Abstract
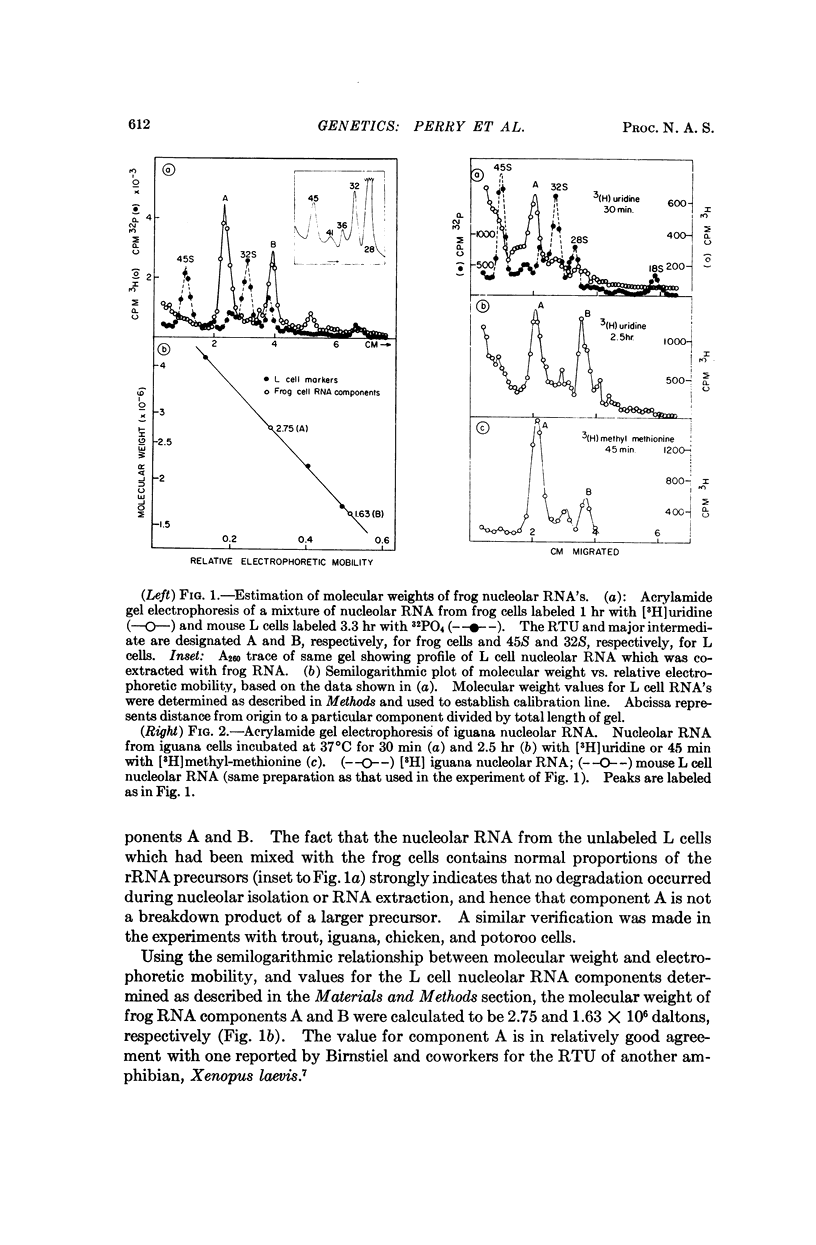
In eukaryotes the two principal RNA components of the ribosomes are initially synthesized as a large complex precursor molecule, which may be thought of as a transcription unit. The precursor is converted, via intermediates, to the mature forms of ribosomal RNA (rRNA). In order to assess the extent of variation in the size of this rRNA transcription unit among different organisms, and to infer its possible mode of evolution, we have determined its molecular weight in several selected species. Pulse-labeled and long-term labeled RNA's were extracted from various types of cells, and analyzed by electrophoresis on acrylamide gels. Identification of particular components as rRNA precursors was made according to several stated criteria. Our results, together with an analysis of previously published data, suggest that in plants and lower animals, up to and including reptiles, the unit of transcription of rRNA is a 2.7-2.8 million dalton molecule, which is only about 25 per cent larger than its combined rRNA products. In contrast, birds, marsupials and placental mammals, exhibit a seemingly less economical form of rRNA synthesis. Their transcription units are 4.0-4.2 million daltons, about 80 per cent larger than the rRNA products. In the organisms with the smaller transcription unit the major intermediate precursor of rRNA is 1.5-1.6 million daltons, as compared to 2.0-2.2 million daltons in birds and mammals. The significance of these findings is discussed in relation to evolutionary changes in the base composition of the ribosomal RNA genes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Meneghini R., Lara F. J. Patterns of ribonucleic acid synthesis in salivary glands of Rhynchosciara angelae larvae during development. Genetics. 1969;61(1 Suppl):351–360. [PubMed] [Google Scholar]
- Birnstiel M., Speirs J., Purdom I., Jones K., Loening U. E. Properties and composition of the isolated ribosomal DNA satellite of Xenopus laevis. Nature. 1968 Aug 3;219(5153):454–463. doi: 10.1038/219454a0. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Edström J. E., Daneholt B. Sedimentation properties of the newly synthesized RNA from isolated nuclear components of Chironomus tentans salivary gland cells. J Mol Biol. 1967 Sep 14;28(2):331–343. doi: 10.1016/s0022-2836(67)80013-5. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Mezger-Freed L. Stable haploid cultured cell lines from frog embryos. Proc Natl Acad Sci U S A. 1970 Feb;65(2):337–344. doi: 10.1073/pnas.65.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Nuclear RNA of the salamander oocyte. Natl Cancer Inst Monogr. 1966 Dec;23:475–488. [PubMed] [Google Scholar]
- Gould M. C. Rna and protein synthesis in the unfertilized eggs of Urechis caupo. Dev Biol. 1969 May;19(5):460–481. doi: 10.1016/0012-1606(69)90083-9. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Synthesis and properties of ribosomal RNA in Drosophila. J Mol Biol. 1969 Nov 28;46(1):85–98. doi: 10.1016/0022-2836(69)90059-x. [DOI] [PubMed] [Google Scholar]
