Abstract
The mechanisms underlying mesial temporal lobe epilepsy (MTLE) remain uncertain. Putative mechanisms should account for several features characteristic of the clinical presentation, and the neurophysiological and neuropathological abnormalities observed in patients with intractable MTLE. Synaptic reorganization of the mossy fiber pathway has received considerable attention over the past two decades as a potential mechanism that increases the excitability of the hippocampal network by the formation of new recurrent excitatory collaterals. Morphological plasticity beyond the mossy fiber pathway has not been as thoroughly investigated. Recently, plasticity of the CA1 pyramidal axons has been demonstrated in acute and chronic experimental models of MTLE. As the hippocampal formation is topographically organized in stacks of slices (lamellas), synaptic reorganization of CA1 axons projecting to subiculum appears to increase the connectivity between lamellas providing a mechanism for translamellar synchronization of cellular hyperexcitability, leading to pharmacologically intractable seizures.
1. Introduction
Pharmacologically intractable partial-onset epilepsy afflicts 20% of patients with epilepsy [26]. However, this subset of patients, about 590,000 Americans, accounts for most of the health care expenditures used for the care of patients with epilepsy. Currently, pharmacological therapy for these patients is aimed to symptomatically relieve seizures by reducing their frequency and severity. Over the past 30 years, almost thirty thousand compounds have been screened using two animal models of acute evoked convulsions to select them for human clinical trial development. Thus, it should not be surprising that this strategy has provided clinicians with excellent anticonvulsants, but that these compounds appear to be less effective against other seizure types, and ineffective in altering the development and natural history of the epilepsies. The development of therapies that alter the natural history of this condition has been hampered by our lack of understanding of the fundamental mechanisms of epileptogenesis, the pathophysiological process underlying the developing of epilepsy. Understanding the mechanisms of epileptogenesis might lead to better experimental models for drug discovery of compounds that are disease modifying, altering the natural history of symptomatic partial-onset epilepsy. Although the mechanisms underlying epileptogenesis remain uncertain, several putative mechanisms observed in limbic structures of patients with partial-onset epilepsy have been explored to explain the progressive nature and development of epilepsy. Our current pharmacological agents fail to target synaptic reorganization. As synaptic reorganization of limbic structures appears to progressively enhance limbic hyperexcitability, it might contribute towards pharmacological intractability in partial-onset epilepsy. This review will critically summarize some of these potential mechanisms including synaptic reorganization in the hippocampal circuitry, aiming to place them within the perspective of the most common pharmacologically intractable partial onset epilepsy, mesial temporal lobe epilepsy.
2. Human Mesial Temporal Lobe Epilepsy
Mesial temporal lobe epilepsy (MTLE) is the most common epilepsy syndrome with pharmacologically intractable partial-onset seizures. This epileptic syndrome has a high association with a remote history of febrile seizures, particularly complex or prolonged febrile seizures, but has also been observed associated with other acute neurological insults such as after an episode of partial-onset status epilepticus, closed head injury, brain tumors, stroke, etc. However, many patients with MTLE have no obvious brain insults, other than perhaps, the cumulative effect of repeated brief partial-onset seizures, which in experimental models have been shown to be associated with neuronal loss in limbic structures [14, 16]. Typically, when there is a history of a prior neurological injury, there is a latency between the initial insult and the onset of MTLE that spans at least several weeks but more commonly several years. It has been observed that severe prolonged insults such as prolonged febrile convulsions or generalized convulsive status epilepticus appear to have a shorter latency to the onset of spontaneous partial-onset seizures, as compared to other less severe precipitating events such as simple febrile seizures [77]. Thus, a latent period between the precipitating event and the onset of epilepsy is one of the hallmarks of the pathophysiological process underlying the progressiveness of epileptogenesis in MTLE.
Most of our understanding of the pathophysiology of MTLE relies from studies of brain tissue obtained surgically from patients with intractable and unilateral MTLE; typically, the tissue is examined after 20 or more years from the onset of their epilepsy. The majority of these highly selected patients dramatically improve after an anterior temporal lobectomy, which includes resection of the hippocampus, amygdala, and adjacent temporal neocortex [21, 37, 38). Thus, it has been presumed that the mechanisms underlying the development and intractability of MTLE must lie within the resected tissue. In a long-term study after temporal lobectomy for unilateral intractable MTLE, about 65% of patients were seizure free or having only auras (Engel’s class I result), and an additional 15% were significantly improved (class II result) in their seizure frequency [23]. However, despite that these patients were not longer having pharmacologically intractable seizures after temporal lobectomy, the majority of them still had experienced rare epileptic seizures. Fifty-five percent of these patients had at least one post-operative partial-onset seizure during an average of 14-years of follow-up after surgery. This and other studies support the view that the mechanisms that led to pharmacologically intractable MTLE lie within the removed temporal structures, but rare partial-onset seizures can be generated elsewhere in the brain of individuals who have suffered intractable MTLE for many years.
Neurophysiological studies have shown that most patients with intractable MTLE have seizure onset within the hippocampal formation [21], and this has led to the study of hippocampal slices from the resected hippocampus obtained during resective surgery in these epileptic patients. The neurophysiological studies demonstrate evidence for cellular hyperexcitability in granule cells and hippocampal pyramidal neurons [41, 57], but only under certain conditions that impair inhibitory mechanisms. Cellular hyperexcitability can be demonstrated by abnormal bursting activity in granule cell and hippocampal pyramidal neurons in response to stimulation, and it is mediated by N-methyl-D-aspartate (NMDA) type of glutamate receptors [24]. To unveil this form of cellular hyperexcitability, the human postsurgical hippocampal slices were pharmacologically manipulated to develop a mild impairment of GABAA mediated inhibition [24]. In addition, these investigators found a general correlation between the degree of cellular hyperexcitability of granule cells under these conditions, and the degree of synaptic reorganization of the mossy fiber pathway, a form of morphological plasticity observed in patients with MTLE and in experimental models of MTLE. Thus, their study suggested that the neurophysiological abnormalities can be associated with neuropathological alterations in patients with MTLE. However, causative relationships between these phenomena can not be established with this approach, which only examines the late stage of these phenomena after many years from the onset of partial seizures.
