Abstract
Targeted molecular imaging techniques have become indispensable tools in modern diagnostics because they provide accurate and specific diagnosis of disease information. Conventional non-specific contrast agents suffer from low targeting efficiency, thus, the use of molecularly targeted imaging probes are needed depending on different imaging modalities. Although recent technologies have yielded various strategies for designing smart probes, utilization of peptide-based probes has been most successful. Phage display technology and combinatorial peptide chemistry have profoundly impacted the pool of available targeting peptides for the efficient and specific delivery of imaging labels. To date, selected peptides that target a variety of disease-related receptors and biomarkers are in place. These targeting peptides can be coupled with the appropriate imaging moieties or nanoplatforms on demand with the help of sophisticated bioconjugation or radiolabeling techniques. This review article examines the current trends in peptide-based imaging probes developed for in vivo applications. We discuss the advantage and challenges in developing peptide-based probes, and summarize current systems with respect to their unique design strategies and applications.
Recent advances in molecular imaging technology have provided numerous opportunities for disease diagnostic and therapeutic procedures (1, 2). Molecular imaging can be used for early disease detection, characterization, and real-time monitoring of therapeutic responses, as well as for investigating drug efficacy. Central to molecular imaging is the development of imaging probes; increasingly, the development of novel probes fuels progress in the field of molecular imaging. In the quest for earlier and more accurate diagnosis of disease and to evaluate response to therapy, several strategies have been developed over the last two decades. Initial efforts utilized radiolabeled small molecules, or macromolecules such as monoclonal antibodies and antibody fragments (3). Although some success has been achieved, the use of these probes has been largely unsuccessful mainly due to their low specificity (small molecules) or limited target permeability (antibodies). For an imaging probe to be clinically useful it should provide a sufficient ‘target-to-background’ ratio to maximize the ‘signal-to-noise’ ratio or contrast in vivo. The ideal imaging compound would have high binding affinity for the target, specific uptake and retention in the target, rapid clearance from non-target tissue, adequate capillary permeability, high stability and integrity in vivo, and would be easy to prepare and safe for use. Considering these criteria, peptides have been increasingly considered as imaging probes, given their distinctive advantages over other small molecules or macromolecules (4–6). Advances in molecular biology have revealed ever-increasing number of potential disease targets including peptide-receptors and peptide-related biomolecules (7). For example, somatostatin (SST), integrin, gastrin-releasing peptide (GRP), cholecystokinin (CCK), neurotensin (NT), glucagon-like peptide-1 (GLP-1), and neuropeptide-Y (NPY) receptors have been successfully identified and characterized for tumor receptor imaging (7–11). Important disease-associated biological processes and regulating factors that occur at the molecular or cellular levels such as cellular pathways of apoptosis and phosphorylation have been fundamentally elucidated (12, 13). Biomarker research has to date discovered various effective and disease-selective biomolecules like proteolytic enzymes (14, 15). Needless to say, these overexpressed receptors and biomolecules represent potential molecular targets for diagnosis and therapy.
Combinatorial peptide chemistry and phage display technology, a molecular genetics approach to ligand discovery, has profoundly impacted the pool of available bioactive synthetic peptides and peptide hormones (16, 17). Selected peptides generally have high affinities and specificity for their target and are active at nanomolar concentrations. Typically, bioactive peptides and peptide hormones have a low molecular weight, containing several to less than 50 amino acids. Small peptides have favorable biodistribution profiles compared to macromolecules, characterized by high uptake in the target and rapid clearance from the blood. In addition, peptides have increased capillary permeability, allowing more efficient penetration into target tissue than macromolecules. Well-established solid-phase peptide synthesis (SPSS) permits reproducible constructs with accurate chemical structures and provides easy scale-up synthesis, handling, and storage. These peptides can be simply synthesized and manipulated to optimize the specificity for the target. Taken together, recent interdisciplinary research at the interface of molecular imaging science and site-specific peptide chemistry has generated highly efficient and stable peptide probes for various different imaging modalities.
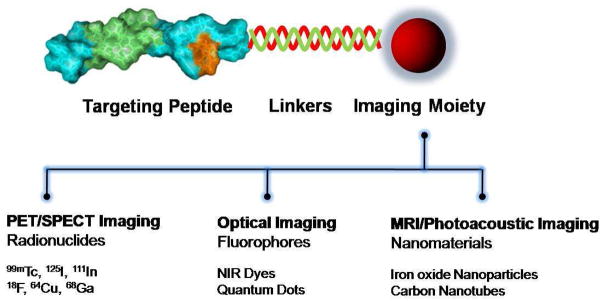
A significant number of selected peptides and peptide hormones have been directly or indirectly labeled with a wide range of imaging moieties according to the imaging modality by various labeling chemistry for use as in vivo probes (Fig. 1). For instance, near-infrared (NIR) fluorescent dyes or quantum dots have been labeled for optical imaging, several radionuclides have been employed for positron emission tomography (PET) or single photon emission computed tomography (SPECT), and paramagnetic agents for magnetic resonance imaging (MRI) (5, 18, 19). Peptides can be labeled with the appropriate moieties on demand with the help of sophisticated bioconjugation and polymer chemistry via organic spacers, macrocyclic or branched chelators, polymers or nanoparticles (20). This review examines the current trends in peptide- and peptide hormone-based imaging probes, and will focus on imaging probes that have been designed for in vivo imaging. We will not discuss probes for cellular imaging or in vitro diagnostics. The use of recently developed key peptide probes is summarized, including probes for PET/SPECT, optical imaging, MRI and multi-modality imaging. We describe design strategies, characteristics and some potential applications of the various peptide-based probes available today.
Figure 1.
A general schematic diagram of peptide-based probes for targeted molecular imaging.
PEPTIDE RECEPTORS AND RADIOLABELED PEPTIDE PROBES
Nuclear imaging methods are widely used for clinical applications because of their high sensitivity and the requirement of injecting a minute quantity of tracer molecules. The most sensitive molecular imaging techniques are the radionuclide-based PET and SPECT imaging modalities (21). Nuclear imaging modalities are able to determine the concentration of specific molecules in the human body in the picomolar range and provide enough sensitivity needed to visualize most interactions between physiological targets and ligands such as disease-related peptide-binding receptors. Most peptide probes are designed based on regulatory peptides, which are naturally occurring peptides with a size ranging from a few to tens of amino acids. These peptides play an important modulatory role in physiological conditions mediated through their specific, high-affinity, peptide-binding receptors. Many of these peptide-binding receptors are massively overexpressed in various diseases, including cancer (7). The increasing evidence of peptide-binding receptor overexpression in specific tumors has accelerated interest in the development of peptide-based probes mostly by using radiolabeling techniques (4, 6). A number of receptor-specific small peptides and their analogs have been screened, synthesized and radiolabeled, and are currently under preclinical or clinical investigation to determine their clinical potential in cancer diagnosis.
Recent advances in combinatorial peptide chemistry and phage display technology have led to the development of robust strategies for the design of receptor-specific small peptides. Typically, naturally occurring peptides have a short biological half-life due to rapid enzymatic degradation in vivo. Once the key amino acid sequences involved in the biological activity have been determined, it might then be possible to engineer the peptide structure to improve biological half-lives and activities in vivo. The common methods used are the introduction of D-amino acids, non-natural amino acids, use of different side-chains, the incorporation of specific hydrophilic and/or hydrophobic amino acids, peptide cyclization, and acetylation and/or amination of peptide. A powerful technique for receptor-specific peptide discovery involves the use of phage display libraries. These libraries contain a vast array of different clones (approximately 107 – 109) that can be rapidly screened in an effort to select target-specific peptides. Specific peptides can be selected, amplified, characterized, and sequenced. Detailed approaches for the discovery and design of targeting peptides have been summarized elsewhere (22–25).
To develop radiolabeled peptide probes, a targeting peptide should be radiolabeled efficiently with high specific radioactivity and be stable under physiological conditions. Various techniques have been developed that allow efficient labeling of peptides with clinically useful radionuclides via chelating moiety or a prosthetic group. Several radionuclides including 99mTc, 123I, 111In, 18F, 64Cu, 68Ga for diagnostic use, or 90Y and 177Lu for therapeutic use have been employed for radiolabeling. The peptide can be labeled with the appropriate radiometals on demand by indirect labeling using the chelating group covalently bound to the peptide, or direct labeling of the peptide when its functional groups are able to act as metal coordinators (4). The most widely-used chelating agents are branched chelators such as diethylenetriaminepentaacetic acid (DTPA) and its analogs (DTPA-like), and macrocyclic chelators such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and its analogs (DOTA-like). These chelating agents utilize carboxylate and amine groups to form stable complexes with metals such as 111In, 64Cu, 68Ga, 86Y, 90Y and 177Lu. Chelating agents including diaminedithiols, activated mercaptoacetylglycilgylcilgylcine (MAG3) and hydrazidonicotinamide (HYNIC) are able to chelate metals like 99mTc and 186Re. Instead of using chelating agents, a prosthetic group such as N-succinimidyl-4-18F-fluorobenzoate (18F-SFB) is necessary for labeling peptides with 18F (6). Due to the relatively short half-life of radionuclides, labeling and purification procedures need to be performed in a controlled and time-restricted fashion, reliable, robust, and high throughput are essential for efficient radiolabeling chemistry. In addition, several other issues also need to be considered. One concerns the physicochemical properties of the radiolabeled peptide. Because of the small size of peptides, attaching a radiolabeled bulky chelating or prosthetic group may influence the biological activity of a peptide. Therefore, site-specific radiochemistry is needed and is important for the preparation of a biologically active peptide probe. In many cases, a spacer is generally present to separate the nuclide complexes from the peptide moiety. However, the incorporation of radionuclides together with chelating group and spacer may bring in alternations to the peptide physical properties. Those can provide unfavorable pharmacokinetic profile for imaging purpose. A wide variety of strategies have been developed in recent years for the convenient and efficient radiolabeling of peptides (4, 6). The physicochemical properties and applications for radiolabeled peptide have been summarized elsewhere. In this section, we discuss the significance of various peptide-receptors in molecular imaging as well as recently-developed radiolabeled peptide-based probes for cancer targeting in vivo systems.
