Abstract
The peripheral nervous system (PNS), including peripheral nerves and dorsal root ganglion (DRG), is involved in numerous neurological disorders, such as peripheral neuropathies (diabetic neuropathy, chronic pain, etc.) and demyelination diseases (multiple sclerosis, congenital muscular dystrophy, Charcot-Marie-Tooth disease, etc.). Effective clinical interventions for those diseases are very limited. Gene therapy represents a novel therapeutic strategy for the PNS diseases, especially with simply and minimally invasive delivery methods. Previously, we have shown that adeno-associated virus type 8 (AAV8) can efficiently transduce muscles body wide by a simple intraperitoneal injection in neonatal mice. In this study, we investigated the capacity of AAV8 in transducing PNS in neonatal mice by intraperitoneal injection and also in adult mice by intramuscular injection. Efficient and long-term gene transfer was found in the white matter of the spinal cord, DRG neurons, and peripheral nerves in both groups, treated either as neonates or as adults, particularly neonates. In the adult mice injected with AAV8 in tibialis anterior and gastrocnemius muscles in one of the hind legs, more neurons were transduced in the lower part of the spinal cord than in the upper part; the DRG neurons were transduced more on the vector-injected side than in the contralateral uninjected side. Few cells in the gray matter of the spinal cord were transduced regardless of the delivery methods and age of the mice. These results support the mechanism of vector retrograde transport and suggest that AAV8 crosses blood–nerve barrier poorly. Our finding should have important implications in gene therapy for peripheral neurological disorders.
Introduction
Peripheral nervous system (PNS) consists of nerves and neurons that are located outside the central nervous system (CNS) or extended outside the CNS from the brain and spinal cord. PNS becomes involved in neuropathies that affect nerve cells reside either in peripheral ganglia or in the brain or spinal cord with axons extending into peripheral nerves. Many inherited and acquired neurological disorders affect PNS rather than CNS, such as peripheral neuropathy (e.g., diabetic neuropathy and chronic pain) as well as demyelination diseases (e.g., multiple sclerosis and laminin-alpha2-deficient congenital muscular dystrophy). Fischer et al. showed that degeneration of neuromuscular junctions occurred before the loss of motor neurons in amyotrophic lateral sclerosis (ALS) animal models (SOD1 mice) and patients, indicating that ALS can be considered a distal axonopathy (Fischer et al., 2004). In congenital muscular dystrophy, demyelinating peripheral neuropathy is a significant problem for laminin α2 chain-null mice (Nakagawa et al., 2001; Gawlik et al., 2006) and MDC1A patient (Cook et al., 1992). The major pathological process of peripheral neuropathy includes demyelination and axonal degeneration, producing symptoms such as pain, burning, pruitis (itchiness), paresthesias (tingling), numbness, and weakness, eventually leading to paralysis. These disorders affect 15–20 million of Americans, and there are very few and limited options for the treatment (Federici and Boulis, 2007). It is urgent to develop an efficient method to deliver therapeutic genes to the PNS to achieve therapeutic efficacy for these disabling diseases (Glorioso et al., 1995; Glorioso and Fink, 2002, 2009).
It has been reported that adeno-associated virus (AAV) vector can efficiently transduce dorsal root ganglion (DRG) neurons, which are sensory neuron cells situated outside of spinal column. However, it needs a microneurosurgical technique to deliver AAV vector to the DRG (Glatzel et al., 2000). Foust et al. reported that AAV could transduce nerve fibers in the dorsal horn and columns, indicating DRG transduction, when AAV serotype8 vector was systemically delivered into neonatal mice (Foust et al., 2008). In this report, we have independently studied the ability of AAV8 vector to transduce DRG, peripheral nerve roots, and CNS by intraperitoneal (i.p.) injection in neonatal mice. In addition, to find more direct evidence that AAV8 vector can enter into spinal cord and PNS via retrograde transport, we also performed direct intramuscular (i.m.) injection of AAV8 vector in adult mice. Our results support the mechanism of retrograde transport, and also provide insight for gene therapy application for dyemyelination diseases, chronic pain, and lower motor neuron diseases.
Materials and Methods
AAV vector production
The recombinant viral vector stocks were produced according to the three-plasmid cotransfection method (Xiao et al., 1998). The viral particles were purified twice through CsCl density gradient ultracentrifugation using the previously published protocol (Snyder et al., 1996). The vector titers of viral particle numbers were determined by the DNA dot blot method. The titer of AAV8-cytomegalovirus (CMV)-LacZ vector was 5 × 1013 vector genomes/ml, and the titer of dsAAV8-CMV-green florescent protein (GFP) vector was 2 × 1012 vector genomes/ml.
Mice and vector injection
All protocol involving animal experiments were approved by the University of North Carolina Animal Care and Use Committee. C57/B10 and Imprinting Control Region (ICR) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The dsAAV-CMV-GFP and AAV-CMV-LacZ vector were delivered into the neonates (3 days old) of C57/B10 mice, respectively, by i.p. injection with 100 μl per mouse (dsAAV8-CMV-GFP: 2 × 1011 vector genomes/neonatal mouse; AAV8-CMV-LacZ: 5 × 1012 vector genomes/neonatal mouse). Three-week-old adult ICR mice were injected with AAV-CMV-LacZ to tibialis anterior (TA) and gastrocnemius (GAS) muscles (2.5 × 1012 vector genomes/muscle). Both genes were driven by CMV promoter.