Studies of the resected tissue from intractable MTLE have shown several neuropathological abnormalities including: 1) a pattern of hippocampal neuronal loss known as mesial hippocampal sclerosis [5, 21], 2) sprouting and reorganization of the mossy fibers in the dentate gyrus [30, 49, 67], 3) hippocampal gliosis [5, 21], and 4) dispersion of granule cells with ectopic locations [30]. These neuropathological abnormalities might be consequences of repeated seizures or the result of the initial precipitating brain insult that led to repeated seizures. However, each of them might be a potential mechanism that contributes toward the pathophysiological process of epileptogenesis, which resulted in pharmacologically intractable MTLE. The relative role of each of these neuropathological abnormalities in epileptogenesis has been considerably improved as they have been replicated in multiple experimental models of MTLE, where the relationships between these phenomena and other potential mechanisms can be systematically tested.
3. Experimental Models of MTLE
There are two general strategies to study the process of epileptogenesis in experimental models of MTLE. The first strategy is to produce an acute excitotoxic insult that induces status epilepticus, which later results in the developing of spontaneous brief seizures. This strategy demonstrates a latency of several days between the insult and the development of seizures, but it is unclear how representative it is of the majority of patients with intractable MTLE who have no identifiable insult earlier in life. Two of the most often used models with this approach are the kainic acid and the pilocarpine models of MTLE. Experimental studies in rats that experienced convulsive status epilepticus induced with kainic acid (KA-SE) or pilocarpine have shown similar neuropathological and neurophysiological abnormalities to those observed in intractable MTLE. Adult rats exposed to KA-SE demonstrate prominent neuronal damage and gliosis in hippocampal pyramidal neurons and the hilar polymorphic neurons of the dentate gyrus [45, 68]. After hippocampal neuronal loss, these regions demonstrate morphological plasticity with sprouting of the mossy fiber pathway into the inner molecular layer of the dentate gyrus [45, 68]. Synaptic reorganization induced by seizures has been studied extensively in the mossy fiber pathway due to the ease of detecting changes in the laminar pattern of this pathway using Timm histochemistry or dynorphin-A immunocytochemistry [14, 15, 17, 25, 30, 49, 66, 67]. Most studies in experimental models of MTLE have shown sprouting in the mossy fiber pathway into the inner molecular layer of the DG and terminal sprouting in CA3 stratum oriens [70]. The second strategy for the study of epileptogenesis in models of MTLE is to provide frequent small insults that only lead to brief repeated seizures using chemoconvulsants [25] or electrical stimulation of limbic pathways (i.e.: kindling) [66]. In general, these chronic models of MTLE (second strategy) show considerable less injury as compared to the acute models that require an initial episode of status epilepticus. Furthermore, the neuropathological and neurophysiological abnormalities evoked in the chronic models are less severe than in the acute models. Although the chronic models more appropriately mimic the seizure burden and frequency of patients with intractable MTLE, they also lead to less frequent late spontaneous seizures than the kainic acid or pilocarpine models of MTLE.
Sprouting and synaptic reorganization have only been investigated in hippocampal pathways; however, neuronal loss has been demonstrated in multiple limbic areas [16]. Experimental models that evoke a more limited degree of hippocampal injury, such as electrical kindling, demonstrate a lesser degree of synaptic reorganization, as compared to the acute models of status epilepticus [14, 16, 18]. An advantage from the acute models of MTLE is that one can study the time course of progression of the neuropathological and neurophysiological abnormalities in relation to the development of spontaneous partial-onset seizures. Following an acute excitotoxic insult, several of these neuropathological phenomena develop during the latency period for several days, before the emergence of cellular hyperexcitability and the onset of spontaneous seizures [9, 27]. During the latency period, there are transitory functional impairments of inhibitory control that recovers during the chronic state. Other neuropathological abnormalities are permanent. It has been hypothesized that these permanent neuropathological alterations might underlie the mechanisms of epileptogenesis in intractable MTLE that leading to late intractable spontaneous seizures (Fig. 1), in particular, several investigators have shown that the progressive development of synaptic reorganization parallels the increased cellular excitability in the dentate gyrus [76], preceding spontaneous seizures. This type of synaptic reorganization of the mossy fiber has been observed in essentially all acute and chronic adult models of MTLE, and in pathological specimens of humans with MTLE [70]. Thus, synaptic reorganization of hippocampal structures is a potential mechanism explaining hippocampal hyperexcitability in patients with intractable MTLE.
Figure 1. Mechanisms of Epileptogenesis.
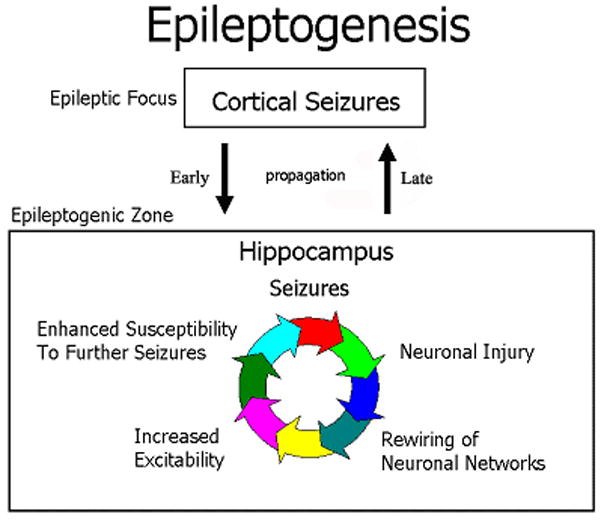
The schematic diagram describes the relationships between pathophysiological phenomena and brain location during epileptogenesis in mesial temporal lobe epilepsy. Partial-onset seizures might initially originate in neocortical areas, but after the establishment of intractability, there is a vicious circle of interrelated pathophysiological phenomena within the hippocampal circuitry that self sustains intractable seizures with propagation to neocortical structures.