Somatostatin receptor (SSTr)
SSTs are regulatory cyclopeptide hormones, which act as a neurotransmitter in the brain. Its hormonal activities are inhibitory and its targets include growth hormone, insulin and glucagon secretion and calcitonin (26). Its biological effects are mediated via specific high-affinity G-protein coupled membrane receptors (GPCrs). Five distinct subtypes of SSTr (SSTr1–SSTr5) have been identified and cloned (27). SSTrs are overexpressed in homogeneous and heterogeneous manners in the majority of tumors such as the neuroendocrine tumors, gliomas, breast cancer, and small cell lung cancer (SCLC), which can serve as a potential target for SST-based imaging probes (28). Unfortunately, the in vivo half-lives of naturally occurring SSTs (SST-28 and SST-14) are extremely short (less than 3 min) mainly due to enzyme degradation. To overcome this problem, a large variety of SST analogues with enhanced resistance to in vivo enzymatic degradation and biological activity has been developed. Cyclic octapeptide octreotide (D-Phe1-Cys2-Phe3-D-Trp4-Lys5-Thr6-Cys7-Thr8-ol) and its analogues, which lack the key enzyme cleavage sites, are more stable than the native SSTs. 111In-DTPA-octreotide (111In-OctreoScan) was approved by the United States Food and Drug Administration (FDA) in 1994 as the first peptide-based radiopharmaceutical agent for scintigraphy of neuroendocrine tumors. Although DTPA-octreotide has moderate binding affinity to SSTr2 and, to a lesser extent, to other SSTrs, and since the DTPA chelating agent is not a suitable chelator for many other nuclides, it has generated tremendous interest in further developing peptides and peptide hormones for imaging SSTrs. Analogs such as [DOTA-Tyr3]-octreotide (DOTA-TOC) and [DOTA-D-Phe1-Tyr3]-Octreotide (DOTA-TATE), HYNIC-TATE and HYNIC-TOC have been designed and several analogs are currently in clinical use (29). 18F-labeld cellobiose (Cel-S-) derivative of TOCA has been developed to improve tracer pharmacokinetics and for clinical application in PET SSTr imaging (30, 31). Recently, a series of radiolabeled SSTr antagonists including 111In-DOTA-[cyclo(D-Cys-Phe-Tyr-D-AgI8(Me,2-naphthoyl)-Lys-Thr-Phe-Cys)] and 111In-DOTA-[4-NO2-Phe-cyclo(D-Cys-Tyr-D-Trp-Lys-Thr-Cys)-D-Tyr-NH2] were prepared and their efficiency was evaluated both in vitro and in SSTr2/SSTr3-expressing tumor models (32). To improve the specificity for SSTrs, analogs with high binding affinity to broader subtypes of somatostatin receptors (such as SSTr2, SSTr3 and SSTr5) have been developed (33) and analogs with high affinity for all receptor subtypes have also been reported (34).
Integrin αvβ3
Integrins are receptors that comprise a family of heterodimeric glycoproteins involved in the extracellular matrix (35). The integrin family plays important roles during the formation of new blood vessels (angiogenesis) in tumors. Of particular interest is Integrin αvβ3. Receptors to αvβ3 are highly expressed on activated and proliferating endothelial cells during tumor angiogenesis and metastasis, but are not readily detectable in resting endothelial cells and most normal organs (8). For these reasons, integrin receptors have been attractive targets for diagnostic imaging. The αvβ3 receptor binds extracellular matrix protein such as vitronectin, which contains the Arg-Gly-Asp (RGD) amino acid sequence (36). RGD peptides strongly bind with the αvβ3 receptor, thus, a variety of peptide probes based on the RGD motif have been developed to target angiogenic vessels (37). Among these, 18F-galacto-RGD and 18F-AH111585 are under clinical investigation (38–40). 18F-galacto-RGD was developed by 18F labeling of the RGD-containing glycopeptides cyclo(Arg-Gly-Asp-D-Phe-Lys(RGDfK, sugar amino acid)), and has been studied in patients with melanoma, sarcoma and breast cancer. 18F-AH111585 is a 18F-labeled small peptide containing multiple disulphide bridges that stabilize the peptide in vivo (41). These probes have produced promising results, however, the relatively low and variable tumor uptake of the peptides among individuals hinders their widespread clinical application. To provide enhanced binding affinity to αvβ3 receptor, various multivalent cyclic RGD-based peptides were designed. For instance, various dimers and tetramers of RGD analogs including Glu-[cyclo(RGDfK)]2, Glu-[Gly-Gly-Gly-cyclo(RGDfK)]2 and Glu-[Gly- [cyclo(RGDfK)]2]2 were prepared and labeled with various radionuclides such as 18F, 64Cu, 68Ga and 99mTc (42–45) (Fig. 2). All the oligomeric peptide probes bound more strongly compared to monomeric RGD peptide in an integrin αvβ3-positive U87MG xenograft model. 18F-labeled RGD dimer, 18F-FPP-[cyclo(RGDyK)]2, has received exploratory investigative new drug application (eIND) approval from FDA and PET/CT imaging studies in a number of healthy volunteers have been conducted. These peptide probes offer new strategies for imaging tumor angiogenesis and its related clinical applications in patients.
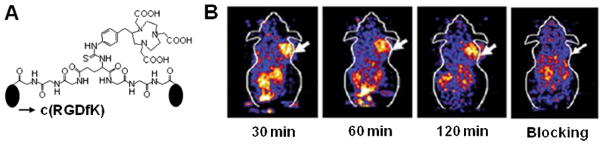
Figure 2.
A) Chemical structure of NOTA-Glu-[Gly-Gly-Gly-cyclo(RGDfK)]2. B) Coronal PET images of the U87MG tumor bearing mouse at 30, 60 and 120 min without and with blocking dose of cyclo(RGDyK) after injection of 68Ga-NOTA-Glu-[Gly-Gly-Gly-cyclo(RGDfK)]2. Modified with permission from ref. 37. Copyright 2009, Springer-Varlag.
Gastrin-releasing peptide receptor (GRPr)
GRPrs are expressed on a variety of cancers including prostate, breast, pancreas, gastrointestinal and SCLC. To date, four mammalian GRPr subtypes have been characterized (7). Bombesin (BBN), pGlu1-Gln2-Arg3-Leu4-Gly5-Asn6-Gln7-Trp8-Ala9-Val10-Gly11-His12-Leu13-Met14-NH2, is an amphibian homologue of mammalian GRP that has high affinity and specificity to GRPrs (46). Because the C-terminal 7-14 amino acid sequence is critical for receptor binding, various BBN(7-14)-based analogs have been developed and coupled with a imaging moiety at the N-terminus of the peptide. An early report showed that 99mTc-RP-527, a tripeptide N3S chelator ((N,N-dimethyl-Gly-Ser-Cys(acm)) coupled to N-terminus of BBN(7-14) via Gly-5-aminovaleric acid linker, can be bound and internalized by BBN/GRP receptor expressing PC-3 cells in vitro and in mice bearing PC-3 tumors (47). Recently, 99mTc-RP-527 identified primary tumors and metastasis in breast carcinoma patients (48). BBN and its analogs have also been labeled with a variety of other radionuclides such as 111In-[DOTA-11-Aun]-BBN(7-14), 64Cu-[DOTA-Lys3]-BBN, 18F-[FB-Lys3]-BBN, and 68Ga-BZH3, (49–52). Furthermore, other BBN agonists with a novel receptor profile have been developed and characterized both in vitro and in vivo. These include, among others, the panbombesin analogs Demobesin 1 and Demobesin 4, and 177Lu-AMBA (53, 54). Because of the broad spectrum of the BBN/GRPr system on various tumors, BBN peptide-based imaging probes are considered as a highly clinically relevant target.
Cholecystokinin-2 (CCK-2)/Gastrin receptor
CCK-2/gastrin receptors are overexpressed in over 90% of human modularly thyroid cancer (MTC) (55). These receptors are also present in other human tumors such as SCLC, astrocytomas, stromal ovarian tumors and gastroenteropancreatic cancer (56). The gastrointestinal peptide CKK and gastrin-related peptide probes were mostly designed based on CCK-8 and minigastrin. CCK-8 is a sulfated octapeptide C-terminus fragment of the biologically active CCK, Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2. Minigastrin is a C-terminal truncated form, having 13-residues with the sequence of Leu1-Glu2-Glu3-Glu4-Glu5-Glu6-Ala7-Tyr8-Gly9-Trp10-Met11-Asp12-Phe13-NH2. CCK and gastrin share an identical five amino acid sequence at their biologically active C-terminus. Chelating agent-conjugated peptide analogs such as 111In or 99mTc-[DTPA-linker]-CCK8 and 111In-[DTPA-D-Glu1 or Leu1]-minigastrin show uptake in CCK-2/gastrin receptor-positive tissues. However, this has not been developed further due to the low tumor-to-kidney ratio or occasional low sensitivity (9). DOTA-linked minigastrins with decreasing numbers of glutamic acid residues improves the binding affinity in gastrin receptor-positive AR4-2J rat pancreatic tumor cells and significantly reduces kidney uptake in AR4-2J bearing rats compared to DTPA-linked minigastrins (57). However, reducing the number of glutamates results in lower metabolic stability. Further developments improve the metabolic stability in vivo should improve the bioavailability of CCK targeted peptide probes.
