Abstract
Direct interaction with the β subunit of the heterotrimeric G protein complex causes voltage-dependent inhibition of N-type calcium channels. To further characterize the molecular determinants of this interaction, we performed scanning mutagenesis of residues 372-387 and 410-428 of the N-type channel α1 subunit, in which individual residues were replaced by either alanine or cysteine. We coexpressed wild type Gβ1γ2 subunits with either wild type or point mutant N-type calcium channels, and voltage-dependent, G protein-mediated inhibition of the channels (VDI) was assessed using patch clamp recordings. The resulting data indicate that Arg376 and Val416 of the α1 subunit, residues which are surface-exposed in the presence of the calcium channel β subunit, contribute significantly to the functional inhibition by Gβ1. To further characterize the roles of Arg376 and Val416 in this interaction, we performed secondary mutagenesis of these residues, coexpressing the resulting mutants with wild type Gβ1γ2 subunits and with several isoforms of the auxiliary β subunit of the N-type channel, again assessing VDI using patch clamp recordings. The results confirm the importance of Arg376 for G protein-mediated inhibition and show that a single amino acid substitution to phenylalanine drastically alters the abilities of auxiliary calcium channel subunits to regulate G protein inhibition of the channel.
Background
The mammalian nervous system expresses nine different genes that encode different types of voltage-gated calcium channel (VGCC) α1 subunits which interact with auxiliary subunits and form classes of VGCCs that are distinct in structure, pharmacology, and physiology [1]. VGCCs containing the α1A and α1B subunits (P/Q- and N-type channels respectively) are distinguished from other types by their localization to pre-synaptic nerve terminals, where they mediate calcium influx which contributes to evoked neurotransmitter release and overall synaptic function [2-4].
Inhibition of P/Q- and N-type channels resulting from activation of G-protein coupled receptors (GPCRs)--a crucial mode of regulation, notably illustrated in the relief of pain sensations in response to opioid drugs [5]--has been studied for over 25 years [6-16]. This mode of regulation is complex and comprises multiple pathways that include direct and indirect actions of G proteins on the channel [17]. During membrane delimited G protein inhibition, GPCR activation releases Gβγ heterodimers which then bind directly to the α1 subunits of P/Q- and N-type channels, and this interaction stabilizes closed channel conformations and culminates in channel inhibition [18,19].
A recent study suggests that interaction of Gβγ with N-type channels can slow the kinetics of channel transition to inactivated states [20]. However, most studies of the direct Gβγ-presynaptic channel interaction have investigated the slowing of transition to activated channel states, and have found this mode of inhibition to be more favored at hyperpolarized potentials, thus allowing for activity dependent dis-inhibition [21-24]. Hence, the term "voltage-dependent inhibition" (VDI) has been used to describe two experimental hallmarks of this Gβγ-mediated regulation: slowing of presynaptic channel activation, and relief of channel inhibition by a strong, depolarizing pre-pulse.
Gβγ-mediated VDI depends on a complex set of structural determinants that contribute to direct interaction between Gβγ and the presynaptic calcium channel. As such, the extent of VDI varies with the isoforms of the channel subunits and the G protein subunits in question [17]. Structure-activity relationship studies of the interaction have revealed roles for three cytosolic regions of the α1 subunit: the N-terminus, the I-II linker domain, and the C-terminus [25-30]. While the C-terminal region of the channel is thought to play a supporting role as an enhancer of overall Gβγ-channel binding affinity [31], both the N-terminus and the I-II linker contribute directly to the inhibitory interaction with Gβγ. Furthermore, contact between the N-terminus and the I-II linker is demonstrated to be necessary for Gβγ-mediated VDI [25]. Efforts to resolve functionally important Gβγ-channel binding interactions have also revealed the direct involvement of two nearby sections of the I-II linker: amino acid residues 372-389 and 410-428 [27,30]. The first of these sections partially overlaps with the α1 subunit alpha interaction domain (AID) and contains residues known to bind the calcium channel β subunit, presumably in a manner that precludes many of them from interaction with Gβγ [32]. However, the contribution of the other I-II linker residues in question--to direct Gβγ-binding and hence to channel inhibition--has remained unclear.
