffic to the tumor sites and, once there, sustain their effectiveness in the presence of an array of immune evasion strategies used by the tumor cells (Zou, 2005).
Many tumor cells or their associated stroma produce cytokines that attract inhibitory rather than effector T cells. Further genetic modification of T lymphocytes to express the relevant chemokine receptor can overcome this difficulty, and ensure that the CAR-modified T cell can efficiently arrive at the tumor. The feasibility of this approach has already been shown for melanomas, which produce Groα and for Hodgkin's lymphoma (HL), which produces TARC (thymus and activation regulated chemokine) (Poppema et al., 1998). T cells engineered to express CXCR2 will preferentially traffic to melanomas (Kershaw et al., 2002), whereas T cells expressing CCR4 will traffic to Hodgkin's lymphoma (Di Stasi et al., 2009). Thus, coexpression of a CAR targeting the CD30 antigen on HL and of CCR4 enhanced antitumor activity in vivo in an HL xenograft model (Di Stasi et al., 2009) (Table 1).
Table 1.
Methods to Overcome Tumor Immune Evasion
| Immune evasion mechanism | Example | Overcoming strategy |
|---|---|---|
| Release of inhibitory molecules | TGF-β | Dominant negative TGF-β receptor (Bollard et al., 2002) |
| FasL | Downregulation of the receptor (Dotti et al., 2005) | |
| Impaired tumor trafficking | TARC/Groα | Forced expression of the specific chemokine receptor (CCR4 [Di Stasi et al., 2009], CXCR2 [Kershaw et al., 2002]) |
| Tumor infiltration by inhibitory cells | Th2 and Treg cells | Second-generation CAR (Loskog et al., 2006) |
| Limited in vivo T cell expansion | Lack of local cytokines | Infusion of exogenous cytokines (IL-2 [Rosenberg et al., 1994], IL-7 [Rosenberg et al., 2006; Sportes et al., 2008], IL-15 [Berger et al., 2009]) |
| Transgenic production of cytokines (IL-2 and IL-15) (Quintarelli et al., 2007; Liu and Rosenberg, 2001) | ||
| Lymphodepletion (Dudley et al., 2008) | ||
| Forced expression of IL-7 receptors (Vera et al., 2009) | ||
| Limited in vivo T cell persistence | Lack of local costimulation | Second- or third-generation CARs (Sadelain et al., 2009) |
| Expression of CAR on antigen-specific CTLs (Pule et al., 2008; Savoldo et al., 2007) |
Abbreviations: CAR, chimeric antigen receptor; CTLs, cytotoxic T lymphocytes; FasL, Fas ligand; IL, interleukin; TARC, thymus and activation regulated chemokine; TGF, transforming growth factor; Th2, helper T cell type 2; Treg, regulatory T cells.
Once at the tumor site, countermeasures to more active immune evasion strategies can be implemented. Both the hypoxic environment, which accompanies large tumors, and the antiinflammatory cytokines they release normally impede T cell function. Inhibitory molecules such as transforming growth factor (TGF)-β, Fas ligand, IL-10, and indoleamine 2,3-dioxygenase (IDO) also attract cells with inhibitory function, such as Th2 cells, suppressor dendritic cells, or regulatory T cells (Zou, 2005). Genetic modification of the CAR itself (Loskog et al., 2006), or of CAR-redirected T cells can overcome all of these (Bollard et al., 2002; Dotti et al., 2005) (Table 1).