Melanocortin-1 receptor (MC-1r)
Over 80% of human metastatic melanoma samples display MC-1rs (58). Thus, these receptors are attractive targets for the diagnosis of melanoma. Alpha-melanocyte stimulating hormone (α-MSH) is a linear tridecapeptide (Ac-Ser1-Tyr2-Ser3-Met4-Glu5-His6-Phe7-Arg8-Trp9-Gly10-Lys11-Pro12-Val13-NH2) that exhibits nanomolar MC-1r binding affinities, making them promising melanoma targeting peptide probes (11). Like other wild-type peptides, the rapid in vivo degradation of α-MSH in plasma limits its use in vivo. To improve the pharmacokinetics, analogs with enhanced stability and biological activity in vivo have been synthesized, largely based on substitution of Met4 with Nle4, and Phe7 with D-Phe7 in α-MSH, the so called α-MSH(NDP). Several radiolabeled α-MSH(NDP) analogs including 111In-[DTPA-α]-MSH(NDP) and 99mTc-[Cys-Gly-Cys-Gly]-α-MSH(NDP) have been introduced. However, these early versions displayed poor tumor targeting mainly due to the persistent accumulation in the liver and kidneys and rapid blood clearance (59). Compared to linear peptide-based probes, metal cyclized α-MSH analogs enhance tumor uptake. For instance, DOTA conjugated rhenium cyclized α-MSH analogs, [Cys3,4,10-D-Phe7-α]-MSH(3-13) (DOTA-ReCCMSH) and DOTA-ReCCMSH(Arg11), have been prepared and labeled with 111In and 64Cu, respectively (60, 61). These ReCCMSH analogs exhibit high tumor concentration and rapid clearance from non-target tissues, compared with linear α-MSH analogs. These results suggest that metal-coordinated cyclized α-MSH peptide analogs have the potential for early detection of malignant melanoma.
Glucagon-like peptide-1 receptor (GLP-1r)
GLP-1-(7-36) is a polypeptide hormone secreted from L-cells in the gastrointestinal (GI) tract in response to the ingestion of nutrients (62). GLP-1 stimulates insulin secretion and inhibits glucagon secretion through an interaction with the GLP-1r, which is expressed on pancreatic beta cells of the islets and also in brain, heart, kidney and the GI tract (63). In vitro studies have demonstrated that high levels of GLP-1r are overexpressed in human insulinomas and gastrinomas (10, 64). To image insulinomas in vivo, radiolabeled GLP-1, 125I-GLP-1(7-36) and its more stable agonist, 111In-[DTPA-Lys40]-exendin-4, have been developed and studied in animal models (65, 66). Exendin-4, a 39 amino acid peptide, is an enzyme resistant GLP-1r agonist approved by the FDA for the treatment of patients with Type 2 diabetes. An 111In-labeled GLP-1 agonist successfully targeted insulinomas in a Rip1-Tag2 mouse model of pancreatic islet beta cell tumors (66). Recently, this 111In-labeled peptide probe was evaluated in patients with insulinomas that were not detected by conventional imaging methods including CT, endoscopic ultrasonography and octreoscan scintigraphy (67). Whole-body SPECT imaging of the abdomen indicated that GLP-1r scan allows precise surgical tumor resection of GLP-1r positive tumors.
Other receptors
Vasoactive intestinal peptide (VIP) receptors have been detected on the normal intestinal and epithelial cell membranes, and are also overexpressed in various tumors cells including colonic adenocarcinoma, pancreatic carcinoma, and carcinoid (68). Various VIP analogs were designed and labeled with radioisotopes, such as 99mTc, 64Cu, and 18F, and have been evaluated in animal models and in humans (69, 70). Neurotensin (NT) is a linear tridecapeptide which can be found in the central nervous system and in peripheral tissues (71). Since many neuroendocrine tumors overexpress NT receptors, NTr can be targeted with radiolabeled NT analogs (72–74). Neuropeptide Y (NPY) receptors are produced in various neuroblastomas, breast cancers, and sarcomas (75, 76). Optimization of NPY analogs has resulted in a number of small NPY analogs and evaluated in animal models (77). Chemokine receptor 4 (CXCR4) is overexpressed in a variety of cancers, including breast, brain, ovarian and prostate (78). The radiolabeled CXCR4 peptide inhibitor has been developed for the imaging of CXCR4 expression in metastatic tumors in animals (79). Peptide antagonists have recently been identified as a promising candidate for in vivo receptor imaging. Until recently, the use of radiolabeled peptide antagonists has not been widely considered for in vivo imaging of GPCRs. Unlike peptide agonists, mostly antagonists do to internalize into cells and accumulation has been assumed to be limited compared to agonists. However, as described in the previous section, several radiolabeled SSTr and GRPr antagonists showed very high tumor accumulation compared to agonists, and these promising results have triggered further development of antagonists with improved binding characteristics (32, 54, 80).
The increased evidence of peptide receptor overexpression in different types of cancers has resulted in the development of a myriad of peptides for in vivo targeted imaging. It is apparent that radiolabeled peptide-based probes have become an important class of molecular imaging probes for the detection of various diseases. Any peptide that has been screened and selected for specific targeting purposes can be directly labeled with radionuclides by well-established techniques, and its potential investigated in vivo. Besides direct labeling, alternative strategies like combinations of peptides labeled with different radionuclides or multi-receptor targeting using hetero-dimer peptides can be applied to obtain optimally-targeted imaging (81). For instance, a radiolabeled BBN-RGD hetero-dimers were synthesized and showed improved tumor targeting efficacy by dual integrin αvβ3 receptor and GRPr recognition in vivo (81, 82).
Although peptide-based therapeutic radiopharmacueticals are not discussed in this review, many of the described peptide imaging probes can be established as a clinically useful class of therapeutic agents (4). This can be done by slight alteration of the amino sequences and the replacement of diagnostic radionuclides to therapeutic moieties such as 90Y and 177Lu. Despite the significant progress in the field of receptor-binding peptides, the application of suitably radiolabeled peptide for targeted molecular imaging is still in the developmental stage (Table 1). The next sections describe different design strategies for the design of optical probes and nanoparticle probes.
Table 1.
A selective list of the radiolabeled peptide probes already investigated in human studies.
| Target receptor | Peptide probe | Condition | Comments | Ref. |
|---|---|---|---|---|
| SSTr | 111In-DTPA-octreotide | Neuroendocrine tumors | Approved | |
| DOTA-TOC | Neuroendocrine tumors | Clinical study | 29 | |
| DOTA-TATE | Neuroendocrine tumors | Clinical study | 29 | |
| 18F-[Gluc-Lys]-TOCA | Neuroendocrine tumors | Clinical study | 31 | |
| Integrin | 18F-galacto-RGD | Head and neck cancer | Clinical study | 38 |
| 18F-AH111585 | Metastatic breast cancer | Phase I trial | 39 | |
| 18F-RGD-K5 | Various cancers | Clinical study | ||
| 18F-FPPRGD2 | eIND | |||
| GRPr | 99mTc-RP-527 | Breast cancer | Clinical study | 47 |
| 68Ga-BZH3 | Gastrointestinal stromal tumor | Clinical study | 51 | |
| GLP-1r | 111In-[DTPA-Lys40]- Exendin-4 | Insulinoma | Clinical study | 66 |
| NTr | 99mTc-NT-XI | Pancreatic adenocarcinoma | Clinical study | 73 |
OPTICAL PEPTIDE PROBES
Optical imaging is one of the most widely used imaging modality in clinical practice and in research. Microscopic optical imaging techniques have already been developed as gold standard tools for in vitro and ex vivo applications in molecular and cellular biology. Recent progress in the field of in vivo optical imaging offers powerful analytical potential in preclinical and clinical applications (83). Compared to other imaging modalities, optical imaging has many advantages, as it enables highly sensitive, non-invasive, and safe detection using readily available instruments at moderate cost (84). Recently developed optical imaging instruments, fluorophores, and sophisticated imaging probes have expanded fluorescence-based imaging techniques, allowing investigations at the whole animal or tissue level in real-time. Combination of fluorophores and materials including peptides, proteins, biopolymers, and novel metals has greatly increased the list of optical imaging probes and significantly improved the performance of whole animal imaging systems. Comprehensive review articles have summarized these recent advancements (20, 85–87). This section discusses the design concepts and recent reports on peptide-based fluorescent probes categorized as fluorophore-labeled and activatable molecular imaging probes.
