Abstract
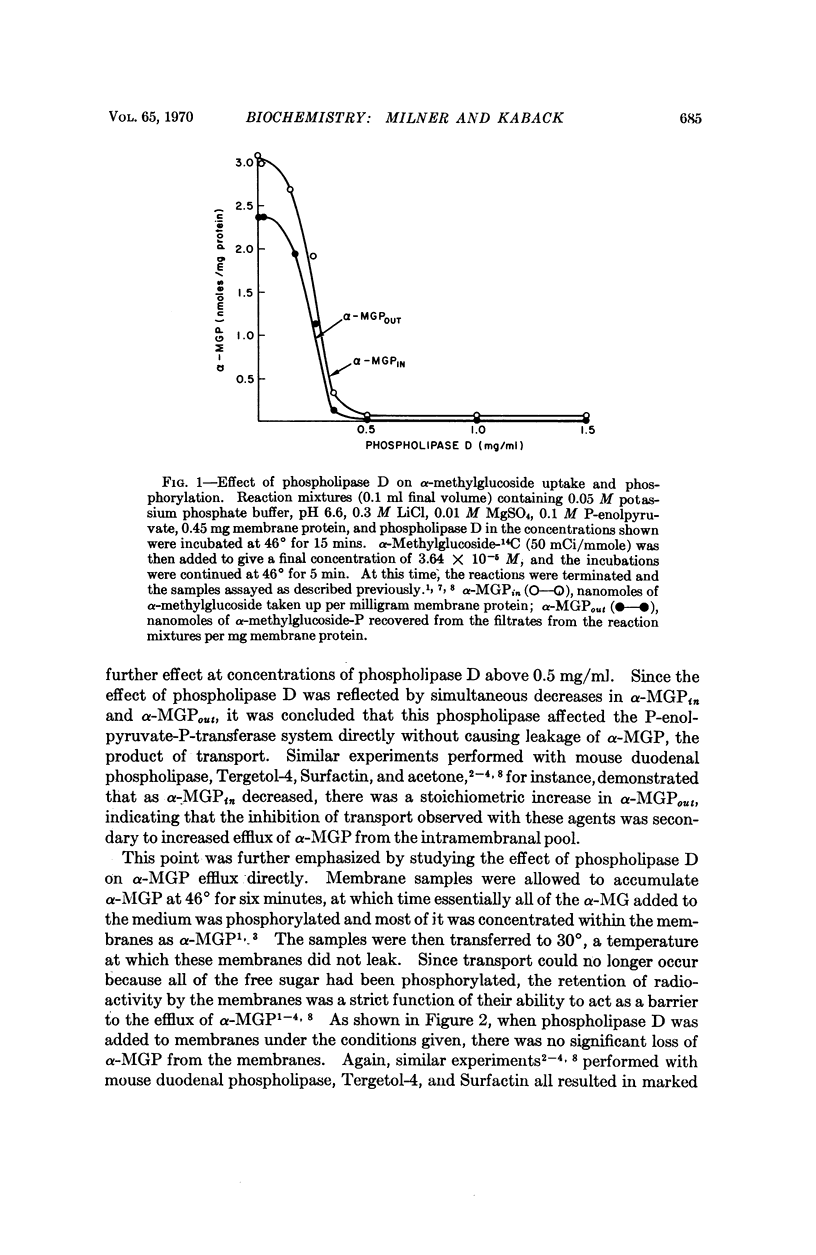
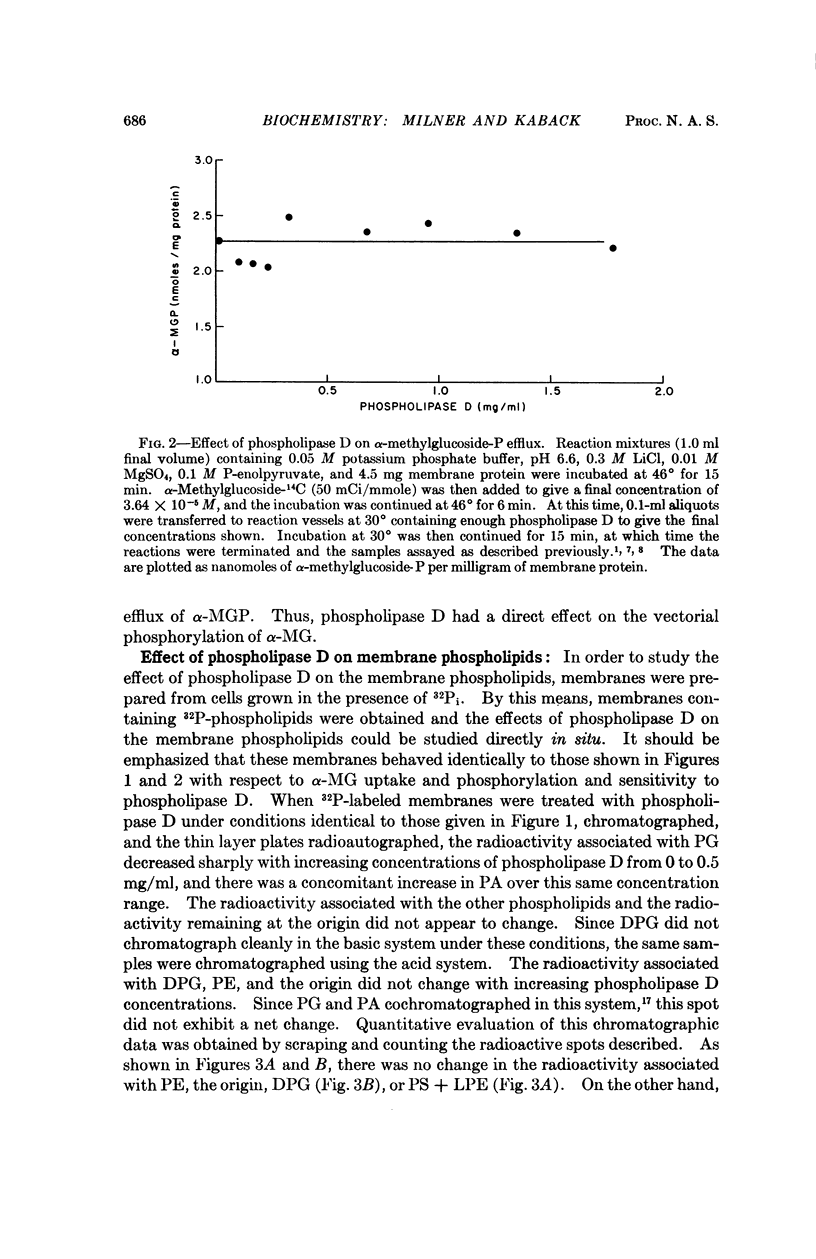
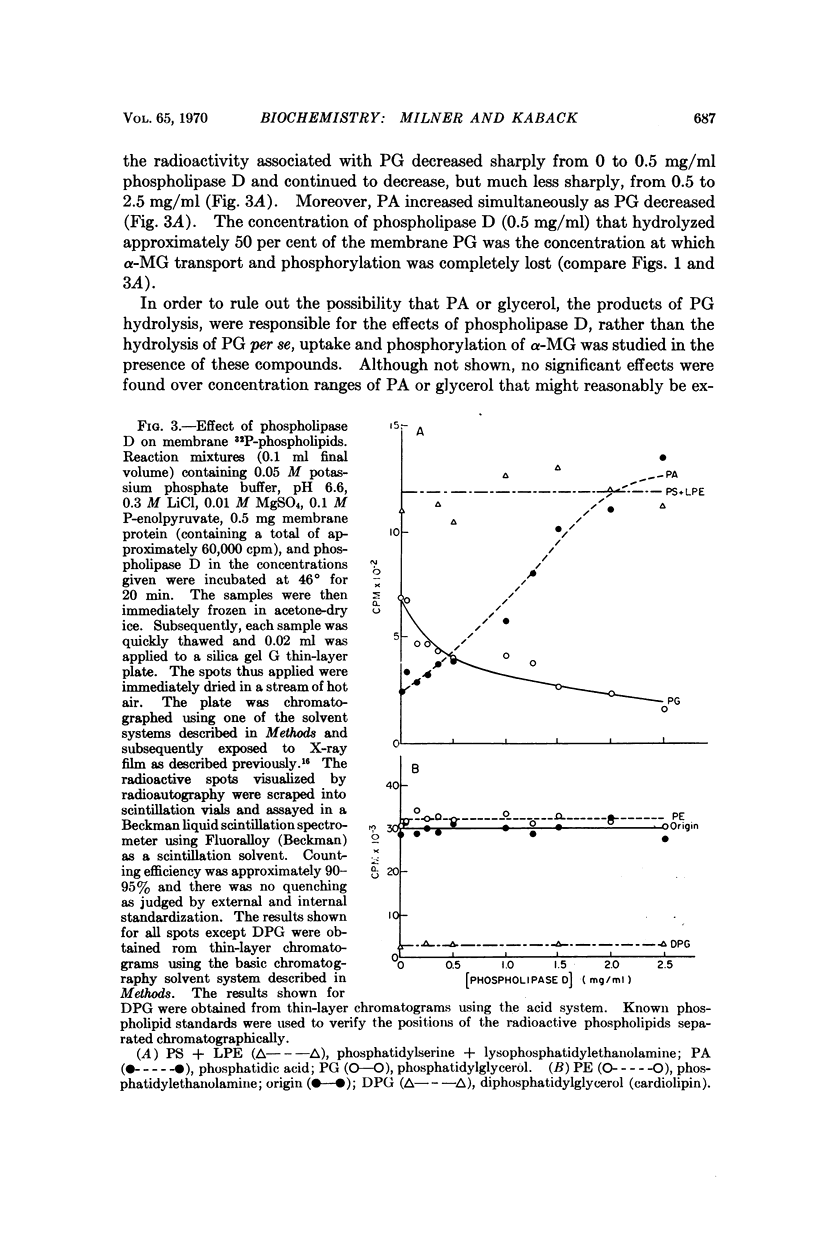
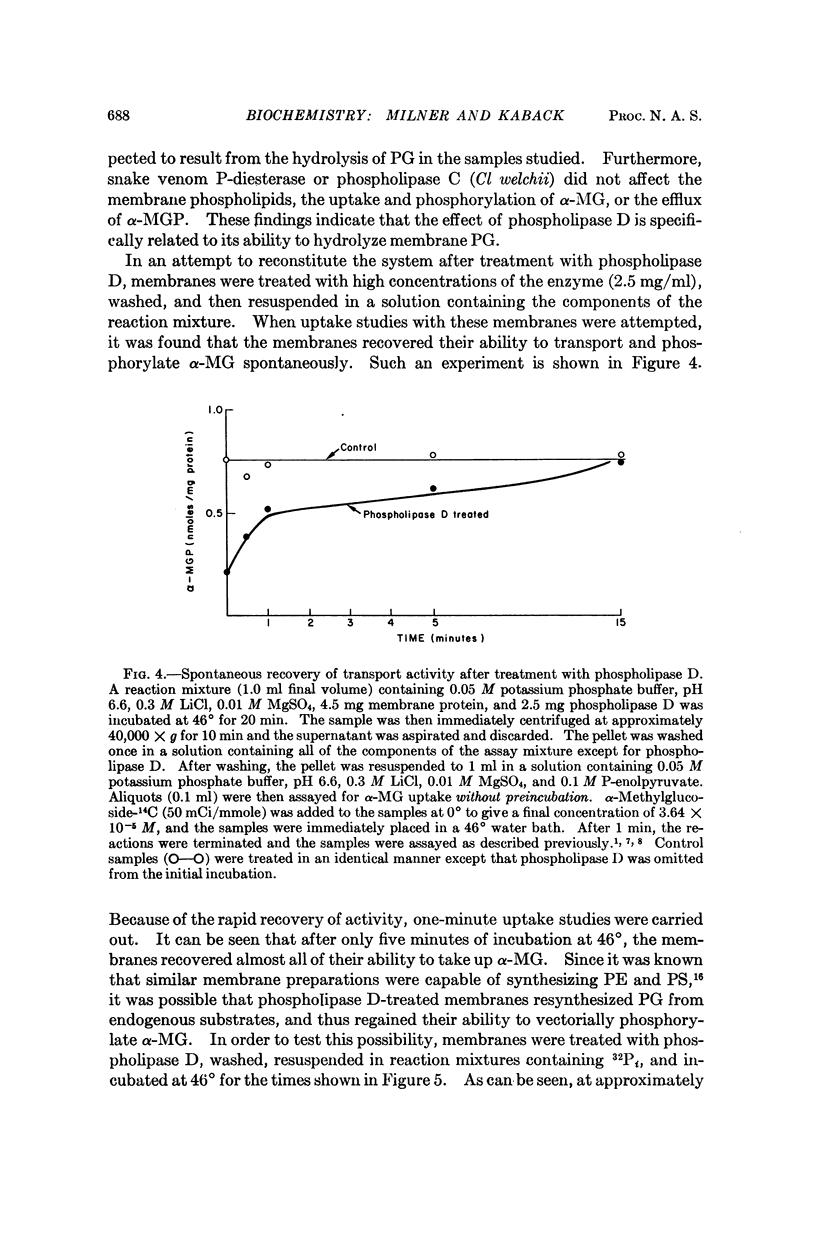
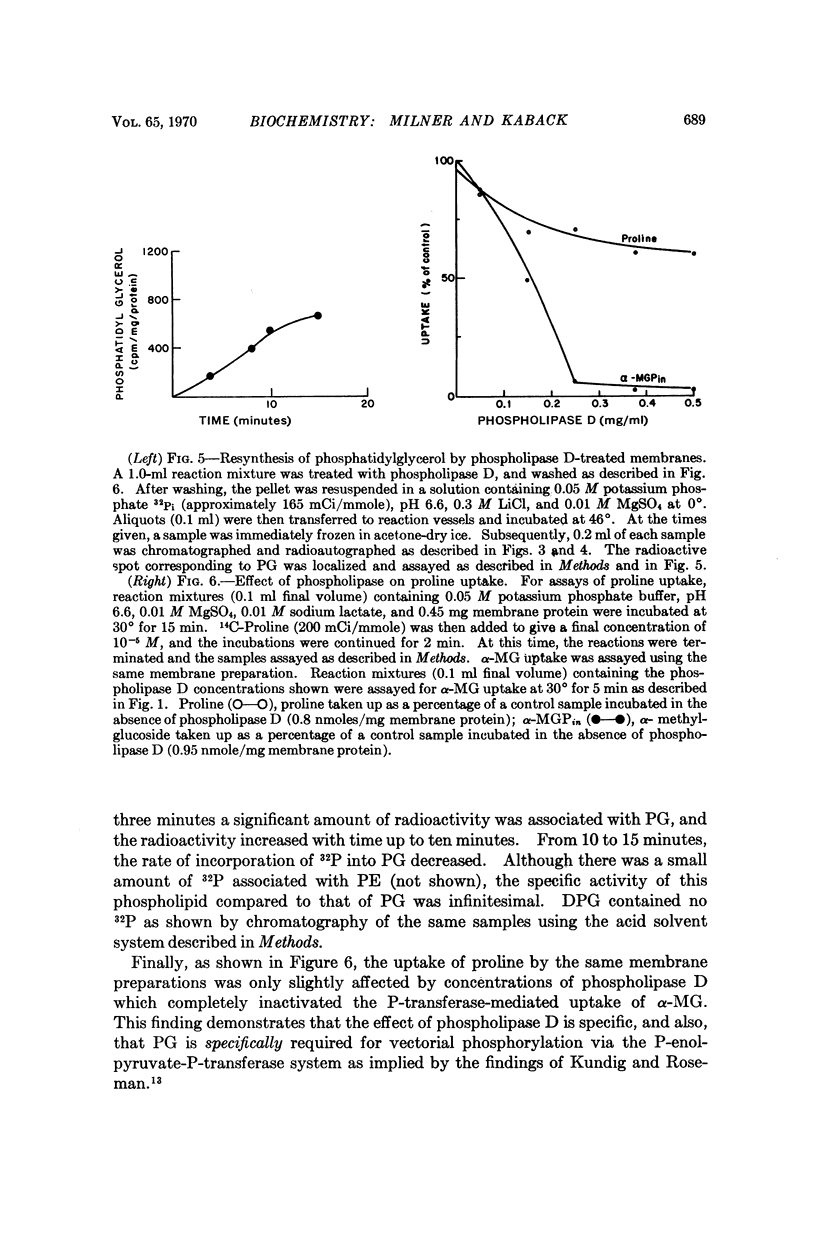
Phospholipase D (cabbage) inhibits the vectorial phosphorylation of α-methylglucoside by isolated membrane preparations from Escherichia coli ML 308-225 without increasing the efflux of intramembranal α-methylglucoside-P. This effect is shown to be related to the ability of phospholipase D to hydrolyze membrane phosphatidylglycerol specifically. After treatment with phospholipase D, the membranes resynthesize phosphatidylglycerol with a return in their ability to take up α-methylglucoside. Since proline uptake by the same preparations is only slightly inhibited by phospholipase D, the data indicate that phosphatidylglycerol is required specifically for transport processes which are mediated by the P-enolpyruvate-P-transferase system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima K., Tsukagoshi N., Tamura G. Inhibition of the synthesis of the alkaline phosphatase of Bacillus megarterium KM by a novel protoplast-bursting factor obtained from Bacillus subtilis 202-7. Biochim Biophys Acta. 1968 Aug;163(1):121–123. doi: 10.1016/0005-2736(68)90042-4. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Deuel F. Proline uptake by disrupted membrane preparations from Escherichia coli. Arch Biochem Biophys. 1969 Jun;132(1):118–129. doi: 10.1016/0003-9861(69)90343-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Regulation of sugar transport in isolated bacterial membrane preparations from Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):724–731. doi: 10.1073/pnas.63.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Glycine uptake in Escherichia coli. II. Glycine uptake, exchange, and metabolism by an isolated membrane preparation. J Biol Chem. 1968 Apr 10;243(7):1390–1400. [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]



