Abstract
Aortic valve calcium (AVC) is common among older adults and shares epidemiologic and histopathologic similarities to atherosclerosis. However, prospective studies have failed to identify meaningful risk-associations with incident (“new”) AVC or its progression. In this study, AVC was quantified from serial computed tomography (CT) images in 5,880 participants (aged 45–84 years) of the Multi-Ethnic Study of Atherosclerosis, using Agatston methodology. Multivariate backwards selection modeling was used to identify risk factors for incident AVC and AVC progression. During a mean follow up of 2.4±0.9 years, 210 subjects (4.0%) developed incident AVC. The incidence rate (mean 1.7 %/year) increased significantly with age (p<0.001). Risk factors for incident AVC included age, male gender, BMI, current smoking, and the use of lipid lowering and antihypertensive medications. Among those with AVC at baseline, the median rate of AVC progression was 2 Agatston units/year [IQR −21, 37]. Baseline Agatston score was a strong, independent predictor of progression, especially among those with high calcium scores at baseline. In conclusion, in this ethnically diverse, pre-clinical cohort, the rate of incident AVC a significantly with age. Incident AVC risk was associated with several traditional cardiovascular risk factors, specifically age, male gender, BMI, current smoking, and the use of both antihypertensive and lipid lowering medications. AVC progression risk was associated with male gender and the baseline Agatston score. Additional research is needed to determine if age- and stage-specific mechanisms underlie risk for AVC progression.
Keywords: valves, calcium, risk factors, epidemiology, imaging
INTRODUCTION
The Multi-Ethnic Study of Atherosclerosis (MESA) is a large, longitudinal investigation focusing on pre-clinical cardiovascular disease in a relatively young, healthy and community-dwelling population. Using quantitative AVC scores obtained from serial CT scans, we sought to characterize the rate of incident (“new”) AVC within MESA, as well as prospective risk-associations with both incident AVC and AVC progression. Because age is an important clinical component of this disease, we also sought to examine the influence of age on both incident AVC and AVC progression.
METHODS
MESA was initiated by the National Heart, Lung and Blood Institute to characterize subclinical cardiovascular disease (CVD) and its progression. A full description of the study design and recruitment process has been reported previously.1 A total of 6,814 free-living individuals without clinically apparent CVD, aged 45 to 84 years, were recruited from six US communities (Baltimore City and County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN) between July 2000 and August 2002. Recruitment targeted four ethnic groups (white, black, Hispanic, or Chinese). Participants were excluded if they self-reported CVD, including angina or prior cardiovascular procedures such as percutaneous coronary interventions, coronary bypass or valve surgery, or pacemaker/defibrillator implantation. The institutional review boards at each participating institution approved the MESA study protocol, and each participant provided informed written consent for study participation and data analysis prior to enrollment.
All MESA participants underwent a baseline examination that included CT examination of the chest. Follow-up testing, including repeat chest CT examination, was performed in two stages. One-half of the cohort returned between September 2002 and January 2004, while the other half returned between March 2004 and July 2005, with average between-scan intervals of 1.6 and 3.2 years, respectively. Of the 6,814 participants initially enrolled in MESA, five were excluded subsequently due to post-enrollment identification of antecedent cardiovascular events. Of this group, the 5,880 MESA participants in whom a follow up CT examination was performed were included in these analyses.
Information regarding the participants’ demographic data and medical history, including age, smoking history and medication use, was self-reported. While participants reporting CVD or valve disease were excluded at baseline, there was no specific screening for subclinical aortic stenosis. Subjects who self-reported their race/ethnicity as white, black, Hispanic, or Chinese were eligible for inclusion; other races/ethnicities were excluded from MESA enrollment.
Additional covariate data were obtained from baseline examination. Continuous variables (body mass index, blood pressure, lipid parameters, fibrinogen and creatinine) were normalized by their standard deviations. Hyperglycemia and impaired fasting glucose were categorized according to the 2003 American Diabetic Association fasting criteria algorithm.2 Plasma lipoprotein parameters were obtained following a twelve hour fast, and sent to a central lipid laboratory (Fairview-University Medical Center, Minneapolis, MN) for analysis, as reported separately.3 Because of skewed data, values for triglycerides and C-reactive protein (CRP) were log-transformed.
Serial CT scans were obtained at each of the four centers using electron beam tomography (EBT) or multidetector CT (MDCT) scanners. To adjust for inter-subject and intra-subject differences in scanner types, images were calibrated individually using calcium phantoms; analyses also included adjustments for scanner pair. Full details concerning the equipment, scanning methods, and quality control in MESA, including image calibration and inter-scanner reproducibility, have been reported previously.4 Additionally, the equivalency of AVC quantification across scanner type has been established.5 Spatial resolution was 1.38 mm3 for EBT (0.68 × 0.68 × 3.00 mm) and 1.15 mm3 for MDCT (0.68 × 0.68 × 2.50 mm).