4. Features of Sprouting and Synaptic Reorganization of the Mossy Fibers
The easier histological method to demonstrate mossy fiber sprouting is to use Timm histochemistry, which depicts the projection pattern of the mossy fibers in the hippocampus due to its high content of Zinc in their synaptic terminals. In the inner molecular layer, there are few Timm granules in normal rats and humans. After many repeated seizures or an episode of status epilepticus, the inner molecular layer demonstrates a dense band of Timm granules in both humans and experimental models of MTLE (Fig. 2). Granule cells axons, the mossy fibers, reorganize and sprout into the inner molecular layer to form new synaptic terminals with dendrites of interneurons and primarily spine and dendrites of granule cells. Timm histochemistry has been used at light and ultrastructural levels to assess the time course of development and permanence of the reorganized projection pattern. Small amounts of mossy fiber sprouting into the inner molecular layer can be recognized as early as 5 days, peaks at 3 weeks, and has been observed to last as long 18 months [14, 15]. After an excitotoxic insult, neuronal degeneration in the hilus can be recognized as early as 6 hours, and at 24 hours, terminal degeneration in the inner molecular layer can be detected prior to the development of mossy fiber sprouting [45, 68]. In contrast, early during development seizure-induced mossy fiber sprouting may occur in the absence of neuronal death [7, 28, 29]. Nevertheless, in adult animals, neuronal loss of the hilar polymorphic neurons is considered the mechanism that triggers mossy fiber sprouting into the inner molecular region of the dentate gyrus [16]. Ultrastructural experiments of acute lesions of pathways projecting to the molecular layer of the dentate gyrus have taught us that the synaptic densities in the molecular layer are restored by 21 days after the lesion [34]. In the kainic acid and pilocarpine models, most of the newly formed synaptic terminals in the inner molecular layer of the DG are on granule cell spines creating primarily a recurrent excitatory collateral circuit [13, 17]. It has been estimated that a sprouted granule cell develops about 500 newly formed synaptic contacts with granule cells and less than 25 contacts upon interneurons [8]. Using the chronic model of repeated kindling seizures to limbic structures, it was also shown that the early degrees of mossy fiber sprouting consists in the development of punctate granules that formed “strings” in the inner molecular layer before spreading to the rest of the layer [14]. These strings were subsequently identified as collections of synaptic contacts upon interneurons including basket cells [17, 50]. Thus, it is likely that mossy fiber sprouting, early on, is a homeostatic compensatory mechanism that is trying to restore inhibitory control by providing feedback synaptic connections upon basket cell interneurons. However, as the repeated kindling seizures continue, or the extent of the inner molecular terminal degeneration from the death of hilar polymorphic neurons is overwhelming, the newly formed synaptic contacts develop primarily upon granule cell spines, dendrites, and soma [8, 17].
Figure 2. Mossy Fiber Sprouting.

These are three photomicrographs from histological sections of the dentate gyrus (DG) stained with Timm histochemistry. Dark punctate granules depict the projection pattern of mossy fiber terminals that originate from granule cell axons. (A) The dentate gyrus from a normal rat shows dense staining in the hilus (area within the U-shape of the DG) and in the CA3 region, with an absence of dark punctate granules in the molecular layer of the DG (arrow). (B) The dentate gyrus from a rat that experienced status epilepticus induced with kainic acid demonstrates prominent staining in the molecular layer of the DG. (C) Human dentate gyrus obtained surgically during a standard anterior temporal lobectomy for the treatment of pharmacologically intractable mesial temporal lobe epilepsy. Note that the molecular layer of the DG has prominent staining demonstrating mossy fiber sprouting into that region.
Seizure-induced plasticity of the mossy fibers is not limited to the formation of aberrant recurrent collaterals into the inner molecular layer of the dentate gyrus. Other alterations of the normal terminal field of the mossy fibers include: a) increased branching within the hilus of the DG, b) development of abnormal connectivity between the two blades of the dentate gyrus, c) increased connectivity to granule cells and hilar region along the septotemporal axis of the hippocampus, and d) formation of aberrant recurrent collaterals into the stratum, oriens of CA3, where the pyramidal neurons extend their basal dendrites [11, 69, 70]. In addition, the dendritic tree of the granule cells of the dentate gyrus also exhibit morphological plasticity remodeling the target surface for synaptic contacts [52]. The dendritic remodeling might have significant consequences in the biophysical properties that allowing for alterations in the temporal and spatial summation of postsynaptic potentials.
To summarize, the latency for the development of morphological plasticity matches the time course of development of spontaneous seizures in acute and chronic experimental models of MTLE. Neuronal loss and terminal degeneration precede the development of mossy fiber sprouting into the inner molecular layer in the acute models of MTLE, and in addition, there is a direct correlation between the degree of neuronal cell loss, mossy fiber sprouting into the inner molecular layer, and granule cell hyperexcitability. However, the physiological consequences of hilar polymorphic neuronal loss and mossy fiber sprouting remain a controversial subject.
5. Functional effects of mossy fiber sprouting
Despite that the evidence shows a direct correlation between the development of mossy fiber sprouting, neuronal loss, and cellular hyperexcitability, there are several alternative hypotheses about the functional effects in the epileptic dentate gyrus after losing hilar neurons besides the formation of recurrent excitatory collaterals upon granule cells. An early hypothesis advanced as potential mechanism of MTLE was that the initial insult or the repeated seizures led to a loss of GABA-ergic inhibitory interneurons, tilting the delicate balance between excitatory and inhibitory forces [6, 48, 51]. The evidence supporting this idea came from immunocytochemical studies of the gamma-aminobutyric decarboxylase (GAD), a critical enzyme in the metabolism of GABA. However, subsequent studies showed that mRNA for this enzyme was still present at normal amounts, with no major changes in the number of those interneurons [12]. It was then identified that the most vulnerable hilar neuron appeared to be the mossy cells. This led to the “dormant cell hypothesis” [60], which states that the epileptic hippocampal network hyperexcitability is due to the loss of neurons that normally excite the more resistant inhibitory basket cells [60, 61]. In several models of MTLE, including the kainic acid model (KA-SE), prominent degeneration of the mossy cells in the hilus ensues immediately during status epilepticus relatively sparing the basket interneurons. The mossy cells normally project excitatory afferents to the basket interneurons, which are the GABA-containing neurons that provide feedback inhibition onto granule cells. The dormant basket cell hypothesis contends that once these mossy cells begin degenerating, the basket cells are not longer providing the normal inhibitory tone required to granule cells leading to disinhibition [60]. There is experimental evidence of immediate granule cell disinhibition at the time when the mossy cells begin degenerating in two models of TLE, but only when granule cells discharged continuously during the status epilepticus [61]. However, several observations have placed significant questions on the validity of this hypothesis. For example, the inhibitory tone of granule cells is restored after resolution of status epilepticus, and appears intact despite the emergence of late spontaneous seizures [55]. Furthermore, a specific prediction of this hypothesis would be that if mossy cells were acutely removed from a normal hippocampal slice preparation, the dentate gyrus would become hyperexcitable. When this experiment was performed by Soltezs and collaborators, there was no evidence of hyperexcitability [53], suggesting that this hypothesis is no longer valid. Thus, after eliminating several alternative hypotheses, we are left with synaptic reorganization of the circuitry as the leading contender to explain the process of epileptogenesis. However, synaptic reorganization might be initially a homeostatic mechanism that tries to restore the inhibitory tone of granule cells [59, 62], but once the process is repeated many times, the overwhelming targets of the sprouted mossy fibers constitute recurrent excitatory collaterals upon granule cells of the dentate gyrus [12, 55].