Here we aimed to further resolve the molecular determinants of Gβγ-mediated channel inhibition by testing the functional contribution of individual residues in the two above-mentioned sections of the α1B I-II linker. Using a combination of alanine/cysteine scanning mutagenesis and whole-cell electrophysiological recordings from tsA-201 cells, we identify two residues of the I-II linker, Arg376 and Val416, as key determinants of Gβγ-mediated, voltage-dependent modulation of N-type channels.
Methods
cDNAs
Wild type (WT) rat calcium channel subunit cDNAs encoding Cav2.2 (α1B), Cavβ1b, Cavβ2a, Cavβ3, and Cavβ4, and α2-δ1 subunits were generously donated by Dr.Terry Snutch (University of British Columbia, Vancouver, BC). The construction of cDNAs encoding WT human Gβ1 and Gγ2 subunits have been described previously [33].
Cav2.2 α1B mutants
cDNAs encoding single-residue Cav2.2 α1B mutants were constructed by overlap extension PCR [34], using WT α1B as the DNA template and Pfu turbo DNA polymerase (Stratagene) according to manufacturer's instructions. AarI and BsiWI restriction sites, found in the native sequence at locations flanking the mutagenized I-II loop-encoding sequence, were included in the 5' ends of the non-mutagenic flanking primers. After mutagenizing and overlap extension reactions, mutant α1B cDNA fragments were digested with AarI and BsiWI, and then sub-cloned into the (AarI-, BsiWI-digested) α1B mammalian expression vector, pCMV30-14G. Codons for 27 residues within amino acid sequence regions 372-389 and 410-428 were individually substituted to alanine, and three naturally occurring alanine codons were substituted to cysteine. These 30 mutations included: F372A, L373A, K374A, L375A, R376A, R377A, Q378A, Q379A, E382A, R383A, N386A, K410A, S411A, P412A, L413A, D414A, A415C, V416A, L417A, K418A, R419A, A420C, A421C, T422A, K423A, K424A, S425A, R426A, N427A, and D428A. cDNAs encoding four additional point mutations, R376E, R376F, V416E, and V416K, and a double alanine mutation, (both R376A and V416A), were also constructed using overlap/extension PCR as described above.
Tissue Culture and Transient Transfection
Human embryonic kidney tsA-201 cells were grown and transiently transfected using the calcium phosphate method as described previously (32). Transfection solutions for individual culture dishes contained a mixture of cDNA expression vectors, with the following quantities of each cDNA expression construct used: calcium channel α1B subunit, 6 μg; Cavβ subunit (6 μg), Cavα2-δ1 subunit (6 μg), Gβ1 subunit, 6 μg; Gγ2 subunit, 6 μg; and 1 μg of a pEGFP marker vector (Clontech). Positive controls contained the WT α1B subunit in place of mutant α1B, and negative controls consisted of the WT α1B subunit in the absence of exogenous Gβ1γ2. 12 hr post-transfection, cells were washed 1× with PBS pH 7.4, supplemented with fresh DMEM, and allowed to recover for an additional 12 hr. To prevent overgrowth, cells were subsequently transferred to a 29°C incubator and maintained for 24-72 hr prior to voltage-clamp recording.
Cav2.2 Voltage Clamp Recordings
Glass coverslips carrying cells expressing mutant or WT Cav2.2 channels were transferred to a 3.5-cm culture dish (Corning) containing external recording solution consisting of 20 mM BaCl2, 1 mM MgCl2, 10 mM HEPES, 40 mM TEA-Cl, 10 mM glucose, and 65 mM CsCl (pH 7.2 adjusted with TEA-OH). Micro-electrode patch pipettes were pulled using a Sutter P-87 micro-electrode puller or a DMZ Universal puller, and manually fire-polished using a Narishige MF-830 Micro Forge to attain a typical resistance of 4-5 MÙ. Internal pipette solution consisted of 108 mM CsMeSO4, 4 mM MgCl2, 9 mM EGTA, and 9 mM HEPES (pH 7.2 adjusted with CsOH).