All studies were processed at a central reading center (Harbor-UCLA Research and Education Institute, Los Angeles, CA) where AVC scores were measured retrospectively by a single, blinded reader (JT). AVC was defined as any calcified lesion residing within the aortic valve leaflets. Calcification of the aortic annulus, sinuses, ascending aorta or coronary arteries was excluded. AVC was quantified using Agatston methodology, which accounts for both lesion area and calcium density (using Hounsfield brightness). Single lesion measurements were summed to give an overall Agatston score. If AVC was not detected, the Agatston score was recorded as zero.
MESA participants were categorized by the presence (Agatston score >0) or absence (Agatston score =0) of AVC on baseline imaging. Those without AVC at baseline were deemed at risk for incident AVC and included in the analyses of risk-associations for incident AVC. All subjects with baseline AVC were included in the AVC progression analyses, regardless of the magnitude of, or direction of change in, Agatston scores. For the main progression analysis, AVC progression was determined by the absolute between-scan change in Agatston scores. Because of right-skew, Agatston score distributions are reported as medians and interquartile ranges (IQR). Comparison of rates of AVC incidence and progression between older and younger subjects was performed using t-tests, with graphic depiction of age-group differences using Lowess smooth curves.
The prevalence of incident calcification in this cohort was <10%, and thus the odds ratio (OR) was used to estimate relative risk. Univariate analyses were adjusted for follow-up time, age and gender. Multivariate logistic (incidence analysis) and linear (progression analysis) regression models were constructed using backwards selection methodology, whereby variables were initially entered into the model, with removal of the least significant variable and iterative reanalysis. Robust linear regression was used to downweight the effects of potential outliers. Follow-up time and scanner pair were forced into all models, and if one component of a categorical variable reached significance, all components of that variable were included. Thresholds for removal and addition were, respectively, p≥0.10 and p<0.05.
Because the best method for modeling AVC progression is unknown, additional exploratory analyses were performed. As prior studies have identified baseline AVC severity as a predictor of progression, analyses were performed including baseline Agatston scores in the model. Additional stratified analyses were performed to assess the influence of baseline Agatston score among those with low and high baseline scores. In addition to considering the absolute change in Agatston score, AVC progression was alternatively modeled multiplicatively as a log transformation of the a difference in Agatston score and as a relative change in Agatston score [defined as difference in log(Agatston score+25)]. This transformation provided a roughly normal distribution of relative progression and was used previously when considering coronary artery calcification in MESA.6
Statistical analyses were performed using Stata 10.1 for Windows (StataCorp LP., College Station, TX). Statistical significance was defined as p<0.05, and RRs are reported with 95% confidence intervals.
RESULTS
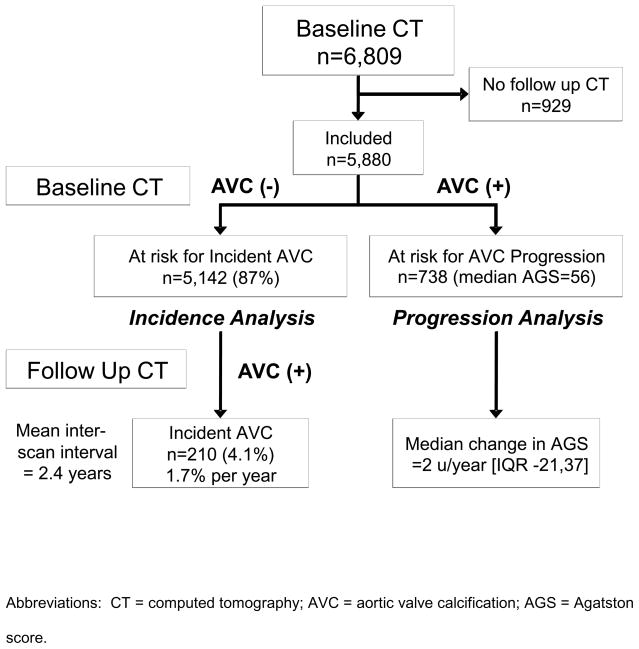
Of the 5,880 subjects with follow up CT examinations, the mean between-scan interval was 2.4±0.9 years. A total of 5,142 subjects (87%) did not have AVC on baseline CT examination, while 738 subjects (13%) had prevalent AVC, with a median Agatston score of 56 [IQR: 19,137]. As shown in Figure 1, the former group comprised the population at risk for incident AVC, while the latter group defined the population at risk for AVC progression. The baseline demographic characteristics of this population are shown in Table 1. Notably, this population was relatively young (age 61.8±10.1 years), ethnically diverse (60% non-white), and rather healthy (74% normoglycemic, 64% normotensive). A total of 16% of the cohort was on lipid lowering therapy, with 15% taking statin medications, and baseline lipid levels were relatively normal (LDL 117±31 mg/dL; HDL 51±15 mg/dL; TG 112 [IQR 77 161]). Renal function was well preserved, with only 1.5% of the population having creatinine levels above 1.5 mg/dL.