X-gal and histology staining
For AAV-CMV-LacZ-injected samples, we performed X-gal staining according to previously described protocol (Sun et al., 2000). Briefly, the cryo-thin tissue sections of the slides were fixed in fix solution (37% formaldehyde and 25% glutaraldehyde in PBS) for 10 min followed by PBS wash. Then, the slides were put in X-gal staining solution overnight at 37°C to incubate, protected from light. All the consecutive cryo-sections of the spinal cord and brain were performed with luxol fast blue/Cresyl Echt Violet staining, and the sections of peripheral nerves and muscles were subjected to hematoxylin and eosin staining.
Immunofluorescent staining
For dsAAV-CMV-GFP-injected samples, we carried out immunofluorescent staining against GFP protein. To avoid high staining background, the mice were perfused with PBS and then 4% paraformaldehyde (under anesthesia) at the time of sacrifice. The primary antibody against GFP was purchased from Abcam (Cambridge, MA) (ab290-50). Immunofluorescent staining of muscle cryo-thin-sections was performed as previously described (Xiao et al., 2000; Watchko et al., 2002).
Mouse anti-neuronal nuclei (NeuN) antibody (Chemicon, Billerica, MA; 1:1000), rabbit anti-neurofilament (NeuF) antibody (Chemicon; 1:300), rabbit anti-myelin basic protein (MBP) antibody (Chemicon; 1:100), mouse anti-beta-galactosidase antibody (Sigma, St. Louis, MO; 1:100), and rabbit anti-beta-galactosidase antibody (Molecular Probes, Carlsbad, CA; 1:500) were used for costaining of peripheral nerves and spinal cord.
Results
Efficient transduction of white matter of spinal cord, DRG, and peripheral nerves in mice treated as neonates
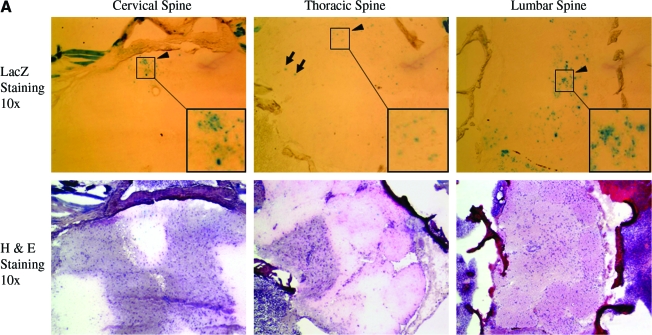
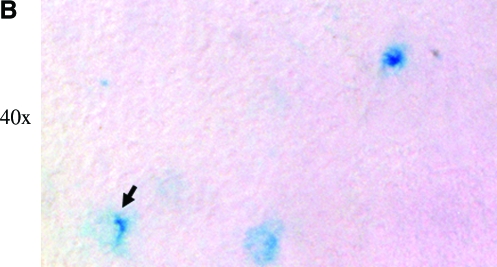
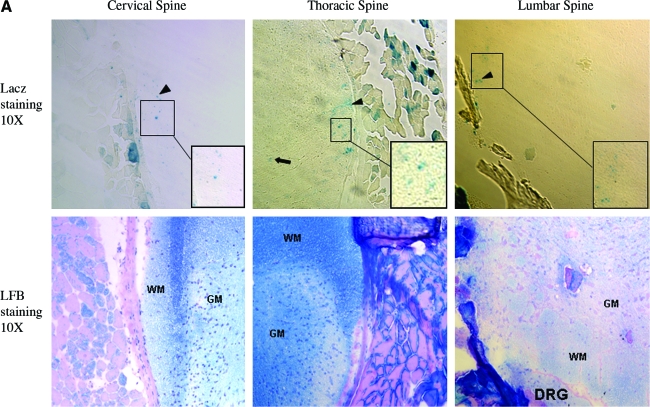
Previously, we have revealed that delivering AAV8 vectors to neonatal mice can efficiently transduce whole-body skeletal and cardiac muscles by a simple i.p. injection (Wang et al., 2005; Qiao et al., 2008, 2009). To explore the feasibility of using AAV vector to infect PNS and spinal cord, we applied the same AAV serotype and similar delivery route in neonatal mice. We first utilized AAV-CMV-LacZ vector. Two months after vector injection, the mice were sacrificed. The whole spinal cord including bone and some adjacent muscles were cut into three segments (cervical, thoracic, and lumbar) and snap-frozen. Cryo-thin-sections were subjected to X-gal staining for LacZ expression and hematoxylin and eosin staining on the consecutive sections to display the histology. As shown in Fig. 1A, the majority of LacZ-positive cells were located in white matter in the spinal cord of AAV-CMV-LacZ-injected mice. We also observed cells expressing LacZ in ventral horns of gray matter (black arrows in Fig. 1A and B), which showed button-like structures in the proximity and overlapping neurons (Fig. 1B). Notably, there were more LacZ-positive cells in the lower spinal cord such as lumbar segment than the higher ones such as cervical segment.