Fluorophore-labeled probes
A peptides can be simply labeled with a fluorophore, which is similar to radiolabeled peptide probe design, except that a fluorophore is used in the place of a radionuclide. Fluorophores with different excitation and emission ranges can be chosen based on experimental conditions for in vitro and in vivo applications. Fluorophores in the visible range with emission between 400 nm and 600 nm such as 7-amino-4-methylcoumarin (AMC), fluorescein isothiocyanate (FITC), and 5-carboxytetramethylrhodamine (TAMRA) are generally used for cellular imaging. However, the use of these fluorophores in vivo has a significant drawback because visible light penetration can be hampered by tissue autofluorescence and can be interfered with by components in the body such as water, hemoglobin, and deoxyhemoglobin (88). Tissue yields reduced autofluorescence at longer wavelength and offer significantly less attenuation of light in the near-infrared (NIR) region (650–900 nm) compared with visible wavelengths. Therefore, fluorophores that operate in the NIR region are beneficial for in vivo imaging applications (19). Chemical structures of most NIR fluorophores are similar to indocyanine green (ICG, emission at 830 nm), a FDA-approved tricarbocyanine dye commonly used as an angiographic agent. To date, a number of NIR fluorophores have been reported and their reactive intermediates for peptide bioconjugation are commercially available (as examples, Cy dyes from GE Healthcare, Alexa Fluor dyes from Invitrogen, IRdye dyes from Li-COR Bioscience, and SRfluor Dyes from Molecular Targeting Technologies). Their specific physicochemical characteristics are summarized elsewhere (85). Because optical imaging is highly sensitive and can detect femtomolar quantities of fluorophores, the approach of targeting tumors by receptor-specific peptides could be successfully adapted when the nuclide is replaced by a NIR fluorophore.
Many biologically active peptide analogs previously introduced in this review have also been labeled with NIR fluorophores. In vivo diagnostic use of a NIR-dye conjugate consisting of indotricarbocyanie (ITCC) dye and the octreotate for tumor imaging has been reported (89). ITCC-octreotate displayed fast and thorough receptor binding properties in SSTr-2 overexpressing RIN38/SSTr-2 cells and provided three-fold higher tumor fluorescence in mice bearing RIN38/SSTr-2 tumors from 3–24 h after intravenous injection. Different libraries of SST analogs were also labeled with fluorophores and tested in SSTr-positive NCI-H69 human SCLC tumors and HT-29 colon tumor bearing mice, respectively (90, 91). For GRPr imaging, Alexa Fluor 680-[Gly-Gly-Gly]-Bombesin(7–14) was synthesized and demonstrated specific GRPr targeting ability in vitro and in mice bearing T-47D breast cancer cells (92). A vast array of optical probes associated with angiogenesis specific targets has been developed using RGD peptide analogs. In one study, Cy5.5 dye was conjugated to the ε-amino group of the lysine residue of c(RGDyK) and used for imaging of integrin αvβ3-positive U87MG tumor xenografts in mice (93). Furthermore, Cy5.5 or Cy7-conjugated mono-, di-, and tetrameric RGD peptides were designed and demonstrated increased receptor binding affinity and imaging efficacy compared to the monomeric compound (94, 95). To use phage as targeted imaging agents, a high-throughput method was developed for identifying and optimizing peptide ligands to map and image biological targets of interest in vivo (96). Using a secreted protein acidic and rich in cystein (SPARC) as a model target for invasive cancer (97) and vascular cell adhesion molecule-1 (VCAM-1) for inflammatory endothelium (98), peptides were selected, simply labeled with fluorophores, and identified in vivo as potent peptide-based imaging probes. Dye-labeled phage clones demonstrated excellent in vivo targeting ability in tumors and VCAM-1 expression vessels, suggesting that fluorophore-based phage clones can be used as in vivo imaging probes. Recently, a clinical trial of phage-selected peptide was reported using dye-labeled peptide conjugate and optical instrument, such as fluorescence endoscopes (99). Although the probe did not use a NIR dye, the use of fluorescein-conjugated human colonic adenoma specific peptide, Val-Arg-Pro-Met-Pro-Leu-Gln, successfully detected and imaged colorectal cancer in humans using a fluorescence confocal microendoscope (99). Fluorophore-conjugated peptides represent powerful tools for high-throughput screening studies and as a potential alternative to nuclear imaging studies in the preclinical settings.
Activatable probes
Activatable optical probes are peptide-based molecules that carry fluorescently quenched fluorophores, and consist of a fluorophore and a quencher attached to the opposite ends of a cleavable peptide linker (86). The fluorophore and quencher, which are usually arranged in close proximity (less than 10 nm apart), induce strong fluorescence quenching by fluorescence resonance energy transfer (FRET) (100) and dark quenching mechanisms (101). These probes are generally activated by peptide cleavage induced by enzymes, which generate a strongly amplified fluorescence signal at a target region such as a tumor. Such probes are also called molecular beacons and have been used in vitro, especially for diagnostic assay purposes. Combination of NIR dyes and small animal imaging instruments have expanded these techniques and allowed in vivo application. Compared to fluorophore-labeled peptide probe, an activatable probe is optically dark in its quenched state and becomes intensely fluorescent in vivo following proteolysis of the substrate linker by the target enzyme. To date, several protease targeted activatable peptide probes are available for in vivo imaging purposes. Proteases are enzymes that hydrolyze specific peptide bonds within proteins and which are overexpressed in a number of pathologies including cancer, inflammation, vascular disease, and infectious diseases (14). The peptide linkers used in the development of activatable probes are possible protease enzyme substrates or their analogs.
A widely-reported activatable probe represents the first biocompatible polymer-associated imaging probe for matrix metalloproteinase (MMP) imaging in vivo (102). MMPs are a family of zinc-dependent endopeptidases that play key roles in several biological processes, and have been used as biomarkers in various diseases including cancer and inflammatory diseases (15). The probe consists of a biopolymer conjugated poly-L-lysine as a backbone and a MMP substrate, Gly-Pro-Leu-Gly-Val-Arg, introduced between the lysine backbone and Cy5.5. A dye-peptide conjugate is attached close enough to permit FRET induced by dye-dye self-quenching. The self-quenched probe produces significantly lower fluorescence in the absence of MMPs and more than 10-fold heightened fluorescence signal in the presence of MMPs. Optical images in various disease models including cancer, atherosclerosis, and myocardial infarction have demonstrated that this probe can image MMP activity in vivo (103–105). Lee et al. described the potential use of a dark-quenched MMP-13 activatable peptide probe to image overexpressed MMP-13 in an osteoarthritis (OA) model (106) (Fig. 3). The probe was designed by a combination of Cy5.5 dye, MMP-13 substrate, and the black hole quencher-3 (BHQ-3) with a sequence as Cy5.5-Gly-Pro-Leu-Gly-Met-Arg-Gly-Leu-Gly-Lys(BHQ-3). The spectrofluorometry study clearly demonstrated significant recovery (>30-fold) of the NIR fluorescence signals in the samples containing MMP-13, with inhibition of the signal in the presence of a MMP-13 inhibitor. When the probe was injected into an OA-induced rat model, the symptoms of the early and late stages of OA could be simply monitored, imaged, and analyzed (106).
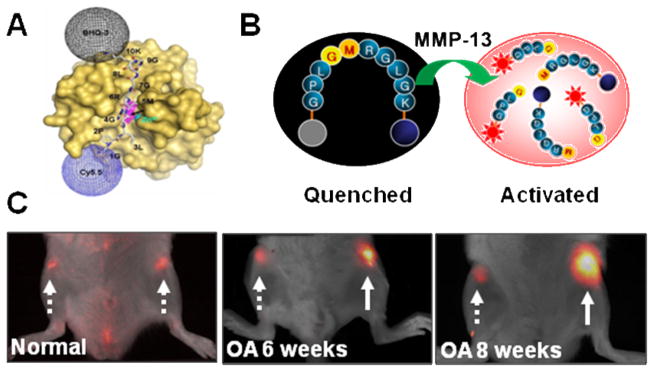
Figure 3.
A dark-quenched activatable MMP-13 probe. A) The molecular surface of human MMP-13 and the modeled binding pockets of the MMP-13 imaging probe. Arrow and italics indicate the cleavage site. B) Schematic diagram of activation process. C) In vivo imaging of overexpressed MMP-13 in normal, six and eight week osteoarthritis (OA)-induced cartilages 1 h after intracartilage-injection of the MMP-13 imaging probe. Arrows; dotted line (normal) and solid line (OA). Modified with permission from ref. 86. Copyright 2008, American Chemical Society.
Apoptosis is a programmed cell death process in organisms that is aberrantly involved in the pathogenesis of many diseases (12). The majority of anticancer drugs initiate apoptosis, thus, the ability to detect the progression of apoptosis could clinically assist in determining whether a patient’s chemotherapy is working properly. To detect apoptosis-associated caspase activity in vivo, a cell-permeable, caspase-activatable NIR probe, TcapQ, has been reported (107). Caspases are a family of cysteine proteases and are crucial mediators of apoptosis. TcapQ consisted of a cell-penetration peptide, Lys-Lys-Lys-Arg-Lys-Val, conjugated to a caspase recognition peptide substrate, Asp-Glu-Val-Asp, which was quenched by a fluorophore-quencher pair, Alexa Fluor 647 and QSY 21, respectively. Incorporation of the activatable strategy onto the cell-permeable caspase substrate enabled imaging caspase activity in apoptotic cells in vitro treated with the anticancer drug in a rat model of glaucoma (107, 108). In contrast to fluorophore-quenched pair probes that become bright after cleavage of the peptide by proteases, Blum et al. developed quenched activity-based probes (qABPs) which become bright after labeling of dye by proteases (109). In this approach, a QSY21 quencher is placed in close proximity to the Cy5 fluorophore via peptide acyloxymethylketone analogs, thereby preventing fluorescence signals. When the qABP encountered their target enzyme, activity-dependent covalent modification of the probe released the fluorescence acceptor, causing the probe to increase fluorescent intensity. These qABPs have been successfully applied for labeling of active cysteine proteases in living cells and show potential for whole-body imaging applications in mice bearing grafted tumors after intravenous injection (109, 110). The unique concepts and applications of activatable probes based on different strategies, for example, the use of small molecules, polymer conjugates, and inorganic nanoparticles, have been extensively described elsewhere (86, 111).