Reorganization of the epileptic circuitry with newly sprouted recurrent excitatory synapses has been advanced as a putative mechanism explaining, at least in part, the cellular hyperexcitability observed in intractable partial-onset epilepsy. This hypothesis is supported by the demonstration in a computer model that even a small increase (4%) in the number of recurrent excitatory collaterals in the dentate gyrus circuitry is sufficient to cause persistent cellular hyperexcitability in the abnormally connected dentate gyrus [40]. A more complex modeling study examined the role of mossy fiber sprouting and the loss of mossy cell using a more elaborate computer model with 500 granule cells, 15 mossy cells, 6 basket cells and 6 interneurons [54]. This latter study examined the effect of mossy fiber sprouting and the loss of mossy cells in the pilocarpine model and assessed the effect of progressive amounts of mossy fiber sprouting from 0 to 50% of the maximum extent of mossy fiber sprouting observed in the pilocarpine model. The study showed that modest amounts (10–15%) of recurrent excitatory connectivity resulted in hippocampal network hyperexcitability [54]. Computer modeling demonstrations are just one of several lines of evidence that strongly support the hypothesis that sprouted mossy fibers plays a key role underlying the intractability of MTLE. Direct paired cell recordings of granule cells have shown monosynaptic potentials between epileptic granule cells [56].
Despite the ubiquity of mossy fiber sprouting in experimental models of MTLE and in human MTLE, some investigators have suggested that mossy fiber sprouting does not appear to be a necessary precondition to establish intractability. There are rare examples of experimental models of MTLE where mossy fiber sprouting was not observed [39]. This apparent discrepancy does not invalidate the hypothesis that circuit reorganization is necessary for epileptic intractability as the only evidence thoroughly investigated in those rare observations was limited to mossy fiber sprouting. Reorganization of synaptic circuitry might be occurring elsewhere in the limbic system initiating pharmacologically intractable temporal lobe epilepsy.
6. Synaptic reorganization in limbic pathways beyond the mossy fibers
In experimental models of epilepsy and in humans with intractable partial-onset epilepsy, the CA1 pyramidal neurons demonstrate persistent cellular hyperexcitability and a pattern of neuronal loss similar to those observed in the epileptic dentate gyrus [35, 36, 42, 43, 63, 64]. Hyperexcitability of epileptic CA1 neurons has been proposed to result from sprouting of recurrent CA1 axon collaterals. Studies investigating local axonal arborizations of the CA1 pyramidal neurons have used primarily the kainic acid model of epilepsy [46, 47, 58, 64]. This observation has been extended to humans with MTLE and the pilocarpine model, confirming the exuberant axonal sprouting of CA1 axons into the stratum oriens of the CA1 region [22, 35, 36]. However, all of these results were demonstrated in hippocampal slice preparations, which have limited depiction of the axonal projection perpendicular to plane of the hippocampal slice, and can not articulate the complexity of the anatomical topography of the CA1 projection to the subiculum.
The examination of the time course of the formation of sprouted CA1 recurrent collaterals in acute models of MTLE suggest that seizure-induced CA1 hyperexcitability is due to the formation of recurrent excitatory collaterals in the CA1 region [22, 35, 36, 42, 43, 63, 64]. Prolonged excitatory post-synaptic potentials (EPSPs) bursts observed in bicuculline treated hippocampal slices from kainic acid (KA) treated rats showed an all or-none behavior [42, 43]. The all-or-none behavior cannot be explained by changes of the intrinsic properties of CA1 pyramidal neurons because the EPSP bursts would then be graded. Thus, the prolonged EPSP bursts are mediated by synaptic transmission reflecting a network-driven hyperexcitability, suggesting a role for synaptic reorganization and sprouting in the CA1. However, the prolonged EPSP bursts were only seen in 28% of the isolated CA1 transverse slices spontaneously [42, 43]. Although this may be due to a sampling error, one possibility is that the critical recurrent CA1 collaterals are cut-away of the hippocampal slice because of the variability in the plane of cutting slices. Subsequently, Smith and Dudek [63, 64] using isolated CA1 transverse slices demonstrated that glutamate microapplication to the CA1 pyramidal cell layer increased excitatory postsynaptic current frequency only in slices of KA-treated rats but not in control slices. These results, along with the work of Esclapez et al. [22] and Lehmann et al. [35, 36], support the hypothesis that the increased recurrent excitatory connections between CA1 pyramidal cells after kainic acid induced status epilepticus are functional connections that increase the excitatory drive of the hippocampal circuitry.
In a recent study, we examined the possibility that the distal axonal projection of the epileptic CA1 pyramidal neurons reorganize outside of its normal laminar boundaries within the subiculum and CA1 hippocampal region (Fig. 3) [18]. If present, sprouting of the CA1 projection to its final output, the subiculum, might provide a mechanism for the enhanced and persistent cellular hyperexcitability in this region that has been observed using a variety of techniques. The major finding of our study is that there was substantial plasticity of the terminal field (distal axonal branches) of the CA1 axonal projection to subiculum in five animal models (three models of acute prolonged seizures and two models of chronic recurrent brief seizures) of intractable partial-onset epilepsy using two histological methods. In three experimental models of epilepsy, our tracing experiments showed that the retrograde labeling extended 42–67% beyond the normal lamellar organization to include lamellas above and below the injection site. This is a demonstration of substantial plasticity of the CA1 projection to subiculum along the septotemporal axis of the hippocampus allowing for increased transverse connectivity among hippocampal lamellas above and below the normal circuitry. The reorganized circuitry might allow epileptic activity in a hippocampal lamella to recruit and synchronize activity in additional hippocampal lamellas, amplifying the epileptic response, and playing a role in the persistent hyperexcitability observed in intractable partial-onset epilepsy (Figs. 4, 5).
Figure 3. Synaptic reorganization in the CA1 projection to subiculum.