FIG. 1.
Intraperitoneal injection of AAV-CMV-LacZ leads to neonatal spinal cord transduction. (A) LacZ-positive cells in white matter. About 5 × 1012 vector genomes of dsAAV8-CMV-LacZ were delivered on 3-day-old neonatal mice, and the mice were sacrificed at 2 months of age. Notably, most of the lacZ-positive spots were in white matter (black arrowheads and insets), and very few LacZ-positive cells were in gray matter (black arrows). Insets show enlarged area. The consecutive sections of H&E staining were used to display the structure of spinal cord. It was also apparent that there were more LacZ-positive cells in lower spine, followed by medium spine, and then the upper one. (B) LacZ-positive cells in gray matter. Button-like structures (black arrow) in gray matter indicate small neuron and axonal communication. AAV, adeno-associated virus; CMV, cytomegalovirus; H&E, hematoxylin and eosin. Color images available online at www.liebertonline.com/hum.
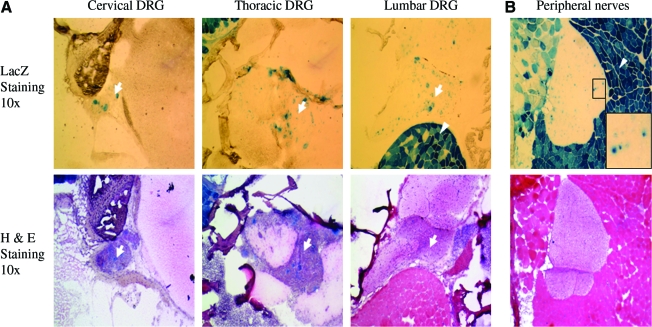
In addition, we noticed that AAV8 vector could efficiently infect DRG neurons that were positioned outside the spinal cord (Fig. 2A, white arrow). Across the entire spine, the DRG neuronal cells were effectively transduced by AAV8 (Fig. 2). Further, we also noticed that the AAV vector could efficiently transduce peripheral nerve cells. As shown in Fig. 2B, we observed LacZ-positive cells in nerve roots of AAV-CMV-LacZ-injected mice. There are several cell types in nerve roots, including Schwann cells, fibroblast, and macrophages (Yaksh, 1999). It is likely that the AAV8 vector infected Schwann cells as we previously observed in the basal lamina of Schwann cells on expression of an extracellular protein, the mini-agrin (Qiao et al., 2005).
FIG. 2.
AAV-CMV-LacZ vector efficiently transduced dorsal root ganglion (DRG) and peripheral nerves in neonatal delivered mice. (A) Transduction of DRG cells. White arrows indicate LacZ-positive cells in DRG, which locates outside the spinal cord, and the consecutive section of H&E staining was utilized to display DRG structure and location. White arrowheads point to the adjacent LacZ-positive muscle cells. (B) Transduction of peripheral nerves by AAV-CMV-LacZ vector. Color images available online at www.liebertonline.com/hum.
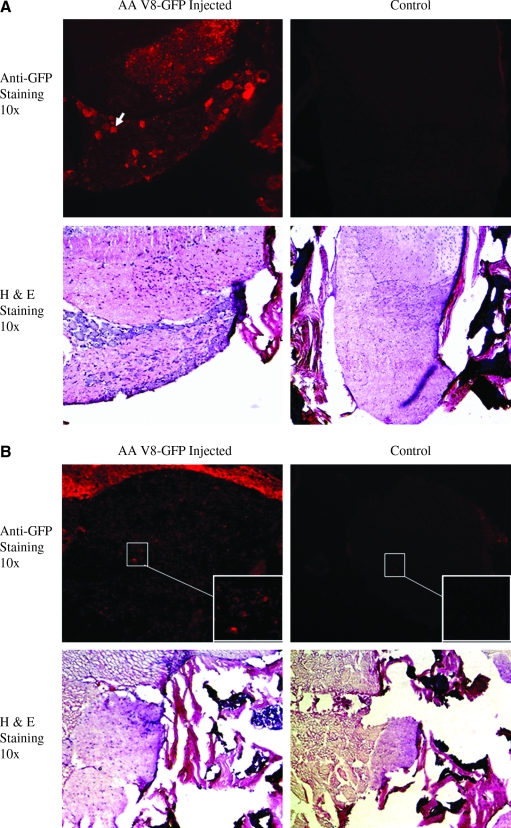
We next examined whether utilizing AAV vector carrying a different reporter gene, such as GFP, would generate the similar results as AAV-CMV-LacZ vector. Similar to AAV-CMV-LacZ vector delivery, dsAAV8-CMV-GFP was delivered into neonatal mice via i.p. injection. The mice were sacrificed 2 months postinjection. Immunofluorescent staining against GFP indicated that most of the GFP-positive cells were located in white matter of spinal cord and DRG (Fig. 3A, white arrow; with greater than 70% positive DRG cells), which is consistent with the LacZ study. Besides white matter of spinal cord and DRG, we also noticed widespread GFP staining in peripheral nerve fibers (Fig. 3B).
FIG. 3.