Whole cell patch clamp recordings were performed in voltage-clamp mode using an Axopatch 200B amplifier (Axon Instruments) linked to a personal computer with pCLAMP version 9.0 or 9.2 software. Series resistance was compensated by 85%, leak currents were negligible, and the data were filtered at 1 kHz. Individual pEGFP-expressing cells were held at -100 mV, and currents were evoked by stepping to a test potential of +20 mV. Only cells with current amplitudes greater than 50 pA and less than 1.5 nA were used for analysis.
Voltage-dependent G protein inhibition was assessed by application of a strong, depolarizing pre-pulse (PP) to +150 mV for 50 ms, immediately prior to the test potential--during alternating sweeps of an assay. Pre-pulse relief of inhibition was quantified as the ratio of peak current amplitudes observed in paired test pulses performed with (I+PP) and without (I-PP) the prepulse (i.e., I+PP/I-PP).
Data Analysis
All data were analyzed using Clampfit version 9.2 (Axon Instruments) and fitted in Sigmaplot 2000 (SPSS Inc.). Statistical analyses were carried out using SigmaStat 2.03 (SPSS Inc.). All sample means are reported +/- SEM. Statistically significant differences between means were assessed using student's t-test, Mann-Whitney rank sum test, or one-way ANOVA at 95% confidence level as appropriate.
Results
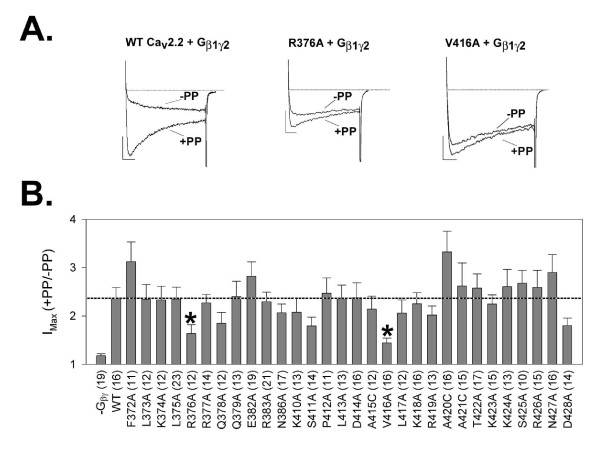
Previously, two sections of the N-type channel I-II linker region, α1B amino acid residues 372-389 and 410-428, were identified as functionally important binding sites for the Gβγ heterodimer [27,30]. To test the contribution of individual residues of these sections to Gβγ-mediated channel inhibition, alanine/cysteine scanning mutagensis was performed. Residues 372-389 include six amino acid residues that are predicted, on the basis of crystallographic data, to be unavailable for interaction with Gβγ, as their access is likely occluded by the calcium channel β subunit [32]. The remaining residues in this section, and in the second region (residues 410-428) were individually substituted to alanine or cysteine. The resulting mutant channels were coexpressed with human Gβ1γ2, and their respective susceptibilities to Gβγ-mediated VDI were quantified using a pre-pulse facilitation (PPF) paradigm (Figure 1).
Figure 1.
A: Three sets of typical current traces from tsA-201 cells expressing WT or mutant rat Cav2.2 calcium channels and Gβ1γ2, as described in METHODS. Each is a matched set of current traces from sequential test pulses, with the second test pulse preceded by a150-mV depolarizing prepulse. (Vertical and horizontal scale bars represent 15 pA and 15 ms, respectively; in each set the trace with larger current values is from the second test pulse.) Left: traces from a cell expressing the WT channel and Gβ1γ2. Center: traces from a cell coexpressing the Cav2.2-R376A mutant channel and Gβ1γ2. Right: traces from a cell coexpressing the Cav2.2-V416A mutant channel and Gβ1γ2. B: Histogram summarizing the results of paired-pulse facilitation (PPF) experiments performed with all Ala/Cys point mutants of Cav2.2; columns show mean PPF values with SE bars for each condition. Respective positions of mutations in the Cav2.2 amino acid sequence are indicated by numbers beneath the corresponding columns (see METHODS for full description of the mutations used). Of the 30 individual amino acid residues examined in the Cav2.2 I-II linker region, Ala mutations at both Arg376 and Val416 (*p < 0.05, t-test) result in a significant loss of Gβγ-mediated channel inhibition, as measured by the degree of pre-pulse relief following a depolarizing pre-pulse, when compared to WT control. Numbers in parentheses indicate numbers of cells tested.