Optical imaging technologies are an important step forward in small animal imaging and are believed to play a significant role in basic research, drug discovery, and preclinical studies. The peptide-based optical probes have a number of advantages including well-defined peptide chemistry, and the use of nonionizing fluorophore labeling technique permit custom synthesis of various imaging probes that can be easily handled, stored, and utilized for repeated studies. Besides the delivery of small imaging moieties such as radionuclides or fluorophores, peptides are attractive vehicles to deliver more complex nanostructures and can be associated with promising nanoplatforms for targeted molecular imaging.
NANOPLATFORM-BASED PEPTIDE PROBES
Modern nanotechnology and molecular imaging science has yielded new strategies for designing nanoplatform-based imaging probes that efficiently detect biomolecules or diagnose diseases (2). The most well-investigated nano-sized materials include magnetic iron oxide nanoparticles (IONPs) for MRI, quantum dots (QDs) for optical imaging, polymeric nanoparticles, carbon nanotubes, gold nanoparticles, and many others. Such nanomaterials provide unique nano-sized scale and physical properties, which afford incomparable probe and target molecule interaction, and allow imaging of biological processes at molecular levels. Besides the physical properties, nanomaterials have large surface areas, which are ideal for efficient modification with a variety of targeting and imaging moieties. Those properties could lead to significantly improved binding properties via a polyvalent effect. Conjugating many targeting and imaging moieties on a single nanomaterial not only enhances binding affinity and specificity, but also will provide amplified signals at the target region. Furthermore, it can be engineered as a nanoplatform for effective and targeted delivery of imaging labels by prolonged plasma half-lives, enhanced stability, improved targeting efficiency, and reduced non-specific binding. Due to the different natures of nanomaterials, the strategies for surface modification modification vary. Over the last decade, there has been significant advancement in the field of nanoplatform-based targeted molecular imaging. Several comprehensive review articles have summarized these recent advances, and have discussed their unique design and applications (2, 18, 20, 87, 112–117). In this section, we discuss innovative nanoplatform-based imaging probes that have been generated by peptide-based approaches. Many platforms described in this section are associated with the RGD peptide as a model targeting system. However, it should be noted that the design platforms for these peptide-based imaging probes can be applied to many other in vivo targets by replacing the specific peptide sequences.
Magnetic nanoparticles for MRI
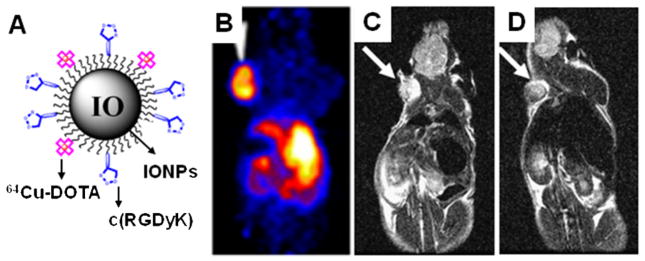
IONPs are one of the most studied nanoparticles in the field of molecular imaging, mainly due to their superior magnetic properties, ease of modification, and biocompatibility. Currently, several IONP formulations have been approved by the FDA and are used in clinics for bowel, liver, spleen, and lymph node MR imaging (18). Furthermore, IONPs have recently been used to label cells and track labeled-cells in vivo (118). However, conventional IONP formulations rely on passive targeting and require nonspecific uptake by cells of the reticuloendotherial system (RES) in vivo, which undermines their targeting specificity. Although IONPs can accumulate at the tumor site due to enhanced permeation retention (EPR) effect (18), non-targeted IONPs seldom attain sufficient amount in the tumor to produce a strong imaging signal. One approach to overcome this limitation is to develop engineered IONPs by conjugating targeting peptides. Peptides can help internalize IOPNs into cells via receptor-mediated endocytosis, providing MRI contrast enhancement. Xie et al. reported the RGD peptide coated ultra-small IONPs as an angiogenesis targeted MRI imaging probe (119). The c(RGDyK) peptides were covalently conjugated to 4-methylcatechol coated IONPs and rendered as stable NPs in physiological condition. c(RGDyK)-IONPs showed 5-fold higher cellular uptake in integrin αvβ3-positive U87MG cells than in MCF-7 human breast cancer cells, which expresses low level of αvβ3. Following systemic injection of c(RGDyK)-IONPs in mice bearing U87MG tumors, peptide-conjugated NPs were found to target the integrin expressing tumor vasculature and tumor cells with little to no macrophage uptake (119). Several other studies of RGD-IONP conjugates have been reported in the literature (120, 121). IONPs that target tumors with high specificity to MMPs were developed by conjugating chlorotoxin (CTX) to poly(ethylene glycol) coated IONPs (122) (Fig. 4). CTX is a 36 amino acid peptide that can specifically bind to MMP-2 on the surface of cells (123). CTX-IONPs showed 10-fold higher internalization in MMP-2-positive 9L glioma cells as compared to that of the non-targeted IONPs in vitro and in mice bearing 9L xenograft tumors. Luteinizing hormone-releasing hormone (LHRH)-conjugated IONPs for detection of breast cancer metastases has been reported (124). LHRH is a decapeptide with the primary sequence of Glu-His-Trp-Ser-Tyr-Leu-Arg-Pro-Gly-NH2; greater than 52% of human breast cancers express receptor binding sites for LHRH (125). The recent progress and the utilization of various targeted IONPs for imaging and therapy are described in detail elsewhere (112, 126). As the major disadvantage of MRI is its inherent low sensitivity, methods and strategies to produce imaging probes with a high specificity and sensitivity are greatly needed. Since IONPs have been used in clinical settings for many years, decorating IONPs with novel targeting peptides will facilitate the applications of targeted molecular MRI.
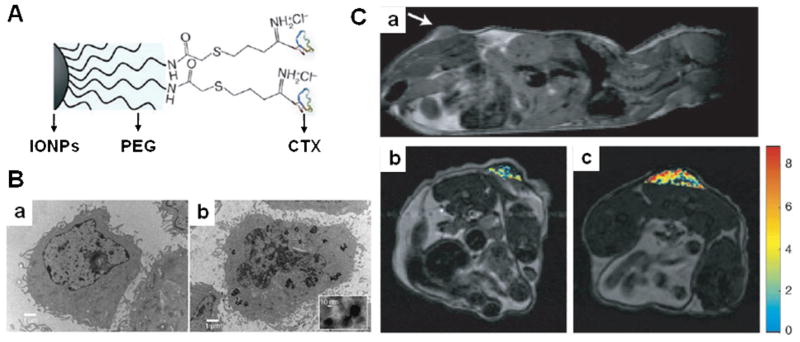
Figure 4.
A) Schematic diagram of CTX peptide conjugation to polyethylene (PEG) coated iron oxide nanoparticles (IONPs). B) TEM images of 9L cells with IONPs a) without and b) with CTX peptides. C) MRI anatomical image of a mouse bearing 9L xenograft tumor. a) Anatomical image in the sagittal plane displaying the location of the tumor (the arrow marks the tumor location). Changes in R2 relaxivity values for the tumor regions, superimposed over anatomical MR images, for mouse receiving IONPs b) without and with CTX peptides after 3 h post injection. Modified with permission from ref. 96. Copyright 2008, Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
QDs for optical imaging
QDs are nanometer-sized inorganic fluorescent semiconductor nanoparticles with a variety of superior properties for optical imaging as compared with organic fluorophores, including high quantum yields, strong resistance to photobleaching and chemical degradation, long fluorescence life time, and composition-tunable fluorescence emission (127). Several biocompatible QDs have been applied to label cells and long-term multicolor cell imaging (128). For in vivo applications, non-targeted QDs have been developed for cell trafficking, vasculature imaging, sentinel lymph node mapping, and neural imaging (2). Although non-targeted NIR QDs have shown potential for imaging in living subjects, targeted QDs are needed to provide effective, specific, and reliable images at target regions (129). As mentioned previously in this review, specific targeting can be achieved by conjugating targeting peptide to the surface of QDs. However, due to the size and short biological half-lives of QD conjugates, there are only a few reports in the literature for successful in vivo applications. Cai et al. reported the first in vivo targeted imaging of tumor vasculature using peptide-conjugated NIR QDs (130, 131). As shown in Fig. 5, c(RGDyK) peptides were conjugated to poly(ethylene glycol) coated QD705. RGD-QD705 exhibited high affinity in integrin αvβ3-positive U87MG cells and in vivo imaging was successfully achieved in mice bearing U87MG tumors, where the tumor NIR fluorescence signal reached maximum at 6 h post injection. A variety of techniques have been explored to label QDs with various targeting peptides, such as cellular nuclei targeting peptide (132), and cell-penetrating peptides (133), but these approaches are currently limited to in vitro use. Recently, QDs modified with antibodies, antigens, aptamers, and targeting proteins for in vivo use have been reviewed (129). Advances in QD technology such as synthesis of multifunctional QDs, improved surface modification, and development of non-Cd based less-toxic QDs, highlights the potential for clinical use of QDs (134).