Immunofluorescent staining of GFP in spinal cord, peripheral nerves, and DRG demonstrates that those cells could be efficiently transduced by AAV8-GFP vector in neonates. Similar to AAV8-CMV-LacZ vector, the 2 × 1012 vector genomes/ml dsAAV8-CMV-GFP vector was also delivered into neonatal mice by intraperitoneal injection (2 × 1011 vector genomes/neonatal mouse). Two months after vector delivery, the mice were sacrificed. (A) Transduction of white matter of spinal cord and DRG by dsAAV-CMV-GFP vector. The same as the results obtained from LacZ staining, most GFP-positive signals (white arrow) were located in white matter of spinal cord and DRG. The neurons located in DRG transduced by AAV vector are about 70–80%. The consecutive sections of H&E staining were used to display the structure. (B) Transduction of peripheral nerves by dsAAV8-CMV-GFP vector. Insets show enlarged area. GFP, green florescent protein.
Efficient retrograde transport of AAV8 vector in adult mice
In our neonatal study by systemic AAV8 delivery, it is possible that the efficient transduction of PNS and spinal cord could be through vector retrograde transport from the muscle as well as directly crossing the blood–nerve barrier. To obtain direct proof that AAV vector can enter into PNS and spinal cord by retrograde transport, we delivered AAV-CMV-LacZ vector to 3-week-old young adult mice by i.m. injection. The vector was injected into TA and GAS muscles of one leg, leaving the contralateral one as an uninjected control. The mice were sacrificed at 1, 2, and 3 months postinjection. Again, the whole spinal cord including bone and adjacent muscles was isolated, divided into cervical, thoracic, and lumbar segments and snap-frozen. After cryo-thin sectioning, the slides were subjected to X-gal staining and luxol fast blue staining, which can clearly differentiate the fiber tracts (white matter) from neuronal cell bodies (gray matter).
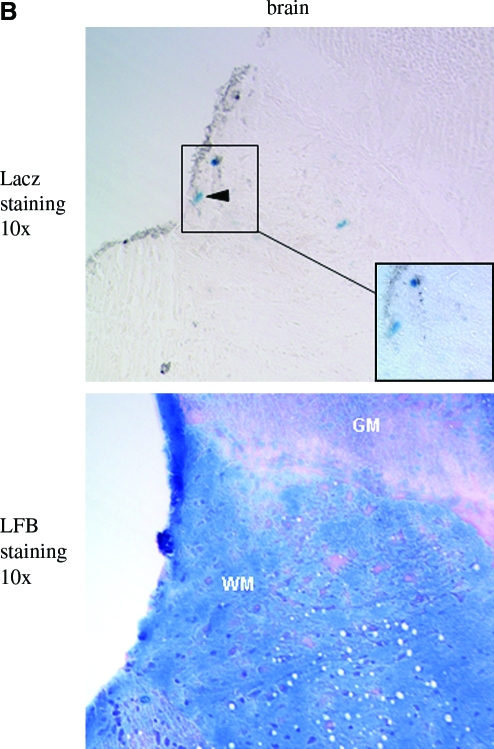
As shown in Fig. 4A, consistent with what we observed in neonatal mice, most LacZ-positive cells were seen in white matter in the spinal cord of AAV-CMV-LacZ-injected mice at 1 month after i.m. injection (black arrowheads). In addition, most LacZ-positive cells were found in lower spine portion, that is, the lumbar segment. Nevertheless, 2 months postinjection, more LacZ-positive cells were detected in the upper portions such as cervical and thoracic segments besides the lower ones (Fig. 4A). In addition, we observed several LacZ-positive cells in the brain 3 months after hind leg i.m. delivery in the adult mice. As shown in Fig. 4B, there were several LacZ-positive cells located in the white matter of the dorsal part of the brain stem, most probably in the gracile fasciculus and cuneate fasciculus. Our results suggest that AAV8 gene transfer and expression in the spinal cord is likely through retrograde transport from peripheral nerve axons.
FIG. 4.
Intramuscular injection of AAV-CMV-LacZ leads to spinal cord transduction. (A) LacZ-positive cells in spinal cord of vector-delivered mice (10 × ). The vector was delivered on 3-week-old adult mice and the mice were sacrificed 1, 2, or 3 months after injection. Obviously, most of the lacZ-positive spots were in white matter (WM; black arrowheads and insets), and very few LacZ-positive cells were in gray matter (GM; black arrows). Insets show enlarged areas of WM (40 × ). LFB staining was used on consecutive sections to display the structure of spinal cord. (B) LacZ staining of brain stem in AAV8-CMV-LacZ-delivered adult mice. The mice were sacrificed 3 months after intramuscular injection. Some LacZ-positive cells were found in the WM of the dorsal part of the left upper brain stem (inset area), most likely in the gracile fasciculus and cuneate fasciculus. Inset shows the enlarged area (40 × ). The consecutive section of LFB staining was used to display the structure of the brain stem. LFB, luxol fast blue. Color images available online at www.liebertonline.com/hum.