“On” and “Off” Target Toxicities
One potential concern about the clinical effects of CAR is toxicity related to the normal tissue distribution of the targeted antigen. Ideally, the targeted antigen should be chosen on the basis of its selective expression by malignant cells, but for many tumor antigens the target is expressed only at differential levels on the malignant cell. For example, carbonic anhydrase IX (CAIX) is frequently overexpressed by clear cell renal carcinoma (RCC) but is also expressed by bile duct epithelial cells. Cholangitis with periductal T cell infiltration was observed when patients with metastatic renal cell carcinoma were treated with autologous T lymphocytes expressing a CAR targeting CAIX (Lamers et al., 2006). One way to obviate this type of problem may involve selection of single-chain antibodies that have low target affinity, so that (normal) low-expressing cells are spared. Alternatively, an inducible system may be used to favor the expression of the transgene at the specific tumor site (Kim et al., 2008). For other tumors, the CAR is deliberately directed toward a lineage-restricted antigen present on both normal and malignant cells. For example, CARs targeting B lymphocyte self-antigens, such as CD19 and CD20, will inevitably target both leukemic cells derived from the B cell compartment and normal lymphocytes, with potential effects on humoral immunity. In this example, infusion of human gamma globulins may compensate for the deficit, which would be deemed acceptable if the approach treats an immediately life-threatening condition. As we increase the potency of CAR-modified T cells and enhance their ability to survive, function, and expand in vivo, it is likely that the adverse consequences of these “on and off” target effects will become more evident. For this reason, there is increasing interest in the possibility of including a suicide gene in the CAR-modified T cells so that progressive or unwanted “on and off” target effects could be more readily controlled (Bonini et al., 1997; Straathof et al., 2005b; Quintarelli et al., 2007).
Conclusion and Future Perspectives
Although a long stretch of CAR-modified T cell development lies ahead, the first glimmers of effectiveness are with us, and the signposts to future success are clearly illuminated. Although we are unfortunately not “nearly there” yet, the second half of the journey should be more clinically productive than the first. Imminent clinical trials will use T cells that have been more extensively engineered, to contain countermeasures to tumor immune evasion strategies such as production of inhibitory cytokines, and to incorporate more effective killing mechanisms such as immunotoxins. A broader range of solid tumors will also be targeted. But beyond these scientific developments, there will also be concomitant technical improvements in cell preparation and testing that will simplify and accelerate administration of these cells to patients with disease, and that will facilitate application outside the current limited number of highly specialized centers. These technological and distributive advances will themselves be driven by demonstrations of the incontrovertible effectiveness of CAR-modified T cells and by confirmation of their superior pharmaco-economics due to fewer adverse effects than conventional agents.
Acknowledgments
This work was supported in part by the Leukemia & Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) (M.K.B.), NIH RO1CA131027 (B.S.), Leukemia & Lymphoma Society Translational Research grants (G.D. and BS), and a Doris Duke Charitable Foundation/Clinical Scientist development award (G.D.). All authors contributed in writing the manuscript.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- Ahmadzadeh M. Rosenberg S.A. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N. Ratnayake M. Savoldo B. Perlaky L. Dotti G. Wels W.S. Bhattacharjee M.B. Gilbertson R.J. Shine H.D. Weiss H.L. Rooney C.M. Heslop H.E. Gottschalk S. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- Alpdogan O. Muriglan S.J. Eng J.M. Willis L.M. Greenberg A.S. Kappel B.J. van den Brink M.R. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J. Clin. Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorsky D.J. Timmerman J.M. Interleukin-21: Biology and application to cancer therapy. Expert Opin. Biol. Ther. 2008;8:1295–1307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- Berger C. Jensen M.C. Lansdorp P.M. Gough M. Elliott C. Riddell S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. Berger M. Hackman R.C. Gough M. Elliott C. Jensen M.C. Riddell S.R. Safety and immunological effects of IL-15 administration in nonhuman primates. Blood. 2009 doi: 10.1182/blood-2008-12-189266. 2009 Jul 15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesteanu A.C. Katsikis P.D. Memory T cells need CD28 costimulation to remember. Semin. Immunol. 2009;21:69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard C.M. Rossig C. Calonge M.J. Huls M.H. Wagner H.J. Massague J. Brenner M.K. Heslop H.E. Rooney C.M. Adapting a transforming growth factor β-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- Bollard C.M. Aguilar L. Straathof K.C. Gahn B. Huls M.H. Rousseau A. Sixbey J. Gresik M.V. Carrum G. Hudson M. Dilloo D. Gee A. Brenner M.K. Rooney C.M. Heslop H.E. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J. Exp. Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini C. Ferrari G. Verzeletti S. Servida P. Zappone E. Ruggieri L. Ponzoni M. Rossini S. Mavilio F. Traversari C. Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- Bonini C. Grez M. Traversari C. Ciceri F. Marktel S. Ferrari G. Dinauer M. Sadat M. Aiuti A. Deola S. Radrizzani M. Hagenbeek A. Apperley J. Ebeling S. Martens A. Kolb H.J. Weber M. Lotti F. Grande A. Weissinger E. Bueren J.A. Lamana M. Falkenburg J.H. Heemskerk M.H. Austin T. Kornblau S. Marini F. Benati C. Magnani Z. Cazzaniga S. Toma S. Gallo-Stampino C. Introna M. Slavin S. Greenberg P.D. Bregni M. Mavilio F. Bordignon C. Safety of retroviral gene marking with a truncated NGF receptor. Nat. Med. 2003;9:367–369. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- Bordignon C. Notarangelo L.D. Nobili N. Ferrari G. Casorati G. Panina P. Mazzolari E. Maggioni D. Rossi C. Servida P. Ugazio A.G. Mavilio F. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- Brenner M.K. Heslop H.E. Is retroviral gene marking too dangerous to use? Cytotherapy. 2003;5:190–193. doi: 10.1080/14653240310001307. [DOI] [PubMed] [Google Scholar]
- Brentjens R. Riviere I. Hollyman D. Taylor C. Nikhamin Y. Stefanski J. Lee J. Yeh R. Santos E. Sadelain M. Unexpected toxicity of cyclophosphamide followed by adoptively transferred CD19-targeted T cells in a patient with bulky CLL [abstract] Mol. Ther. 2009;17(Suppl. 1) 2009. [Google Scholar]
- Cavalieri S. Cazzaniga S. Geuna M. Magnani Z. Bordignon C. Naldini L. Bonini C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- Comoli P. Pedrazzoli P. Maccario R. Basso S. Carminati O. Labirio M. Schiavo R. Secondino S. Frasson C. Perotti C. Moroni M. Locatelli F. Siena S. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J. Clin. Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- Cooper L.J. Topp M.S. Serrano L.M. Gonzalez S. Chang W.C. Naranjo A. Wright C. Popplewell L. Raubitschek A. Forman S.J. Jensen M.C. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- Cooper M.A. Fehniger T.A. Turner S.C. Chen K.S. Ghaheri B.A. Ghayur T. Carson W.E. Caligiuri M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- Dieli F. Vermijlen D. Fulfaro F. Caccamo N. Meraviglia S. Cicero G. Roberts A. Buccheri S. D'Asaro M. Gebbia N. Salerno A. Eberl M. Hayday A.C. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A. De Angelis B. Rooney C.M. Zhang L. Mahendravada A. Foster A.E. Heslop H.E. Brenner M.K. Dotti G. Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G. Savoldo B. Pule M. Straathof K.C. Biagi E. Yvon E. Vigouroux S. Brenner M.K. Rooney C.M. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Rosenberg S.A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Wunderlich J.R. Yang J.C. Sherry R.M. Topalian S.L. Restifo N.P. Royal R.E. Kammula U. White D.E. Mavroukakis S.A. Rogers L.J. Gracia G.J. Jones S.A. Mangiameli D.P. Pelletier M.M. Gea-Banacloche J. Robinson M.R. Berman D.M. Filie A.C. Abati A. Rosenberg S.A. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Yang J.C. Sherry R. Hughes M.S. Royal R. Kammula U. Robbins P.F. Huang J. Citrin D.E. Leitman S.F. Wunderlich J. Restifo N.P. Thomasian A. Downey S.G. Smith F.O. Klapper J. Morton K. Laurencot C. White D.E. Rosenberg S.A. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy J. Burrows J.M. Bharadwaj M. Burrows S.R. Cooper L. Pimtanothai N. Khanna R. Ex vivo analysis of T-cell responses to Epstein-Barr virus-encoded oncogene latent membrane protein 1 reveals highly conserved epitope sequences in virus isolates from diverse geographic regions. J. Virol. 2003;77:7401–7410. doi: 10.1128/JVI.77.13.7401-7410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z. Waks T. Gross G. Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. U.S.A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney H.M. Lawson A.D. Bebbington C.R. Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- Gattinoni L. Finkelstein S.E. Klebanoff C.A. Antony P.A. Palmer D.C. Spiess P.J. Hwang L.N. Yu Z. Wrzesinski C. Heimann D.M. Surh C.D. Rosenberg S.A. Restifo N.P. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M. Le Deist F. Wulffraat N. McIntyre E. Radford I. Villeval J.L. Fraser C.C. Cavazzana-Calvo M. Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P. Soulier J. Lim A. Morillon E. Clappier E. Caccavelli L. Delabesse E. Beldjord K. Asnafi V. Macintyre E. Dal C.L. Radford I. Brousse N. Sigaux F. Moshous D. Hauer J. Borkhardt A. Belohradsky B.H. Wintergerst U. Velez M.C. Leiva L. Sorensen R. Wulffraat N. Blanche S. Bushman F.D. Fischer A. Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop H.E. Ng C.Y. Li C. Smith C.A. Loftin S.K. Krance R.A. Brenner M.K. Rooney C.M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Hombach A. Heuser C. Sircar R. Tillmann T. Diehl V. Pohl C. Abken H. Characterization of a chimeric T-cell receptor with specificity for the Hodgkin's lymphoma-associated CD30 antigen. J. Immunother. 1999;22:473–480. doi: 10.1097/00002371-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Hu W.S. Pathak V.K. Design of retroviral vectors and helper cells for gene therapy. Pharmacol. Rev. 2000;52:493–511. [PubMed] [Google Scholar]
- Huang X. Guo H. Kang J. Choi S. Zhou T.C. Tammana S. Lees C.J. Li Z.Z. Milone M. Levine B.L. Tolar J. June C.H. Scott M.R. Wagner J.E. Blazar B.R. Zhou X. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol. Ther. 2008;16:580–589. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C. Mihara K. Andreansky M. Nicholson I.C. Pui C.H. Geiger T.L. Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- Imai C. Iwamoto S. Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.C. Cooper L.J. Wu A.M. Forman S.J. Raubitschek A. Engineered CD20-specific primary human cytotoxic T lymphocytes for targeting B-cell malignancy. Cytotherapy. 2003;5:131–138. doi: 10.1080/14653240310001028. [DOI] [PubMed] [Google Scholar]
- Kaech S.M. Tan J.T. Wherry E.J. Konieczny B.T. Surh C.D. Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kershaw M.H. Wang G. Westwood J.A. Pachynski R.K. Tiffany H.L. Marincola F.M. Wang E. Young H.A. Murphy P.M. Hwu P. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- Kershaw M.H. Westwood J.A. Parker L.L. Wang G. Eshhar Z. Mavroukakis S.A. White D.E. Wunderlich J.R. Canevari S. Rogers-Freezer L. Chen C.C. Yang J.C. Rosenberg S.A. Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Peng G. Hicks J.M. Weiss H.L. Van Meir E.G. Brenner M.K. Yotnda P. Engineering human tumor-specific cytotoxic T cells to function in a hypoxic environment. Mol. Ther. 2008;16:599–606. doi: 10.1038/sj.mt.6300391. [DOI] [PubMed] [Google Scholar]