Figure 1.
Flow diagram for MESA participants, categorized by aortic valve calcification status.
Table 1.
Baseline characteristics of the study population stratified by aortic valve calicum progression.
| At risk for incident AVC* | At risk for | ||
|---|---|---|---|
| Characteristic | No Incident AVC (n=4932) | Incident AVC (n=210) | AVC progression† (n=738) |
| Follow up time (years) | 2.42 ± 0.85 | 2.46 ± 0.88 | 2.42 ± 0.85 |
| Age (years) | 60.4 ± 9.6 | 67.0 ± 8.4 | 70.1 ± 8.1 |
| Men | 2223 (45%) | 117 (55%) | 453 (61%) |
| Race/Ethnicity | |||
| White | 1903 (38.6%) | 87 (41.4%) | 356 (48.2%) |
| Chinese | 618 (12.5%) | 13 (6.2%) | 55 (7.5%) |
| Black | 1350 (27.4%) | 61 (29.0%) | 173 (23.4%) |
| Hispanic | 1061 (21.5%) | 49 (23.3%) | 154 (20.9%) |
| Education | |||
| < High school | 773 (15.7%) | 40 (19.1%) | 159 (21.7%) |
| High school degree | 2310 (46.9%) | 98 (46.9%) | 335 (45.7%) |
| College degree | 1840 (37.4%) | 71 (34.0%) | 239 (32.6%) |
| Body mass index (kg/m2) | 28.3 (5.5%) | 29.2 (5.2%) | 28.5 (4.9%) |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 124 ± 20 | 131 ± 23 | 134 ± 22 |
| Diastolic blood pressure | 72 ± 10 | 73 ± 10 | 72 ± 10 |
| Hypertension therapy | 1625 (33%) | 107 (51%) | 398 (54%) |
| Diabetes mellitus | 519 (10.5%) | 41 (19.5%) | 134 (18.3%) |
| Smoker | |||
| Never | 2545 (51.7%) | 94 (45.0%) | 318 (43.3%) |
| Former | 1739 (35.3%) | 85 (40.7%) | 346 (47.1%) |
| Current | 639 (13.0%) | 30 (14.4%) | 70 (9.5%) |
| Pack-years smoking‡ | 15 [5, 30] | 15 [7, 28] | 18 [6, 40] |
| Cholesterol (mg/dl) | |||
| Low density lipoprotein | 117 ± 31 | 119 ± 33 | 118 ± 33 |
| High density lipoprotein | 51 ± 15 | 50 ± 16 | 49 ± 14 |
| Triglycerides‡ | 109 [77, 159] | 119 [83, 171] | 121 [84, 171] |
| TC:HDL ratio | 4.04 ± 1.25 | 4.21 ± 1.29 | 4.22 ± 1.14 |
| Lipid lowering therapy | 712 (14%) | 55 (26%) | 187 (25%) |
| Statin therapy | 658 (13%) | 50 (24%) | 170 (23%) |
| C-reactive protein (mg/dl)‡ | 1.86 [0.81, 4.21] | 1.91 [0.94, 4.29] | 1.99 [0.95, 3.95] |
| Fibrinogen (mg/dl) | 343 ± 71 | 355 ± 81 | 358 ± 74 |
| Creatinine (mg/dl) | 0.94 ± 0.21 | 0.98 ± 0.27 | 1.04 ± 0.44 |
Among those free of AVC at baseline.
Among those with baseline Agatston score >0.
Median [IQR]. For pack-years smoking, among current and former smokers only.
Proportions may not total 100% in all cases due to rounding. Counts may not equal cohort sample size due to missing data.
Abbreviations: TC:HDL ratio is the ratio of total cholesterol to high-density lipoprotein cholesterol.
Of the 5,142 participants without AVC at baseline, 210 (4.1%) developed AVC during the follow up period, with an annualized incidence rate of 1.7%/year. For those with incident AVC, the median Agatston score on the follow up scan was 13 [IQR: 6, 39]. The rates for incident AVC were increased in those with, as compared to those without, male gender, diabetes and hypertension (Table 2). Additionally, there was a marked increase in the AVC incidence rate in the older age groups (Figure 2). Subjects aged 70–79 years had a 6-fold higher rate of incident AVC than did subjects aged 50–54 years (3.5%/year vs. 0.6%/year, p<0.001). Males had slightly higher incidence rates across all age groups, but there was no significant age-gender interaction on the incident rate of AVC (Figure 2).