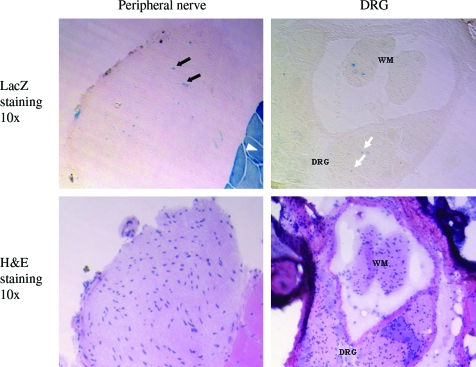
To further define the route through which AAV8 vector travels to the PNS and CNS from the injection site in the muscle rather than systemically, we analyzed LacZ gene expression in different muscle groups and peripheral nerves including sciatic and peroneal nerves, on both sides of the body. Our results showed that strong LacZ-positive cells were found in the vector-injected TA and GAS muscles and, to a less degree, the other muscle groups on the same hind leg (Fig. 5, white arrowhead). However, very few and faint LacZ-positive muscle cells were found in the contralateral side (data not shown), suggesting that systemic dissemination of AAV8 is very limited after i.m. injection in the lower hind leg. The sciatic nerve on the injected side was lacZ positive (Fig. 5 black arrow), but the contralateral one was negative (data not shown). Interestingly, LacZ-positive cells were found in bilateral DRG (Fig. 5, white arrow), bilateral spinal cord (Fig. 4A), and brain stem (Fig. 4B). This result demonstrated that the retrograde transport is probably the mechanism of LacZ transduction to CNS by AAV8 vector via i.m. injection.
FIG. 5.
Transduction of peripheral nerves and DRG by AAV-CMV-LacZ vector via intramuscular delivery in adult mice. LacZ-positive cells were found in peripheral nerves (black arrows) and DRG (white arrows), which was located outside of sacrococcygeal spine. LacZ-positive cells were found in skeletal muscles (white arrowheads). H&E staining was used to display the structure of peripheral nerve and DRG. WM, white matter. Color images available online at www.liebertonline.com/hum.
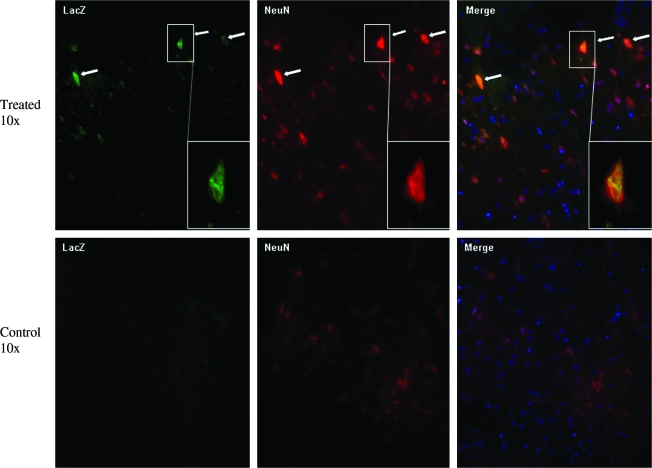
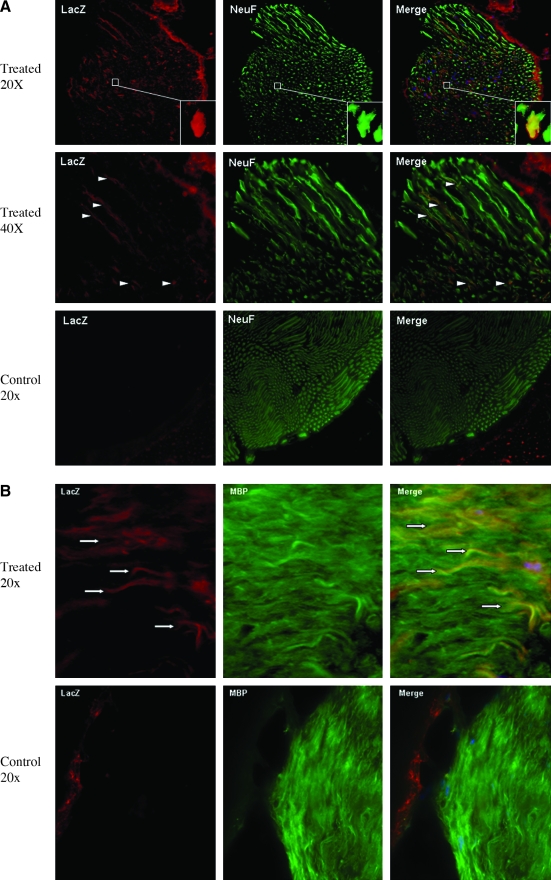
To determine which cell type in spinal cord and PNS is transduced by AAV8 vector, we performed double immunofluorescent staining with anti-beta-galactosidase (LacZ) and antibodies specific for neural cell, nerve axon, as well as Schwann cell markers. As we described earlier, in the spinal cord, we observed a number of LacZ-positive cells in dorsal horns of gray matter. To find out whether those AAV-transduced cells are neurons, we performed costaining against LacZ antibody and anti-NeuN protein antibody, a marker confined to almost exclusively neurons (Mullen et al., 1992; Wolf et al., 1996). Our results indicated colocalization of LacZ signal and NeuN signal (Fig. 6, white arrow and inset area), demonstrating transduction of neurons in the spinal cord by AAV8 vector through retrograde via i.m. delivery in adult mice. To identify which cell types were transduced by AAV8 vector in peripheral nerves, we did costaining of LacZ antibody and one of the major CNS myelin protein marker synthesized by oligodendrites (central) and Schwann cells (peripheral) (Morell, 1984)—anti-MBP (Grima et al., 1992), as well as the axoplasm marker in dendrites and axons—anti-NeuF (Karlsson et al., 1989). As shown in Fig. 7A, most LacZ-positive signals colocalized with anti-NeuF staining, indicating the presence of LacZ in nerve fibers transported from the neuronal axons. Additionally, some LacZ-positive cells were also stained positive in MBP, suggesting the transduction of myelinating Schwann cells in peripheral nerve by AAV8 vector (Fig. 7B).
