Abstract
The DNA-dependent RNA polymerase activity present in rat liver nuclei has been solubilized and purified from whole nuclei and from subnuclear fractions. As reported earlier (Roeder, R. G., and W. J. Rutter, Nature, 224, 234 (1969)), two major chromatographically distinct enzymatic species (I and II) are present in whole nuclei. Subfractionation of whole nuclei into nucleolar and nucleoplasmic fractions had little effect on the total recovery of activity. Purified nucleoli contain predominantly polymerase I, whereas the nucleoplasmic fraction is greatly enriched for polymerase II. A third minor peak of activity has also been resolved in the nucleoplasmic preparations. We conclude that the RNA polymerases are specifically localized within the nucleus and may, therefore, play specific roles in the regulation of genetic transcription.
Full text
PDF
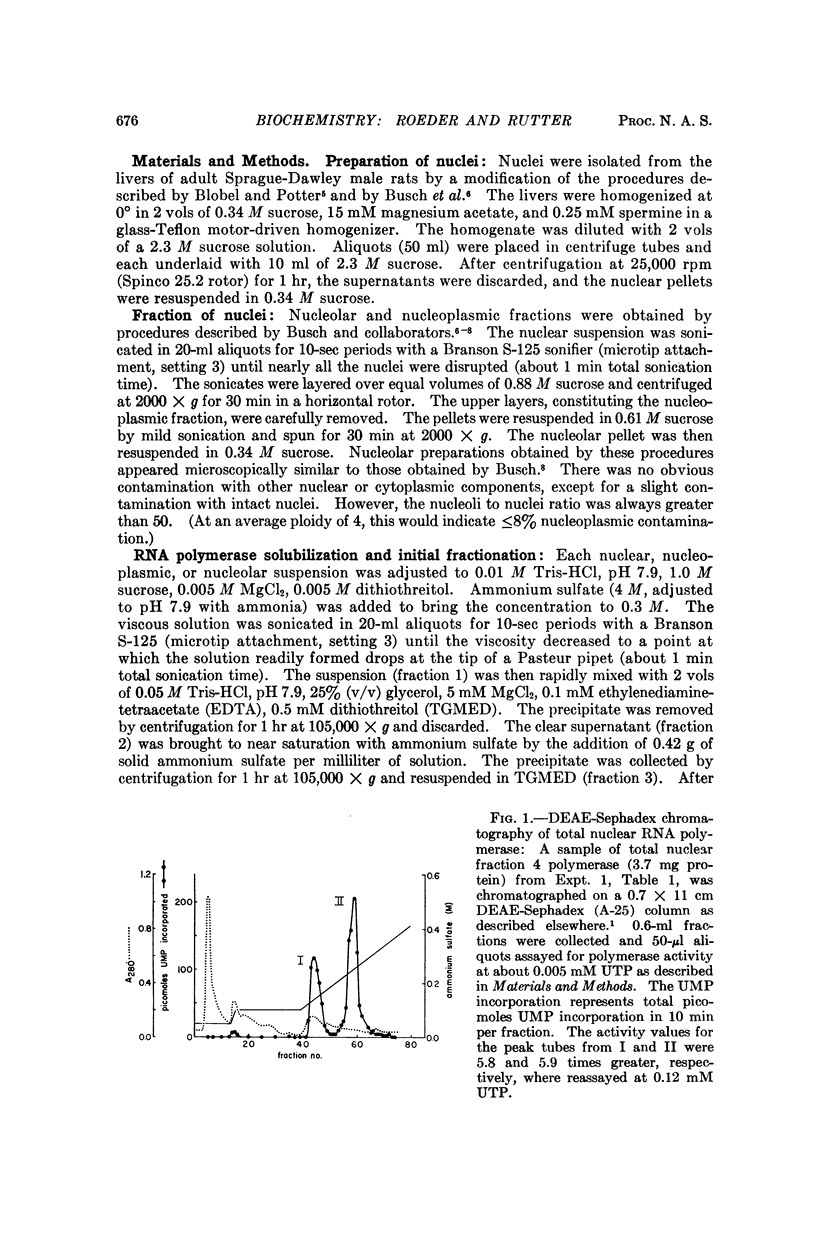

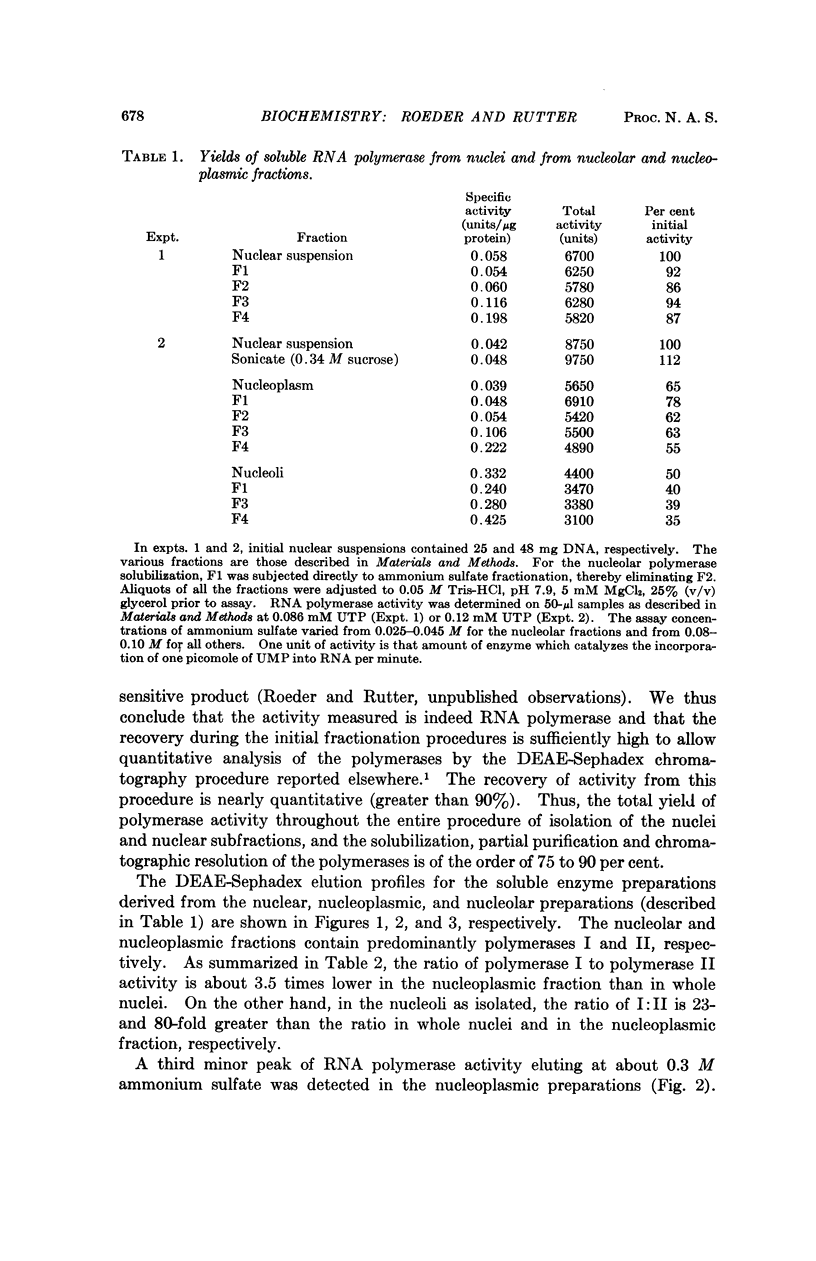
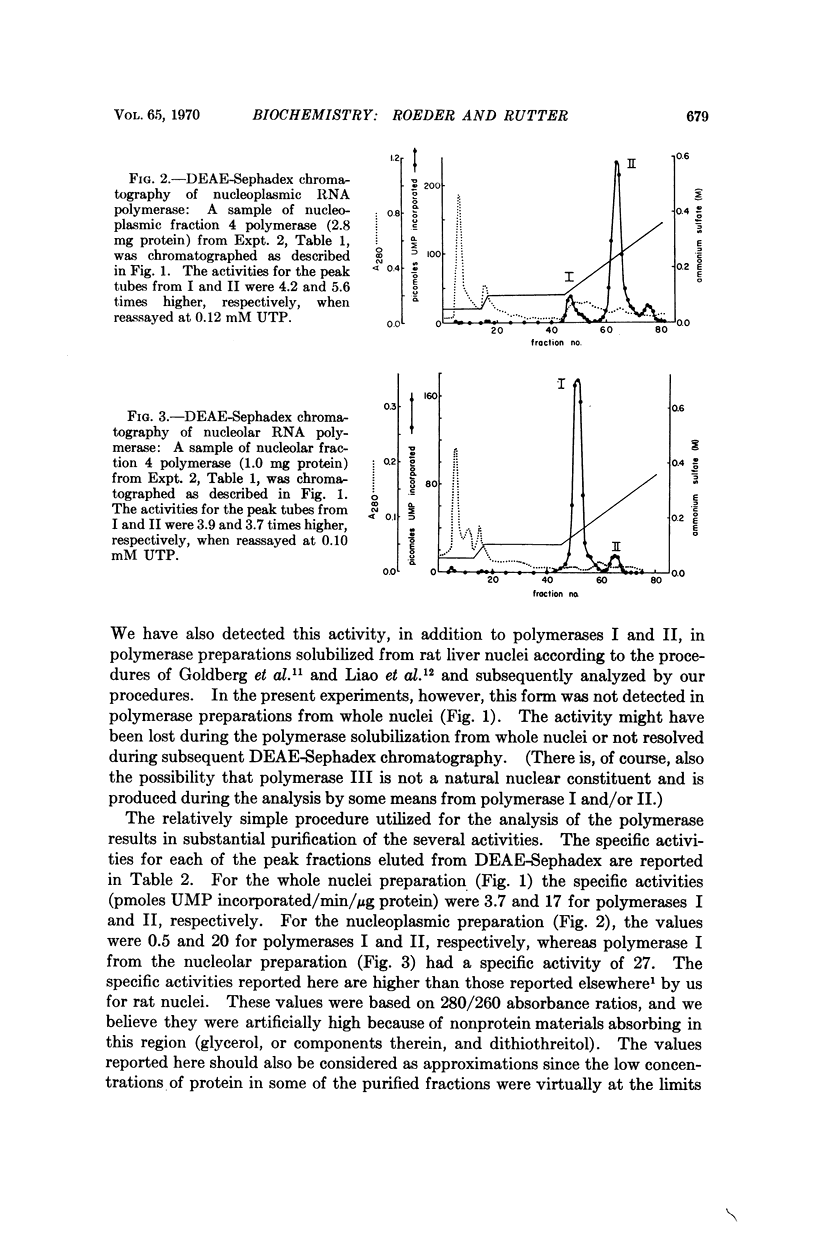