WT channels displayed the hallmark characteristics of Gβγ-mediated channel inhibition (Figure 1A, left), including kinetic slowing of activation and relief of inhibition by a strong depolarizing pre-pulse (PPF ratio for WT channel assays: 2.36 +/- 0.23). When examining PPF ratios obtained with mutant and WT channels, two of the 30 mutants examined, R376A and V416A, showed a significant loss of Gβγ-mediated inhibition when compared to wild type channels (PPF ratios 1.64 +/- 0.18 and 1.44 +/- 0.01, respectively; *P = 0.028 and 0.001, respectively; see Figure 1B). To test whether or not the effect of these mutations on Gβγ-mediated channel inhibition were additive, a double Cav2.2 α1B mutant containing both the R376A and V416A substitutions was engineered. Co-expression of this double mutant with exogenous Gβ1γ2, and subsequent electrophysiological analysis using the PPF protocol, found the degree of Gβγ-mediated inhibition to be significantly less than that of WT channels, but similar to that observed in the presence of either one of the individual mutations alone (PPF ratio: 1.71 +/- 0.15, *t-test, P = 0.023) (Figure 2A).
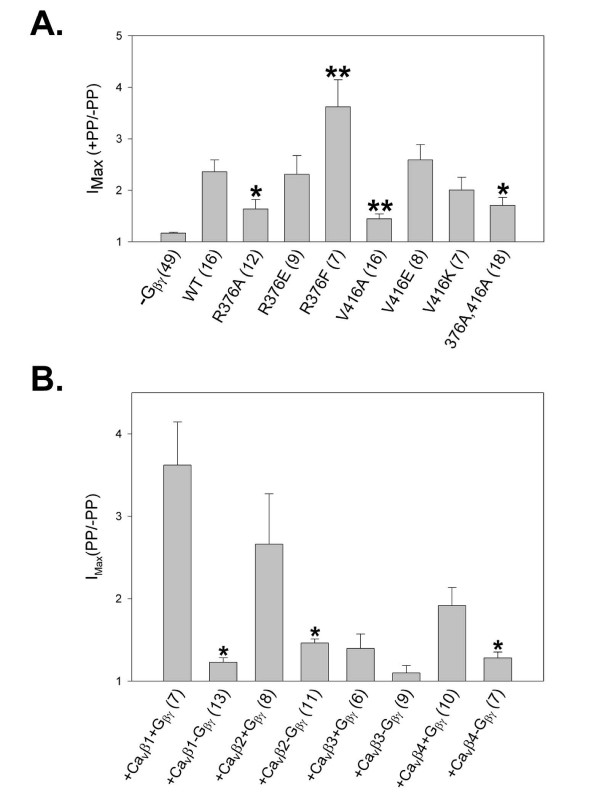
Figure 2.
A: Histogram summarizing the results of PPF experiments performed with Cav2.2 isoforms carrying mutations of α1B residues Arg376 and Val416. Columns show mean PPF values with SE bars for each condition. Human Gβ1γ2 was co-expressed in tsA-201 cells with the Cav2.2 isoforms for each condition presented except for the negative control ("-Gβ1γ2"). Respective mutations in the Cav2.2 amino acid sequence are indicated beneath the corresponding columns (see METHODS for full description of the mutations used). Of the various conditions examined only the mutations R376A, R376F, V416A, and the double mutation R376A, V416A resulted in a significant loss of Gβγ-mediated channel inhibition, as measured by the degree of pre-pulse relief following a depolarizing pre-pulse, when compared to WT control (*p < 0.05, t-test, **p < 0.05 one-way ANOVA, Dunnett's method, or Kruskal-Wallis one-way ANOVA on ranks). Numbers in parentheses indicate numbers of cells tested for the respective condition. B: Histogram summarizing the results of PPF experiments using tsA-201 cells co-transfected to express α1B mutant R376F with Cavβ isoforms β1B, β2, β3, and β4, with or without heterologous human Gβ1γ2 as indicated by labels beneath columns. Columns show mean PPF values with SE bars for each condition (see METHODS for full description of the mutations used). Of the conditions examined, coexpression of Cavβ1B and Cavβ2a, and Cavβ4 resulted in statistically significant differences in mean current density for R376F channels expressed with and without heterologous Gβγ (*p < 0.05 using t-test, Mann-Whitney rank sum test, and t-test, respectively).