FIG. 6.
Transduction of neuronal cells of spinal cord via retrograde transport of AAV-CMV-LacZ vector. Costaining of anti-beta-galactosidase (LacZ) and anti-neuronal nuclei (NeuN) of lumbar spinal cord showed some colocalization of NeuN-positive cells with LacZ (white arrows)-positive signal, demonstrating that neurons in the spinal cord were transduced. The inset is a magnification of an LacZ-positive neuron. Color images available online at www.liebertonline.com/hum.
FIG. 7.
Characterization of LacZ-positive cells in peripheral nerves in mice injected intramuscularly. (A) Neuron axon was transduced by AAV-CMV-LacZ vector. Colocalization of LacZ and neurofilament (nerve fiber marker) indicated that neuronal axons in peripheral nerves were transduced by AAV-CMV-LacZ vector (white arrowheads). The inset is an enlarged magnification of an LacZ-positive neuronal axon (cross section). (B) Schwann cells were also transduced by AAV-CMV-LacZ vector via retrograde. Colocalization of LacZ and the myelin basic protein (MBP) indicated that myelinating Schwann cells were also transduced (white arrows).
Discussion
Gene therapy for peripheral neuropathies invokes therapeutic gene delivery to the spinal cord and PNS. In this study, we found that efficient gene transfer to the spinal cord and PNS could be achieved by simple gene delivery methods—thus, injection of AAV8 vectors in neonatal mice intraperitoneally and in adult mice intramuscularly. While our data obtained from neonatally injected mice are consistent with a previous report (Foust et al., 2008), data from adult mice intramuscularly injected with AAV8 vectors provide new evidence supporting retrograde transport of AAV vectors in the nervous system (Kaspar et al., 2003; Lu et al., 2003; Xu et al., 2005; Pirozzi et al., 2006; Foust et al., 2008; Hollis et al., 2008). These findings should have valuable implications in gene therapy for neurological disorders.
Retrograde transport of AAV vectors from muscle to the spinal cord was first reported by Kaspar et al. using AAV2 vectors in an ALS mouse model (Kaspar et al., 2003). Subsequently, to find vectors with higher efficiency, more serotypes of AAV vectors have been explored for retrograde transport from muscle to nerve (Burger et al., 2004; Xu et al., 2005; Federici and Boulis, 2007; Foust et al., 2008; Hollis et al., 2008; Foust et al., 2009). A recent report by Hollis et al. demonstrated that AAV1 is effective in retrograde transport (Hollis et al., 2008). Previously, several groups, including our own (Inagaki et al., 2006), have shown that certain AAVs like AAV8 and AAV9 exhibit superior capability of widespread gene transfer to skeletal muscles throughout the body. This prompted us to investigate AAV8 in this aspect. Gene expression in PNS and spinal cord via retrograde transport by AAV8 was not reported until recently in neonatal mice (Foust et al., 2008). We have done similar studies on AAV8 in neonatal mice and observed consistent results. Nonetheless, if AAV8 readily crossed blood-brain barrier (BBB), we should have seen more neurons expressing LacZ in gray matter than we observed, but we found only very few positive neurons throughout different ages and time points. These observations suggest that AAV8 vector crosses BBB poorly even in neonatal stage before the closure of the BBB and that AAV8 gene transfer in gray matter is most likely from retrograde transport through nerve terminals rather than BBB crossing. In contrast, a recent study revealed that AAV9 is superior in crossing the BBB and renders efficient gene transfer to the brain and spinal cord after intravenous vector injection (Foust et al., 2009). However, AAV9 is much less effective in retrograde transport from muscle to the nerve than AAV8 (Zheng et al., unpublished observation).
Systemic neonatal vector delivery is only useful for certain diseases that require early interventions, whereas retrograde gene transfer to nervous system by i.m. injection in adult is more applicable to a broad range of peripheral neurological and neuromuscular disorders. In the current study, we are able to demonstrate that AAV8 vector could also efficiently transduce spinal cord, DRG neurons, as well as peripheral nerve in adult mice after i.m. injection. We have two important observations that further support the notion of retrograde transport from nerve endings in the muscle to the neuron cell bodies, rather than systemically crossing the BBB by AAV8. First, more nerve cells were transduced in the proximity to the vector-injected muscles (TA and GAS on the lower hind leg) than the distal sites, for example, upper segment of the spinal cord. Second, LacZ-positive muscle cells are predominantly localized in the vector-injected side and few positive cells were found in the contralateral side, indicating that local trafficking other than systemic trafficking is a primary mechanism. As a result, it is not surprising that the DRG cells were transduced by AAV8 vectors because the perineurium of DRG has fewer layers of perineurial cells and larger gaps than the peripheral nerves (Yaksh, 1999), making DRG more accessible to AAV8 particles (Yaksh, 1999).