Table 2.
Cumulative incidence and mean annualized incidence rates of aortic valve calcium by demographic subgroup, among those free of aortic valve calcium at baseline.
| Number at risk (n) | Incident AVC (n) | Cumulative Incidence (%) | Incidence Rate (%/y) | |
|---|---|---|---|---|
| Total | 5142 | 210 | 4.0 | 1.7 |
| Female | 2802 | 93 | 3.3 | 1.4 |
| Male | 2340 | 117 | 5.0 | 2.1 |
| Hypertension | 2104 | 115 | 5.5 | 2.3 |
| Diabetes mellitus | 560 | 41 | 7.3 | 3.2 |
| White | 1990 | 87 | 4.4 | 1.8 |
| Chinese | 631 | 13 | 2.1 | 0.8 |
| Black | 1411 | 61 | 4.3 | 1.9 |
| Hispanic | 1110 | 49 | 4.4 | 1.9 |
Abbreviations: AVC=aortic valve calcium; IFG=impaired fasting glucose
Figure 2. Average incidence rate of AVC (% per year) by yearly age-increments, among those free of AVC at baseline (n=5,142), for both men (squares) and women (circles).
The size of the scatter points is weighted for number at risk at each age category, and non-linear Lowess smooth curves are displayed for the full cohort (solid), women (dotted) and men (dashed). There is a marked increase in AVC incidence rate with advancing age.
In univariate analyses (controlling for between-scan interval, age and gender), risk factors associated with incident AVC were age, male gender, BMI, antihypertensive therapy, diabetes, current smoking, the Total:HDL cholesterol ratio, and the use of lipid lowering therapies (Table 3). Interestingly, participants with incident AVC had higher triglycerides and Total:HDL cholesterol ratios, but similar levels of LDL and HDL cholesterol (Table 1). There were no significant associations between incident AVC and CRP, fibrinogen or creatinine.
Table 3.
Risk of incident aortic valve calcium among those free of aortic valve calcium at baseline (n=5,142): univariate and multivariate stepwise backward logistic regression models.
| Characteristic | Univariate model* | Fully adjusted model† | ||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Age | 2.03 (1.78, 2.32) | <0.001 | 2.19 (1.84, 2.61) | <0.001 |
| Male | 1.59 (1.20, 2.11) | 0.001 | 1.87 (1.31, 2.69) | 0.001 |
| Race/Ethnicity | ||||
| White | Reference | |||
| Chinese | 0.43 (0.24, 0.79) | 0.006 | ||
| Black | 1.02 (0.73, 1.44) | 0.89 | ||
| Hispanic | 1.08 (0.75, 1.55) | 0.69 | ||
| BMI | 1.33 (1.17, 1.51) | <0.001 | 1.26 (1.08, 1.46) | 0.004 |
| Blood pressure | ||||
| Systolic blood pressure | 1.05 (0.98, 1.13) | 0.16 | ||
| Diastolic blood pressure | 1.11 (0.96, 1.28) | 0.16 | ||
| Antihypertensive therapy | 1.60 (1.20, 2.14) | 0.001 | 1.40 (1.02 1.92) | 0.04 |
| Diabetes mellitus | 1.80 (1.25, 2.60) | 0.002 | ||
| Smoker | ||||
| Never | Reference | |||
| Former | 1.17 (0.85, 1.60) | 0.32 | 1.43 (0.99, 2.09) | 0.06 |
| Current | 1.65 (1.07, 2.54) | 0.02 | 2.49 (1.49, 4.15) | <0.001 |
| Pack-years smoking‡ | 0.93 (0.85, 1.01) | 0.10 | 0.92 (0.83, 1.01) | 0.08 |
| TC:HDL Ratio | 1.19 (1.05, 1.35) | 0.007 | ||
| Lipid lowering therapy | 1.73 (1.25, 2.41) | 0.001 | 1.76 (1.25, 2.49) | 0.001 |
| C-reactive protein | 1.08 (0.96, 1.22) | 0.20 | ||
| Fibrinogen | 1.13 (0.97, 1.33) | 0.12 | ||
| Creatinine | ||||
| ≤0.9 mg/dL | 0.93 (0.63, 1.37) | 0.72 | 0.97 (0.65, 1.45) | 0.90 |
| 1.0 mg/dL | Reference | Reference | ||
| ≥1.1 mg/dL | 0.69 (0.46, 1.05) | 0.09 | 0.62 (0.40, 0.96) | 0.03 |
Univariate model includes adjustment for age, gender and between-scan interval.