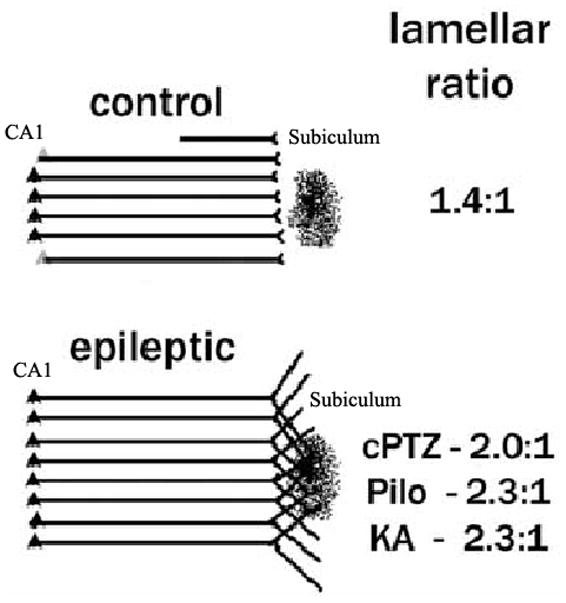
There is a prominent reorganization of the lamellar projection of the CA1 axonal pathway to the subiculum in several animal models of mesial temporal lobe epilepsy using retrograde tracers. In control rats, the extent of CA1 retrograde labeling from an injection site is limited to a couple of lamellas above and below of the injection site in subiculum. In contrast, in epileptic rats, the retrograde labeling extends beyond several CA1 lamellas above and below the normal projection. This is direct evidence that axonal terminals from neurons in those layers extend their axons into the area of injection (Figure modified from ref. 18).
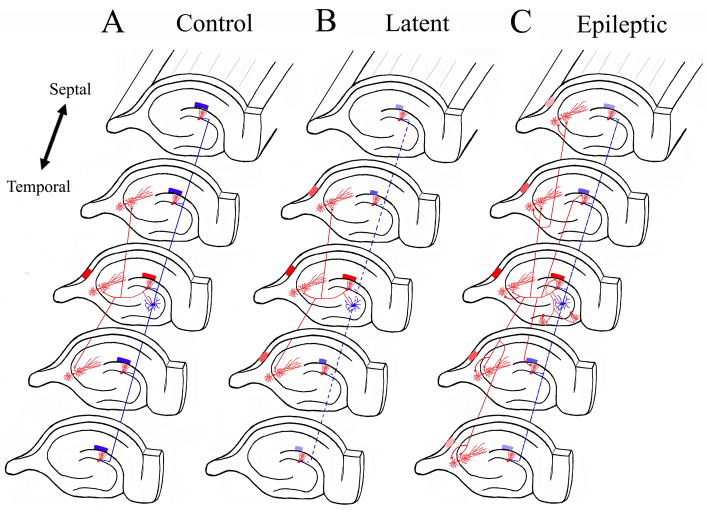
Figure 4. Synaptic Reorganization in mossy fiber pathway results in translamellar hyperexcitability in the hippocampal formation.
The schematic drawings illustrate the normal hippocampal circuitry, the abnormal circuitry during the latent state, and after spontaneous seizures develop in the kainic acid model of mesial temporal lobe epilepsy. The red neurons and axons are excitatory neurons, while the blue neurons are inhibitory interneurons. A. In the normal dentate gyrus, activation of granule cells in a lamella results in a limited activation of the CA3 pyramidal region shown also in red, and the hilar neurons inhibit dentate granule cells in lamellas above and below the activated lamella. B. During the latent state, inhibitory mechanisms are functionally impaired with slow improvement in the inhibitory tone (perhaps, in part, due to synaptic reorganization of inhibitory pathways). There is a mild degree of disinhibition in lamellas above and below the activate lamella (shown as smaller blue block in those lamellas). Furthermore, mild disinhibition results in a greater degree of activation in the CA3 pyramidal region (shown as a greater number of activated red CA3 lamellas). C. Once spontaneous seizures develop in epileptic models, there is prominent synaptic reorganization of the mossy fiber into the inner molecular layer of the DG, across DG blades, and into the basal dendrites of CA3 pyramidal regions. The resulting increased recurrent excitatory connectivity between principal neurons in the hippocampus and within hippocampal lamellas results in translamellar sprouting.
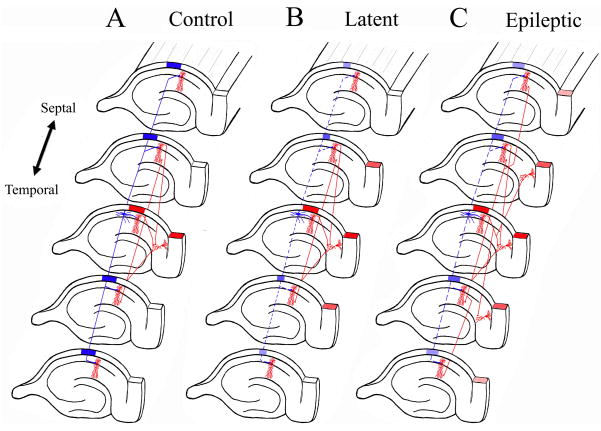
Figure 5. Synaptic Reorganization in CA1 projection to the subiculum results in translamellar hyperexcitability in the hippocampal formation.
The schematic drawings illustrate the normal hippocampal circuitry, the abnormal circuitry during the latent state, and after spontaneous seizures develop in the kainic acid model of mesial temporal lobe epilepsy. The red neurons and axons are excitatory neurons, while the blue neurons are inhibitory interneurons. A. In the normal hippocampus, activation of the CA1 pyramidal neurons in a lamella results in a limited activation of subicular neurons shown also in red, and the CA1 interneurons inhibit CA1 pyramidal neurons from lamellas above and below the activated lamella (shown as a blue block over those lamellas). B. During the latent state, inhibitory mechanisms are functionally impaired with slow improvement in the inhibitory tone (perhaps, in part, due to synaptic reorganization of inhibitory pathways). There is a mild degree of disinhibition in lamellas above and below the activate lamella (shown as smaller blue block in those lamellas). Furthermore, mild disinhibition results in a greater degree of activation in subiculum (shown as a greater number of activated subiculum sections). C. Once spontaneous seizures develop in epileptic models, there is prominent synaptic reorganization of the CA1 pyramidal axons making synaptic contacts with additional CA1 pyramidal neurons and subicular neurons in sections (lamellas) above and below their normal projection pattern. The resulting increased recurrent excitatory connectivity between principal neurons in the hippocampus and within hippocampal lamellas results in translamellar sprouting.