To further characterize the roles of Arg376 and Val416, additional single mutant α1B subunits, containing R376E, R376F, V416E, and V416K substitutions, were engineered and co-expressed with exogenous Gβ1γ2. Neither of the latter Val416 substitutions resulted in significant changes in PPF ratio as compared to WT channels, Fig. 2A). However, the phenylalanine substitution at Arg376 significantly increased the PPF ratio for channels coexpressed with Gβ1γ2 (mean values of 2.36 and 3.62 for WT and R376F, respectively, t-test p < 0.017, Fig. 2A), suggesting that this amino acid substitution actually increased Gβγ-mediated channel inhibition. These data thus further support the notion of residue 376 being an important determinant of VD G protein modulation of N-type channels.
The enhancement of Gβγ-mediated VDI by the R376F mutation, and the proximity of this mutation to critical sites of interaction between the α1B and β subunits of the Cav2.2 channel, led us to ask whether the nature of the Cavβ subunit might affect this enhancement. To examine this issue, we coexpressed the α2-δ subunit and the R376F mutant with different isoforms of the Cavβ subunit in tsA-201 cells, and for each resulting population of Cav2.2 channels, we tested the effect of coexpression of heterologous Gβ1γ2 on current densities and PPF ratios. As shown in Fig. 2B, little VD modulation was observed in the absence of exogenously coexpressed Gβ1γ2 irrespective of the type of calcium channel β subunit that was present. For channels containing either Cavβ2a or Cavβ4, coexpression with Gβ1γ2 induced robust VD modulation of channel activity, whose magnitude was, however, smaller than that observed with channels containing Cavβ1. Strikingly, virtually no VD modulation was evident in R376F channels containing the Cavβ3 subunit (i.e., there was no significant difference in PPF in the presence and the absence of G proteins). These data are in striking contrast to our previous findings showing that with WT Cav2.2, Gβ1γ2 most strongly inhibited channels containing Cavβ2a, followed by Cavβ3, Cavβ4 and Cavβ1B [35]. Hence, a single amino acid substitution in the Cav2.2 I-II linker drastically alters the Cavβ subunit dependence of Gβ1γ2 inhibition of the channel.
Discussion
In this manuscript we have narrowly focused on the contribution of individual amino acids in the Cav2.2 I-II linker region to voltage dependent G protein inhibition of the channel. Among thirty amino acids in the I-II linker of the Cav2.2 channel, we have identified two, Arg376 and Val416, that serve as determinants of G-protein mediated VDI of Cav2.2 channels, suggesting a highly localized interaction of Gβ1γ2 with the I-II loop. The impact of single amino acid substitutions on G protein inhibition is reminiscent of our earlier findings showing that phosphorylation of a single I-II linker residue, Thr422, can disrupt modulation of Cav2.2 channels by Gβ1 [36,37].