Variables chosen from backwards selection and also included follow up time. Variables without risk estimates were not independently associated with incident AVC and were not retained in the final model.
Model for pack-years includes adjustment for current and former smokers.
Relative risks for continuous variables are expressed as per standard deviation increase in predictor, with the following exceptions: age (per decade increase), blood pressure (per 10 mmHg increase), pack-years (per 10 unit increase), and c-reactive protein (per log increase).
Abbreviations: TC:HDL ratio is the ratio of total cholesterol to high-density lipoprotein cholesterol.
In the fully adjusted model (Table 3), positive associations with incident AVC were found for age, male gender, BMI, current smoking, and the use of both antihypertensive and lipid lowering medications. In contrast, creatinine >1.1 mg/dL demonstrated a negative association with incident AVC. Repeat sensitivity analyses using a cutoff definition for incident AVC of Agatston score >10 showed similar findings.
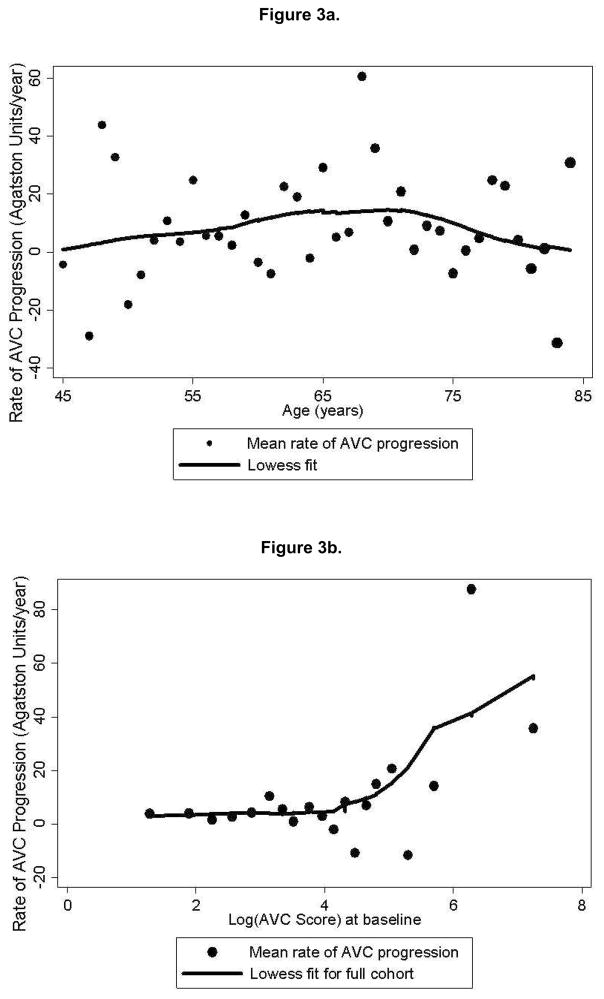
Of the 738 subjects with prevalent AVC at baseline, the median rate of change in Agatston scores was 2 [IQR: −21, 37] units/year. In contrast to incident AVC, there did not appear to be a correlation between age and AVC progression. (Figure 3a). In univariate analyses (controlling for between-scan interval, scanner type, age and gender), risk factors associated with greater AVC progression included only male gender and baseline Agatston score. In the primary backward regression analysis (Table 4), which did not include baseline Agatston score in the model, only male gender was associated with an increased rate of AVC progression, although age was borderline significant. In contrast, higher diastolic blood pressures were associated with a lower rate of AVC progression.
Figure 3.
Figure 3a. Mean annualized rate of AVC progression (Agatston units/year) by yearly age increments, among those with detectable AVC at baseline (n=738). The size of the scatter points is weighted for number at risk at each age category, and a non-linear Lowess smooth curve (solid) is displayed.
Figure 3b. Mean annualized rate of AVC progression (Agatston units/year) grouped by baseline Agatston scores, among those with detectable AVC at baseline (n=738). Baseline Agatston scores are log-transformed to reduce skew.
Table 4.