Figure 5.
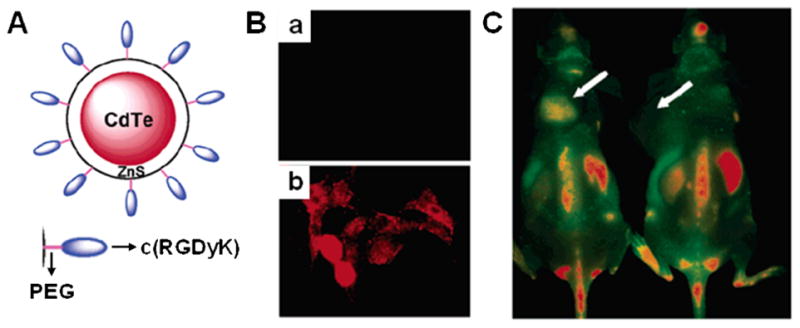
A) Schematic diagram of QD750-PEG2k-RGD. B) In vitro staining of human breast MCF and human glioblastoma U87MG cells (low and high integrin αvβ3 expression, respectively) using QD750-PEG2k-RGD. C) In vivo NIR fluoresnce imaging of U87MG tumor bearing mice (left shoulder, pointed by white arrows) injected with QD750-PEG2k-RGD (left ) and QD750-PEG2k (right), respectively. Modified with permission from ref. 105. Copyright 2006, American Chemical Society.
Dual-modality imaging probes
Targeting peptides have also been successfully adapted to dual-modality probes for in vivo molecular imaging. Each imaging modality has its own unique strengths and weaknesses. For example, PET has high target sensitivity but relatively poor spatial resolution, whereas MRI can provide good spatial resolution with three-dimensional tomography but limited target sensitivity. Similarly, optical imaging has high sensitivity but suffers from low tissue penetration in vivo. Therefore, the combination of two popular imaging modalities might offer the prospect of improved diagnostic ability (135). To date, significant progress has been made towards the preparation of dual-modality imaging probes, and targeting peptides also play important roles as specific ligands in these unique systems. For instances, dual-modality probe have been reported for PET/MRI, PET/optical and MRI/optical imaging. A probe for PET/MRI has been prepared by combination of radionuclide and IONPs; labeling 64Cu-DOTA on the surface of polyaspartic acid coated IONPs (136) (Fig. 6). Similarly, QD-based dual-modality probes for both PET and optical imaging have been reported by conjugating 64Cu-DOTA on the surface of biocompatible QDs (137). For MRI and optical dual-imaging applications, a probe has been prepared by loading Cy5.5 dye into cross-linked IONPs (138). To make these dual-modality imaging probes useful for targeted imaging applications, RGD peptides were labeled on the surface of each nanoparticle. All the RGD-conjugated dual-modality probes showed specific binding in tumor regions and images acquired by different imaging techniques were consistent (136–138). Various combinations for different imaging modalities can be designed such as for PET/MRI, PET/optical, PET/CT, PET/ultra sound, or MRI/optical. The design strategies, characteristics, and applications of the various dual-modality molecular imaging probes available today can be found elsewhere (135).
Figure 6.
A) Schematic diagram of an integrin αvβ3 targeting 64Cu-DOTA-IONP-RGD PET/MRI dual-modality probe. B) A 2D projection PET image of a mouse bearing U87MG tumor at 4 h post-injection of the probe. T2-weighted MR images of mice (the arrow indicates the tumor) C) before and D) 4 h after intravenous injection of the probe. Modified with permission from ref. 111. Copyright 2008, by the Society of Nuclear Medicine, Inc.
Other nanoplatforms
Recent developments in polymer chemistry have provided a significant number of biocompatible polymer structures including dendrimers, multivalent, branched, graft, and block-co-polymers, and have enabled the development of a new generation of imaging probes (20). An optical imaging probe associated with polymeric nanoparticle and peptide has been investigated for atherosclerotic lesion imaging. Park et al. reported an atherosclerosis imaging probe by conjugating phage-selected atherosclerotic plaque-homing peptide (the AP peptide, Cys-Arg-Lys-Arg-Leu-Asp-Arg-Asn-Cys) to hydrophobically modified glycol chitosan (HGC) nanoparticles (139). The AP peptide selectively bound to atherosclerotic plaque in vivo by adhering to the IL-4 receptor on endothelial cells, macrophages, and smooth muscle cells (140). Cy5.5 dye-labeled HGC nanoparticle has previously been developed as a tumor imaging probe, which displayed higher tumor targeting efficiency based on the EPR effect (141). In vitro binding characteristics of nanoparticles have shown that Cy5.5-HGC-AP binds more avidly to cytokine (TNF-α)-activated bovine aortic endothelial cells (BAECs) than Cy5.5-HGC and to unactivated BAECs. In vivo optical imaging demonstrated that Cy5.5-HGC-AP binds better to atherosclerotic lesions in a low-density lipoprotein receptor-deficient (Ldlr−/−) atherosclerotic mouse than to such lesions in a normal mouse. In one study, a polyacrylamide-based nanoformulation containing iron oxide nanoparticles were labeled with a vascular homing peptide, F3, and were investigated in rat 9L gliomas following intravenous injection (142). F3 is a 31 amino acid sequence of the N-terminal fragment of human high-mobility group protein 2, which was discovered using phage-displayed cDNA libraries (143). F3 peptides successfully delivered polymeric nanoparticles to the surface of MDA-MB-435 cells in vitro and conferred a greater amount of nanoparticle accumulation and longer duration within the tumor in rats bearing 9L gliomas. Recently, the surface of single-walled carbon nanotubes (SWNTs) were coated with poly(ethylene glycol) linked RGD peptides; these efficiently targeted integrin positive tumors in mice (144). SWNT-RGDs were stable in serum and their accumulation in U87MG tumors were evaluated by 64Cu-labeled SWNT-RGD. In vivo efficacy of SWNT-RGDs was further analyzed by Raman imaging and photoacoustic imaging techniques (145, 146).
Nanotechnologies have become increasingly important for targeted molecular imaging, and all evidences suggest that their importance will continue to grow. A number of targeting peptides can be engineered as nanoplatforms for targeted delivery of imaging agents by enhanced targeting efficacy, improved stability, and reduced non-specific binding. There are numerous unexplored possibilities to expand the use of nanoplatform-based imaging probes. For instance, the nanoplatforms can provide an all-in-one theranostic approach that combines different targeting peptides, imaging probes, and therapeutic drugs. It may allow simultaneous disease diagnosis, therapy, and monitoring therapeutic responses. Despite the unique characteristics of polymeric or inorganic-based nanomaterials, they have generally suffered from low targeting efficiency in vivo. Various selected peptides can simply provide researchers with potential tools to overcome many of the current limitations related to targeted delivery. However, efforts are needed to unveil the chronic toxicity and metabolism mechanisms of nanoplatform-based imaging probes in the body.
CONCLUSIONS
Molecular imaging-guided diagnostics is becoming increasingly directed and specific, taking advantage of various imaging modalities and probes. These powerful techniques will offer valuable opportunities to study and image the dynamics of disease-related biological processes at the molecular level. Driven by the success of octreotide, peptide-based probes have become established methods in nuclear medicine. Progress in molecular biology has elucidated a number of receptors and other cell surface molecules that can be utilized as molecular targets for peptide probes. More recently, interdisciplinary molecular imaging and material sciences research has generated various novel constructs, and the use of selected peptides allowed the efficient and targeted molecular imaging. In this review article, we have presented and introduced numerous approaches by the term ‘peptide-based probes’ with respect to their probe design strategies and applications. Although many of these sophisticated probes reportedly show promising results, especially in small animal models, very few have a clinical impact so far. However, the improved practical potency of attractive peptide-based probes highlights their potential as novel tools for targeted molecular imaging.