The ability of AAV vector to transduce peripheral nerve roots and white matter of spinal cord indicated that AAV vector could cross the perineurium and achieve gene transfer there. Nerve roots include both sensory (dorsal) and motor (ventral) bundles. We believe AAV8 vector can cross not only sensory perineurium but also motor perineurium, though with much lower efficiency in the latter. Previously, we have shown that AAV1-mini-agrin, encoding an extracellular protein, can be expressed in the basal lamina of peripheral nerves when the vector was delivered in neonates (Qiao et al., 2005). Those data are consistent with our current findings.
Directing gene transfer to neurons has a number of options: (1) directly injecting the brain and spinal cord to bypass the BBB (Alisky and Davidson, 2000); (2) using vectors that can cross the BBB (Foust et al., 2009); (3) taking advantage of retrograde transport (Eusebio et al., 2003; Kaspar et al., 2003; Foust et al., 2008). The ability of AAV8 to direct gene transfer mainly to axons and myelin of peripheral nerve and white matter of spinal cord makes it a useful tool for demyelinating diseases or other disease with demyelinating process, such as CMD. Although AAV8 vector transduces lower motor neurons poorly, systemic delivery of AAV vector encoding secreted protein to spinal cord through DRG will still be an attractive therapeutic approach for motor neuron disease such as ALS. DRG is a group of sensory nerve bodies. In addition to sending sensory information to the brain via the dorsal columns, the DRG sends projections to lower motor neuron or interneuron as a part of reflex arc (Yaksh, 1999). Synapses on lower motor neuron could be an efficient means to deliver neurotrophic factors to diseased cells. Our study also indicates that if a therapeutic protein is intracellular and needs to be delivered to a large portion of lower motor neurons, such as in the case of spinal muscular atrophy, alternative delivery route and more efficient vectors have to be further pursued.
In addition, DRG transduction could have potential benefit in models of chronic pain (Federici and Boulis, 2007; Storek et al., 2008; Beutler and Reinhardt, 2009). Xu et al. have achieved strong GFP expression with AAV vector in DRG, dorsal roots, and peripheral axons (Xu et al., 2003a). They have also obtained very encouraging therapeutic efficacy in pain treatment (Xu et al., 2003b). However, they utilized direct DRG injection and sciatic nerve injection, which were more sophisticated than i.m. injection. Systemic delivery with AAV serotype 8 described in our study could be a more convenient alternative route to deliver genes to DRG for treatment of neuropathic pains and other neuromuscular diseases.
Acknowledgments
Hui Zheng is a recipient of 2007 Chinese national predoctoral program award organized by China Scholarship Council. This work was supported by NIH ARO45967 to Xiao Xiao.
Author Disclosure Statement
No competing financial interests exist.
References
- Alisky J.M. Davidson B.L. Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum. Gene. Ther. 2000;11:2315–2329. doi: 10.1089/104303400750038435. [DOI] [PubMed] [Google Scholar]
- Beutler A.S. Reinhardt M. AAV for pain: Steps towards clinical translation. Gene Ther. 2009;16:461–469. doi: 10.1038/gt.2009.23. [DOI] [PubMed] [Google Scholar]
- Burger C. Gorbatyuk O.S. Velardo M.J. Peden C.S. Williams P. Zolotukhin S. Reier P.J. Mandel R.J. Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cook J.D. Gascon G.G. Haider A. Coates R. Stigsby B. Ozand P.T. Banna M. Congenital muscular dystrophy with abnormal radiographic myelin pattern. J Child Neurol. 1992;(7 Suppl):S51–S63. doi: 10.1177/08830738920070010811. [DOI] [PubMed] [Google Scholar]
- Eusebio A. Oliveri F. Barzaghi P. Ruegg M.A. Expression of mouse agrin in normal, denervated and dystrophic muscle. Neuromuscul. Disord. 2003;13:408–415. doi: 10.1016/s0960-8966(03)00036-1. [DOI] [PubMed] [Google Scholar]
- Federici T. Boulis N. Gene therapy for peripheral nervous system diseases. Curr. Gene Ther. 2007;7:239–248. doi: 10.2174/156652307781369083. [DOI] [PubMed] [Google Scholar]
- Fischer L.R. Culver D.G. Tennant P. Davis A.A. Wang M. Castellano-Sanchez A. Khan J. Polak M.A. Glass J.D. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Foust K.D. Nurre E. Montgomery C.L. Hernandez A. Chan C.M. Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Poirier A. Pacak C.A. Mandel R.J. Flotte T.R. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum. Gene Ther. 2008;19:61–70. doi: 10.1089/hum.2007.093. [DOI] [PubMed] [Google Scholar]
- Gawlik K.I. Li J.Y. Petersen A. Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum. Mol. Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- Glatzel M. Flechsig E. Navarro B. Klein M.A. Paterna J.C. Bueler H. Aguzzi A. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc. Natl. Acad. Sci. U. S. A. 2000;97:442–447. doi: 10.1073/pnas.97.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J.C. Deluca N.A. Fink D.J. Development and application of herpes simplex virus vectors for human gene therapy. Annu. Rev. Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- Glorioso J.C. Fink D.J. Use of HSV vectors to modify the nervous system. Curr. Opin. Drug Discov. Dev. 2002;5:289–295. [PubMed] [Google Scholar]