Risk of aortic valve calcium progression among those with aortic valve calcium > 0 at baseline (n=738): univariate and multivariate stepwise backward linear regression models.
| Characteristic | Univariate model* | Fully Adjusted Model† | ||
|---|---|---|---|---|
| β‡ (95% CI) | p-value | β‡ (95% CI) | p-value | |
| Baseline Agatston score | 0.31 (0.19, 0.42) | <0.001 | Not Included | |
| Age | 14.4 (−1.9,30.8) | 0.08 | 18.3 (−0.1, 36.8) | 0.05 |
| Male | 32.9 (0.7, 65.1) | 0.04 | 37.5 (6.6, 68.5) | 0.02 |
| Race/Ethnicity | ||||
| White | Reference | |||
| Chinese | −52.2 (−86.6, −17.7) | 0.003 | ||
| Black | −33.5 (−69.8, 2.7) | 0.07 | ||
| Hispanic | −21.9 (−63.6, 19.9) | 0.30 | ||
| BMI | 12.7 (−5.7, 31.1) | 0.18 | 15.0 (−1.5, 31.4) | 0.08 |
| Blood pressure | ||||
| Systolic blood pressure | −5.0 (−14.2, 4.3) | 0.29 | ||
| Diastolic blood pressure | −16.1 (−33.7, 1.5) | 0.07 | −15.2 (−28.9, −1.5) | 0.03 |
| Antihypertensive therapy | −12.8 (−46.8, 21.2) | 0.46 | ||
| Diabetes mellitus | −27.2 (−60.5, −6.2) | 0.11 | ||
| Smoking status | ||||
| Never | Reference | |||
| Former | 33.7 (−1.5, 69.0) | 0.06 | ||
| Current | 5.3 (−35.6, 46.1) | 0.80 | ||
| Pack-years smoking|| | 7.1 (−4.0, 18.2) | 0.20 | ||
| TC:HDL ratio | −6.1 (−21.7, 9.5) | 0.44 | ||
| Lipid lowering therapy | −1.6 (−37.8, 34.6) | 0.93 | ||
| C-reactive protein | 9.0 (−10.9, 29.0) | 0.38 | ||
| Fibrinogen | 17.5 (−14.8, 49.8) | 0.29 | ||
| Creatinine | ||||
| ≤0.9 mg/dL | −7.3 (−49.8, 35.1) | 0.73 | ||
| 1.0 mg/dL | Reference | |||
| ≥1.1 mg/dL | −12.5 (−60.2, 35.2) | 0.61 | ||
Univariate model includes adjustment for age, gender, between-scan interval and scanner pair.
Variables chosen from backwards selection and also included follow up time. Variables without risk estimates were not independently associated with AVC progression and were not retained in the final model.
β = difference in average progression comparing groups (categorical variables) or per standard deviation increase in predictor (continuous variables), with the following exceptions: age (per decade increase), blood pressure (per 10 mmHg increase), pack-years (per 10 unit increase), and c-reactive protein (per log increase).
Model for pack-years includes adjustment for current and former smoker
Abbreviations: TC:HDL ratio is the ratio of total cholesterol to high-density lipoprotein cholesterol.
To further assess the impact of baseline Agatston score on risk-associations for AVC progression, additional exploratory analyses were performed. The relationship between baseline Agatston score and AVC progression is shown in Figure 3b. When baseline Agatston score was included as a predictor of absolute AVC progression (Table 5), baseline Agatston score was strongly associated with progression and was the only variable identified in the stepwise regression model. Baseline Agatston scores showed no association with progression among those with baseline Agatston scores below the median (56 Agatston units), but showed strong association those with baseline Agatston scores above the median. When progression was defined in relative terms (thereby also controlling for baseline Agatston scores), none of the risk factors examined showed significant associations.
Table 5.
Exploratory analyses of AVC progression among those with AVC > 0 at baseline: Linear regression models of absolute AVC progression incorporating baseline Agatston score (AGS) as a predictor, stratified above and below median baseline AVC, and of relative AVC progression.
| Absolute Progression |
Relative Progression* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Full Cohort (n=738) | Below Median (AGS <59, n=366) | Above Median (AGS >59, n=372) | Full Cohort (n=738) | ||||
| β‡ (95% CI) | p-value | β‡ (95% CI) | p-value | β‡ (95% CI) | p-value | β‡ (95% CI) | p-value | |
| Baseline AGS | 0.20 (0.05, 0.35) | 0.009 | 0.21 (0.05, 37.2) | 0.01 | Not Included | |||
| Age | ||||||||
| Gender | 0.1 (−0.01, 0.22) | 0.07 | ||||||
| Blood Pressure | ||||||||
| Systolic BP | ||||||||
| Diastolic BP | −12.6 (−27.2, 2.0) | 0.09 | −23.0 (−49.6, 3.6) | 0.09 | ||||
| Creatinine | ||||||||
| ≤0.9 mg/dL | 3.7 (−5.2, 12.6) | 0.41 | ||||||
| 1.0 mg/dL | reference | |||||||
| ≥1.1 mg/dL | 11.3 (1.2, 21.3) | 0.03 | ||||||
Variables chosen from backwards selection and also included adjustments for follow up time and scanner types. Variables without risk estimates were not independently associated with AVC progression and were not retained in the final model. Other variables included in the analysis included male gender, family history of MI, the use of antihypertensive medications, diabetes category (none, impaired fasting glucose, diabetes), smoking status (never, former, current), pack years, cholesterol (LDL, HDL and triglycerides), CRP (log), fibrinogen and creatinine category (≤0.9, 1.0, ≥1.1).