Arg376 is particularly interesting because the R376F mutation drastically altered the impact of Cavβ subunit coexpression on the degree of VDI: whereas Gβ1-mediated inhibition of WT channels is reported to be strongest for channels containing Cavβ2a, followed by Cavβ3, Cavβ4 and Cavβ1B, respectively [35], we report here that Gβ1-mediated regulation of R376F channels is strongest for channels containing Cavβ1B, followed by Cavβ2a, Cavβ4, Cavβ3, respectively, with no significant voltage-dependent inhibition observed for the latter. At this point we do not know how the mutation of residue 376 to phenylalanine increases VD G protein inhibition. When coexpressed with Cavβ1B in the absence of heterologous Gβ1γ2, the R376F mutant had a half activation potential that did not differ significantly from that of the wild type channels (data not shown); moreover, at the majority of test potentials examined, the mutation yielded no significant changes in the rates of activation and inactivation (versus WT channels, data not shown), suggesting that the effects of the mutation on Gβγ modulation are not complicated by changes in biophysical properties of the channel. An increased degree of prepulse relief could occur as a result of several mechanisms. First, the mutation could destabilize the binding of Gβγ to the channel, thus resulting in more effective dissociation of Gβγ from the channel in response to membrane depolarization. This however seems unlikely, because the kinetics of the facilitated current were found to be similar for both the wild type and the mutant channels, indicating that both channels are completely dis-inhibited (and thus dissociated from Gβγ) following the application of a prepulse (data not shown). Agler and colleagues [25] reported that the N-terminus of Cav2.2 is capable of interacting with the domain I-II linker, and that this interaction contributes to G protein inhibition of the channel. It is thus conceivable that the nature of residue 376 could affect G protein inhibition indirectly by virtue of altering the binding of the N-terminus to the I-II linker.
Alternatively, it is possible that residue 376 is involved in transducing Gβγ binding to alter channel gating, such that a stronger voltage dependent inhibition is observed in the mutant channel. Residue 376 is three amino acid residues just upstream of an alpha helical structure (the AID, comprising residues 379-396) that is involved in binding of the Cavβ subunit [38], and could potentially serve as a hinge that links G protein binding to the gating machinery of the channel. However, it has also been proposed for Cav2.1 channels that I-II linker residues 357-378 are all part of a stable continuation of the alpha helical structure of the AID, and that stability and continuity of this helical structure is required for VD G protein inhibition of the channel [39]. In the latter case the R376F mutation may simply create a more stable binding pocket for Gβγ, perhaps in part by eliminating one of the eight positive charges carried by the side chains of I-II residues 357-396, which may render this section of the I-II linker less likely to move in response to a membrane depolarization event. Whatever the actual case, the proximity of Arg376 to the Cavβ subunit interaction site also provides for a mechanism by which the nature of the Cavβ subunit could affect the extent of G protein inhibition that is observed.
Although alanine mutagenesis of residues 376 and 416 significantly reduced the effects of Gβγ, VDI was not completely eliminated, and the effects of the individual amino acid substitutions were not additive. This suggests that either other amino acid residues in the Cav2.2 α1 subunit might help stabilize the binding of Gβγ to the channel (such as for example, residue in the N-terminus), or that the Cavβ subunit may contribute directly to anchoring Gβγ to the channel. The latter would be consistent with recent findings showing that the presence of the Cavβ subunit is required to permit VDI of Cav2.1 channels [39].
Altogether, our data further implicate the domain I-II linker region as an important contributor to voltage dependent G protein modulation of N-type calcium channels. Furthermore, our results suggest that the regulation of N-type calcium channels by G proteins involves complex interactions between Gβγ, the Cav2.2 α1 subunit, and the auxiliary Cavβ subunit, and reveal that substitution of a single amino acid residue that is conserved in all HVA calcium channels is sufficient to significantly alter the interactions among these players. Although the precise molecular mechanism by which residue 376 couples Gβγ interactions to alterations in channel function remains unknown, the observation that highly localized alteration of a single amino acid residue increased G protein inhibition of the channel may offer potential avenues to enhance the efficacy of therapeutics acting on N-type channels via GPCRs.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HWT performed molecular biology, cell transfection, electrophysiology, data analysis, and proofreading. AEK performed cell transfection, most of the electrophysiology recordings, and contributed data analysis and proofreading. LC performed tissue culture and electrophysiology recordings. LBV and DL performed cell transfection, electrophysiology, and data analysis. GWZ designed and supervised the research project, and provided analysis and proofreading. All authors read and approved the final manuscript.
Contributor Information
Hugo W Tedford, Email: hwtedford@vestaron.com.
Alexandra E Kisilevsky, Email: aekisile@ucalgary.ca.