- Jeanteur P., Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. II. Evidence for sequences of non-ribosmal type in 45 and 32 s ribosomal RNA precursors. J Mol Biol. 1968 May 14;33(3):757–775. doi: 10.1016/0022-2836(68)90318-5. [DOI] [PubMed] [Google Scholar]
- Leick V. Formation of subribosomal particles in the macronuclei of Tetrahymena pyriformis. Eur J Biochem. 1969 Mar;8(2):221–228. doi: 10.1111/j.1432-1033.1969.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Osawa S. Biosynthesis of ribosomes in bacterial cells. Prog Nucleic Acid Res Mol Biol. 1965;4:161–188. doi: 10.1016/s0079-6603(08)60787-4. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E., Greenberg H. RNA metabolism in the HeLa cell nucleus and nucleolus. Natl Cancer Inst Monogr. 1966 Dec;23:489–512. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Persistent synthesis of 5S RNA when production of 28S and 18S ribosomal RNA is inhibited by low doses of actinomycin D. J Cell Physiol. 1968 Dec;72(3):235–246. doi: 10.1002/jcp.1040720311. [DOI] [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. The nucleolus and the synthesis of ribosomes. Natl Cancer Inst Monogr. 1965 Dec;18:325–340. [PubMed] [Google Scholar]
- Petermann M. L., Pavlovec A. The subunits and structural ribonucleic acids of Jensen sarcoma ribosomes. Biochim Biophys Acta. 1966 Feb 21;114(2):264–276. doi: 10.1016/0005-2787(66)90308-x. [DOI] [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele W. J. Localization of deoxyribonucleic acid complementary to ribosomal ribonucleic acid and preribosomal ribonucleic acid in the nucleolus of rat liver. J Biol Chem. 1968 Jun 25;243(12):3333–3341. [PubMed] [Google Scholar]
- Taber R. L., Jr, Vincent W. S. The synthesis and processing of ribosomal RNA precursor molecules in yeast. Biochim Biophys Acta. 1969 Aug 20;186(2):317–325. doi: 10.1016/0005-2787(69)90009-4. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C., PYLE E. A., DEXTER R. P. Preparation of monolayer cell cultures from tissues of some lower vertebrates. Science. 1960 Dec 23;132(3443):1890–1891. doi: 10.1126/science.132.3443.1890. [DOI] [PubMed] [Google Scholar]
- Wallace H., Birnstiel M. L. Ribosomal cistrons and the nucleolar organizer. Biochim Biophys Acta. 1966 Feb 21;114(2):296–310. doi: 10.1016/0005-2787(66)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Loening U., Willems M., Penman S. Acrylamide gel electrophoresis of HeLa cell nucleolar RNA. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1088–1095. doi: 10.1073/pnas.58.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Wagner E., Laing R., Penman S. Base composition of ribosomal RNA precursors in the HeLa cell nucleolus: further evidence of non-conservative processing. J Mol Biol. 1968 Mar 14;32(2):211–220. doi: 10.1016/0022-2836(68)90005-3. [DOI] [PubMed] [Google Scholar]