Relative progression defined as difference in log(AGS+25).
β = difference in average progression per one unit change in predictor or in comparison to reference group.
Abbreviations: AGS = Agatston score, BP = blood pressure.
In unadjusted analyses, Chinese participants appeared to have a substantially lower rate of incident AVC as compared to whites, blacks or Hispanics. However, in the fully adjusted analyses (Table 3), there were no significant racial or ethnic differences in risk for incident AVC. Chinese, black and Hispanic participants all had lower rates of AVC progression as compared to white participants, although only the Chinese showed a significantly lower rate of progression. Mean rates of AVC progression (in Agatston units/year) were 12.7 for whites, 3.5 for Chinese, 8.4 for blacks, and 9.7 for Hispanics. However, fully adjusted analyses (Table 4) did not identify significant racial or ethnic differences in the rate of AVC progression.
DISCUSSION
This analysis of the MESA cohort, using quantitative measures of AVC from serial CT imaging, provides important insights into early-stage AVC in a primary-prevention population. While it is well-known that AVC prevalence increases with age,7 ours is the first to show that the incidence rate of AVC increases with age and that age is independently associated with disease initiation. Thus AVC is more common with age due to both the accumulation of cases and an increase in the rate of disease formation. The biology of aging is complex, involving many changes at the chromosomal, mitochondrial and cellular levels. While it is possible that age serves only as a marker of duration of risk factor exposure, it is plausible that age-related cellular and metabolic changes promote the formation of AVC.
Prior epidemiologic investigations have reported cross-sectional associations between AVC and traditional risk factors, including age, male gender, smoking, hypertension, hyperlipidemia, diabetes, metabolic syndrome and height.7–10 However, only age and LDL cholesterol were associated with incident AVC in the Cardiovascular Health Study (CHS) population.11 Our study supports and extends the prior cross-sectional risk associations to prospective analyses, thereby supporting a causal inference for these factors in disease formation. In multivariate models, we found positive associations with age, male gender, BMI, current smoking, and use of antihypertensive and lipid lowering medications. We also recently reported an association between metabolic syndrome and incident AVC in this MESA cohort.12
Because our study likely contains confounding by indication, it seems reasonable to infer that hypertension and dyslipidemia, rather than their therapies, play a causal role in AVC formation. Moreover, consistent with guideline recommendations for statin therapy,13–15 statin treatment was more prevalent in MESA for those with diabetes (26%) than in those without diabetes (12%). Thus the observed association between lipid lowering therapy and incident AVC may, in part, reflect the impact of diabetes on incident AVC risk.12 In exploratory analyses using imputed LDL cholesterol levels for statin users (modeling a 30% LDL cholesterol reduction as treatment effect), diabetes showed a borderline association (β=1.49 [0.99,2.23], p=0.05) with incident AVC, while the Total:HDL cholesterol ratio showed a strong association (β=1.24 [1.07,1.43], p=0.004). Due to this confounding, we cannot determine the effects of lipid lowering therapy itself, and it remains possible that statin use is protective against incident AVC.
Prior studies examining potential risk factors for AVC progression have demonstrated variable results. Smaller CT-based studies of early-stage calcific aortic valve disease by Messika-Zeitoun and Pohle either found no associations with CVD risk factors, or associations with LDL cholesterol only.16,17 Prior echocardiographic studies, performed in hospital-based cohorts involving older subjects with later stage calcific aortic valve disease (i.e., aortic stenosis) and higher rates of renal dysfunction and CVD, have shown variable associations.18–21 Other, community-based studies using echocardiography have suggested that lipids were not associated with progression of late-stage calcific aortic valve disease,22 but have identified baseline valve calcium severity as a primary determinant of hemodynamic progression.23,24
Since it is unknown if the biology of calcification itself promotes calcification, we evaluated two models of AVC progression, one examining only CVD risk factors and the other incorporating baseline AVC content. In the first, we found age and male gender to be associated with the rate of AVC progression. In the second, we failed to find significant associations between traditional CVD risk factors and calcium progression. This finding can be interpreted in two manners. First, baseline Agatston scores, high baseline Agatston scores may simply be a marker of higher rates of past progression. The clinical factors causing “fast” progression lead to high baseline calcium levels, and these factors continue to cause higher rates of progression prospectively. In this light, baseline Agatston scores may represent a summary marker of CVD risk, thus displacing CVD risk factors from the multiplicative model.