7. Cellular hyperexcitability in subiculum
The main projection pathway of the CA1 region is to the subiculum, which is the final output structure of the hippocampal formation. The principal neurons in the subiculum are electrophysiologically characterized by their firing properties: regular spiking or bursting action potentials [65]. Regular spiking neurons are further subdivided into tonic-spiking, with regularly timed spikes, whereas adapting neurons have slow increase in latency between spikes. Bursting neurons can also be further divided into weak bursting and strong bursting action potentials. The first burst in a weak bursting neuron has two to three spikes, where a strong bursting neuron contains three or more spikes [44]. These action potentials can be spontaneous in the isolated subiculum or evoked by orthodromic stimulation of efferent CA1 neurons. The relative ratio of pyramidal subicular neurons is controversial and varies in several studies, from 69 % bursting neurons [65, 72] to 37% bursting [44], but Menendez de la Prida [44] found that neurons with a larger soma are more likely to be bursting and may contribute to a bias in the method to classify neurons because it is easier to impale larger pyramidal neurons.
Synaptic reorganization of the CA1 axons projecting to the subiculum might have a major role explaining the persistent cellular hyperexcitability in the epileptic hippocampal formation circuitry. In the pilocarpine and kainic acid models of MTLE, there is some degree of neuronal degeneration in the subiculum that is less than in CA1 or the hilus of the dentate gyrus. In a study using the pilocarpine model, there was only 30% neuronal loss in the subiculum, while over 60% of CA1 pyramidal and hilar polymorphic neurons degenerated [31]. This selective vulnerability has also been observed in patients with MTLE [19, 20]. Neuronal loss results in a change of the relative ratio of bursting behavior of pyramidal neurons in the subiculum, which has also been studied in the pilocarpine model. After status epilepticus induced with pilocarpine, there is a prominent increase in the number of bursting neurons in the subiculum from 40% in normal rats to 82% of neurons [73], perhaps due to selective vulnerability of the non-bursting pyramidal neurons in the subiculum. Nevertheless, the presence of increased numbers of intrinsically bursting neurons in the output gate of the hippocampus might allow the reorganized subiculum to play potentially a critical role in modulating the transition between interictal and ictal events [74, 75].
8. Translamellar hyperexcitability
The observation that epileptic models reorganize the CA1 projection to subiculum with an increased lamellar ratio might be better understood in the context of the lamellar hypothesis of hippocampal organization. The hypothesis of the lamellar organization of the hippocampus was derived from mapping of the hippocampal formation using extracellular field evoked recordings [2, 3]. The lamellar hypothesis proposes that the hippocampal formation consists of a stack of hippocampal slices that are functionally connected uni-directionally along the rostro-caudal axis. In this manner, the entorhinal cortex projects to the dentate gyrus via the perforant pathway. The dentate gyrus then projects to the CA3 pyramidal region via the mossy fiber pathway. The CA3 region projects to the CA1 pyramidal region through the Schaffer collaterals. The CA1 region projects to the subiculum, which in turn, projects back to the entorhinal cortex completing the loop within the hippocampal formation. The myelinated fibers of these synaptic pathways project in the rostro-caudal axis following the alvear surface of the hippocampus [71]. In essence, the function of the structure is primarily organized topographically into hippocampal slices or lamellas, and interconnections with lamellas above or below have no major significant importance for its primary function. However, there is some divergence of the projections within lamellas. For example, experiments have shown that an injection of 10% of the ventrodorsal axis of the entorhinal cortex projected to about 25% of the dentate gyrus [1]. Furthermore, some types of hilar polymorphic neurons give rise to highly dense projections to the dentate gyrus for almost two thirds of the entire ventrodorsal axis [32, 33]. Despite that the presence, density and extent of these associational interconnections seem to invalidate the lamellar hypothesis, detailed functional studies are lacking. The function of some of the hilar polymorphic neurons could be activating local inhibitory circuits tuning out lamellas above or below the activated lamella [78, 79]. Nevertheless, the projections in the hippocampal formation clearly follow a topographical organization that is reorganized in experimental models of MTLE providing additional pathways for further activation of hippocampal circuitry that do not normally receive these projections [18]. The functional consequences of these alterations are not completely understood, but might contribute to the state of hypersynchrony and hyperexcitability observed in epilepsy.
A revision of the lamellar hypothesis using extracellular field potentials in vivo [4] showed that it still “remains a useful concept for understanding of hippocampal connectivity.” Andersen et al. [4] showed that the amplitude of the compound action potential was largest in a slightly oblique transverse band across the CA1 toward the subiculum with decreasing activation in the lamellas above and below the activated lamella despite the larger dorso-ventral extent of the neuroanatomical projection. Recent experiments [10, 78, 79] have also shown that the larger extent of dorsoventral projections actually serves to inhibit the surrounding lamellas to limit the spread of activation. In the dentate gyrus, the excitatory associational projections play a major role for activating inhibitory hilar circuits in the lamellas away from the source of the associational projection. This pattern of organization strengthens the lamellar hypothesis by fine-tuning the activation to the “on lamella” while there is increase inhibition in the “off lamella” (see Figs. 4, 5). Furthermore, Zappone and Sloviter [79] suggest that hilar neuronal loss in epileptic models leads to translamellar disinhibition in the dentate gyrus. Although the functional consequences of remodeling of the CA1 projection to the subiculum in acute and chronic epilepsy models is not known, we suggest that the enhanced excitatory spread of CA1 axonal collaterals that make contacts with spines profiles would enhance translamellar hyperexcitability in the subiculum (Fig. 5), the output gate of the hippocampal formation [18]. Early during the process of epileptogenesis, it is likely that in chronic models of MTLE, the primary effect of translamellar sprouting is to aim to restore inhibitory tone such as been observed in isolated hippocampal slices. However, as the number of potential targets for the sprouted fibers decreases, translamellar sprouting results in primarily recurrent excitatory collaterals that with cellular hyperexcitability. There are many unanswered questions deserving further investigation.
8. CONCLUSIONS
Synaptic reorganization of hippocampal pathways induced by excitotoxicity or repeated seizures is not limited to the mossy fiber pathway. Increased connectivity of the epileptic hippocampal network allows for increased synchrony and faster propagation of epileptic discharges within the structure. Synaptic reorganization of mossy fiber projection of the dentate gyrus has been shown to play a significant role underlying persistent cellular hyperexcitability in the epileptic circuitry of the hippocampal formation. As the subiculum is the output gate of the hippocampus, its critical location in the limbic pathways, the presence of intrinsically bursting neurons, and the increased lamellar interconnectivity in epileptic models might also explain the persistent cellular hypersynchrony and hyperexcitability in the limbic system observed in humans with intractable MTLE. Although synaptic reorganization of limbic pathways is not a necessary precondition for the occurrence of partial onset seizures, it might be the cellular mechanism underlying the pharmacological intractability of human MTLE.