- Kowolik C.M. Topp M.S. Gonzalez S. Pfeiffer T. Olivares S. Gonzalez N. Smith D.D. Forman S.J. Jensen M.C. Cooper L.J. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- Lamers C.H. Sleijfer S. Vulto A.G. Kruit W.H. Kliffen M. Debets R. Gratama J.W. Stoter G. Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Landmeier S. Altvater B. Pscherer S. Eing B.R. Kuehn J. Rooney C.M. Juergens H. Rossig C. Gene-engineered varicella-zoster virus reactive CD4+ cytotoxic T cells exert tumor-specific effector function. Cancer Res. 2007;67:8335–8343. doi: 10.1158/0008-5472.CAN-06-4426. [DOI] [PubMed] [Google Scholar]
- Liu K. Rosenberg S.A. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J. Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskog A. Giandomenico V. Rossig C. Pule M. Dotti G. Brenner M.K. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- Ma A. Koka R. Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Maher J. Brentjens R.J. Gunset G. Riviere I. Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Miller D.G. Adam M.A. Miller A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone M.C. Fish J.D. Carpenito C. Carroll R.G. Binder G.K. Teachey D. Samanta M. Lakhal M. Gloss B. net-Desnoyers G. Campana D. Riley J.L. Grupp S.A. June C.H. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A. Karikari I. Cui X. Xie W. Schmittling R. Sampson J.H. Selective modification of antigen-specific T cells by RNA electroporation. Hum. Gene Ther. 2008;19:511–521. doi: 10.1089/hum.2007.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyasu R.T. Anton P.A. Deeks S.G. Scadden D.T. Connick E. Downs M.T. Bakker A. Roberts M.R. June C.H. Jalali S. Lin A.A. Pennathur-Das R. Hege K.M. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M. Sanvito F. Ponzoni M. Bartholomae C. Sergi S.L. Benedicenti F. Ambrosi A. Di Serio C. Doglioni C. Von Kalle C. Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R. Hughes M.S. Yang J.C. Sherry R.M. Royal R.E. Topalian S.L. Kammula U.S. Restifo N.P. Zheng Z. Nahvi A. de Vries C.R. Rogers-Freezer L.J. Mavroukakis S.A. Rosenberg S.A. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gallay P. Ory D. Mulligan R. Gage F.H. Verma I.M. Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nanjappa S.G. Walent J.H. Morre M. Suresh M. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J. Clin. Invest. 2008;118:1027–1039. doi: 10.1172/JCI32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanno M. Shiohara T. Yamamoto H. Kawakami K. Ishikawa H. γδ T cells: Firefighters or fire boosters in the front lines of inflammatory responses. Immunol. Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Park J.R. Digiusto D.L. Slovak M. Wright C. Naranjo A. Wagner J. Meechoovet H.B. Bautista C. Chang W.C. Ostberg J.R. Jensen M.C. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Paulos C.M. Suhoski M.M. Plesa G. Jiang T. Basu S. Golovina T.N. Jiang S. Aqui N.A. Powell D.J., Jr. Levine B.L. Carroll R.G. Riley J.L. June C.H. Adoptive immunotherapy: Good habits instilled at youth have long-term benefits. Immunol. Res. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S. Potters M. Visser L. van den Berg A.M. Immune escape mechanisms in Hodgkin's disease. Ann. Oncol. 1998;9(Suppl. 5):S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Powell D.J., Jr. Dudley M.E. Robbins P.F. Rosenberg S.A. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule M.A. Straathof K.C. Dotti G. Heslop H.E. Rooney C.M. Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pule M.A. Savoldo B. Myers G.D. Rossig C. Russell H.V. Dotti G. Huls M.H. Liu E. Gee A.P. Mei Z. Yvon E. Weiss H.L. Liu H. Rooney C.M. Heslop H.E. Brenner M.K. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintarelli C. Vera J.F. Savoldo B. Giordano Attianese G.M. Pule M. Foster A.E. Heslop H.E. Rooney C.M. Brenner M.K. Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rischer M. Pscherer S. Duwe S. Vormoor J. Jurgens H. Rossig C. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 2004;126:583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- Robbins P.F. Dudley M.E. Wunderlich J. El-Gamil M. Li Y.F. Zhou J. Huang J. Powell D.J., Jr. Rosenberg S.A. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C.M. Smith C.A. Ng C.Y. Loftin S. Li C. Krance R.A. Brenner M.K. Heslop H.E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Rooney C.M. Smith C.A. Ng C.Y. Loftin S.K. Sixbey J.W. Gan Y. Srivastava D.K. Bowman L.C. Krance R.A. Brenner M.K. Heslop H.E. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Rosenberg S.A. Aebersold P. Cornetta K. Kasid A. Morgan R.A. Moen R. Karson E.M. Lotze M.T. Yang J.C. Topalian S.L. Merino M.J. Culver K. Miller A.D. Blaese R.M. Anderson W.F. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Yannelli J.R. Yang J.C. Topalian S.L. Schwartzentruber D.J. Weber J.S. Parkinson D.R. Seipp C.A. Einhorn J.H. White D.E. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Sportes C. Ahmadzadeh M. Fry T.J. Ngo L.T. Schwarz S.L. Stetler-Stevenson M. Morton K.E. Mavroukakis S.A. Morre M. Buffet R. Mackall C.L. Gress R.E. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Restifo N.P. Yang J.C. Morgan R.A. Dudley M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig C. Bollard C.M. Nuchtern J.G. Merchant D.A. Brenner M.K. Targeting of GD2-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int. J. Cancer. 2001;94:228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- Rossig C. Bollard C.M. Nuchtern J.G. Rooney CM. Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- Ruggeri L. Mancusi A. Burchielli E. Capanni M. Carotti A. Aloisi T. Aversa F. Martelli M.F. Velardi A. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol. Dis. 2008;40:84–90. doi: 10.1016/j.bcmd.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Sadelain M. Brentjens R. Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B. Rooney C.M. Di Stasi A. Abken H. Hombach A. Foster A.E. Zhang L. Heslop H.E. Brenner M.K. Dotti G. Epstein-Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S. Kieper W.C. Jameson S.C. Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Singh H. Manuri P.R. Olivares S. Dara N. Dawson M.J. Huls H. Hackett P.B. Kohn D.B. Shpall E.J. Champlin R.E. Cooper L.J. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C. Hakim F.T. Memon S.A. Zhang H. Chua K.S. Brown M.R. Fleisher T.A. Krumlauf M.C. Babb R.R. Chow C.K. Fry T.J. Engels J. Buffet R. Morre M. Amato R.J. Venzon D.J. Korngold R. Pecora A. Gress R.E. Mackall C.L. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny M.J. Brown C.E. Ruel C. Jensen M.C. Medulloblastomas expressing IL13Rα2 are targets for IL13-zetakine+ cytolytic T cells. J. Pediatr. Hematol. Oncol. 2007;29:669–677. doi: 10.1097/MPH.0b013e3181468c68. [DOI] [PubMed] [Google Scholar]
- Straathof K.C. Bollard C.M. Popat U. Huls M.H. Lopez T. Morriss M.C. Gresik M.V. Gee A.P. Russell H.V. Brenner M.K. Rooney C.M. Heslop H.E. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood. 2005a;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- Straathof K.C. Pule M.A. Yotnda P. Dotti G. Vanin E.F. Brenner M.K. Heslop H.E. Spencer D.M. Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005b;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till B.G. Jensen M.C. Wang J. Chen E.Y. Wood B.L. Greisman H.A. Qian X. James S.E. Raubitschek A. Forman S.J. Gopal A.K. Pagel J.M. Lindgren C.G. Greenberg P.D. Riddell S.R. Press O.W. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. Savoldo B. Vigouroux S. Biagi E. Pule M. Rossig C. Wu J. Heslop H.E. Rooney C.M. Brenner M.K. Dotti G. T lymphocytes redirected against the κ light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J.F. Hoyos V. Savoldo B. Quintarelli C. Giordano Attianese G.M. Leen A.M. Liu H. Foster A.E. Heslop H.E. Rooney C.M. Brenner M.K. Dotti G. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol. Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R.E. Bechtel C.M. Natarajan V. Baseler M. Hege K.M. Metcalf J.A. Stevens R. Hazen A. Blaese R.M. Chen C.C. Leitman S.F. Palensky J. Wittes J. Davey R.T., Jr. Falloon J. Polis M.A. Kovacs J.A. Broad D.F. Levine B.L. Roberts M.R. Masur H. Lane H.C. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96:467–474. [PubMed] [Google Scholar]
- Wilson M.H. Coates C.J. George A.L., Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Yee C. Thompson J.A. Byrd D. Riddell S.R. Roche P. Celis E. Greenberg P.D. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]