- Glorioso J.C. Fink D.J. Herpes vector-mediated gene transfer in the treatment of chronic pain. Mol. Ther. 2009;17:13–18. doi: 10.1038/mt.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B. Zelenika D. Pessac B. A novel transcript overlapping the myelin basic protein gene. J. Neurochem. 1992;59:2318–2323. doi: 10.1111/j.1471-4159.1992.tb10126.x. [DOI] [PubMed] [Google Scholar]
- Hollis E.R. 2nd, Kadoya K.Hirsch M.Samulski R.J.Tuszynski M.H.2008Efficient retrograde neuronal transduction utilizing self-complementary AAV1 Mol. Ther. 16296–301. [DOI] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A. Gibson G.A. Mctiernan C.F. Kay M.A. Nakai H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J.E. Rosengren L.E. Haglid K.G. Polyclonal antisera to the individual neurofilament triplet proteins: A characterization using ELISA and immunoblotting. J. Neurochem. 1989;53:759–765. doi: 10.1111/j.1471-4159.1989.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Kaspar B.K. Llado J. Sherkat N. Rothstein J.D. Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Lu Y.Y. Wang L.J. Muramatsu S. Ikeguchi K. Fujimoto K. Okada T. Mizukami H. Matsushita T. Hanazono Y. Kume A. Nagatsu T. Ozawa K. Nakano I. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci. Res. 2003;45:33–40. doi: 10.1016/s0168-0102(02)00195-5. [DOI] [PubMed] [Google Scholar]
- Morell P. Myelin. Plenum Press; New York: 1984. [Google Scholar]
- Mullen R.J. Buck C.R. Smith A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nakagawa M. Miyagoe-Suzuki Y. Ikezoe K. Miyata Y. Nonaka I. Harii K. Takeda S. Schwann cell myelination occurred without basal lamina formation in laminin alpha2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35:101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- Pirozzi M. Quattrini A. Andolfi G. Dina G. Malaguti M.C. Auricchio A. Rugarli E.I. Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia. J. Clin. Invest. 2006;116:202–208. doi: 10.1172/JCI26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C. Li J. Zhu T. Draviam R. Watkins S. Ye X. Chen C. Li J. Xiao X. Amelioration of laminin-α2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11999–12004. doi: 10.1073/pnas.0502137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C. Li J. Jiang J. Zhu X. Wang B. Li J. Xiao X. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum. Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- Qiao C. Li J. Zheng H. Bogan J. Li J. Yuan Z. Zhang C. Bogan D. Kornegay J. Xiao X. Hydrodynamic limb vein injection of adeno-associated virus serotype 8 vector carrying canine myostatin propeptide gene into normal dogs enhances muscle growth. Hum. Gene Ther. 2009;20:1–10. doi: 10.1089/hum.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. Xiao X. Samulski R.J. Production of recombinant adeno-associated viral vectors. In: Dracopoli N., editor. In Current Protocals in Human Genetics. John Wiley; New York: 1996. [DOI] [PubMed] [Google Scholar]
- Storek B. Reinhardt M. Wang C. Janssen W.G. Harder N.M. Banck M.S. Morrison J.H. Beutler A.S. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Li J. Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat. Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- Wang Z. Zhu T. Qiao C. Zhou L. Wang B. Zhang J. Chen C. Li J. Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Watchko J. O'day T. Wang B. Zhou L. Tang Y. Li J. Xiao X. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum. Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- Wolf H.K. Buslei R. Schmidt-Kastner R. Schmidt-Kastner P.K. Pietsch T. Wiestler O.D. Blumcke I. NeuN: A useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Xiao X. Li J. Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X. Li J. Tsao Y.P. Dressman D. Hoffman E.P. Watchko J.F. Full functional rescue of a complete muscle (TA) in dystrophic hamsters by adeno-associated virus vector-directed gene therapy. J. Virol. 2000;74:1436–1442. doi: 10.1128/jvi.74.3.1436-1442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Ma C. bass C. Terwilliger E.F. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341:203–214. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Xu Y. Gu Y. Wu P. Li G.W. Huang L.Y. Efficiencies of transgene expression in nociceptive neurons through different routes of delivery of adeno-associated viral vectors. Hum. Gene Ther. 2003a;14:897–906. doi: 10.1089/104303403765701187. [DOI] [PubMed] [Google Scholar]
- Xu Y. Gu Y. Xu G.Y. Wu P. Li G.W. Huang L.Y. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: A strategy to increase opioid antinociception. Proc. Natl. Acad. Sci. U. S. A. 2003b;100:6204–6209. doi: 10.1073/pnas.0930324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T.L. Spinal Drug Delivery. Elsevier Health Sciences; The Netherlands: 1999. [Google Scholar]