Acknowledgments
This review paper was supported by a grant from NINDS (NS02078 - JEC). We appreciate the administrative assistance of Laura Moreno.
References
- 1.Amaral DG, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–38. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Bland BH, Dudar JD. Organization of the hippocampal output. Exp Brain Res. 1973;17:152–68. doi: 10.1007/BF00235025. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Soleng AF, Raastad M. The hippocampal lamella hypothesis revisited. Brain Res. 2000;886:165–71. doi: 10.1016/s0006-8993(00)02991-7. [DOI] [PubMed] [Google Scholar]
- 5.Babb TL, Lieb JP, Brown WJ, Pretorius J, Crandall PH. Distribution of pyramidal cell density and hyperexcitability in the epileptic human hippocampal formation. Epilepsia. 1984;25:721–8. doi: 10.1111/j.1528-1157.1984.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 6.Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989;9:2562–74. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender RA, Dube C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyett JM, Buckmaster PS. Somatostatin-immunoreactive interneurons contribute to lateral inhibitory circuits in the dentate gyrus of control and epileptic rats. Hippocampus. 2001;11:418–22. doi: 10.1002/hipo.1056. [DOI] [PubMed] [Google Scholar]
- 9.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41(Suppl 6):S144–52. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 10.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- 11.Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophys. 1999;81:712–21. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- 12.Buckmaster PS, Jongen-Relo AL, Davari SB, Wong EH. Testing the disinhibition hypothesis of epileptogenesis in vivo and during spontaneous seizures. J Neurosci. 2000;20:6232–40. doi: 10.1523/JNEUROSCI.20-16-06232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–8. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavazos JE, Golarai G, Sutula TP. Septotemporal Variation of the Supragranular Projection of the Mossy Fiber Pathway in the Dentate Gyrus of Normal and Kindled Rats. Hippocampus. 1992;2:363–72. doi: 10.1002/hipo.450020404. [DOI] [PubMed] [Google Scholar]
- 16.Cavazos JE, Das I, Sutula T. Kindling Induces a Pattern of Neuronal Loss in the Hippocampus that Resembles Human Hippocampal Sclerosis. J Neurosci. 1994;14:3106–21. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid treated rats. J Comp Neurol. 2003;458:272–92. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- 18.Cavazos JE, Jones SM, Cross DJ. Sprouting and Synaptic Reorganization in the Subiculum and CA1 Region the Hippocampus in Acute and Chronic Models of Partial-Onset Epilepsy. Neuroscience. 2004;126:677–88. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 20.Cohen I, Navarro V, Huberfeld G, Clemenceau S, Baulac M, Miles R. Response to Comment on “On the Origin of Interictal Activity in Human Temporal Lobe Epilepsy in Vitro”. Science. 2003;301:463. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 21.Engel J., Jr Update on surgical treatment of the epilepsies. Clin Exp Neurol. 1992;29:32–48. [PubMed] [Google Scholar]
- 22.Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408:449–60. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Foldvary N, Nashold B, Mascha E, Thompson EA, Lee N, McNamara JO, Lewis DV, Luther JS, Friedman AH, Radtke RA. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54:630–4. doi: 10.1212/wnl.54.3.630. [DOI] [PubMed] [Google Scholar]
- 24.Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–58. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 25.Golarai G, Cavazos JE, Sutula TP. Activation of the dentate gyrus by pentylenetetrazol evoked seizures induces mossy fiber synaptic reorganization. Brain Res. 1992;593:257–64. doi: 10.1016/0006-8993(92)91316-7. [DOI] [PubMed] [Google Scholar]
- 26.Hauser WA. The natural history of drug resistant epilepsy: epidemiologic considerations. Epilepsy Res. 1992;(Suppl 5):25–8. [PubMed] [Google Scholar]
- 27.Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 28.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 29.Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. J Comp Neurol. 1999;404:537–53. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Houser C, Miyashiro J, Swartz B, Walsh G, Rich J, Delgado-Escueta A. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–82. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- 32.Laurberg S. Commissural and intrinsic connections of the rat hippocampus. J Comp Neurol. 1979;184:685–708. doi: 10.1002/cne.901840405. [DOI] [PubMed] [Google Scholar]
- 33.Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentatae and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- 34.Laurberg S, Zimmer J. Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J Comp Neurol. 1981;200:433–59. doi: 10.1002/cne.902000310. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann TN, Gabriel S, Kovacs R, Eilers A, Kivi A, Schulze K, Lanksch WR, Meencke HJ, Heinemann U. Alterations of neuronal connectivity in area CA1 of hippocampal slices from temporal lobe epilepsy patients and from pilocarpine-treated epileptic rats. Epilepsia. 2000;41(Suppl 6):S190–4. doi: 10.1111/j.1528-1157.2000.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann TN, Gabriel S, Eilers A, Njunting M, Kovacs R, Schulze K, Lanksch WR, Heinemann U. Fluorescent Tracer in Pilocarpine-Treated Rats Shows Widespread Aberrant Hippocampal Neuronal Connectivity. Eur J Neurosci. 2001;14:83–95. doi: 10.1046/j.0953-816x.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- 37.Lieb JP, Engel J, Jr, Babb TL. Interhemispheric propagation time of human hippocampal seizures. I. Relationship to surgical outcome. Epilepsia. 1986;27:286–93. doi: 10.1111/j.1528-1157.1986.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 38.Lieb JP, Babb TL, Engel J., Jr Quantitative comparison of cell loss and thiopental-induced EEG changes in human epileptic hippocampus. Epilepsia. 1989;30:147–56. doi: 10.1111/j.1528-1157.1989.tb05447.x. [DOI] [PubMed] [Google Scholar]
- 39.Longo BM, Mello LE. Effect of long-term spontaneous recurrent seizures or reinduction of status epilepticus on the development of supragranular mossy fiber sprouting. Epilepsy Res. 1999;36:233–41. doi: 10.1016/s0920-1211(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 40.Lytton WW, Hellman KM, Sutula TP. Computer models of hippocampal circuit changes of the kindling model of epilepsy. Artif Intell Med. 1998;13:81–97. doi: 10.1016/s0933-3657(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 41.Masukawa LM, Higashima M, Kim JH, Spencer DD. Epileptiform discharges evoked in hippocampal brain slices from epileptic patients. Brain Res. 1989;493:168–74. doi: 10.1016/0006-8993(89)91012-3. [DOI] [PubMed] [Google Scholar]