ABBREVIATIONS
- 18F-SFB
N-succinimidyl-4-18F-fluorobenzoate
- AMC
7-amino-4-methylcoumarin
- AP
atherosclerotic plaque
- BAECs
bovine aortic endothelial cells
- BBN
bombesin
- BHQ-3
black hole quencher-3
- CCK
cholecystokinin
- CT
computed tomography
- CTX
chlorotoxin
- CXCR4
chemokine receptor 4
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DTPA
diethylenetriaminepentaacetic acid
- eIND
emergency investigational new drug application
- EPR
enhanced permeation retention
- FITC
fluorescein isothiocyanate
- FRET
fluorescence resonance energy transfer
- GLP-1
glucagon-like peptide-1
- GPCrs
G-protein coupled membrane receptors
- GRP
gastrin-releasing peptide
- HGC
hydrophobically modified glycol chitosan
- HYNIC
hydrazidonicotinamide
- ICG
indocyanine green
- IONPs
iron oxide nanoparticles
- ITCC
indotricarbocyanie
- LHRH
Luteinizing hormone-releasing hormone
- MAG3
mercaptoacetylglycilgylcilgylcine
- MC-1
melanocortin-1
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- NIR
near-infrared
- NPY
neuropeptide-Y
- NT
neurotensin
- OA
osteoarthritis
- PET
positron emission tomography
- qABPs
quenched activity-based probes
- QDs
quantum dots
- r
receptor
- ReCCMSH
[Cys3,4,10-D-Phe7-α]-MSH(3-13)
- RES
reticuloendotherial system
- RGD
Arg-Gly-Asp
- SCLC
small cell lung cancer
- SPARC
secreted protein acidic and rich in cystein
- SPECT
single photon emission computed tomography
- SPSS
solid-phase peptide synthesis
- SST
somatostatin
- SWNTs
single-walled carbon nanotubes
- TAMRA
5-carboxytetramethylrhodamine
- VCAM-1
vascular cell adhesion molecule-1
- VIP
vasoactive intestinal peptide
- α-MSH
alpha-melanocyte stimulating hormone
References
- 1.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244:39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 2.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 3.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14:191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 4.Aloj L, Morelli G. Design, synthesis and preclinical evaluation of radiolabeled peptides for diagnosis and therapy. Curr Pharm Des. 2004;10:3009–3031. doi: 10.2174/1381612043383511. [DOI] [PubMed] [Google Scholar]
- 5.Reubi JC, Maecke HR. Peptide-based probes for cancer imaging. J Nucl Med. 2008;49:1735–1738. doi: 10.2967/jnumed.108.053041. [DOI] [PubMed] [Google Scholar]
- 6.Okarvi SM. Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Med Res Rev. 2004;24:357–397. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 7.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 9.Reubi JC. Targeting CCK receptors in human cancers. Curr Top Med Chem. 2007;7:1239–1242. doi: 10.2174/156802607780960546. [DOI] [PubMed] [Google Scholar]
- 10.Korner M, Stockli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48:736–743. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 11.Miao Y, Quinn TP. Alpha-melanocyte stimulating hormone peptide-targeted melanoma imaging. Front Biosci. 2007;12:4514–4524. doi: 10.2741/2406. [DOI] [PubMed] [Google Scholar]
- 12.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 13.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 14.Edwards DR, Murphy G. Cancer. Proteases--invasion and more. Nature. 1998;394:527–528. doi: 10.1038/28961. [DOI] [PubMed] [Google Scholar]
- 15.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 16.Aina OH, Liu R, Sutcliffe JL, Marik J, Pan CX, Lam KS. From combinatorial chemistry to cancer-targeting peptides. Mol Pharm. 2007;4:631–651. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 17.Petrenko V. Evolution of phage display: from bioactive peptides to bioselective nanomaterials. Expert Opin Drug Deliv. 2008;5:825–836. doi: 10.1517/17425247.5.8.825. [DOI] [PubMed] [Google Scholar]
- 18.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim J-H, Park K, Nam HY, Lee S, Kim K, Kwon IC. Polymers for bioimaging. Progress in Polymer Science. 2007;32:1031–1053. [Google Scholar]
- 21.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 22.Newton J, Deutscher SL. Phage peptide display. Handb Exp Pharmacol. 2008:145–163. doi: 10.1007/978-3-540-77496-9_7. [DOI] [PubMed] [Google Scholar]
- 23.Brown KC. New approaches for cell-specific targeting: identification of cell-selective peptides from combinatorial libraries. Curr Opin Chem Biol. 2000;4:16–21. doi: 10.1016/s1367-5931(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 24.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti E, Morelli G, Accardo A, Mansi R, Tesauro D, Aloj L. Criteria for the design and biological characterization of radiolabeled peptide-based pharmaceuticals. BioDrugs. 2004;18:279–295. doi: 10.2165/00063030-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 27.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 28.Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84–91. doi: 10.1053/snuc.2002.31022. [DOI] [PubMed] [Google Scholar]
- 29.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–247. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Schottelius M, Poethko T, Herz M, Reubi JC, Kessler H, Schwaiger M, Wester HJ. First 18F-labeled tracer suitable for routine clinical imaging of sst receptor-expressing tumors using positron emission tomography. Clin Cancer Res. 2004;10:3593–3606. doi: 10.1158/1078-0432.CCR-03-0359. [DOI] [PubMed] [Google Scholar]
- 31.Meisetschlager G, Poethko T, Stahl A, Wolf I, Scheidhauer K, Schottelius M, Herz M, Wester HJ, Schwaiger M. Gluc-Lys([18F]FP)-TOCA PET in patients with SSTR-positive tumors: biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. J Nucl Med. 2006;47:566–573. [PubMed] [Google Scholar]
- 32.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Macke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–16441. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginj M, Chen J, Walter MA, Eltschinger V, Reubi JC, Maecke HR. Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res. 2005;11:1136–1145. [PubMed] [Google Scholar]
- 34.Ginj M, Zhang H, Eisenwiener KP, Wild D, Schulz S, Rink H, Cescato R, Reubi JC, Maecke HR. New pansomatostatin ligands and their chelated versions: affinity profile, agonist activity, internalization, tumor targeting. Clin Cancer Res. 2008;14:2019–2027. doi: 10.1158/1078-0432.CCR-07-1687. [DOI] [PubMed] [Google Scholar]
- 35.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 36.Horton MA. The αvβ3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 37.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 38.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 39.Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, Watzlowik P, Wester HJ, Haubner R, Schwaiger M. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 40.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui AM, Palmieri C, Osman S, Glaser M, Turton D, Al-Nahhas A, Aboagye EO. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 41.Indrevoll B, Kindberg GM, Solbakken M, Bjurgert E, Johansen JH, Karlsen H, Mendizabal M, Cuthbertson A. NC-100717: a versatile RGD peptide scaffold for angiogenesis imaging. Bioorg Med Chem Lett. 2006;16:6190–6193. doi: 10.1016/j.bmcl.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Niu G, Shi J, Liu S, Wang F, Chen X. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin alphavbeta3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 44.Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug Chem. 2009;20:750–759. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51:7980–7990. doi: 10.1021/jm801134k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733–740. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Van de Wiele C, Dumont F, Dierckx RA, Peers SH, Thornback JR, Slegers G, Thierens H. Biodistribution and dosimetry of 99mTc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor-expressing malignancies. J Nucl Med. 2001;42:1722–1727. [PubMed] [Google Scholar]
- 48.Van de Wiele C, Phonteyne P, Pauwels P, Goethals I, Van den Broecke R, Cocquyt V, Dierckx RA. Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry. J Nucl Med. 2008;49:260–264. doi: 10.2967/jnumed.107.047167. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, Volkert WA. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med. 2003;44:823–831. [PubMed] [Google Scholar]
- 50.Zhang X, Cai W, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen X. 18F-labeled bombesin analogs for targeting GRP receptor-expressing prostate cancer. J Nucl Med. 2006;47:492–501. [PubMed] [Google Scholar]
- 51.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33:371–380. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. 68Ga-labeled bombesin studies in patients with gastrointestinal stromal tumors: comparison with 18F-FDG. J Nucl Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- 53.Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, Thomas R, Eaton SM, Bogdan NJ, Arunachalam T, Reubi JC, Raju N, Metcalfe EC, Lattuada L, Linder KE, Swenson RE, Tweedle MF, Nunn AD. 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J Nucl Med. 2006;47:1144–1152. [PubMed] [Google Scholar]
- 54.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49:318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 55.Reubi JC, Waser B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int J Cancer. 1996;67:644–647. doi: 10.1002/(SICI)1097-0215(19960904)67:5<644::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 56.Koopmans KP, Neels ON, Kema IP, Elsinga PH, Links TP, de Vries EG, Jager PL. Molecular imaging in neuroendocrine tumors: Molecular uptake mechanisms and clinical results. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Good S, Walter MA, Waser B, Wang X, Muller-Brand J, Behe MP, Reubi JC, Maecke HR. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur J Nucl Med Mol Imaging. 2008;35:1868–1877. doi: 10.1007/s00259-008-0803-4. [DOI] [PubMed] [Google Scholar]
- 58.Tatro JB, Wen Z, Entwistle ML, Atkins MB, Smith TJ, Reichlin S, Murphy JR. Interaction of an a-melanocyte-stimulating hormone-diphtheria toxin fusion protein with melanotropin receptors in human melanoma metastases. Cancer Res. 1992;52:2545–2548. [PubMed] [Google Scholar]
- 59.Chen J, Giblin MF, Wang N, Jurisson SS, Quinn TP. In vivo evaluation of 99mTc/188Re-labeled linear alpha-melanocyte stimulating hormone analogs for specific melanoma targeting. Nucl Med Biol. 1999;26:687–693. doi: 10.1016/s0969-8051(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- 61.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of α-MSH. J Med Chem. 2005;48:2985–2992. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 62.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 63.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 64.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 65.Gotthardt M, Fischer M, Naeher I, Holz JB, Jungclas H, Fritsch HW, Behe M, Goke B, Joseph K, Behr TM. Use of the incretin hormone glucagon-like peptide-1 (GLP-1) for the detection of insulinomas: initial experimental results. Eur J Nucl Med Mol Imaging. 2002;29:597–606. doi: 10.1007/s00259-002-0761-1. [DOI] [PubMed] [Google Scholar]
- 66.Wild D, Behe M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Macke HR. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–2033. [PubMed] [Google Scholar]