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Busch H., Narayan K. S., Hamilton J. Isolation of nucleoli in a medium containing spermine and magnesium acetate. Exp Cell Res. 1967 Aug;47(1):329–336. doi: 10.1016/0014-4827(67)90235-2. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Moon H. D., Rosenau W. Partial purification of RNA polymerase from rat liver nuclei. Biochim Biophys Acta. 1969 Jan 7;171(1):192–194. doi: 10.1016/0005-2744(69)90120-x. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Presence of two RNA polymerase activities in liver nucleoli. Biochim Biophys Acta. 1968 Apr 22;157(2):421–423. doi: 10.1016/0005-2787(68)90098-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao S., Sagher D., Fang S. M. Isolation of chromatin-free RNA polymerase from mammalian cell nuclei. Nature. 1968 Dec 28;220(5174):1336–1337. doi: 10.1038/2201336a0. [DOI] [PubMed] [Google Scholar]
- MCCONKEY E. H., HOPKINS J. W. THE RELATIONSHIP OF THE NUCLEOLUS TO THE SYNTHESIS OF RIBOSOMAL RNA IN HELA CELLS. Proc Natl Acad Sci U S A. 1964 Jun;51:1197–1204. doi: 10.1073/pnas.51.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Hamilton T. H. The intranuclear localization of two DNA-dependent RNA polymerase activities. Proc Natl Acad Sci U S A. 1967 May;57(5):1371–1378. doi: 10.1073/pnas.57.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo A. O., Littau V. C., Allfrey V. G., Mirsky A. E. Modification of ribonucleic Acid synthesis in nuclei isolated from normal and regenerating liver: some effects of salt and specific divalent cations. Proc Natl Acad Sci U S A. 1967 Mar;57(3):743–750. doi: 10.1073/pnas.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Hormones and the synthesis and utilization of ribonucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:191–250. doi: 10.1016/s0079-6603(08)60235-4. [DOI] [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. EVIDENCE FOR TWO DNA-DEPENDENT RNA POLYMERASE ACTIVITIES IN ISOLATED RAT-LIVER NUCLEI. Biochim Biophys Acta. 1964 Jul 22;87:531–533. doi: 10.1016/0926-6550(64)90133-1. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]