- 42.Meier CL, Obenaus A, Dudek FE. Persistent Hyperexcitability in Isolated Hippocampal CA1 of Kainate-Lesioned Rats. J Neurophys. 1992;68:2120–7. doi: 10.1152/jn.1992.68.6.2120. [DOI] [PubMed] [Google Scholar]
- 43.Meier CL, Dudek FE. Spontaneous and Stimulation-Induced Synchronized Bursts Afterdischarges in the Isolated CA1 of Kainate-Treated Rats. J Neurophys. 1996;76:2231–9. doi: 10.1152/jn.1996.76.4.2231. [DOI] [PubMed] [Google Scholar]
- 44.Menendez de la Prida L, Suarez F, Pozo MA. Electrophysiological and morphological diversity of neurons from the rat subicular complex in vitro. Hippocampus. 2003;13:728–44. doi: 10.1002/hipo.10123. [DOI] [PubMed] [Google Scholar]
- 45.Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980;182:1–9. doi: 10.1016/0006-8993(80)90825-2. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima S, Franck JE, Bilkey D, Schwartzkroin PA. Local circuit synaptic interactions between CA1 pyramidal cells and interneurons in the kainate-lesioned hyperexcitable hippocampus. Hippocampus. 1991;1:67–78. doi: 10.1002/hipo.450010107. [DOI] [PubMed] [Google Scholar]
- 47.Perez Y, Morin F, Beaulieu C, Lacaille JC. Axonal sprouting of CA1 pyramidal cells in hyperexcitable hippocampal slices of kainate-treated rats. Eur J Neurosci. 1996;8:736–48. doi: 10.1111/j.1460-9568.1996.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 48.Peterson GM, Ribak CE. Hippocampus of the seizure-sensitive gerbil is a specific site for anatomical changes in the GABAergic system. J Comp Neurol. 1987;261:405–22. doi: 10.1002/cne.902610306. [DOI] [PubMed] [Google Scholar]
- 49.Represa A, Le Gal La Salle G, Ben-Ari Y. Hippocampal plasticity in the kindling model of epilepsy in rats. Neurosci Lett. 1989;99:345–50. doi: 10.1016/0304-3940(89)90471-0. [DOI] [PubMed] [Google Scholar]
- 50.Ribak CE, Peterson GM. Intragranular mossy fibers in rats and gerbils form synapses with the somata and proximal dendrites of basket cells in the dentate gyrus. Hippocampus. 1991;1:355–64. doi: 10.1002/hipo.450010403. [DOI] [PubMed] [Google Scholar]
- 51.Ribak CE, Joubran C, Kesslak JP, Bakay RA. A selective decrease in the number of GABAergic somata occurs in pre-seizing monkeys with alumina gel granuloma. Epilepsy Res. 1989;4:126–38. doi: 10.1016/0920-1211(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 52.Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–53. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Ratzliff AH, Howard AL, Santhakumar V, Osapay I, Soltesz I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci. 2004;24:2259–69. doi: 10.1523/JNEUROSCI.5191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophys. 2005;93:437–53. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- 55.Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci. 2003;23:2759–68. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophys. 2003;90:2536–47. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- 57.Schwartzkroin PA. Hippocampal slices in experimental and human epilepsy. Adv Neurol. 1986;44:991–1010. [PubMed] [Google Scholar]
- 58.Shao LR, Dudek FE. Increased excitatory synaptic activity and local connectivity of hippocampal CA1 pyramidal cells in rats with kainate-induced epilepsy. J Neurophys. 2004;92:1366–73. doi: 10.1152/jn.00131.2004. [DOI] [PubMed] [Google Scholar]
- 59.Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–6. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- 60.Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–54. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- 61.Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- 62.Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: Possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–60. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. J Neurophys. 2001;85:1–9. doi: 10.1152/jn.2001.85.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Smith BN, Dudek FE. Network Interactions mediated by new excitatory connections between ca1 pyramidal cells in rats with kainate-induce epilepsy. J Neurophys. 2002;87:1655–8. doi: 10.1152/jn.00581.2001. [DOI] [PubMed] [Google Scholar]
- 65.Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophys. 2000;84:2398–408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- 66.Sutula T, He XX, Cavazos J, Scott G. Synaptic Reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–50. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- 67.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–30. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 68.Sutula T, Cavazos J, Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosc. 1992;12:4173–87. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutula T, Zhang P, Lynch M, Sayin U, Golarai G, Rod R. Synaptic and axonal remodeling of mossy fibers in the hilus and supragranular region of the dentate gyrus in kainate-treated rats. J Comp Neurol. 1998;390:578–94. doi: 10.1002/(sici)1096-9861(19980126)390:4<578::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 70.Sutula T. Seizure-Induced Axonal Sprouting: Assessing Connections Between Injury, Local Circuits, and Epileptogenesis. Epilepsy Curr. 2002;2:86–91. doi: 10.1046/j.1535-7597.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamamaki N, Nojyo Y. Disposition of the slab-like modules formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. J Comp Neurol. 1990;291:509–19. doi: 10.1002/cne.902910403. [DOI] [PubMed] [Google Scholar]
- 72.Taube JS. Electrophysiological properties of neurons in the rat subiculum in vitro. Exp Brain Res. 1993;96:304–18. doi: 10.1007/BF00227110. [DOI] [PubMed] [Google Scholar]
- 73.Wellmer J, Su H, Beck H, Yaari Y. Long-lasting modification of intrinsic discharge properties in subicular neurons following status epilepticus. Eur J Neurosci. 2002;16:259–66. doi: 10.1046/j.1460-9568.2002.02086.x. [DOI] [PubMed] [Google Scholar]
- 74.Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003;301:463. doi: 10.1126/science.1084237. [DOI] [PubMed] [Google Scholar]
- 75.Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005;46 (Suppl 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- 76.Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophys. 2001;85:1067–77. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- 77.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–26. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 78.Zappone CA, Sloviter RS. Commissurally projecting inhibitory interneurons of the rat hippocampal dentate gyrus: a colocalization study of neuronal markers and the retrograde tracer Fluoro-gold. J Comp Neurol. 2001;441:324–44. doi: 10.1002/cne.1415. [DOI] [PubMed] [Google Scholar]
- 79.Zappone CA, Sloviter RS. Translamellar disinhibition in the rat hippocampal dentate gyrus after seizure-induced degeneration of vulnerable hilar neurons. J Neurosci. 2004;24:853–64. doi: 10.1523/JNEUROSCI.1619-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]