- 67.Wild D, Macke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–768. doi: 10.1056/NEJMc0802045. [DOI] [PubMed] [Google Scholar]
- 68.Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–3112. [PubMed] [Google Scholar]
- 69.Thakur ML, Aruva MR, Gariepy J, Acton P, Rattan S, Prasad S, Wickstrom E, Alavi A. PET imaging of oncogene overexpression using 64Cu-vasoactive intestinal peptide (VIP) analog: comparison with 99mTc-VIP analog. J Nucl Med. 2004;45:1381–1389. [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C, Kim C, McCue PA, Wickstrom E, Thakur ML. PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med. 2008;49:112–121. doi: 10.2967/jnumed.107.043703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vincent JP. Neurotensin receptors: binding properties, transduction pathways, and structure. Cell Mol Neurobiol. 1995;15:501–512. doi: 10.1007/BF02071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maes V, Garcia-Garayoa E, Blauenstein P, Tourwe D. Novel 99mTc-labeled neurotensin analogues with optimized biodistribution properties. J Med Chem. 2006;49:1833–1836. doi: 10.1021/jm051172f. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Garayoa E, Blauenstein P, Bruehlmeier M, Blanc A, Iterbeke K, Conrath P, Tourwe D, Schubiger PA. Preclinical evaluation of a new, stabilized neurotensin(8–13) pseudopeptide radiolabeled with 99mTc. J Nucl Med. 2002;43:374–383. [PubMed] [Google Scholar]
- 74.Buchegger F, Bonvin F, Kosinski M, Schaffland AO, Prior J, Reubi JC, Blauenstein P, Tourwe D, Garcia Garayoa E, Bischof Delaloye A. Radiolabeled neurotensin analog, 99mTc-NT-XI, evaluated in ductal pancreatic adenocarcinoma patients. J Nucl Med. 2003;44:1649–1654. [PubMed] [Google Scholar]
- 75.Korner M, Reubi JC. NPY receptors in human cancer: a review of current knowledge. Peptides. 2007;28:419–425. doi: 10.1016/j.peptides.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 76.Korner M, Waser B, Reubi JC. High expression of neuropeptide Y1 receptors in ewing sarcoma tumors. Clin Cancer Res. 2008;14:5043–5049. doi: 10.1158/1078-0432.CCR-07-4551. [DOI] [PubMed] [Google Scholar]
- 77.Zwanziger D, Khan IU, Neundorf I, Sieger S, Lehmann L, Friebe M, Dinkelborg L, Beck-Sickinger AG. Novel chemically modified analogues of neuropeptide Y for tumor targeting. Bioconjug Chem. 2008;19:1430–1438. doi: 10.1021/bc7004297. [DOI] [PubMed] [Google Scholar]
- 78.Wong D, Korz W. Translating an Antagonist of Chemokine Receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 79.Hanaoka H, Mukai T, Tamamura H, Mori T, Ishino S, Ogawa K, Iida Y, Doi R, Fujii N, Saji H. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006;33:489–494. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Cescato R, Erchegyi J, Waser B, Piccand V, Maecke HR, Rivier JE, Reubi JC. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem. 2008;51:4030–4037. doi: 10.1021/jm701618q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J Med Chem . 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 82.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 83.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 84.Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 85.Licha K, Olbrich C. Optical imaging in drug discovery and diagnostic applications. Adv Drug Deliv Rev. 2005;57:1087–1108. doi: 10.1016/j.addr.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 86.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem Commun (Camb) 2008:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 87.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal Bioanal Chem. 2008;391:2469–2495. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 88.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 89.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grotzinger C. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol. 2001;19:327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 90.Kostenich G, Livnah N, Bonasera TA, Yechezkel T, Salitra Y, Litman P, Kimel S, Orenstein A. Targeting small-cell lung cancer with novel fluorescent analogs of somatostatin. Lung Cancer. 2005;50:319–328. doi: 10.1016/j.lungcan.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Kostenich G, Oron-Herman M, Kimel S, Livnah N, Tsarfaty I, Orenstein A. Diagnostic targeting of colon cancer using a novel fluorescent somatostatin conjugate in a mouse xenograft model. Int J Cancer. 2008;122:2044–2049. doi: 10.1002/ijc.23353. [DOI] [PubMed] [Google Scholar]
- 92.Ma L, Yu P, Veerendra B, Rold TL, Retzloff L, Prasanphanich A, Sieckman G, Hoffman TJ, Volkert WA, Smith CJ. In vitro and in vivo evaluation of Alexa Fluor 680-bombesin[7–14]NH2 peptide conjugate, a high-affinity fluorescent probe with high selectivity for the gastrin-releasing peptide receptor. Mol Imaging. 2007;6:171–180. [PubMed] [Google Scholar]
- 93.Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Cancer Res. 2004;64:8009–8014. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Z, Wu Y, Xiong Z, Gambhir SS, Chen X. Near-infrared fluorescent RGD peptides for optical imaging of integrin αvβ3 expression in living mice. Bioconjug Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y, Cai W, Chen X. Near-infrared fluorescence imaging of tumor integrin αvβ3 expression with Cy7-labeled RGD multimers. Mol Imaging Biol. 2006;8:226–236. doi: 10.1007/s11307-006-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly KA, Waterman P, Weissleder R. In vivo imaging of molecularly targeted phage. Neoplasia. 2006;8:1011–1018. doi: 10.1593/neo.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 98.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 99.Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu P, Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 101.Johansson MK, Cook RM. Intramolecular dimers: a new design strategy for fluorescence-quenched probes. Chemistry. 2003;9:3466–3471. doi: 10.1002/chem.200304941. [DOI] [PubMed] [Google Scholar]
- 102.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 103.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 104.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 105.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S, Park K, Lee SY, Ryu JH, Park JW, Ahn HJ, Kwon IC, Youn IC, Kim K, Choi K. Dark quenched matrix metalloproteinase fluorogenic probe for imaging osteoarthritis development in vivo. Bioconjug Chem. 2008;19:1743–1747. doi: 10.1021/bc800264z. [DOI] [PubMed] [Google Scholar]
- 107.Maxwell D, Chang Q, Zhang X, Barnett EM, Piwnica-Worms D. An improved cell-penetrating, caspase-activatable, near-infrared fluorescent Peptide for apoptosis imaging. Bioconjug Chem. 2009;20:702–709. doi: 10.1021/bc800516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc Natl Acad Sci U S A. 2009;106:9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 110.Blum G, Mullins SR, Keren K, Fonovic M, Jedeszko C, Rice MJ, Sloane BF, Bogyo M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol. 2005;1:203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 111.Tung CH. Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers. 2004;76:391–403. doi: 10.1002/bip.20139. [DOI] [PubMed] [Google Scholar]
- 112.Xie J, Huang J, Li X, Sun S, Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem. 2009;16:1278–1294. doi: 10.2174/092986709787846604. [DOI] [PubMed] [Google Scholar]
- 113.Li ZB, Cai W, Chen X. Semiconductor quantum dots for in vivo imaging. J Nanosci Nanotechnol. 2007;7:2567–2581. doi: 10.1166/jnn.2007.628. [DOI] [PubMed] [Google Scholar]
- 114.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 115.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 116.Bahr JL, Tour JM. Covalent chemistry of single-wall carbon nanotubes. Journal of Materials Chemistry. 2002;12:1952–1958. [Google Scholar]
- 117.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 118.Budde MD, Frank JA. Magnetic tagging of therapeutic cells for MRI. J Nucl Med. 2009;50:171–174. doi: 10.2967/jnumed.108.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie J, Chen K, Lee HY, Xu C, Hsu AR, Peng S, Chen X, Sun S. Ultrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin alpha(v)beta3-rich tumor cells. J Am Chem Soc. 2008;130:7542–7543. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 122.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 124.Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99:163–176. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 125.Chatzistamou L, Schally AV, Nagy A, Armatis P, Szepeshazi K, Halmos G. Effective treatment of metastatic MDA-MB-435 human estrogen-independent breast carcinomas with a targeted cytotoxic analogue of luteinizing hormone-releasing hormone AN-207. Clin Cancer Res. 2000;6:4158–4165. [PubMed] [Google Scholar]
- 126.Peng XH, Qian X, Mao H, Wang AY, Chen ZG, Nie S, Shin DM. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3:311–321. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 129.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 131.Cai W, Chen X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat Protoc. 2008;3:89–96. doi: 10.1038/nprot.2007.478. [DOI] [PubMed] [Google Scholar]
- 132.Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Letters. 2004;4:1827–1832. [Google Scholar]
- 133.Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J Am Chem Soc. 2007;129:14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 134.Cai WB, Hsu AR, Li ZB, Chen XY. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Research Letters. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee S, Chen X. Dual-modality probes for in vivo molecular imaging. Mol Imaging. 2009;8:87–100. [PubMed] [Google Scholar]
- 136.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 137.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 138.Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, Koning GA, Griffioen AW, Nicolay K. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19:2008–2010. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- 139.Park K, Hong HY, Moon HJ, Lee BH, Kim IS, Kwon IC, Rhee K. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J Control Release. 2008;128:217–223. doi: 10.1016/j.jconrel.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 140.Hong HY, Lee HY, Kwak W, Yoo J, Na MH, So IS, Kwon TH, Park HS, Huh S, Oh GT, Kwon IC, Kim IS, Lee BH. Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atherosclerosis. J Cell Mol Med. 2008;12:2003–2014. doi: 10.1111/j.1582-4934.2008.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J Control Release. 2007;122:305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 142.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 143.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci U S A. 2002;99:7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 145.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zavaleta C, de la Zerda A, Liu Z, Keren S, Cheng Z, Schipper M, Chen X, Dai H, Gambhir SS. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Letters. 2008;8:2800–2805. doi: 10.1021/nl801362a. [DOI] [PMC free article] [PubMed] [Google Scholar]