Alternatively, these data could be interpreted as supporting the hypothesis that traditional CVD risk factors contribute to lesion formation and early stage progression, while later stage progression is influenced more heavily by direct paracrine or systemic regulators of calcification. Histopathological studies have documented myofibroblast transdifferentiation toward an osteoblastic phenotype in later stage disease,25 giving biologic plausibility to this hypothesis. When the analyses were stratified by baseline Agatston score, dissimilar results were found for those above and below the median. Only in the upper half of the cohort was the severity of baseline calcification a predictor of AVC progression. Thus our results are consistent with the hypothesis of overlapping biologic processes, but this epidemiologic investigation is limited in its ability to draw mechanistic conclusions.
The MESA study was in part designed to investigate the influence of race/ethnicity on subclinical cardiovascular disease. In multivariate analyses accounting for traditional CVD risk factors, we found no significant racial or ethnic differences in incident AVC or in AVC progression among Chinese, black and Hispanic as compared to white participants. This is in contrast to that previously observed in MESA regarding CAC,6 and in CHS for AVC progression in African-American subjects (the only non-white race or ethnicity studied).11
Our study has several limitations. First, MESA excluded subjects with known CVD and comprised subjects with low baseline AVC scores. Thus our findings are directly applicable only to this healthier population and to early-stage calcific aortic valve disease. Additionally, this exclusion may cause CVD-survival bias and diminish the perceived strength of associations, especially among the older cohorts. Second, AVC was not pre-specified variable during MESA design and thus the trial was not specifically powered for this analysis. However, the sample size of 738 for progression analyses is much larger than in previous studies,16,17 and therefore provides greater power to detect true associations and to minimize Type 2 errors. Third, baseline and follow up scans were measured separately, which may cause differential inclusion of annular calcium in the overall score. This is the most likely explanation for the high number of negative progressors seen in this study.
Acknowledgments
Role of the Sponsor: The NHLBI participated in the design and conduct of the MESA study, and the NHLBI Project Office reviewed and approved the manuscript prior to submission.
Funding/Support: This research was supported by R01-HL-63963-01A1, and by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Clinical Trial Registration: ClinicalTrials.gov Registry Unique Identifier: NCT00005487; URL: http://www.clinicaltrials.gov/ct2/show/NCT00005487
Drs. Owens, Katz and O’Brien had full access to the data and take responsibility for its integrity. All authors have read and approve the manuscript as submitted.
The authors thank Susan Larsen for assistance in manuscript preparation and the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial Disclosure: Dr. Budoff has received honoraria from and is on the speakers’ bureau of General Electric. Dr. O’Brien has received honoraria from and is on the speakers’ bureaus of AstraZeneca, Bristol-Myers Squibb and Merck.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi- ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 2.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 3.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 5.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, O’Brien KD, Carr JJ. Effect of scanner type on the reproducibility of extracoronary measures of calcification: the multi-ethnic study of atherosclerosis. Acad Radiol. 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 7.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 8.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 9.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 10.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors--a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 11.Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 12.Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O’Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 (Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 16.Pohle K, Dimmler A, Feyerer R, Feger S, Ropers D, Daniel WG, Achenbach S. Quantification of aortic valve calcification with electron beam tomography: a histomorphometric validation study. Invest Radiol. 2004;39:230–234. doi: 10.1097/01.rli.0000115749.08234.6a. [DOI] [PubMed] [Google Scholar]
- 17.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 18.Nassimiha D, Aronow WS, Ahn C, Goldman ME. Association of coronary risk factors with progression of valvular aortic stenosis in older persons. Am J Cardiol. 2001;87:1313–1314. doi: 10.1016/s0002-9149(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 19.Palta S, Pai A, Gill K, Pai R. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101:2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- 20.Faggiano P, Aurigemma GP, Rusconi C, Gaasch WH. Progression of valvular aortic stenosis in adults: literature review and clinical implications. Am Heart J. 1996;132:408–417. doi: 10.1016/s0002-8703(96)90440-8. [DOI] [PubMed] [Google Scholar]
- 21.Faggiano P, Antonini-Canterin F, Erlicher A, Romeo C, Cervesato E, Pavan D, Piazza R, Huang G, Nicolosi G. Progression of aortic valve sclerosis to aortic stenosis. Am J Cardiol. 2003;91:99–101. doi: 10.1016/s0002-9149(02)03011-4. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 23.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 24.Bahler RC, Desser DR, Finkelhor RS, Brener SJ, Youssefi M. Factors leading to progression of valvular aortic stenosis. Am J Cardiol. 1999;84:1044–1048. doi: 10.1016/s0002-9149(99)00496-8. [DOI] [PubMed] [Google Scholar]
- 25.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]