Lucienne B Vieira, Email: lubvieira@yahoo.com.
Diego Varela, Email: dvarela@bitmed.med.uchile.cl.
Lina Chen, Email: linchen@ucalgary.ca.
Gerald W Zamponi, Email: zamponi@ucalgary.ca.
Acknowledgements
This work was supported by an operating grant to GWZ from the Canadian Institutes of Health Research. GWZ is a Scientist of the Alberta Heritage Foundation for Medical Research (AHFMR) and a Canada Research Chair in Molecular Neurobiology. HWT, DV and LBV were supported by AHFMR Fellowships and by Fellowships from the Heart and Stroke Foundation of Canada. AEK was supported by an AHFMR Studentship award and a studentship from the Natural Sciences and Engineering Research Council of Canada.
References
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-P. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliot EM, Hell JW, Starr TVB, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin H, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Zamponi GW. Targeting calcium channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004;25:465–470. doi: 10.1016/j.tips.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Bernheim L, Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992;8:97–106. doi: 10.1016/0896-6273(92)90111-P. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bernheim L, Mathie A, Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1991;88:652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol. 1994;477(Pt 3):415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golard A, Siegelbaum SA. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993;13:3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol. 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Kongsamut S, Tsien RW. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Hille B. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G protein pathways. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-L. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G Protein Modulation of Cav2 Calcium Channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- McDavid S, Currie KP. G-Proteins Modulate Cumulative Inactivation of N-Type (Cav2.2) Calcium Channels. J Neurosci. 2006;26:13373–13383. doi: 10.1523/JNEUROSCI.3332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/S0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc Natl Acad Sci USA. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agler HL, Evans J, Tay LH, Anderson MJ, Colecraft HM, Yue DT. G protein-gated inhibitory module of N-type (Ca(v)2.2) Ca2+ channels. Neuron. 2005;46:891–904. doi: 10.1016/j.neuron.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of residues in the N terminus of alpha1B critical for inhibition of the voltage-dependent calcium channel by Gbeta gamma. J Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein beta-gamma complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- Page KM, Stephens GJ, Berrow NS, Dolphin AC. The intracellular loop between domains I and II of the B-type calcium channel confers aspects of G-protein sensitivity to the E-type calcium channel. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of gbeta-gamma with a C-terminal gbeta-gamma-binding domain of the Ca2+ channel alpha1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- Li B, Zhong H, Scheuer T, Catterall WA. Functional role of a C-terminal Gbetagamma-binding domain of Ca(v)2.2 channels. Mol Pharmacol. 2004;66:761–769. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot MI, Stotz SC, Jarvis SE, Zamponi GW. Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms. J Physiol. 2000;527(Pt 2):203–212. doi: 10.1111/j.1469-7793.2000.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Arnot MI, Doering CJ, Zamponi GW. Calcium channel beta subunits differentially regulate the inhibition of N-type channels by individual Gbeta isoforms. J Biol Chem. 2001;276:45051–45058. doi: 10.1074/jbc.M107784200. [DOI] [PubMed] [Google Scholar]
- Hamid J, Nelson D, Spaetgens R, Dubel SJ, Snutch TP, Zamponi GW. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. J Biol Chem. 1999;274:6195–6202. doi: 10.1074/jbc.274.10.6195. [DOI] [PubMed] [Google Scholar]
- Cooper CB, Arnot MI, Feng ZP, Jarvis SE, Hamid J, Zamponi GW. Cross-talk between G-protein and protein kinase C modulation of N-type calcium channels is dependent on the G-protein beta subunit isoform. J Biol Chem. 2000;275:40777–40781. doi: 10.1074/jbc.C000673200. [DOI] [PubMed] [Google Scholar]
- Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel beta-subunits: structural insights AID our understanding. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y-H, Bangaru SD, He L, Abele K, Tanabe S, Kozasa T, Yang J. Origin of the Voltage Dependence of G-Protein Regulation of P/Q-type Ca2+ Channels. J Neurosci. 2008;28:14176–14188. doi: 10.1523/JNEUROSCI.1350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]